Abstract
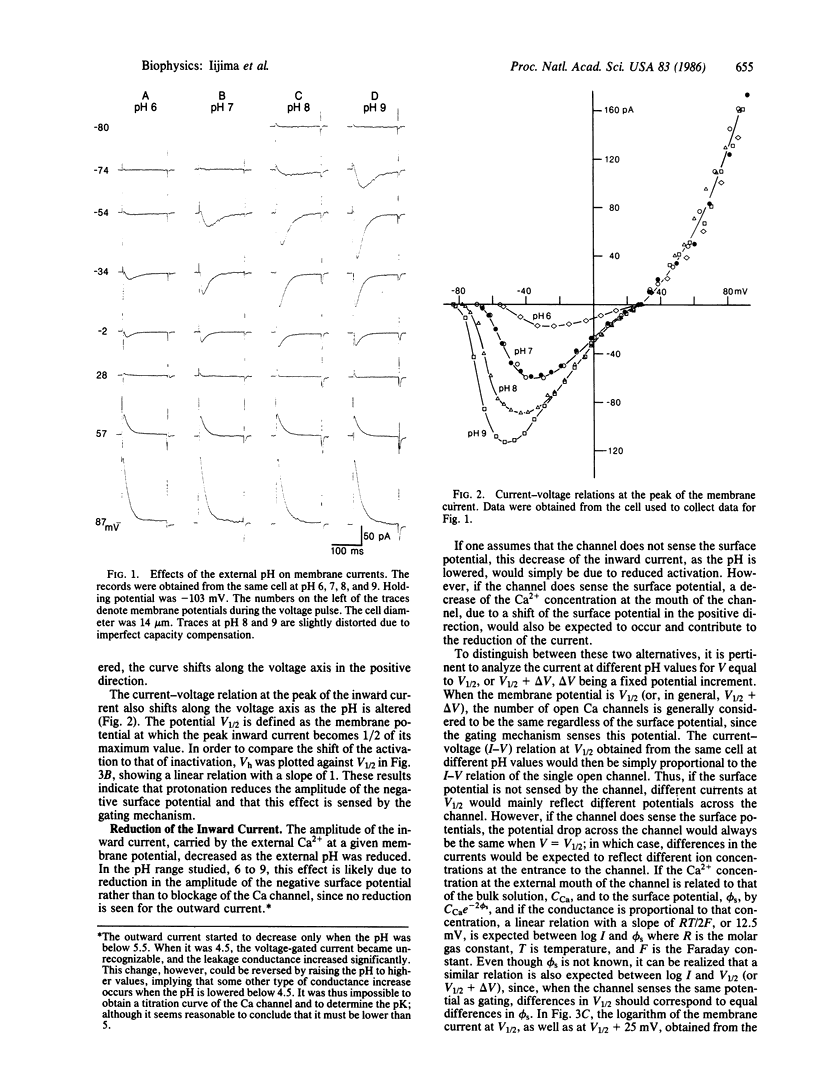
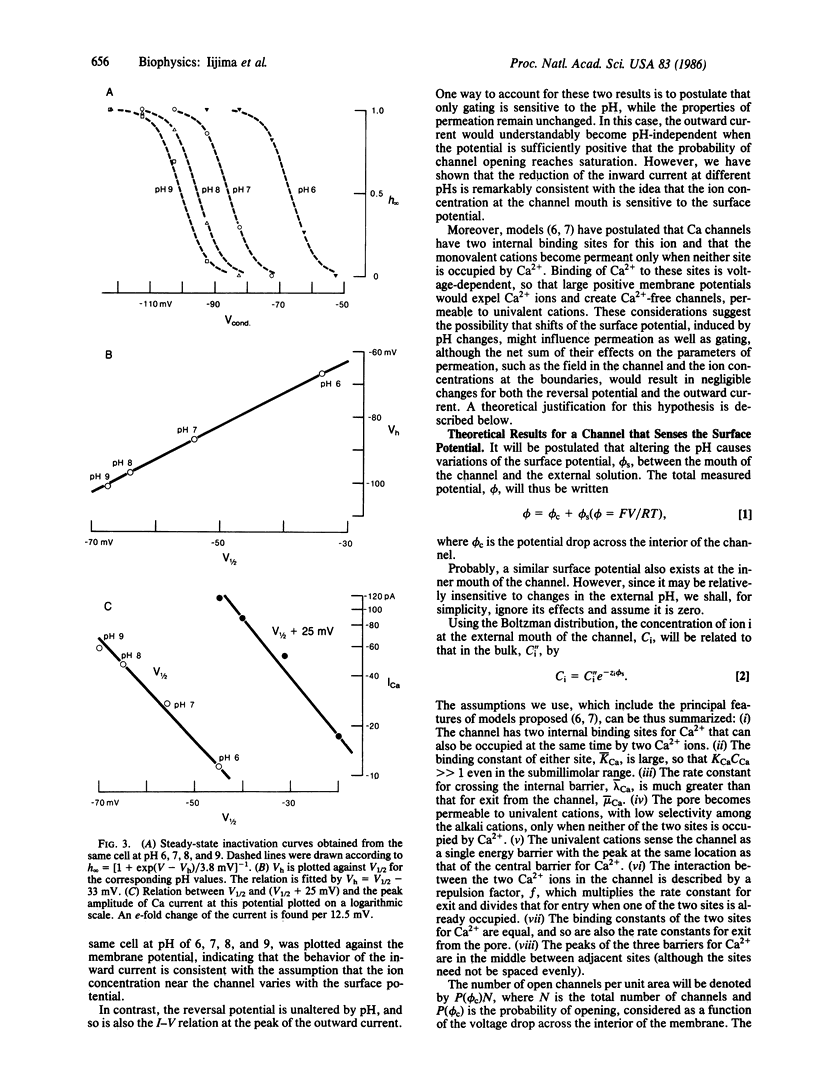
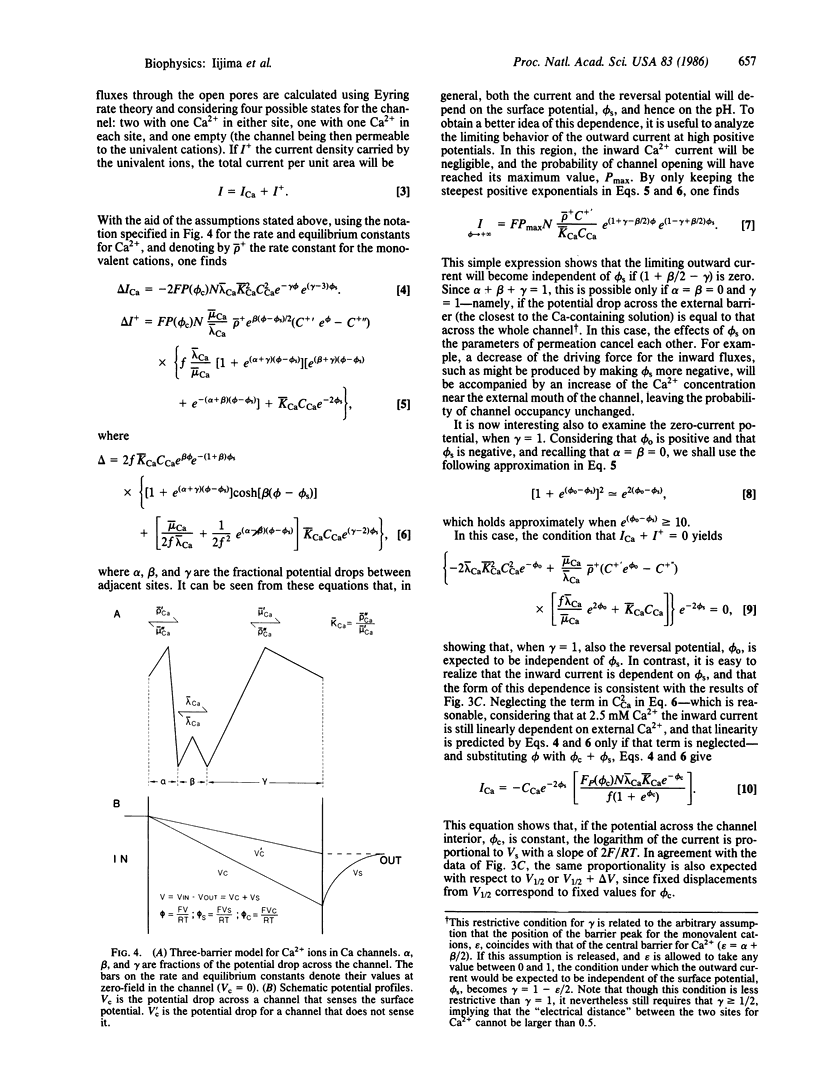
Some effects of the external pH on Ca channels were studied in a hybridoma cell line (mAb-7B), by using the whole-cell configuration of the patch-clamp technique. As the pH was lowered, both the activation and the inactivation curves shifted toward less negative membrane potentials, suggesting a pH-induced decrease of an external negative surface potential, sensed by the mechanism of gating. The potential for half-activation, V1/2, and that for half-inactivation, Vh, were related by a straight line with a slope of one. The inward current varied exponentially with V1/2, as would be expected if the field inside the channel and the Ca2+ concentration at the entrance were sensitive to the surface potential. However, the reversal potential and the outward current were unaltered by changes in the pH. Under the hypothesis that the channel senses the surface potential, all these results, as well as the nernstian behavior of the reversal potential with respect to Ca2+, observed in previous studies, are accounted for by a three-barrier, two-ion model for a channel, provided it is assumed that the potential in the channel drops almost entirely across the barrier adjacent to the external solution.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Almers W., McCleskey E. W. Non-selective conductance in calcium channels of frog muscle: calcium selectivity in a single-file pore. J Physiol. 1984 Aug;353:585–608. doi: 10.1113/jphysiol.1984.sp015352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fukushima Y., Hagiwara S. Currents carried by monovalent cations through calcium channels in mouse neoplastic B lymphocytes. J Physiol. 1985 Jan;358:255–284. doi: 10.1113/jphysiol.1985.sp015550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fukushima Y., Hagiwara S., Saxton R. E. Variation of calcium current during the cell growth cycle in mouse hybridoma lines secreting immunoglobulins. J Physiol. 1984 Oct;355:313–321. doi: 10.1113/jphysiol.1984.sp015421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hagiwara S., Ohmori H. Studies of calcium channels in rat clonal pituitary cells with patch electrode voltage clamp. J Physiol. 1982 Oct;331:231–252. doi: 10.1113/jphysiol.1982.sp014371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hess P., Tsien R. W. Mechanism of ion permeation through calcium channels. 1984 May 31-Jun 6Nature. 309(5967):453–456. doi: 10.1038/309453a0. [DOI] [PubMed] [Google Scholar]
- Hille B., Woodhull A. M., Shapiro B. I. Negative surface charge near sodium channels of nerve: divalent ions, monovalent ions, and pH. Philos Trans R Soc Lond B Biol Sci. 1975 Jun 10;270(908):301–318. doi: 10.1098/rstb.1975.0011. [DOI] [PubMed] [Google Scholar]
- Ohmori H., Yoshii M. Surface potential reflected in both gating and permeation mechanisms of sodium and calcium channels of the tunicate egg cell membrane. J Physiol. 1977 May;267(2):429–463. doi: 10.1113/jphysiol.1977.sp011821. [DOI] [PMC free article] [PubMed] [Google Scholar]


