Abstract
The silver-mediated C–H trifluoromethylation of aromatic substrates using TMSCF3 is described. The development, optimization, and scope of these transformations are reported. AgCF3 intermediates are proposed.
Trifluoromethylated aromatic compounds are widely prevalent in pharmaceuticals, agrochemicals, and organic materials.1 As a result, the development of transition metal-mediated/catalyzed methods for introducing CF3 groups into organic molecules has been the subject of intense research. Over the past 5 years a variety of Pd2,3 and Cu4,5-based protocols have been developed for the trifluoromethylation of aryl halides, aryl boronic acids, and aromatic carbon–hydrogen bonds. In addition, several free-radical approaches are available for arene trifluoromethylation.6,7 Despite this extensive progress, current trifluoromethylation methods have significant limitations. Some systems utilize expensive trifluoromethylating reagents (e.g., S-(trifluoromethyl)thiophenium salts,3a,4f,5d Togni’s reagent,5b,e or TESCF3).3b,5a Others involve temperatures greater than 100 °C2b,3a,b,4d and/or exhibit modest substrate scope/generality.3c,5a,b,6 Free radical-based methods often require inconvenient electrochemical or photochemical activation procedures6b,d,e or utilize potentially explosive reagents like peroxides at elevated temperatures.6c,g Additionally, C–H bond trifluoromethylation methods (which are particularly attractive because they do not require pre-functionalized starting materials) remain especially limited in substrate scope.3a,d,6
We were interested in the possibility of addressing some of these limitations by identifying metals other than Cu or Pd that could promote the formation of benzotrifluorides. We were attracted to Ag based on the fact that it is readily available, is directly below Cu on the periodic table (suggesting the potential for similar reactivity), and has recently proven a useful promotor for other organometallic reactions.8 There are also a number of reports describing the synthesis of AgCF3 complexes.9,10 However, the reactivity of these species has not been explored extensively,9,10 thereby suggesting opportunities for new reaction discovery. We report herein that the combination of AgOTf, KF, and TMSCF3 can be used for the C–H trifluoromethylation of aromatic substrates under mild conditions. The development, scope, and mechanism of this transformation are discussed herein.
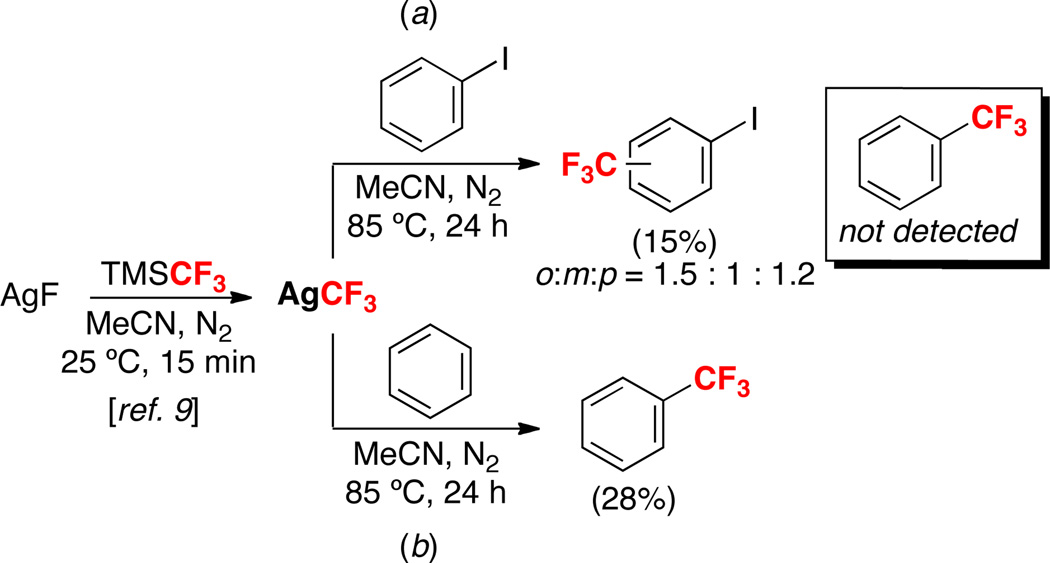
CuCF3 complexes are well-known to react with aryl iodides to afford benzotrifluoride products.4 Thus, we first sought to examine the reactivity of AgCF3 with PhI (Scheme 1a). AgCF3 was generated in situ from the reaction of AgF with TMSCF3 in MeCN for 15 min at 25 °C using the procedure of Tyrra and Naumann.9 PhI (20 equiv) was then added, and the reaction was heated at 85 °C for 24 h. 19F NMR spectroscopic analysis of the crude reaction mixture did not show the presence of PhCF3. Instead, three isomeric C–H trifluoromethylation products were observed in 15% total yield based on TMSCF3 (o : m : p ratio = 1.5 : 1 : 1.2). This result clearly demonstrates the orthogonal reactivity of AgCF3 and CuCF3 reagents with aryl–H versus aryl–I bonds. Conducting this same procedure with benzene in place of PhI afforded the C–H trifluoromethylation product PhCF3 in 28% yield (Scheme 1b).
Scheme 1.
Reaction of AgCF3 with PhI and Benzene
This Ag-mediated C–H trifluoromethylation reaction was optimized using benzene (20 equiv) as the substrate and DCE as the solvent (see Supporting Information for evaluation of other solvents). Since this is a net 2e− oxidation reaction (where AgI is presumably acting as the terminal oxidant), our optimization studies began with 2 equiv of various AgI salts. As shown in Table 1, the use of 2 equiv of AgF, AgNO3, or AgOTf in the presence of 2 equiv of KF afforded trifluorotoluene in modest yield, with AgOTf providing the best result (entries 3–5). In contrast, AgOAc and Ag2O generated <10% product under analogous conditions (entries 1 and 2). Moving from 2 equiv to 4 equiv of AgOTf/KF improved the yield from 68 to 87% (entries 5 and 6). Importantly, KF is required for the AgOTf reaction (Table 1, entry 7), presumably to activate the TMSCF3. For comparison, we also examined the reactivity of CuI salts like [CuOTf]2·C6H6 and CuI under these conditions. As shown in entries 9 and 10, they provided none of the C–H trifluoromethylation product, again highlighting the complementarity of this Ag-based method versus more traditional Cu-mediated trifluoromethylation protocols.
Table 1.
 | |||
|---|---|---|---|
| entry | metal salt | metal salt/ KF equiv |
yield (%) |
| 1 | AgOAc | 2/2 | 6 |
| 2 | Ag2O | 2/2 | 6 |
| 3 | AgNO3 | 2/2 | 40 |
| 4 | AgF | 2/2 | 45 |
| 5 | AgOTf | 2/2 | 68 |
| 6 | AgOTf | 4/4 | 87 |
| 7 [c] | AgOTf | 4/0 | 0 |
| 8 [d] | AgOTf | 4/4 | 53 |
| 9 | [CuOTf]2·C6H6 | 2/2 | 0 |
| 10 | CuI | 4/4 | 0 |
General conditions: C6H6 (20 equiv), TMSCF3 (1 equiv) in DCE at 85 °C for 24 h.
Yields determined by 19F NMR analysis.
No KF.
Conditions: C6H6 (1 equiv), TMSCF3 (5 equiv), AgOTf (4 equiv), KF (4 equiv) in DCE at 85 °C for 24 h.
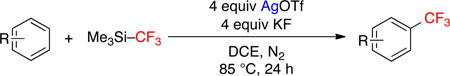
The final optimized conditions (20 equiv of benzene, 4 equiv of AgOTf and KF, 1 equiv of TMSCF3 at 85 °C for 24 h) were readily scalable, affording trifluorotoluene in 87%, 84%, and 87% yield on 0.08, 0.5, and 1 mmol scales, respectively. Notably, the use of benzene as the limiting reagent (1 equiv) along with 5 equiv of TMSCF3 also led to an acceptable 53% yield (Table 1, entry 8).
This Ag-mediated C–H trifluoromethylation reaction was applicable to a variety of different arene substrates. As shown in Table 2, arenes bearing electron-donating alkyl or alkoxy substituents reacted in good to excellent yield (entries 1–10). In general, these transformations proceeded with a modest preference for trifluoromethylation at C–H sites ortho and para to the electron-donating alkyl and alkoxy groups. Heteroaromatics like N-methyl pyrrole, thiophene, and caffeine were also good substrates for C–H trifluoromethylation and reacted with moderate to excellent site selectivity (entries 12, 13, and 15).11 Under our optimal conditions PhI afforded a mixture of the o, m, and p-trifluoromethylated isomers in 46% total yield (entry 11). The trifluoromethylation of naphthalene proceeded in good yield with modest selectivity for the α-position (entry 14).
Table 2.
 | ||||
|---|---|---|---|---|
| entry | substrate | major product | yield (%) | isomer ratio |
| 1[c] |  |
87 | --- | |
| 2 | 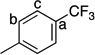 |
81 | a : b : c 2.7 : 1.4 : 1 |
|
| 3 | 76 | --- | ||
| 4 | 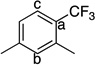 |
76 | a : b : c 5.2 : 3.5 : 1 |
|
| 5 |  |
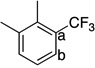 |
65 | a : b 1.4 : 1 |
| 6 | 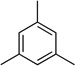 |
 |
78 | --- |
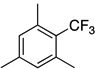
| 7 |  |
 |
87 | a : b : c 2.7 : 1.2 : 1 |
| 8[c] | 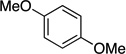 |
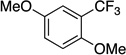 |
88 | --- |
| 9 | 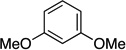 |
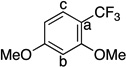 |
85 | a : b : c 13 : 7.3 : 1 |
| 10 | 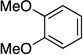 |
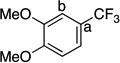 |
70 | a : b 4 : 1 |
| 11[c] | 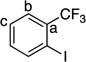 |
46 | a : b : c 1.7 : 1.2 : 1 |
|
| 12[c] | 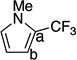 |
44 | a : b >20 : 1 |
|
| 13 | 72 | a : b 8 : 1 |
||
| 14[d] | 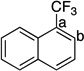 |
70 | a : b 4.8 : 1 |
|
| 15[d] | 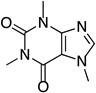 |
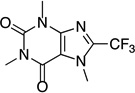 |
42 | --- |
General conditions: substrate (10 equiv), TMSCF3 (1 equiv) in DCE at 85 °C for 24 h.
Yield and selectivity determined by 19F NMR analysis of the crude reaction mixtures.
20 equiv substrate used.
5 equiv substrate used.
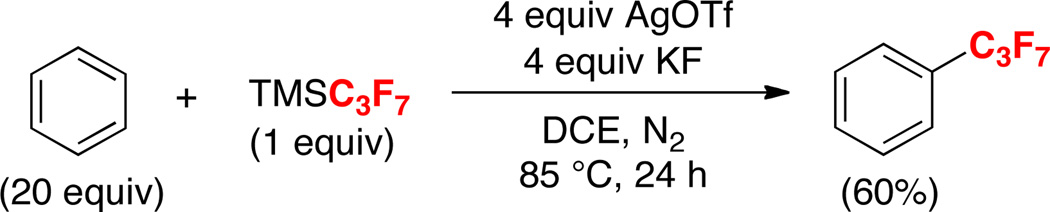
The optimal reaction conditions were also effective for transfer of other perfluoroalkyl groups. For example, the AgOTf/KF-mediated reaction of benzene with TMSC3F7 afforded (heptafluoropropyl)benzene in 60% yield (Scheme 2).
Scheme 2.
Ag-Mediated Perfluoroalkylation of Benzene
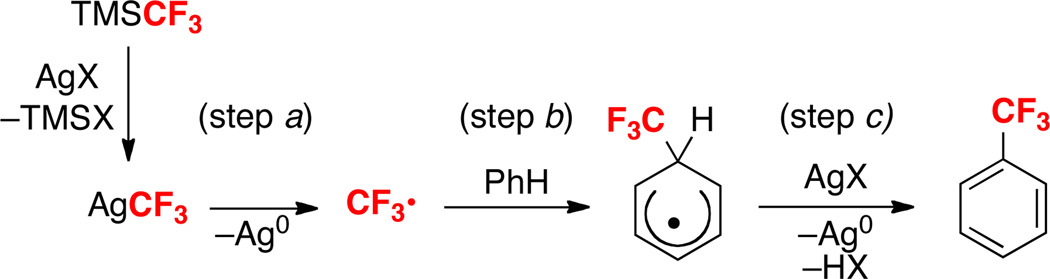
We initially hypothesized that this transformation proceeded via a pathway involving Ag-promoted generation of a trifluoromethyl radical (CF3•) (Scheme 3, step a), which then participates in a radical aromatic substitution reaction. Addition of CF3• to the aromatic ring to generate intermediate A (step b) followed by SET from A to a second equivalent of AgI (step c) would release the product along with HOTf and Ag0. Importantly, free radical arene trifluoromethylation6 and perfluoroalkylation7 has significant precedent in the literature. Most commonly, CF3• is generated from CF3Br or CF3I either photochemically or electrochemically.6b,d,e A more recent report by Yamakawa and coworkers demonstrated radical trifluoromethylation of aromatic compounds using CF3I, FeII and H2O2.6g However, to our knowledge, the use of TMSCF3 as a precursor to radical arene trifluoromethylation has not been reported.
Scheme 3.
Possible Free Radical Pathway for Ag-Mediated Trifluoromethylation
To test for the possibility of CF3• intermediates, we examined the AgOTf/KF-promoted reaction of benzene with TMSCF3 in the presence of a variety of radical initiators/inhibitors. In the presence of light (which is frequently used to promote radical reactions), the reaction proceeded in slightly lower yield (75% versus 87%). This may be due to the light sensitivity of Ag salts.12 The addition of azobisisobutyronitrile (AIBN), a radical initiator, led to a moderate decrease in the overall yield of the reaction. The use of 20 mol % of AIBN resulted in 77% yield of PhCF3, while a 57% yield was obtained in upon addition of 1 equiv of this additive.12 Nitrobenzene has been employed previously as an inhibitor of SET transfer steps (like step c in Scheme 3) during free radical trifluoromethylation.6a Interestingly, the addition of 1 equiv of NO2Ph had little effect on the Ag-mediated reaction of benzene with TMSCF3 (85% versus 87% yield in the absence of this additive). TEMPO has been utilized in the literature as a trap for CF3•. 3c The addition of of 1 equiv of TEMPO led to a dramatic reduction in yield (to 7%) under otherwise analogous conditions.
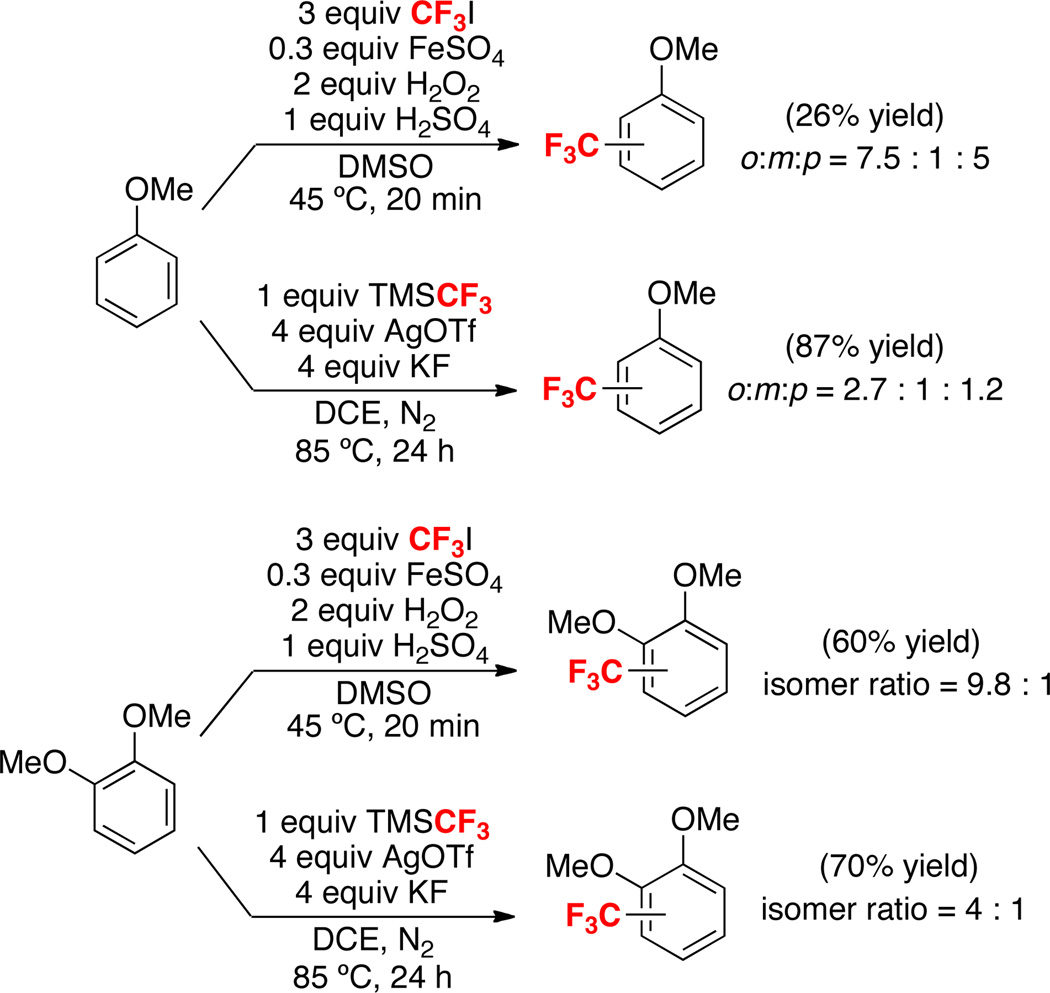
Because the results with the radical inhibitors/initiators were somewhat ambiguous, we next sought to compare the site selectivity of TMSCF3/AgOTf/KF-mediated trifluoromethylation to that of a known CF3• reaction. Under the reaction conditions described by Yamakawa and coworkers,6g anisole reacted with in situ-generated CF3• to form a 7.5 : 1 : 5 ratio of o:m:p trifluoromethylated products (Scheme 4). This reaction shows significantly higher o/p selectivity compared to our Ag-mediated transformation (where o:m:p = 2.7 : 1 : 1.2). Veratrole also reacted with different site selectivity for trifluoromethylation with CF3• versus TMSCF3/AgOTf/KF (Scheme 4).13 While further studies are needed to gain a complete mechanistic picture of the TMSCF3/AgOTf/KF-mediated reaction, these results suggest against a purely free-radical pathway. The involvement of caged and/or Ag-associated radicals is a likely possibility. Notably, Kamigata has proposed a mechanism involving “radical intermediates confined in the coordination sphere of Ru” for related transformations.6f
Scheme 4.
Comparison of Reactivity and Selectivity of Radical Trifluoromethylation
In conclusion, this report describes the silver-mediated trifluoromethylation of aromatic substrates with TMSCF3. These reactions are proposed to proceed via a AgCF3 intermediate, and preliminary studies suggest against free CF3• as an intermediate. Importantly, these Ag-mediated reactions proceed with complementary reactivity to analogous transformations of CuCF3 reagents. Ongoing studies are focused on probing the mechanism and developing related Ag-catalyzed trifluoromethylation reactions.
Supplementary Material
Acknowledgment
We thank the NIH NIGMS (GM073836) for financial support. We also thank Dr. Rebecca Loy (post-doc in MSS group), Brannon Gary (graduate student in MSS group), and Dr. Marion Emmert (post-doc in MSS group) for helpful discussions.
Footnotes
Supporting Information Available. Experimental procedures along with experimental and spectroscopic data for new compounds. This material is available free of charge via the Internet at http://pubs.acs.org.
References
- 1.(a) Schlosser M. Angew. Chem. Int. Ed. 2006;45:5432. doi: 10.1002/anie.200600449. [DOI] [PubMed] [Google Scholar]; (b) Müller K, Faeh C, Diederich F. Science. 2007;317:1881. doi: 10.1126/science.1131943. [DOI] [PubMed] [Google Scholar]; (c) Hangman WK. J. Med. Chem. 2008;51:4359. [Google Scholar]; (d) Kirk KL. Org. Process Res. Dev. 2008;12:305. [Google Scholar]; (e) Purser S, Moore PR, Swallow S, Gouverneur V. Chem. Soc. Rev. 2008;37:320. doi: 10.1039/b610213c. [DOI] [PubMed] [Google Scholar]; (f) Grushin VV, Tomashenko OA. Chem. Rev. 2011;111:4475. doi: 10.1021/cr1004293. [DOI] [PubMed] [Google Scholar]; (g) Roy S, Gregg BT, Gribble GW, Le V-D, Roy S. Tetrahedron. 2011;67:2161. [Google Scholar]
- 2.Recent examples of palladium-mediated arene trifluoromethylation reactions: Grushin VV, Marshall WJ. J. Am. Chem. Soc. 2006;128:4632. doi: 10.1021/ja0602389. Grushin VV, Marshall WJ. J. Am. Chem. Soc. 2006;128:12644. doi: 10.1021/ja064935c. Grushin VV. Acc. Chem. Res. 2010;43:160. doi: 10.1021/ar9001763. Ball ND, Kampf JW, Sanford MS. J. Am. Chem. Soc. 2010;132:2878. doi: 10.1021/ja100955x. Ye Y, Ball ND, Kampf JW, Sanford MS. J. Am. Chem. Soc. 2010;132:14682. doi: 10.1021/ja107780w. Ball ND, Gary JB, Ye Y, Sanford MS. J. Am. Chem. Soc. 2011;133:7577. doi: 10.1021/ja201726q.
- 3.Recent examples of Pd-catalyzed arene trifluoromethylation reactions: Wang X, Truesdale L, Yu JQ. J. Am. Chem. Soc. 2010;132:3648. doi: 10.1021/ja909522s. Cho EJ, Senecal TD, Kinzel T, Zhang Y, Watson DA, Buchwald SL. Science. 2010;328:1679. doi: 10.1126/science.1190524. Mu X, Chen S, Zhen X, Liu G. Chem. Eur. J. 2011;17:6039. doi: 10.1002/chem.201100283. Loy RN, Sanford MS. Org. Lett. 2011;13:2548. doi: 10.1021/ol200628n.
- 4.Recent examples of Cu-mediated trifluoromethylation reactions: Dubinina GG, Furutachi H, Vicic DA. J. Am. Chem. Soc. 2008;130:8600. doi: 10.1021/ja802946s. Dubinina GG, Ogikubo J, Vicic DA. Organometallics. 2008;27:6233. Chu L, Qing F-L. Org. Lett. 2010;12:5060. doi: 10.1021/ol1023135. McReynolds KA, Lewis RS, Ackerman LKG, Dubinina GG, Brennessel WW, Vicic DA. J. Fluorine Chem. 2010;131:1108. Senecal TD, Parsons AT, Buchwald SL. J. Org. Chem. 2011;76:1174. doi: 10.1021/jo1023377. Zhang C-P, Wang Z-L, Chen Q-Y, Zhang C-T, Gu Y-C, Xiao J-C. Angew. Chem. Int. Ed. 2011;50:1896. doi: 10.1002/anie.201006823. Morimoto H, Tsubogo T, Litvinas ND, Hartwig JF. Angew. Chem. Int. Ed. 2011;50:3793. doi: 10.1002/anie.201100633. Tomashenko OA, Escudero EC, Belmonte MM, Grushin VV. Angew. Chem. Int. Ed. 2011;50:7655. doi: 10.1002/anie.201101577.
- 5.Recent examples of Cu-catalyzed trifluoromethylation reactions: Oishi M, Konda H, Amii H. Chem. Commun. 2009:1909. doi: 10.1039/b823249k. Shimizu R, Egami H, Nagi T, Chae J, Hamashima Y, Sodeoka M. Tetrahedron Lett. 2010;51:5947. Knauber T, Arikan F, Röschenthaler G-V, Gooßen LJ. Chem. Eur. J. 2011;17:2689. doi: 10.1002/chem.201002749. Xu J, Luo D-F, Xiao B, Liu Z-J, Gong T-J, Fu Y, Liu L. Chem. Commun. 2011;47:4300. doi: 10.1039/c1cc10359h. Liu T, Shen Q. Org. Lett. 2011;13:2342. doi: 10.1021/ol2005903.
- 6.Examples of arene/heteroarene trifluoromethylation with CF3•: Wakselman C, Tordeux M. J. Chem. Soc. Chem. Commun. 1987:1701. Akiyama T, Kato K, Kajitani M, Sakaguchi Y, Nakamura J, Hayashi H, Sugimori A. Bull. Chem. Soc. Jpn. 1988;61:3531. Sawada H, Nakayama M. J. Fluorine Chem. 1990;46:423. Langlois BR, Laurent E, Roidot M. Tetrahedron Lett. 1991;32:7525. McClinton MA, McClington DA. Tetrahedron. 1992;48:6555. Kamigata N, Ohtsuka T, Fukushima T, Yoshida M, Shimizu T. J. Chem. Soc. Perkin Trans. 1. 1994:1339. Kino T, Nagase Y, Ohtsuka Y, Yamamoto K, Uraguchi D, Tokuhisa K, Yamakawa T. J. Fluorine Chem. 2010;131:98. Ji Y, Brueckl T, Baxter RD, Fujiwara Y, Seiple IB, Su S, Blackmond DG, Baran PS. Proc. Nat. Acad. Sci. 2011;108:14411. doi: 10.1073/pnas.1109059108.
- 7.Examples of arene/heteroarene perfluoroalkylation with RF•: Cowell A, Tamborski C. J. Fluorine Chem. 1981;17:345. Dolbier WR. Chem. Rev. 1996;96:1557. doi: 10.1021/cr941142c. Bravo A, Bjørsvik H-R, Fontana F, Liguori L, Mele A, Minisci F. J. Org. Chem. 1997;62:7128. doi: 10.1021/jo970302s. Huang X-T, Long Z-Y, Chen Q-Y. J. Fluorine Chem. 2001;111:107. Li Y, Li C, Yue W, Jiang W, Kopecek R, Qu J, Wang Z. Org. Lett. 2010;12:2374. doi: 10.1021/ol1007197.
- 8.(a) Teverovskiy G, Surry DS, Buchwald SL. Angew. Chem. Int. Ed. 2011;50:7312. doi: 10.1002/anie.201102543. [DOI] [PMC free article] [PubMed] [Google Scholar]; (b) Huang C, Liang T, Harada S, Lee E, Ritter T. J. Am. Chem. Soc. 2011;133:13308. doi: 10.1021/ja204861a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.For a review on silver perfluoroalkyl complexes: Tyrra WE, Naumann D. J. Fluorine Chem. 2004;125:823.
- 10.Wang Z, Lee R, Jia W, Yuan Y, Wang W, Feng X, Huang KW. Organometallics. 2011;30:3229. [Google Scholar]
- 11.Several other heteroaromatics, including furan (16% yield), 2-methylfuran (7% yield), pyridine (2% yield, 2 isomers), and 1-methylimidazole (3% yield, 3 isomers), afforded low yield of mono-trifluoromethylated products under our standard conditions.
- 12.It is possible that light/AIBN do not have an effect on this system because they are just not capable of promoting the Ag–CF3 bond homolysis (step a in Scheme 3).
- 13.The different selectivity with CF3• does not appear to be a temperature or solvent effect. For example, when the Fe-catalyzed reaction of CF3• with veratrole ([a] in Scheme 4) was conducted at 85 °C, it afforded 10.2 : 1 selectivity (67% yield). Similarly, when DCE was used as the solvent in place of DMSO, the product was obtained with 7.7 : 1 selectivity (4% yield).
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.