Abstract
LFA-1, an antigen involved in cytolytic T lymphocyte-mediated killing, and Mac-1, the receptor for complement component C3bi, constitute a family of structurally and functionally related cell surface glycoproteins involved in cellular interactions. In both mouse and man, Mac-1 (OKM1) and LFA-1 share a common 95-kDa beta subunit but are distinguished by their alpha chains, which have different cellular distributions, apparent molecular masses (165 and 177 kDa, respectively), and peptide maps. We report the isolation of a genomic clone from a human genomic library that on transfection into mouse fibroblasts produced a molecule(s) reactive with monoclonal antibodies to OKM1, to LFA-1, and to platelet glycoprotein IIb-IIIa. This gene was cloned by several cycles of transfection of L cells with a human genomic library cloned in lambda phage Charon 4A and subsequent "rescue" of the lambda phage. Transfection with the purified recombinant lambda DNA yielded a transfectant that expressed the three human alpha chains of OKM1, LFA-1, and glycoprotein IIb-IIIa, presumably in association with the murine beta chain.
Full text
PDF

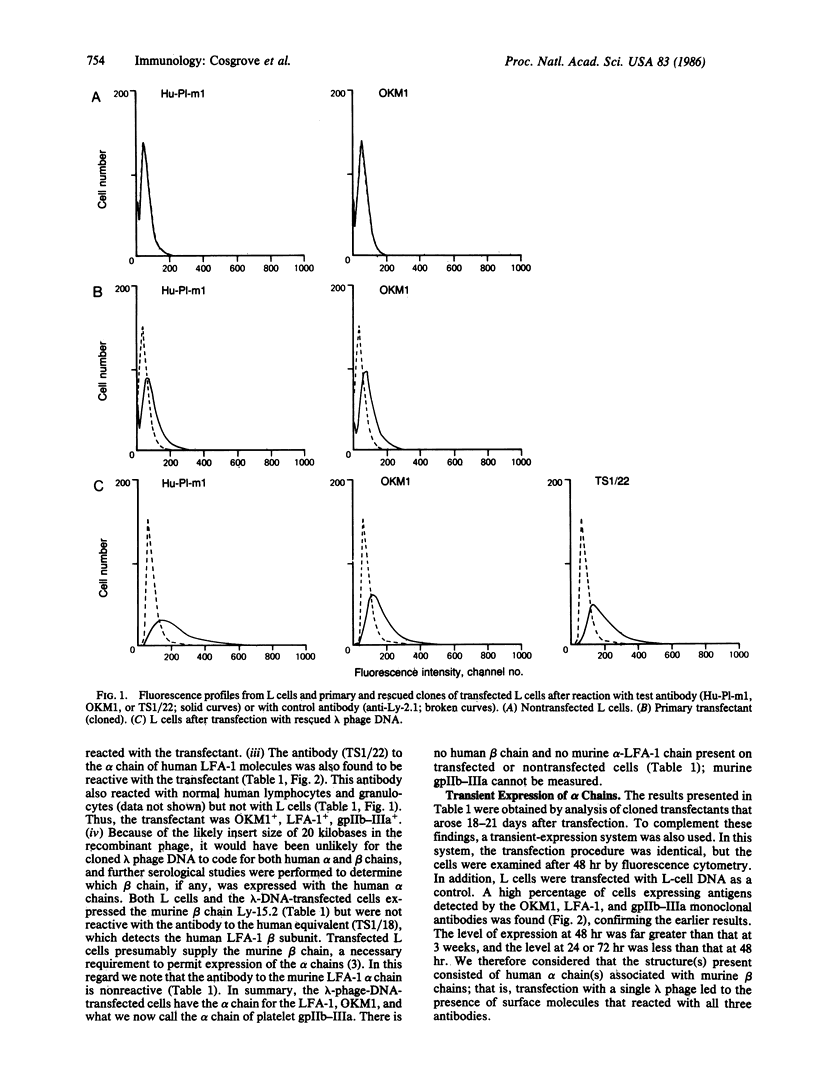

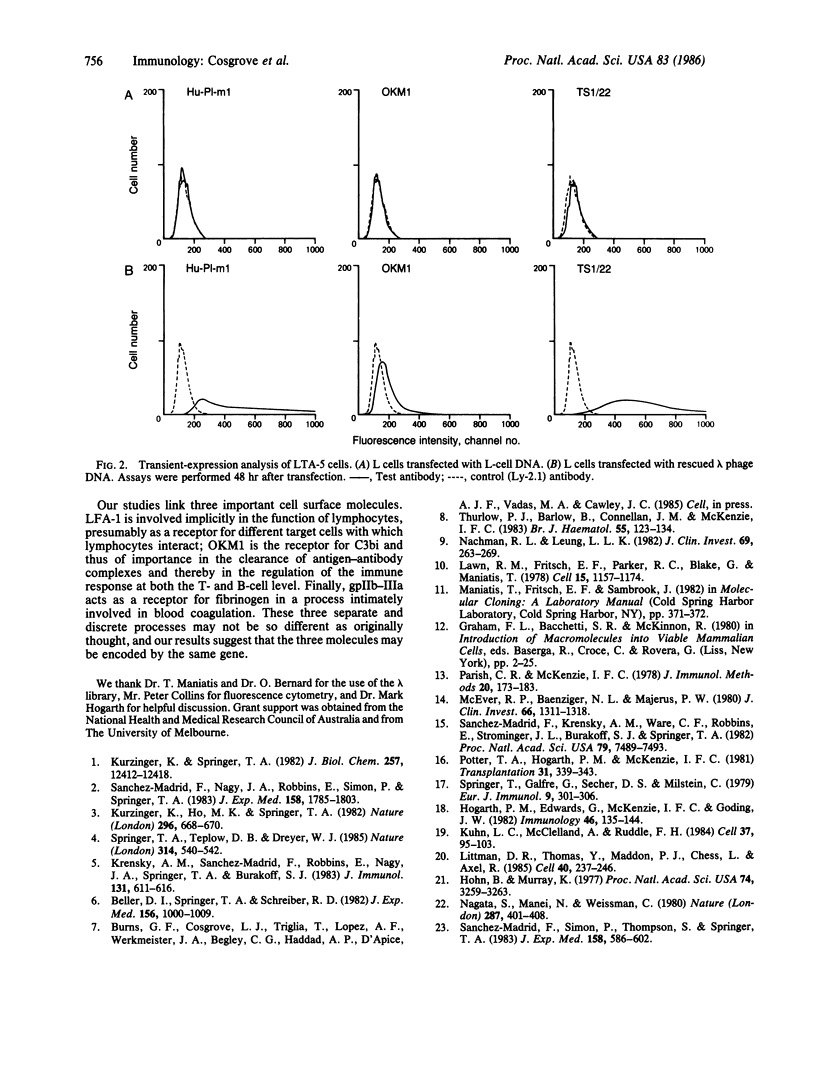
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Beller D. I., Springer T. A., Schreiber R. D. Anti-Mac-1 selectively inhibits the mouse and human type three complement receptor. J Exp Med. 1982 Oct 1;156(4):1000–1009. doi: 10.1084/jem.156.4.1000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hogarth P. M., Edwards J., McKenzie I. F., Goding J. W., Liew F. Y. Monoclonal antibodies to the murine Ly-2.1 cell surface antigen. Immunology. 1982 May;46(1):135–144. [PMC free article] [PubMed] [Google Scholar]
- Hohn B., Murray K. Packaging recombinant DNA molecules into bacteriophage particles in vitro. Proc Natl Acad Sci U S A. 1977 Aug;74(8):3259–3263. doi: 10.1073/pnas.74.8.3259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krensky A. M., Sanchez-Madrid F., Robbins E., Nagy J. A., Springer T. A., Burakoff S. J. The functional significance, distribution, and structure of LFA-1, LFA-2, and LFA-3: cell surface antigens associated with CTL-target interactions. J Immunol. 1983 Aug;131(2):611–616. [PubMed] [Google Scholar]
- Kühn L. C., McClelland A., Ruddle F. H. Gene transfer, expression, and molecular cloning of the human transferrin receptor gene. Cell. 1984 May;37(1):95–103. doi: 10.1016/0092-8674(84)90304-0. [DOI] [PubMed] [Google Scholar]
- Kürzinger K., Ho M. K., Springer T. A. Structural homology of a macrophage differentiation antigen and an antigen involved in T-cell-mediated killing. Nature. 1982 Apr 15;296(5858):668–670. doi: 10.1038/296668a0. [DOI] [PubMed] [Google Scholar]
- Kürzinger K., Springer T. A. Purification and structural characterization of LFA-1, a lymphocyte function-associated antigen, and Mac-1, a related macrophage differentiation antigen associated with the type three complement receptor. J Biol Chem. 1982 Oct 25;257(20):12412–12418. [PubMed] [Google Scholar]
- Lawn R. M., Fritsch E. F., Parker R. C., Blake G., Maniatis T. The isolation and characterization of linked delta- and beta-globin genes from a cloned library of human DNA. Cell. 1978 Dec;15(4):1157–1174. doi: 10.1016/0092-8674(78)90043-0. [DOI] [PubMed] [Google Scholar]
- Littman D. R., Thomas Y., Maddon P. J., Chess L., Axel R. The isolation and sequence of the gene encoding T8: a molecule defining functional classes of T lymphocytes. Cell. 1985 Feb;40(2):237–246. doi: 10.1016/0092-8674(85)90138-2. [DOI] [PubMed] [Google Scholar]
- McEver R. P., Baenziger N. L., Majerus P. W. Isolation and quantitation of the platelet membrane glycoprotein deficient in thrombasthenia using a monoclonal hybridoma antibody. J Clin Invest. 1980 Dec;66(6):1311–1318. doi: 10.1172/JCI109983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nachman R. L., Leung L. L. Complex formation of platelet membrane glycoproteins IIb and IIIa with fibrinogen. J Clin Invest. 1982 Feb;69(2):263–269. doi: 10.1172/JCI110448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagata S., Mantei N., Weissmann C. The structure of one of the eight or more distinct chromosomal genes for human interferon-alpha. Nature. 1980 Oct 2;287(5781):401–408. doi: 10.1038/287401a0. [DOI] [PubMed] [Google Scholar]
- Parish C. R., McKenzie I. F. A sensitive rosetting method for detecting subpopulations of lymphocytes which react with alloantisera. J Immunol Methods. 1978;20:173–183. doi: 10.1016/0022-1759(78)90254-5. [DOI] [PubMed] [Google Scholar]
- Potter T. A., Hogarth P. M., McKenzie I. F. Ly-15: a new murine lymphocyte alloantigenic locus. Transplantation. 1981 May;31(5):339–342. [PubMed] [Google Scholar]
- Sanchez-Madrid F., Krensky A. M., Ware C. F., Robbins E., Strominger J. L., Burakoff S. J., Springer T. A. Three distinct antigens associated with human T-lymphocyte-mediated cytolysis: LFA-1, LFA-2, and LFA-3. Proc Natl Acad Sci U S A. 1982 Dec;79(23):7489–7493. doi: 10.1073/pnas.79.23.7489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanchez-Madrid F., Nagy J. A., Robbins E., Simon P., Springer T. A. A human leukocyte differentiation antigen family with distinct alpha-subunits and a common beta-subunit: the lymphocyte function-associated antigen (LFA-1), the C3bi complement receptor (OKM1/Mac-1), and the p150,95 molecule. J Exp Med. 1983 Dec 1;158(6):1785–1803. doi: 10.1084/jem.158.6.1785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanchez-Madrid F., Simon P., Thompson S., Springer T. A. Mapping of antigenic and functional epitopes on the alpha- and beta-subunits of two related mouse glycoproteins involved in cell interactions, LFA-1 and Mac-1. J Exp Med. 1983 Aug 1;158(2):586–602. doi: 10.1084/jem.158.2.586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Springer T. A., Teplow D. B., Dreyer W. J. Sequence homology of the LFA-1 and Mac-1 leukocyte adhesion glycoproteins and unexpected relation to leukocyte interferon. Nature. 1985 Apr 11;314(6011):540–542. doi: 10.1038/314540a0. [DOI] [PubMed] [Google Scholar]
- Springer T., Galfré G., Secher D. S., Milstein C. Mac-1: a macrophage differentiation antigen identified by monoclonal antibody. Eur J Immunol. 1979 Apr;9(4):301–306. doi: 10.1002/eji.1830090410. [DOI] [PubMed] [Google Scholar]
- Thurlow P. J., Barlow B., Connellan J. M., McKenzie I. F. Detection of glycoprotein IIb and IIIa by monoclonal antibodies. Br J Haematol. 1983 Sep;55(1):123–134. doi: 10.1111/j.1365-2141.1983.tb01230.x. [DOI] [PubMed] [Google Scholar]


