Abstract
Mechanisms by which Wnt pathways integrate the organization of receptors, organelles, and cytoskeletal proteins to confer cell polarity and directional cell movement are incompletely understood. We show that acute responses to Wnt5a involve recruitment of actin, myosin IIB, Frizzled 3, and melanoma cell adhesion molecule into an intracellular structure in a melanoma cell line. In the presence of a chemokine gradient, this Wnt-mediated receptor–actin–myosin polarity (W-RAMP) structure accumulates asymmetrically at the cell periphery, where it triggers membrane contractility and nuclear movement in the direction of membrane retraction. The process requires endosome trafficking, is associated with multivesicular bodies, and is regulated by Wnt5a through the small guanosine triphosphatases Rab4 and RhoB. Thus, cell-autonomous mechanisms allow Wnt5a to control cell orientation, polarity, and directional movement in response to positional cues from chemokine gradients.
Wnt signaling controls cell polarity and directional cell movement in developmental systems, as well as cell invasion in certain cancers. Features shared between noncanonical Wnt pathways include recruitment of Frizzled (Fz) receptors to the posterior end of cells, and asymmetric distribution of atypical cell adhesion molecules, often associated with Fz (1, 2). Thus, receptors redistribute, in response to Wnt, to define an axis of asymmetry. In developmental systems, these processes can be regulated by interactions with adjacent cells, which confer orientation with respect to surrounding tissues (1, 3). For example, during endoderm specification in Caenorhabditis elegans, the division plane in the four-cell blastomere is determined by a positional Wnt signal from a near-by P2 cell (4). In contrast, Wnt pathway mutations in C. elegans that disrupt neuronal cell migration and polarity can be rescued by Wnt overexpression without requiring a localized source of ligand (5, 6). This suggests that directional presentation of Wnt to cells is not always needed for cell polarization, rather that Wnt ligands act permissively to allow cells to respond to environmental cues for longitudinal guidance and directional movement. In such cases, intracellular events responding directly to Wnt may be difficult to identify and distinguish from responses to cell-cell contact or paracrine signaling.
To address this, we examined acute responses to Wnt5a in dispersed cells. Wnt5a expression correlates with high-grade, invasive human melanomas and promotes invasiveness in melanoma cell lines (7). Treatment of WM239A melanoma cells with purified Wnt ligands showed expected signaling selectivity, where Wnt3a enhanced β-catenin–dependent transcription and nuclear translocation, while Wnt5a enhanced protein kinase C (PKC) autophosphorylation with little effect on β-catenin–dependent signaling (fig. S1) (8).
WM239A cells treated with Wnt5a were immunostained for melanoma cell adhesion molecule (MCAM, also known as MUC18 and CD146), an immunoglobulin G–family cell adhesion receptor implicated in melanoma tumorigenesis and metastasis (9). In untreated cells, MCAM was distributed uniformly. However, treatment of cells with Wnt5a for 30 min led to redistribution of MCAM into a polarized structure, which we call the Wnt5a-mediated receptor–actin–myosin polarity (W-RAMP) structure, for reasons described below (Fig. 1, A and B). The W-RAMP structure was also observed in some untreated cells, but this was suppressed by depleting Wnt5a with RNA interference (RNAi) (Fig. 1B), which indicated that the basal signal results from autocrine responses to endogeneous ligand. Similar W-RAMP structures were observed in WM1789 melanoma cells, as well as in xenograft tumors derived from WM239A cells (Fig. 1A and fig. S2A). In contrast, Wnt3a did not induce the W-RAMP structure over that of the controls did (Fig. 1B).
Fig. 1.
An asymmetric “W-RAMP structure” forms in response to Wnt5a and defines cell polarization and orientation. (A) Examples of cells with uniform MCAM localization versus asymmetric W-RAMP structures, observed by indirect immunofluorescence in WM239A cells treated for 30 min with or without Wnt5a or in WM1789 cells treated for 30 min with Wnt5a. Nuclei are shown by 4′,6′-diamidino-2-phenylindole (DAPI) staining. (B) Wnt5a significantly increases the number of WM239A cells displaying asymmetric MCAM compared with control or Wnt3a-treated cells. [Data show means ± SEM. *P < 0.005 by Student’s t test. Number of experiments (n) = 4. Cells counted: control, 1391; Wnt5a, 1380; Wnt3a, 1408.] RNAi knockdown of endogenous Wnt5a reduces the number of cells exhibiting the W-RAMP structure under untreated conditions, from 6% to less than 0.9% (Data show means with highest and lowest values, n = 2. Cells counted: control, 648; Wnt5a-RNAi, 1051). (C) In the presence of CXCL12, Wnt5a increased the percentage of cells with W-RAMP structures (red) positioned distal to Golgi (green) (means ± SEM; *P < 0.005, n = 4. Cells counted: control, 99; Wnt5a, 136). In the absence of CXCL12, Wnt5a did not affect the W-RAMP structure position relative to Golgi (n = 3. Cells counted: control, 134; Wnt5a, 131). (D) Wnt5a increased the number of cells with Golgi pointed toward or away from the gradient (class 1 and 3), with little directional bias.
In order to address its relation to cell polarization and orientation, the position of the W-RAMP structure was examined relative to Golgi, which in polarized motile cells typically localizes to the anterior end. To provide a localized cue for positional orientation, melanoma cells were exposed to a gradient of CXCL12, a chemokine that signals through the CXCR4 receptor to promote cell invasion (10, 11). WM239A cells were placed in a chemotaxis chamber in the presence or absence of Wnt5a and with CXCL12 in the chemo-attractant source chamber. After 30 min, cells were immunostained for MCAM and a Golgi marker.
Three patterns were observed: (i) W-RAMP structures were located distal to Golgi with respect to the nucleus, consistent with a posterior location; (ii) W-RAMP structures were proximal to Golgi; or (iii) Golgi were not clearly polarized. Wnt5a treatment increased the percentage of cells with Golgi postioned distal to the W-RAMP structures and decreased the percentages of other patterns (Fig. 1C). This indicates that Wnt5a enhances cell polarization, directing MCAM to the distal end. It is noteworthy that the response completely depended on the presence of chemokine, because without CXCL12, distal juxtapositioning between MCAM and Golgi did not increase and, in fact, slightly decreased following Wnt5a treatment (Fig. 1C). Thus, Wnt5a promotes cell polarity in a manner that requires the CXCL12 gradient.
We examined the orientation of Golgi with respect to the CXCL12 gradient source (Fig. 1D). Wnt5a increased the number of cells in which Golgi were pointed either toward or away from the CXCL12 gradient and decreased the number of cells in which Golgi were not clearly polarized (Fig. 1D). However, Wnt5a did not substantially affect proximity of the W-RAMP structure relative to the gradient source, and cells became polarized with Golgi and MCAM at distal ends, whether or not they were oriented toward CXCL12. In no case were cells oriented with their long axis perpendicular to the gradient, when Golgi and MCAM were distal to each other. Thus, Wnt5a induces polarization in dispersed cells, and cell polarization and directionality can be defined by the formation of W-RAMP structures and their localization relative to Golgi. Chemotaxis toward CXCL12 is not an underlying cause of Wnt5a-induced polarization, although polarization requires interactions with external factors that confer cues for positional orientation.
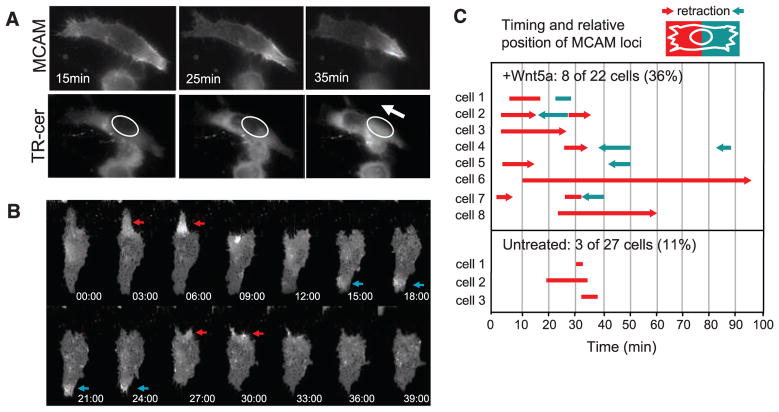
Live-cell imaging showed that the W-RAMP structure is dynamic. Endogeneous MCAM, labeled with antibody-coupled fluorophore, appeared polarized in cells within 25 min after Wnt5a addition. Enrichment of MCAM at one edge of the cell was in each case accompanied by rapid retraction of the membrane toward the cell body and nuclear movement in the same direction, revealing coordination between membrane retraction and directional cell movement (Fig. 2A and movies S1 to S4). Similar behavior was observed in cells transfected with MCAM fused to green fluorescent protein (MCAM-GFP) and actin fused to monomeric red fluorescent protein (mRFP-actin) (fig. S3 and movies S5 and S6).
Fig. 2.
Formation of the W-RAMP structure is dynamic and associated with membrane retraction. (A) Live WM239A cells were incubated with AlexaFluor-488–conjugated MCAM antibody and Texas Red (TR)–ceramide. In response to Wnt5a (in absence of CXCL12) MCAM decreases at the left end of the cell and increases at the right, followed by membrane retraction toward the W-RAMP structure. TR-ceramide staining reveals nuclear translocation in the direction of membrane retraction (arrow). (B) The W-RAMP structure forms in successive waves. MCAM-GFP expressed in WM239A cells accumulates at one cell end (red arrow) followed by membrane retraction (after 9 min). A second W-RAMP structure forms at the opposite cell end (blue arrow), followed by membrane retraction (after 27 min). A third W-RAMP structure (red arrow) is followed by membrane retraction at 30 min. (C) Live cells were monitored continuously for MCAM-GFP localization. The x axis represents time after addition of Wnt5a. The length of each bar represents the duration of each wave of polarized accumulation of MCAM. Red versus blue lines distinguish the relative end of the cell at which the wave forms. Arrowheads indicate cases where membrane retraction follows formation of a wave, and the relative end of the cell at which the retraction occurs. Representative cells can be viewed in movies S7 to S12.
The sequence of events in cells responding to Wnt5a was distinct from untreated cells. In a random sampling of live cells, each monitored for 100 min, the W-RAMP structure appeared in 36% of cells treated with Wnt5a (8 of 22 cells), with an average duration of 16 min. Wnt5a-treated cells often showed multiple waves, with the first wave appearing within 10 min, and the majority associated with membrane retraction (Fig. 2, B and C, and movies S7 to S9). In contrast, W-RAMP structures were found in only 11% of untreated cells (3 of 27 cells), none of which formed multiple waves. Single waves appeared after 20 min with an average duration of 7 min and were never associated with membrane retraction (Fig. 2C, and movies S10 to S12). This revealed ~40% penetrance of the Wnt5a response within an asynchronous cell population and confirmed that, in untreated cells, the lower frequency quantified at 30 min (12 to 15%) can be ascribed to the transient properties of the W-RAMP structure.
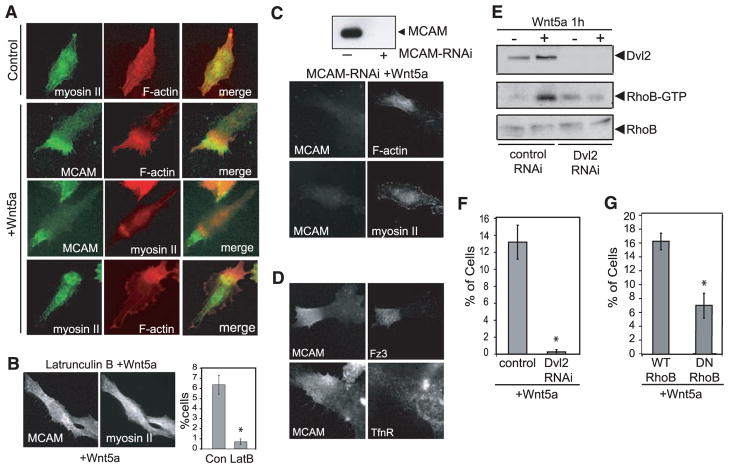
In epithelial cells, tail-end recruitment of F-actin and myosin IIB triggers membrane retraction (12). We therefore examined the distribution of these proteins with respect to MCAM. In untreated melanoma cells, F-actin and myosin IIB were uniformly distributed throughout the cell. However, after Wnt5a treatment, F-actin formed a dense band near each W-RAMP structure, with close associations between F-actin and MCAM extending to the cell edge (Fig. 3A). Myosin IIB also localized to this region, overlapping MCAM and the dense actin band (Fig. 3A). Asymmetric F-actin and myosin IIB structures, similar to MCAM, were also observed in xenograft tumors (fig. S2B). Thus, the organization of the W-RAMP structure involves close associations between cytoskeletal components and cell adhesion receptors. This parallels behavior in Drosophila wing epithelia, where actin and myosin II organize asymmetrically to regulate wing hair formation in response to Wnt signaling (13).
Fig. 3.
The W-RAMP structure is formed through reciprocal interactions with actin and myosin IIB. (A) In Wnt5a-treated cells, myosin IIB and F-actin form asymmetric structures that partially overlap with the W-RAMP structure. (B) Latrunculin B (10 nM) pretreatment of cells strongly inhibits Wnt5a-dependent formation of the W-RAMP structure (means ± SEM; *P < 0.005. Cells counted: dimethylsulfoxide-treated, 1362; LatB, 1538). (C) RNAi knockdown of MCAM expression leads to actin structures that are more diffuse and disorganized than controls, as well as complete abrogation of myosin IIB structures. (D) Indirect immunofluorescence of WM239A cells shows that Fz3 colocalizes with the W-RAMP structure, but transferrin receptor does not. (E) Pull-down assay with immobilized glutathione S-transferase–rhotekin reveals elevated RhoB-GTP in response to Wnt5a. Dvl2 knockdown blocks the activation of RhoB by Wnt5a. (F) Dvl2 RNAi knockdown abolishes W-RAMP structure in Wnt5a-treated cells (*P < 0.05, n = 3. Cells counted: control RNAi, 1002; Dvl2-RNAi, 1139). (G) Transient transfection of cells with DN-RhoB blocks Wnt5a-dependent formation of the W-RAMP structure (*P < 0.05, n = 3. Cells counted: WT-RhoB, 608; DN-RhoB, 590).
We examined the role of F-actin and myosin IIB in forming the W-RAMP structure. Cell treatment with latrunculin B at concentrations high enough to block dynamic actin reorganization, but low enough to preserve actin stress fibers, completely prevented Wnt5a-regulated asymmetry of MCAM and myosin IIB without affecting overall cell morphology (Fig. 3B). Conversely, blocking MCAM expression by treating cells with MCAM-RNAi before adding Wnt5a completely abolished the dense actin and myosin IIB bands in all cells, while allowing a more diffuse asymmetry of actin (Fig. 3C and fig. S4). This demonstrates that polarization of the W-RAMP structure requires actin polymerization and that reciprocal interactions between MCAM and actin mediate recruitment of myosin IIB. We hypothesize that the transient and dynamic movement of the W-RAMP structure triggers localized membrane retraction by directing recruitment of actin and myosin IIB. Membrane retraction subsequently promotes nuclear movement, establishing the directionality of cell locomotion.
Fz3, a noncanonical Wnt receptor, was also recruited to the W-RAMP structure, but transferrin receptor, which is not involved in Wnt signaling, was not (Fig. 3D). Depletion of disheveled-2 (Dvl2) by RNAi and inhibition of PKC completely blocked the W-RAMP structure (Fig. 4, E and F, and fig. S5A). Thus, formation of the W-RAMP structure requires known effectors of non-canonical Wnt signaling.
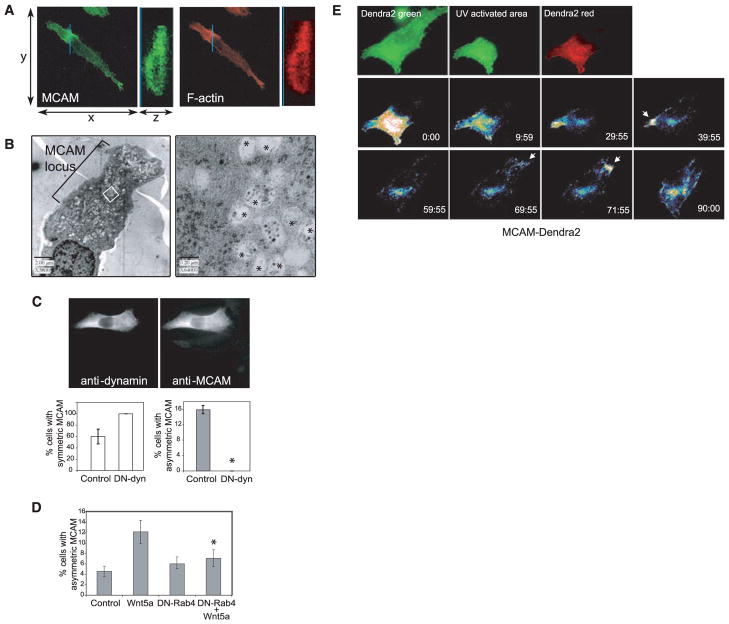
Fig. 4.
Formation and dynamic movement of the W-RAMP structure requires membrane internalization and endosome recycling and is associated with multivesicular bodies. (A) Confocal Z-series reconstructed in the region of the MCAM structure (blue line), showed that MCAM (MCAM-specific antibody, green) and F-actin (phalloidin, red) are localized to both cytosolic and membrane regions and are excluded from the nucleus. (B) Electron micrographs show accumulation of membrane vesicles associated with the W-RAMP structure. (Right) Higher magnification reveals that vesicles consist of multivesicular bodies (asterisks) interspersed among cytoskeletal filaments. (C) Cells expressing DN-dynamin, easily distinguished by their higher immunoreactivity, showed higher percentages with uniform symmetric MCAM localization and lower percentages with asymmetric MCAM localization (*P < 0.005, n = 3. Cells counted: control, 167; DN-dynamin, 203). (D) DN-Rab4 blocks the ability of Wnt5a to promote formation of W-RAMP structures, decreased significantly compared with Wnt5a alone (*P < 0.05, n = 4. Cells counted: control, 1019; Wnt5a, 1244; DN-Rab4, 1262; DN-Rab4+Wnt5a, 1034). (E) Red fluorescence from photoactivated MCAM-Dendra2 accumulates transiently in a perinuclear pool by 60 min, then at the opposite end of the cell by 71 min (arrow), followed by membrane retraction. Colors from lowest to highest intensity are black, blue, green, yellow, orange, red, and white.
We also examined Rho guanosine triphosphatases (GTPases), because RhoA, in many instances, is important for Wnt signaling (14). In vitro pull-down assays showed that Wnt5a enhanced the abundance of guanosine triphosphate (GTP)–bound active RhoB within 1 hour of stimulation, but did not affect the amount of RhoA-GTP (Fig. 3E and fig. S5, B and C). Expression of dominant-negative mutant (DN) RhoB(T19N) (in which Thr19 is replaced by Asn) in WM239A cells followed by Wnt5a treatment suppressed the number of cells forming W-RAMP structures, down to amounts in untreated cells (Fig. 3G). Furthermore, inhibition of Dvl2 by RNAi blocked activation of RhoB, which confirmed that RhoB is a downstream effector of Wnt (Fig. 3E). These findings reveal the requirement for RhoB in Wnt5a signaling, for controlling the polarized distribution of MCAM and associated cytoskeletal proteins.
Because RhoB directs endosome trafficking, we asked whether MCAM is localized to intracellular membrane compartments and whether W-RAMP structures are regulated by Wnt5a-responsive vesicle trafficking (15). Wnt5a promotes membrane internalization (16), and such behavior would parallel events in zebrafish gastrulation, in which directional mesendodermal cell movement in response to Wnt11 requires endocytosis of E-cadherin and can be blocked by GTPase-defective mutant dynamin or DN Rab5 (17). Confocal imaging showed that polarized MCAM and F-actin structures were excluded from nuclei, but were otherwise present throughout the cell body and not restricted to the cell membrane (Fig. 4A). Visualizing W-RAMP–containing cells by electron microscopy revealed that the cell region that included the W-RAMP structure was dense with multivesicular bodies (MVBs) decorated with filamentous structures (Fig. 4B), which suggested that the W-RAMP structure is composed of MVBs associated with MCAM and polymerized cytoskeletal proteins. These findings are consistent with the requirement of the W-RAMP structure for RhoB, which is known to localize to and direct trafficking of MVBs (15).
We tested whether formation of the W-RAMP structure depended on receptor internalization and endosomal trafficking. A DN of dynamin (K44A, in which Lys44 is replaced by Ala) blocks receptor-mediated endocytosis by inhibiting plasma membrane cleavage at the neck of invaginating vesicles (18). Cells transfected with DN dynamin completely suppressed MCAM asymmetry, which resulted in a uniform distribution of MCAM across the cell (Fig. 4C). Thus, membrane internalization, an initial step in endosomal trafficking, was necessary to form the W-RAMP structure.
Because Rab4 directs early endosomes to the recycling endosome compartment, cells were transfected with DN-Rab4 (N121I) (in which Asn121 is replaced by Ile) fused to GFP in order to block endosome trafficking. DN-Rab4 blocked formation of W-RAMP structures down to control levels (Fig. 4D), which indicated that Rab4 mediates Wnt5a signaling, as observed with Dvl2, dynamin, and RhoB. Although GFP-Rab4 was not observed within W-RAMP structures, MCAM partially overlapped GFP-Rab4 within the perinuclear region, consistent with its association with recycling endosomes (fig. S6). The results indicate that dynamic movement and intracellular translocation of MCAM is mediated via internalization of MCAM and trafficking of receptor endosomes. This might occur through a linear pathway with Wnt5a upstream of Rab4 and other effectors, or through parallel pathways involving convergence between Wnt5a and endosomal trafficking effectors.
Cells forming W-RAMP structures typically displayed only one structure in each cell at any moment. This suggested that dynamic redistribution of W-RAMP structures may involve release of MCAM from one region of the cell, movement to distal regions, and assembly into another structure. To test this, cells were transfected with MCAM fused to Dendra2, a photoactivatable protein, which, upon ultraviolet (UV) illumination, switches from green to red fluorescence (19). One-third of the cell area was UV-illuminated, followed by Wnt5a addition (Fig. 4E and movie S13). Within 15 to 30 min, photoactivated MCAM-Dendra2 decreased within the illuminated region and, over 30 to 60 min, accumulated within a perinuclear compartment consistent with the location of Rab4-positive recycling endosomes. By 60 min, MCAM-Dendra2 appeared in punctate cytosolic patterns within the non–UV-illuminated region, followed by its accumulation at the cell edge and subsequent membrane retraction. This confirms that the W-RAMP structure is subject to turnover, where proteins are recycled in a process of disassembly, intracellular redistribution, and reassembly. Given the requirement for RhoB, dynamin, and Rab4, as well as the association of MVBs with the W-RAMP structure, we speculate that turnover involves movement of MCAM through recycling endosomes and MVBs and intracellular translocation of MVBs.
In conclusion, we report a mechanism by which dispersed cells respond acutely to non-canonical Wnt signaling; it involves recruitment and redistribution of cellular proteins into an intracellular structure that integrates receptors for cell adhesion and cell signaling with components of the cytoskeletal architecture. In the presence of gradient cues from secreted factors, the W-RAMP structure asymmetrically distributes in a polarized manner, where it directs membrane retraction and thus influences the direction of cell movement. This allows Wnt5a to control polarity and directional orientation, even in cells lacking positional information from cell-cell contacts. The W-RAMP structure requires Dvl2 and PKC, involves membrane internalization and endosome trafficking and is regulated by RhoB and formation of MVBs. This contrasts with other mechanisms, in which Wnt regulates cytoskeletal architecture via RhoA and receptor distribution via endocytic pathways. Our findings add insight to the understanding of acute intracellular events mediated by Wnt signaling.
Supplementary Material
Acknowledgments
We are indebted to D. Chan and Z. Zhang for help with xenograft tumor growth, N. Camp for preliminary data collection, and T. Giddings for guidance with TEM. This work was supported by NIH grants F32-CA112847 (E.S.W.), F32-CA105796 (G.M.A.), and R01-CA118972 (N.G.A.).
Footnotes
References and Notes
- 1.Adler PN. Dev Cell. 2002;2:525. doi: 10.1016/s1534-5807(02)00176-4. [DOI] [PubMed] [Google Scholar]
- 2.Shimada Y, Yonemura S, Ohkura H, Strutt D, Uemura T. Dev Cell. 2006;10:209. doi: 10.1016/j.devcel.2005.11.016. [DOI] [PubMed] [Google Scholar]
- 3.Park FD, Tenlen JR, Priess JR. Curr Biol. 2004;14:2252. doi: 10.1016/j.cub.2004.12.019. [DOI] [PubMed] [Google Scholar]
- 4.Goldstein B, Takeshita H, Mizumoto K, Sawa H. Dev Cell. 2006;10:391. doi: 10.1016/j.devcel.2005.12.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hilliard MA, Bargmann CI. Dev Cell. 2006;10:379. doi: 10.1016/j.devcel.2006.01.013. [DOI] [PubMed] [Google Scholar]
- 6.Whangbo J, Kenyon C. Mol Cell. 1999;4:851. doi: 10.1016/s1097-2765(00)80394-9. [DOI] [PubMed] [Google Scholar]
- 7.Weeraratna AT, et al. Cancer Cell. 2002;1:279. doi: 10.1016/s1535-6108(02)00045-4. [DOI] [PubMed] [Google Scholar]
- 8.Materials and methods are available as supporting material on Science Online.
- 9.Satyamoorthy K, Muyrers J, Meier F, Patel D, Herlyn M. Oncogene. 2001;20:4676. doi: 10.1038/sj.onc.1204616. [DOI] [PubMed] [Google Scholar]
- 10.Bartolome RA, et al. Cancer Res. 2004;64:2534. doi: 10.1158/0008-5472.can-03-3398. [DOI] [PubMed] [Google Scholar]
- 11.Murakami T, et al. Cancer Res. 2002;62:7328. [PubMed] [Google Scholar]
- 12.Kolega J. Mol Biol Cell. 2003;14:4745. doi: 10.1091/mbc.E03-04-0205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Winter CG, et al. Cell. 2001;105:81. doi: 10.1016/s0092-8674(01)00298-7. [DOI] [PubMed] [Google Scholar]
- 14.Veeman MT, Axelrod JD, Moon RT. Dev Cell. 2003;5:367. doi: 10.1016/s1534-5807(03)00266-1. [DOI] [PubMed] [Google Scholar]
- 15.Ellis S, Mellor H. Trends Cell Biol. 2000;10:85. doi: 10.1016/s0962-8924(99)01710-9. [DOI] [PubMed] [Google Scholar]
- 16.Chen W, et al. Science. 2003;301:1391. doi: 10.1126/science.1082808. [DOI] [PubMed] [Google Scholar]
- 17.Ulrich F, et al. Dev Cell. 2005;9:555. doi: 10.1016/j.devcel.2005.08.011. [DOI] [PubMed] [Google Scholar]
- 18.Van der Bliek AM, et al. J Cell Biol. 1993;122:553. doi: 10.1083/jcb.122.3.553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gurskaya NG, et al. Nat Biotechnol. 2006;24:461. doi: 10.1038/nbt1191. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.