Abstract
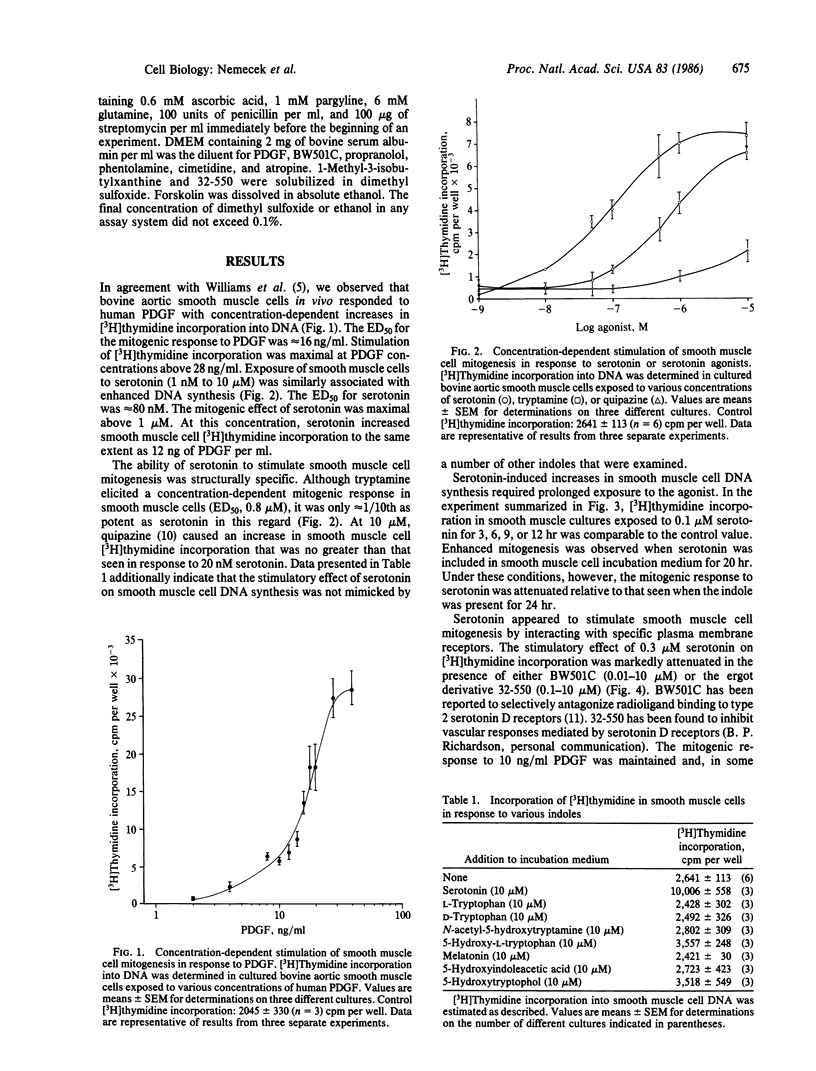
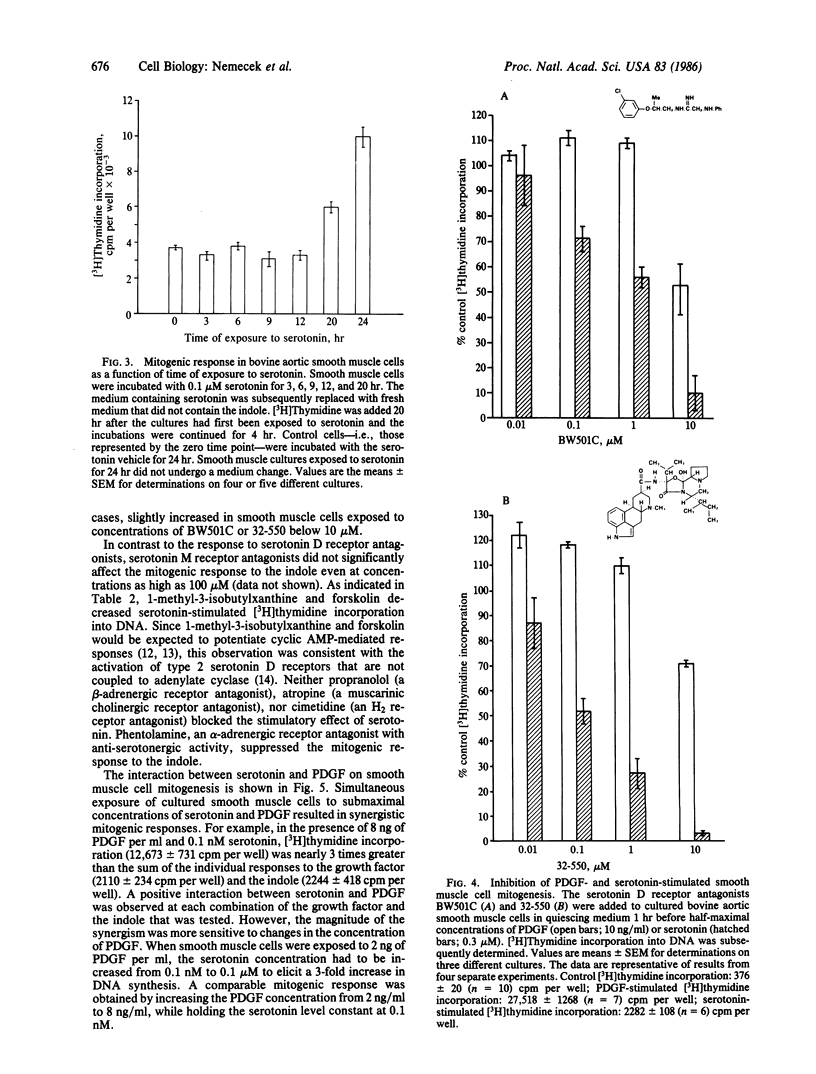
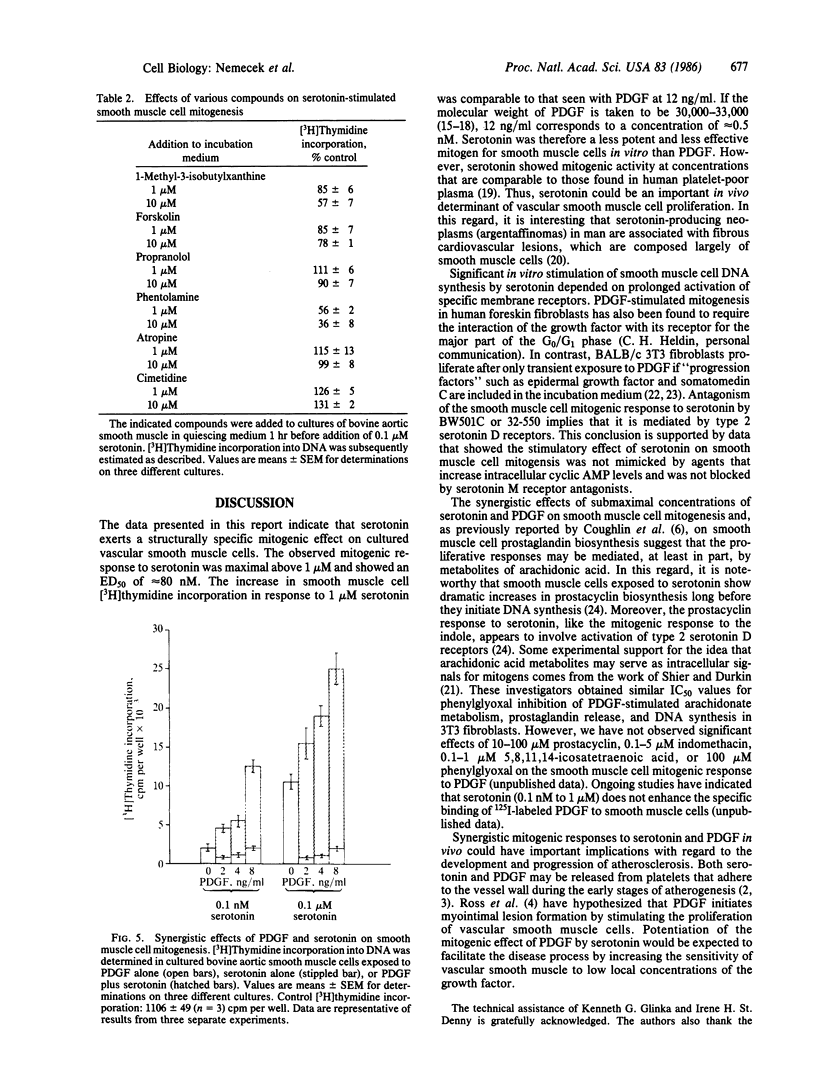
Bovine aortic smooth muscle cells in vitro responded to 1 nM to 10 microM serotonin with increased incorporation of [3H]thymidine into DNA. The mitogenic effect of serotonin was half-maximal at 80 nM and maximal above 1 microM. At a concentration of 1 microM, serotonin stimulated smooth muscle cell mitogenesis to the same extent as human platelet-derived growth factor (PDGF) at 12 ng/ml. Tryptamine was approximately 1/10th as potent as serotonin as a mitogen for smooth muscle cells. Other indoles that are structurally related to serotonin (D- and L-tryptophan, 5-hydroxy-L-tryptophan, N-acetyl-5-hydroxytryptamine, melatonin, 5-hydroxyindoleacetic acid, and 5-hydroxytryptophol) and quipazine were inactive. The stimulatory effect of serotonin on smooth muscle cell DNA synthesis required prolonged (20-24 hr) exposure to the agonist and was attenuated in the presence of serotonin D receptor antagonists. When smooth muscle cells were incubated with submaximal concentrations of serotonin and PDGF, synergistic rather than additive mitogenic responses were observed. These data indicate that serotonin has a significant mitogenic effect on smooth muscle cells in vitro, which appears to be mediated by specific plasma membrane receptors.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Antoniades H. N. Human platelet-derived growth factor (PDGF): purification of PDGF-I and PDGF-II and separation of their reduced subunits. Proc Natl Acad Sci U S A. 1981 Dec;78(12):7314–7317. doi: 10.1073/pnas.78.12.7314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coughlin S. R., Moskowitz M. A., Antoniades H. N., Levine L. Serotonin receptor-mediated stimulation of bovine smooth muscle cell prostacyclin synthesis and its modulation by platelet-derived growth factor. Proc Natl Acad Sci U S A. 1981 Nov;78(11):7134–7138. doi: 10.1073/pnas.78.11.7134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coughlin S. R., Moskowitz M. A., Levine L. Identification of a serotonin type 2 receptor linked to prostacyclin synthesis in vascular smooth muscle cells. Biochem Pharmacol. 1984 Feb 15;33(4):692–695. doi: 10.1016/0006-2952(84)90330-7. [DOI] [PubMed] [Google Scholar]
- Deuel T. F., Huang J. S., Proffitt R. T., Baenziger J. U., Chang D., Kennedy B. B. Human platelet-derived growth factor. Purification and resolution into two active protein fractions. J Biol Chem. 1981 Sep 10;256(17):8896–8899. [PubMed] [Google Scholar]
- Faggiotto A., Ross R., Harker L. Studies of hypercholesterolemia in the nonhuman primate. I. Changes that lead to fatty streak formation. Arteriosclerosis. 1984 Jul-Aug;4(4):323–340. doi: 10.1161/01.atv.4.4.323. [DOI] [PubMed] [Google Scholar]
- Faggiotto A., Ross R. Studies of hypercholesterolemia in the nonhuman primate. II. Fatty streak conversion to fibrous plaque. Arteriosclerosis. 1984 Jul-Aug;4(4):341–356. doi: 10.1161/01.atv.4.4.341. [DOI] [PubMed] [Google Scholar]
- Ferrans V. J., Roberts W. C. The carcinoid endocardial plaque; an ultrastructural study. Hum Pathol. 1976 Jul;7(4):387–409. doi: 10.1016/s0046-8177(76)80054-8. [DOI] [PubMed] [Google Scholar]
- Frattini P., Cucchi M. L., Santagostino G., Corona G. L. A sensitive fluorimetric method for determination of platelet-bound and plasma free serotonin. Clin Chim Acta. 1979 Mar 15;92(3):353–360. doi: 10.1016/0009-8981(79)90213-4. [DOI] [PubMed] [Google Scholar]
- Heldin C. H., Westermark B., Wasteson A. Platelet-derived growth factor. Isolation by a large-scale procedure and analysis of subunit composition. Biochem J. 1981 Mar 1;193(3):907–913. doi: 10.1042/bj1930907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leof E. B., Wharton W., van Wyk J. J., Pledger W. J. Epidermal growth factor (EGF) and somatomedin C regulate G1 progression in competent BALB/c-3T3 cells. Exp Cell Res. 1982 Sep;141(1):107–115. doi: 10.1016/0014-4827(82)90073-8. [DOI] [PubMed] [Google Scholar]
- Peroutka S. J., Lebovitz R. M., Snyder S. H. Two distinct central serotonin receptors with different physiological functions. Science. 1981 May 15;212(4496):827–829. doi: 10.1126/science.7221567. [DOI] [PubMed] [Google Scholar]
- Pledger W. J., Stiles C. D., Antoniades H. N., Scher C. D. Induction of DNA synthesis in BALB/c 3T3 cells by serum components: reevaluation of the commitment process. Proc Natl Acad Sci U S A. 1977 Oct;74(10):4481–4485. doi: 10.1073/pnas.74.10.4481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raines E. W., Ross R. Platelet-derived growth factor. I. High yield purification and evidence for multiple forms. J Biol Chem. 1982 May 10;257(9):5154–5160. [PubMed] [Google Scholar]
- Raines E. W., Ross R. Platelet-derived growth factor. I. High yield purification and evidence for multiple forms. J Biol Chem. 1982 May 10;257(9):5154–5160. [PubMed] [Google Scholar]
- Rodríguez R., Rojas-Ramírez J. A., Drucker-Colín R. R. Serotonin-like actions of quipazine on the central nervous system. Eur J Pharmacol. 1973 Nov;24(2):164–171. doi: 10.1016/0014-2999(73)90067-8. [DOI] [PubMed] [Google Scholar]
- Ross R., Glomset J. A. The pathogenesis of atherosclerosis (second of two parts). N Engl J Med. 1976 Aug 19;295(8):420–425. doi: 10.1056/NEJM197608192950805. [DOI] [PubMed] [Google Scholar]
- Ross R., Glomset J., Kariya B., Harker L. A platelet-dependent serum factor that stimulates the proliferation of arterial smooth muscle cells in vitro. Proc Natl Acad Sci U S A. 1974 Apr;71(4):1207–1210. doi: 10.1073/pnas.71.4.1207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seamon K. B., Daly J. W. Forskolin: a unique diterpene activator of cyclic AMP-generating systems. J Cyclic Nucleotide Res. 1981;7(4):201–224. [PubMed] [Google Scholar]
- Shier W. T., Durkin J. P. Role of stimulation of arachidonic acid release in the proliferative response of 3T3 mouse fibroblasts to platelet-derived growth factor. J Cell Physiol. 1982 Aug;112(2):171–181. doi: 10.1002/jcp.1041120204. [DOI] [PubMed] [Google Scholar]
- Weinstein R., Stemerman M. B., Maciag T. Hormonal requirements for growth of arterial smooth muscle cells in vitro: and endocrine approach to atherosclerosis. Science. 1981 May 15;212(4496):818–820. doi: 10.1126/science.7013068. [DOI] [PubMed] [Google Scholar]
- Williams L. T., Tremble P., Antoniades H. N. Platelet-derived growth factor binds specifically to receptors on vascular smooth muscle cells and the binding becomes nondissociable. Proc Natl Acad Sci U S A. 1982 Oct;79(19):5867–5870. doi: 10.1073/pnas.79.19.5867. [DOI] [PMC free article] [PubMed] [Google Scholar]