Abstract
Candida albicans colonises numerous niches within humans and thus its success as a pathogen is dependent on its ability to adapt to diverse growth environments within the host. Two component signal transduction is a common mechanism by which bacteria respond to environmental stimuli and, although less common, two component-related pathways have also been characterised in fungi. Here we report the identification and characterisation of a novel two component response regulator protein in C. albicans which we have named CRR1 (Candida Response Regulator 1). Crr1 contains a receiver domain characteristic of response regulator proteins, including the conserved aspartate that receives phosphate from an upstream histidine kinase. Significantly, orthologues of CRR1 are present only in fungi belonging to the Candida CTG clade. Deletion of the C. albicans CRR1 gene, or mutation of the predicted phospho-aspartate, causes increased sensitivity of cells to the oxidising agent hydrogen peroxide. Crr1 is present in both the cytoplasm and nucleus, and this localisation is unaffected by oxidative stress or mutation of the predicted phospho-aspartate. Furthermore, unlike the Ssk1 response regulator, Crr1 is not required for the hydrogen peroxide-induced activation of the Hog1 stress-activated protein kinase pathway, or for the virulence of C. albicans in a mouse model of systemic disease. Taken together, our data suggest that Crr1, a novel response regulator restricted to the Candida CTG clade, regulates the response of C. albicans cells to hydrogen peroxide in a Hog1-independent manner that requires the function of the conserved phospho-aspartate.
Introduction
Two component signal transduction is a primary mechanism utilised by bacteria to respond to environmental stimuli. These signalling modules are comprised of a sensor histidine kinase and a response regulator protein containing a receiver domain [1]. Upon stimulation, phosphate is transferred from a histidine residue in the kinase to an aspartate residue located in the receiver domain of the response regulator protein. This phosphorylation influences the activity of the response regulator protein to trigger the appropriate response to the environmental stimulus. Two component-related signal transduction mechanisms are also utilised, although less extensively, in certain eukaryotes including fungi, slime mould and plants [2]. Interestingly, in contrast to the bacterial systems, a more complex multi-step phosphorelay involving three components appears to predominate in eukaryotic systems. Such eukaryotic pathways typically consist of a hybrid sensor histidine kinase, containing both kinase and receiver domains, an intermediary phosphorelay protein and a response regulator protein containing a receiver domain. In these cases phosphate is transferred from a histidine residue in the kinase domain to an aspartate residue located in the receiver domain of the histidine kinase. This phosphate is then transferred to a histidine residue in the phosphorelay protein which then completes transfer to an aspartate residue in the receiver domain of the response regulator.
A function unique to eukaryotic two component-related signalling pathways is to relay stress signals to stress-activated protein kinase (SAPK) pathways, which are important stress signalling modules solely found in eukaryotes [3]. In the model yeast Saccharomyces cerevisiae, osmotic stress-induced activation of the Hog1 SAPK is regulated by a multi-step two component-related system consisting of the Sln1 histidine kinase, the Ypd1 phosphorelay protein and the Ssk1 response regulator, which functions in parallel with a second pathway that contains the Sho1 transmembrane protein [4]. In response to osmotic stress, the Sln1 histidine kinase is inactivated due to loss of turgor pressure within the membrane [5]. This subsequently halts phosphorelay through Ypd1 leading to a rapid dephosphorylation of Ssk1 [6]. Dephosphorylated Ssk1 activates the MAPKKKs Ssk2/Ssk22 [7], which subsequently activate Hog1. Interestingly, in the distantly related yeast Schizosaccharomyces pombe, an analogous system comprising of the histidine kinases, Mak2 (Phk1) and Mak3 (Phk2) [8], [9], the phosphorelay protein Mpr1 (Spy1) [10] and the response regulator Mcs4 [8], functions to relay hydrogen peroxide, but not osmotic, stress signals to the Hog1-related Sty1 (Spc1) SAPK pathway. Peroxide sensing by the S. pombe two component-related pathway is mediated by GAF and PAS domains present in the Mak2 and Mak3 kinases [9].
In addition to Ssk1/Mcs4, S. cerevisiae and S. pombe both contain a second response regulator protein termed Skn7 [11] and its homologue Prr1 [12], respectively. However, unlike Ssk1/Mcs4, the Skn7 and Prr1 response regulators are transcription factors that do not regulate the Hog1/Sty1 SAPK pathways. In S. cerevisiae, Skn7 regulates the expression of genes involved in the cell wall and the oxidative stress response [13], [14] yet, interestingly, two component-mediated phosphorylation of Skn7 is only required for the cell wall functions of this transcription factor [13], [14]. In contrast, recent studies illustrated that Prr1 is required for the transcriptional response of S. pombe cells to a wide range of hydrogen peroxide concentrations [9], [15] and that two component-mediated phosphorylation of Prr1 is required for the response to high but not low levels of hydrogen peroxide [9].
Two component proteins, related to those in S. cerevisiae and S. pombe, have also been identified in the major fungal pathogen of humans, Candida albicans [16]. Stress responses are intimately linked with the virulence of this medically important fungus [17], and notably several of these two component proteins have been implicated in pathogenesis [16]. C. albicans contains three structurally distinct histidine kinases; Sln1 is most similar to the Sln1 osmosensor in S. cerevisiae [18], Chk1 is the closest homologue of the Mak2 and Mak3 hydrogen peroxide stress sensors in S. pombe [19], and Nik1/Cos1 is related to the Nik-1 histidine kinase in Neurospora crassa [18], [20], [21]. C. albicans also contains a single phosphorelay protein, Ypd1 [22], and homologues of the Ssk1 and Skn7 response regulators [23], [24]. Indeed, similar to Ssk1 and Mcs4 in S. cerevisiae and S. pombe, respectively, Ssk1 is important for the regulation of the Hog1 SAPK in C. albicans. Specifically, Ssk1 is required for efficient oxidative stress-induced activation of Hog1 in C. albicans [25], [26], which is reminiscent of Mcs4 regulation of the Sty1 SAPK in S. pombe. However, the identity of the histidine kinase(s) responsible for sensing and signalling oxidative stress signals to Ssk1 in C. albicans remains elusive [27], [28]. C. albicans also contains Skn7, a homologue of the Skn7/Prr1 response regulators in S. cerevisiae and S. pombe and, similar to findings in these model yeasts, C. albicans cells lacking Skn7 display impaired resistance to oxidative stress-inducing agents [24].
Here, we describe the identification and characterisation of a novel response regulator in C. albicans, which we name Crr1 (Candida Response Regulator 1), that is not conserved in S. cerevisiae or S. pombe. We demonstrate that Crr1 is specifically involved in the response of C. albicans to hydrogen peroxide stress, but not to other oxidising agents or a range of other stress conditions. Furthermore, our data suggests that Crr1 functions in a Hog1-independent pathway and that the role of the protein in hydrogen peroxide responses is regulated by the phosphorylation of the conserved aspartic acid residue within the receiver domain. Collectively, our data suggests that the novel response regulator protein Crr1 functions in a hitherto unidentified two component signal transduction pathway to specifically regulate the response of C. albicans to hydrogen peroxide.
Materials and Methods
Ethics statement
The animal experiments carried out were approved by the University of Aberdeen local ethical review committee and under Project Licence PPL 60/4135, granted by the UK Home Office. All work conformed to UK Home Office regulations.
Strains and growth conditions
The C. albicans strains used in this study are listed in Table 1. Cells were grown at 30°C in either YPD media (2% yeast extract, 1% bactopeptone, 2% glucose) or SD media (6.79 g/l yeast nitrogen base without amino acids, 2% glucose) supplemented with the required nutrients for auxotrophic mutants [29].
Table 1. Strains used in this study.
| Strain | Genotype | Source |
| RM1000 | ura3::λ imm434/ura3::λimm434, his1::hisG/his1::hisG | [51] |
| JC50 | RM1000 hog1::LoxP-ura3-LoxP, hog1::LoxP-HIS1-LoxP+CIp20 | [36] |
| JC52 | RM1000 hog1::LoxP-ura3-LoxP, hog1::LoxP-HIS1-LoxP+CIp20-HOG1 | [36] |
| JC806 | RM1000+CIp20 | This study |
| JC528 | RM1000 crr1::hisG/crr1::hisG | This study |
| JC566 | RM1000 crr1::hisG/crr1::hisG+CIp20 | This study |
| JC803 | RM1000 crr1::hisG/crr1::hisG+CIp20-CRR1 | This study |
| JC804 | RM1000 crr1::hisG/crr1::hisG+CIp20-CRR1 | This study |
| JC784 | RM1000 ssk1::LoxP-URA3-LoxP/ssk1::LoxP-HIS1-LoxP | This study |
| JC787 | RM1000 crr1::hisG/crr1::hisG, ssk1::LoxP-URA3-LoxP/ssk1::LoxP-HIS1-LoxP | This study |
| JC924 | RM1000 crr1::hisG/crr1::hisG+pACT1-CRR1GFP | This study |
| JC926 | RM1000 crr1::hisG/crr1::hisG+pACT1-CRR1(D209N)GFP | This study |
| SN148 | arg4Δ/arg4Δ leu2Δ/leu2Δ his1Δ/his1Δ ura3Δ::imm434/ura3Δ::imm434 iro1Δ::imm434/iro1Δ::imm434 | [32] |
| JC747 | SN148+CIp30 | [52] |
| JC1552 | SN148 ssk1::LoxP-ARG4 -LoxP/ssk1::LoxP-HIS1-LoxP+CIp20 | This study |
| JC1571 | SN148 crr1::LoxP-ARG4 -LoxP/crr1::LoxP-HIS1-LoxP | This study |
| JC1572 | SN148 crr1::LoxP-ARG4 -LoxP/crr1::LoxP-HIS1-LoxP+CIp20 | This study |
| JC1574 | SN148 crr1::LoxP-ARG4 -LoxP/crr1::LoxP-HIS1-LoxP+CIp20-CRR1 | This study |
| JC1576 | SN148 crr1::LoxP-ARG4 -LoxP/crr1::LoxP-HIS1-LoxP+pACT1-CRR1GFP | This study |
| JC1578 | SN148 crr1::LoxP-ARG4 -LoxP/crr1::LoxP-HIS1-LoxP+pACT1-CRR1(D209N)GFP | This study |
Strain construction
The oligonucleotide primers used for generating the constructs described below are listed in Table 2.
Table 2. Oligonucleotides used in this study.
| Name | Sequence 5′ to 3′ |
| CRR1PromF | gcgcggatccgcgaaagttcacagttattgtg |
| CRR1TermR | gcgcggatcctataaacacgacaaacctccttgg |
| CRR1delF | aaattgcctccccctgttgcaagtaatttttcctcctttttttttgatttgtatatttttacaaccaataagttattattgaattcattgtacacactaaccagggttttcccagtcacg |
| CRR1delR | aaacatcgtagaacaacgtagaaacaaccataaaccattcaaagaaacaagatacaaaacaaaaatataagtcaaacaaaaaacccgctctgaatgcatctcactaaagggaacaaaagc |
| SSK1delF | ctaggggaaccaaaaaaaaaaatattaaaaataaccaagaaagaaataaagaaacaagaattctgcttataaaacgaatataaaaaaaaaataataactcccagggttttcccagtcacg |
| SSK1delR | aattttatcaatcattaaaagcaaaaactgaaaaaaaccgaaaacctaatttattccaacgactcatcttagtggcatttcataaatccgtttttttcttctcactaaagggaacaaaagc |
| CRR1HindIIIF | cggcccaagcttatgatatccatgaacccaattatg |
| CRR1GFPHindIIIR | cggcccaagcttgctattttgttttttcttg |
| CRR1DNmutF | cattccatatttatcaacattgagatgcctgatg |
| CRR1MHR | gaattcgctagcttaatgatggtgatgatggtgaagtcctcctcgctgatcaatttttgttcttcagccatggacaaatcttcttcagaaattaacttttgctcctctattttgttttttcttgttataattatatc |
| CRR1PstIF | aatgtctgcagccatcaatcggtatataatttggaag |
Deletion of CRR1
The CRR1 locus was disrupted by Ura-blasting [30] in RM1000 to generate strain JC528 (crr1Δ). The crr1::hisG-URA3-hisG disruption cassette deleted codons 2–281 of the 282 codon predicted open reading frame. Gene disruptions were confirmed by PCR. To construct re-integrant control strains the CRR1 gene plus 1000 bp of the promoter region and 214 bp of the terminator region were amplified by PCR, using the oligonucleotide primers CRR1PromF and CRR1TermR, and ligated into the BamHI site of CIp20 to create Clp20-CRR1 [31]. The CIp20-CRR1 plasmid was digested with StuI and integrated at the RPS10 locus in the crr1Δ mutant to generate strains JC803 and JC804. To generate a crr1Δ deletion mutant that was auxotrophically identical to the reconstituted strain, the CIp20 vector was integrated at the RPS10 locus in the crr1Δ mutant to generate strain JC566. CRR1 was also deleted in a second strain background, SN148 [32]. CRR1 disruption cassettes, comprising either the ARG4 gene or the HIS1 gene flanked by loxP sites and 100 nucleotides corresponding to regions 5′ and 3′ of the CRR1 open reading frame, were generated by PCR using the oligonucleotide primers CRR1delF and CRR1delR and the plasmid templates pLAL2 or pLHL2 [33], respectively. These CRR1 disruption cassettes replaced the entire 282 codon open reading frame of CRR1. To construct the re-integrant control strain, the CIp20-CRR1 plasmid was digested with StuI as above and integrated at the RPS10 locus in the crr1Δ mutant (JC1571) to generate strain JC1574. As above, a crr1Δ deletion mutant that was auxotrophically identical to the reconstituted strain was generated by integrating the CIp20 vector at the RPS10 locus in the crr1Δ mutant to generate strain JC1572.
Deletion of SSK1
SSK1 disruption cassettes, comprising either the URA3 gene or HIS1 gene flanked by loxP sites and 100 nucleotides of DNA sequence corresponding to regions 5′ and 3′ of the SSK1 open reading frame, were generated by PCR using the oligonucleotide primers SSK1delF and SSK1delR, and the plasmid templates pLUL2 or pLHL2, respectively [33]. These SSK1 disruption cassettes, which deleted the entire 674 codon open reading frame, were sequentially introduced into C. albicans RM1000 (CRR1) or crr1Δ (JC528) cells to disrupt both alleles of SSK1 and generate strains JC784 and JC787, respectively. Gene disruptions were confirmed by PCR. SSK1 was also deleted in a second strain background, SN148 [32]. SSK1 disruption cassettes, comprising either the ARG4 gene or the HIS1 gene flanked by loxP sites and 100 nucleotides corresponding to regions 5′ and 3′ of the SSK1 open reading frame, were generated by PCR using the oligonucleotide primers SSK1delF and SSK1delR and the plasmid templates pLAL2 or pLHL2 [33], respectively. The CIp20 vector was integrated at the RPS10 locus in the resulting ssk1Δ mutant to generate strain JC1552.
GFP-tagging and mutagenesis of Crr1
To tag Crr1 at the C-terminus with GFP, the CRR1 gene was amplified by PCR using the oligonucleotide primers CRR1HindIIIF and CRR1GFPHindIIIR, and ligated into the HindIII site adjacent to the GFP sequence in pACT1-GFP [34] to create pACT1-CRR1GFP. The pACT1-CRR1GFP plasmid was linearised by digestion with StuI to target integration at the RPS10 locus in C. albicans crr1::hisG/crr1::hisG (JC528) and crr1::HIS1/crr1::ARG4 (JC1571) cells. In the resulting strains, JC924 and JC1576 respectively, expression of CRR1GFP is controlled by the ACT1 promoter. Mutagenesis of CRR1 to create the crr1D209N allele was performed by a two-stage PCR in which a mega primer, generated using the oligonucleotides CRR1DNmutF and CRR1MHR and the plasmid CIp10-CRR1 as template, was subsequently used with the oligonucleotide CRR1PstIF and the plasmid CIp10-CRR1 as template. The resulting 1.2 kb PCR fragment was digested with PstI and NheI and ligated into CIp-C-ZZ [35] digested with PstI and NheI to remove the TEV-protein A-encoding sequence. The resulting plasmid CIp-CRR1(D209N) was used as template for PCR, using the oligonucleotide primers CRR1HindIIIF and CRR1GFPHindIIIR, and the PCR fragment produced was then ligated into the HindIII site of pACT1-GFP as above. The resulting pACT1-CRR1(D209N)GFP plasmid was linearised and targeted to the RPS10 locus in C. albicans crr1::hisG/crr1::hisG (JC528) and crr1::HIS1/crr1::ARG4 (JC1571) cells, as described above, to generate strains JC926 and JC1578, respectively. The integrated open reading frames and the correct chromosomal insertion of the GFP-tagged derivatives of CRR1 were confirmed by PCR and DNA sequencing.
Stress sensitivity tests
C. albicans strains to be tested were grown in liquid culture at 30°C to exponential phase and then 10 fold serial dilutions were spotted onto YPD plates containing the indicated compounds, using a 48-well replica plater (Sigma-Aldrich). Plates were incubated at 30°C for 24 h.
Hog1 phosphorylation assays
Cells were grown to mid-exponential phase at 30°C and exposed to either 5 mM hydrogen peroxide or 1 M NaCl for the indicated times. Protein extracts were prepared and phosphorylated Hog1 was detected by western blot with an anti-phospho-p38 antibody (New England Biolabs) as described previously [36]. Blots were stripped and total levels of Hog1 were determined by probing with an anti-Hog1 antibody (Santa Cruz Biotechnology).
Microscopy
Cells were fixed in 3.7% para-formaldehyde, washed in PEM (100 mM PIPES pH 7.6, 1 mM EGTA, 1 mM MgSO4) and spread onto poly-L-lysine-coated slides as described previously [36]. Cover slips were mounted onto slides using Vectashield mounting medium containing DAPI (Vector Laboratories, Burlingame, CA). DAPI and GFP fluorescence were captured by exciting cells with 365 nm and 450–490 nm wavelengths, respectively, using a Zeiss Axioscope microscope, with a 63× oil immersion objective, and Axiovision imaging system.
Virulence analysis
The standard 28-day survival method was employed to examine the potential role of Crr1 in mediating C. albicans virulence. Female BALB/c mice (6–8 weeks; Harlan, UK) were housed in groups of 6 with food and water provided ad libitum. C. albicans strains RM1000+CIp20 (JC806), crr1Δ+CIp20 (JC566), and crr1Δ+CIp20-CRR1 (JC803), were grown in NGY medium (0.1% neopeptone, 0.4% glucose, 0.1% yeast extract) for 18 h at 30°C with constant agitation. Cells were harvested in sterile saline, washed twice, and resuspended to produce inoculants containing approximately 2.5×106 cfu/ml. Mice were injected with 100 µl of each strain, with inoculants ranging from 1.2–1.4×104 cfu/g mouse body weight. All experimental work was carried out under UK Home Office licence regulations and conformed to the requirements of the Ethical Review Committee of the University of Aberdeen. Mouse condition and weight were monitored daily, with mice culled either when they showed signs of severe infection or if weight decreased by more than 20% from the initial body weight. For all culled mice, death was recorded as occurring on the following day. At the time of death, the left kidney and spleen were aseptically removed and homogenised in saline for organ burden determination. Mouse survival was plotted and compared by Kaplan-Meier survival plots and kidney/spleen counts compared by Kruskall-Wallis non-parametric test.
Results
Identification of a novel response regulator in C. albicans
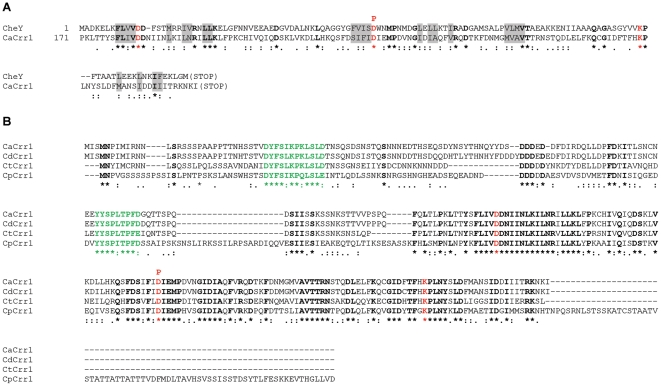
To shed more insight into the roles of two component signal transduction pathways in C. albicans we analysed the genome database (http://www.candidagenome.org/) for potential hitherto unidentified two component signal transduction proteins. This analysis revealed an uncharacterised open reading frame (orf19.5843) in C. albicans which we have named CRR1 (Candida Response Regulator 1), that encodes a potential novel two component response regulator protein. Analysis of the predicted sequence of Crr1 revealed a potential receiver domain, that contains all the key residues found in such domains, including two highly conserved aspartate residues, one of which receives phosphate from an upstream histidine kinase, and a highly conserved lysine residue (Fig. 1A, indicated in red bold). Moreover, the similarity of the potential receiver domain of Crr1 to the prototypical bacterial response regulator CheY, extends to a large number of hydrophobic residues that are components of the hydrophobic core of CheY (Fig. 1A, indicated in grey shading; [37]). Interestingly, homologues of Crr1 are not found in either of the well characterised model yeasts, S. cerevisiae or S. pombe. Indeed, the only closely related homologues of Crr1 are encoded by uncharacterised open reading frames present in other members of the Candida CTG clade [38] (Figs. 1B; S1). Moreover, it is noteworthy that the homology to Crr1 extends outside of the receiver domain only in the diploid members of the Candida CTG clade [38]; Candida dubliniensis, Candida tropicalis, Candida parapsilosis and Lodderomyces elongisporus (Fig. 1B and data not shown), whereas significant homology is restricted to the receiver domain in members of the haploid subclade [38]; Debaromyces hansenii, Candida guilliermondii and Candida lusitaniae (Fig. S1). It is also interesting to note that sequence analysis of the proteins in the haploid subclade did not reveal any obvious homology outside of the potential receiver domain within this subgroup of proteins (Fig. S1B). Taken together our analysis has revealed a novel family of response regulator proteins that appears to be confined to the Candida CTGclade.
Figure 1. Sequence analysis of the response regulator protein Crr1 in C. albicans and identification of homologues in other diploid members of the Candida CTG clade.
(A) Clustal alignment of the receiver domain located in the C-terminal region of Crr1 (orf19.5843) of C. albicans (CaCrr1) and the CheY response regulator protein of Escherichia coli which essentially consists of a receiver domain. Residues that are identical between the receiver domains are indicated by bold, the aspartate and lysine residues conserved in all receiver domains are shown in red bold, and the aspartate residue which is predicted to be phosphorylated by two component signal transduction by a bold red “P”. Hydrophobic residues that are components of the hydrophobic core of CheY are indicated by grey shading. Note that the homology between the receiver domains extends to the replacement of amino acids with others with similar chemical properties. A colon indicates a highly similar substitution and a full stop a similar substitution. (B) Clustal alignment of potential Crr1 homologues in C. albicans (CaCrr1), C. dubliniensis (CD36_30940; CdCrr1), C. tropicalis (CTRG_00590; CtCrr1), and C. parapsilosis (CPAG_04104; CpCrr1). Residues shared by all four proteins are highlighted as described in (A) above. Two conserved regions were identified (green bold) that lie N-terminal to the potential receiver domain in each protein. The predicted protein sequences of the Crr1 homologues in the diploid members of the Candida CTG clade were obtained by BLAST analyses at the C. albicans genome web site (http://candidagenome.org/).
Crr1 is required for hydrogen peroxide resistance in C. albicans
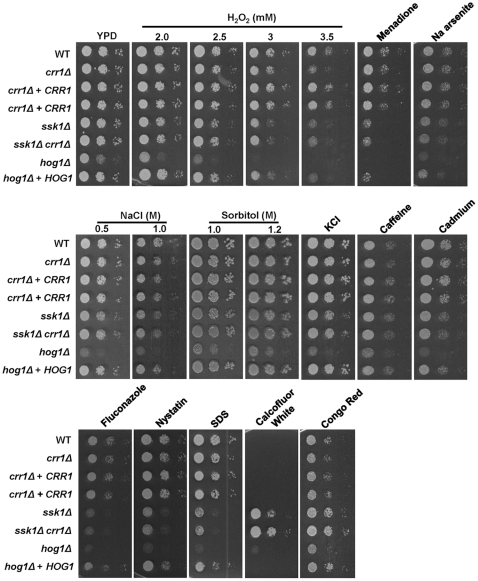
To examine the function(s) of Crr1 in C. albicans, a homozygous null mutant was generated. Each of the two copies of the CRR1 allele in this diploid fungus was inactivated using the ura-blaster gene disruption system, which deleted codons 2–281 of the predicted 282 codon reading frame in strain RM1000. Previous studies in C. albicans have implicated the other response regulator proteins, Ssk1 and Skn7, in the oxidative stress response. For example, cells lacking SSK1 display increased sensitivity to a range of oxidative stress-inducing agents including hydrogen peroxide, menadione and potassium superoxide [25], whilst cells lacking SKN7 display increased sensitivity to hydrogen peroxide and t-BOOH but not to menadione or potassium superoxide [24]. Hence, to examine the potential role of Crr1 in the response of C. albicans to oxidative and other stress conditions, we compared the sensitivity of wild-type, crr1Δ, and reintegrant (crr1Δ+CRR1) cells to an extensive panel of stress-inducing agents (Fig. 2). Deletion of CRR1 did not impair the growth of C. albicans under non-stress conditions. Notably, however, deletion of CRR1 specifically resulted in impaired resistance to the oxidative stress-inducing agent hydrogen peroxide and, importantly, this phenotype was reversed upon reintroduction of the wild-type CRR1 gene into the crr1Δ strain (Fig. 2). In contrast, no notable increase in stress sensitivity was observed in response to other oxidative stress-inducing agents, such as menadione, a variety of osmotic stress-inducing agents, such as NaCl, KCl or sorbitol, heavy metals such as cadmium or arsenic, caffeine, or antifungal drugs such as fluconazole or nystatin (Fig. 2). Deletion of CRR1 in the SN148 [32] background replicated such findings (Fig. S2A).
Figure 2. The Crr1 response regulator is required for the resistance of cells to hydrogen peroxide.
2×103 cells, and 10-fold dilutions thereof, of exponentially-growing wild-type (WT, JC806), crr1Δ (JC566), crr1Δ+CRR1 (JC803, JC804), ssk1Δ (JC784), ssk1Δ/crr1Δ (JC787), hog1Δ (JC50) and hog1Δ+HOG1 (JC52) strains were spotted onto YPD plates containing the following agents; hydrogen peroxide (2, 2.5, 3, 3.5 mM), 250 mM menadione, 2.5 mM Na arsenite, NaCl (0.5, 1.0 M), sorbitol (1.0, 1.2 M), 0.6 M KCl, 12.5 mM caffeine, 1 mM cadmium, 5 µg/ml fluconazole, 5 µg/ml nystatin, 0.02% SDS, 50 µg/ml Calcofluor White and 200 µg/ml Congo Red. Plates were incubated at 30°C for 24 h.
In C. albicans the Hog1 SAPK is activated in response to a range of stress conditions, including hydrogen peroxide and, moreover, the Ssk1 response regulator plays an important role in the relay of hydrogen peroxide signals to Hog1 [25], [26], [28]. In S. cerevisiae, the analogous Ssk1 response regulator has been shown to relay osmotic, but not oxidative, stress signals to the Hog1 SAPK and it does this in parallel with a second, Sho1-mediated, osmosensing pathway (reviewed in [4]). Intriguingly, although the analogous Sho1 pathway does not appear to relay osmotic stress signals to Hog1 in C. albicans [28], both C. albicans single ssk1Δ or double ssk1Δsho1Δ mutants retain wild-type levels of Hog1 activation following osmotic stress [25], [28]. Thus, there is a distinct mechanism of Hog1 activation in response to osmotic stress in C. albicans that is independent of both Sho1 and Ssk1. The identification of Crr1 raised the possibility that this novel response regulator functions redundantly with Ssk1 to relay osmotic and possibly other stress signals to the Hog1 SAPK. To investigate this hypothesis, first a double ssk1Δcrr1Δ mutant was created and the stress sensitive phenotypes exhibited by ssk1Δ, crr1Δ, ssk1Δcrr1Δ and hog1Δ mutants compared. Significantly, the single ssk1Δ and crr1Δ mutants displayed a similar level of sensitivity to hydrogen peroxide, intermediate to that displayed by hog1Δ cells, and this was not further increased in the double ssk1Δcrr1Δ mutant (Fig. 2). Cells lacking SSK1 also displayed intermediate sensitivity to a range of other stress conditions such as the superoxide generator menadione, heavy metals, SDS and various drugs compared to that exhibited by hog1Δ cells (Fig. 2). However, deletion of CRR1 did not result in increased sensitivity to any of these conditions either in the presence or absence of SSK1 (Fig. 2). Similarly, whilst deletion of SSK1 increased the resistance of cells to cell wall damaging agents as previously reported [39], such as the cell wall biogenesis inhibitors Calcofluor White and Congo Red, this was not exacerbated in the ssk1Δcrr1Δ double mutant (Fig. 2). Collectively, these data suggest that, although Ssk1 is involved in the response to multiple stress conditions, Crr1 is specifically required for the response of cells to hydrogen peroxide.
Ssk1, but not Crr1, regulates Hog1 phosphorylation in response to hydrogen peroxide but both response regulators are dispensable for NaCl-induced Hog1 phosphorylation
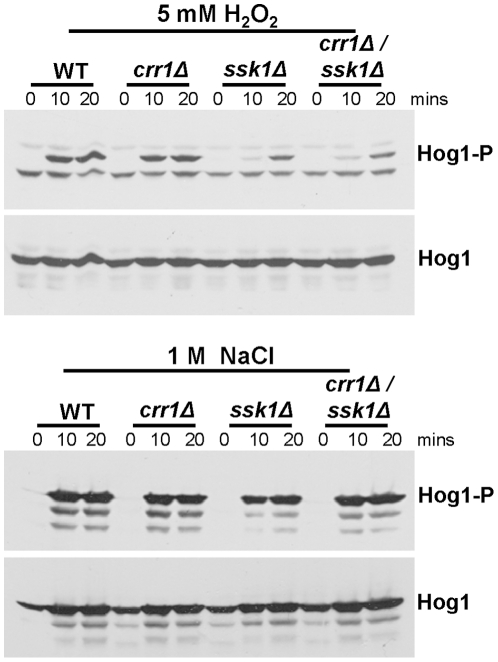
As cells lacking the Crr1 response regulator displayed increased sensitivity to hydrogen peroxide, in a manner that links the protein to Ssk1 function, we next examined whether, like Ssk1 [25], Crr1 relays oxidative stress signals to the Hog1 SAPK. Consistent with previous reports [25], [28], western blot analysis revealed that hydrogen peroxide-induced activation of the Hog1 SAPK was impaired in cells lacking Ssk1 (Fig. 3). However, in contrast, wild-type levels of hydrogen peroxide-induced Hog1 phosphorylation were observed in crr1Δ cells (Figs. 3; S2B), and the level of hydrogen peroxide-induced Hog1 activation associated with loss of SSK1 was not further impaired in ssk1Δcrr1Δ double mutant cells (Fig. 3). Furthermore, consistent with the wild-type levels of osmotic stress resistance exhibited by ssk1Δ, crr1Δ and ssk1Δcrr1Δ cells (Figs. 2; S2A), Hog1 activation was not impaired in any of these mutants following NaCl treatment (Figs. 3; S2B).
Figure 3. The Ssk1, but not the Crr1, response regulator is required for Hog1 activation in response to hydrogen peroxide.
Western blot analysis of whole cell extracts isolated from wild-type (WT, JC806), crr1Δ (JC566), ssk1Δ (JC784), and crr1Δssk1Δ (JC787) cells after treatment with 5 mM hydrogen peroxide or 1 M NaCl for the specified times. Western blots were probed with an anti-phospho-p38 antibody, which specifically recognises the phosphorylated, active form of C. albicans Hog1 (Hog1-P). Total levels of Hog1 protein were determined by stripping the blot and reprobing with an anti-Hog1 antibody which recognises both phosphorylated and unphosphorylated forms of Hog1 (Hog1).
Taken together, our data show that, whilst both response regulators Ssk1 and Crr1 are important for the oxidative stress response in C. albicans, Crr1 influences the response of cells to hydrogen peroxide in a pathway that is independent of Hog1 phosphorylation. Moreover, together with the observations that ssk1Δcrr1Δ cells display wild-type levels of osmotic stress resistance and osmotic stress-induced Hog1 activation, these data suggest that Ssk1 and Crr1 do not function redundantly to regulate Hog1 activation in response to osmotic stress.
Mutation of the putative phospho-aspartate of Crr1 does not impact on the cellular localisation of Crr1, but does result in impaired resistance to hydrogen peroxide
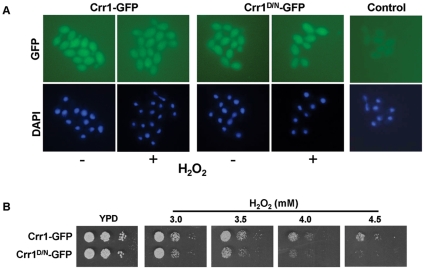
Previous studies in the model yeasts S. cerevisiae and S. pombe revealed that, whilst the Ssk1/Mcs4 response regulators are cytoplasmic [9], [40], the Skn7/Prr1 response regulator transcription factors are predominantly nuclear [9], [40]. Hence, to further characterise the novel response regulator Crr1 in C. albicans the cellular location of a Crr1-GFP fusion protein was determined by fluorescence microscopy. To facilitate this analysis a strain was created in which a CRR1-GFP fusion gene was expressed from the ACT1 promoter. This was necessary as previous experiments using epitope-tagged Crr1-fusions expressed at the CRR1 locus, indicated that Crr1 is a very low abundance protein (unpublished obs.). In addition, to investigate whether two component-mediated phosphorylation of Crr1 may impact on either the localisation and/or function of this response regulator, the conserved aspartic acid (D209) residue located in the receiver domain, that is predicted to be phosphorylated by two component phosphorelay (Fig. 1) was substituted with asparagine (Crr1D/N) which is predicted to mimic hypo-phosphorylation [41]. The Crr1-GFP and Crr1D/N-GFP fusion proteins were found to be present in both the cytoplasmic and nuclear compartments of the cell, with no obvious nuclear or cytoplasmic exclusion (Fig. 4A). Furthermore, treatment of cells with hydrogen peroxide did not alter this diffuse cellular localisation pattern (Fig. 4A). Thus, Crr1 has a distinct cellular localisation pattern to that previously documented for the Ssk1/Mcs4 and Skn7/Prr1 response regulators in S. cerevisiae and S. pombe and, moreover, this pattern is not affected by oxidative stress or mutation of the predicted phospho-aspartate residue.
Figure 4. Mutation of the putative phospho-aspartate of Crr1 impairs hydrogen peroxide resistance, but does not affect the cellular localisation of the protein.
(A) The localisation of GFP-tagged wild-type Crr1 (Crr1-GFP) and mutant Crr1, in which the putative phospho-aspatate residue in the receiver domain was mutated to asparagine (Crr1D/N-GFP), were determined by fluorescence microscopy of JC924 (Crr1-GFP) and JC926 (Crr1D/N-GFP) cells before (−) and after (+) treatment with 5 mM hydrogen peroxide (GFP) for 10 min. Nuclei were visualised by DAPI staining (DAPI). The control panel illustrates the level of background fluorescence observed in wild-type cells (JC806) expressing untagged Crr1. (B) 103 cells, and 10-fold dilutions thereof, of exponentially-growing crr1Δ cells expressing either CRR1-GFP (JC924) or CRR1D/N-GFP (JC926) were spotted onto YPD plates containing the indicated concentrations of hydrogen peroxide and incubated at 30°C for 24 h.
To further investigate whether two component-mediated phosphorylation of Crr1 is important for the function of the protein, the hydrogen peroxide sensitivities of crr1Δ cells expressing either Crr1-GFP or Crr1D/N-GFP were compared. Strikingly, cells expressing Crr1D/N-GFP were found to be reproducibly more sensitive to hydrogen peroxide than the isogenic ‘wild-type’ cells expressing Crr1-GFP and, moreover, displayed similar sensitivity to crr1Δ cells (compare Figs. 2 and 4B). This phenotype associated with Crr1D/N-GFP was replicated in SN148 cells (Fig. S2C). Based on the mutational analyses of other two component signal transduction proteins in bacteria and fungi these results strongly suggest that two component-mediated phosphorylation of Crr1 is important for the function of the protein in contributing to hydrogen peroxide resistance in C. albicans.
Crr1 is not required for C. albicans virulence
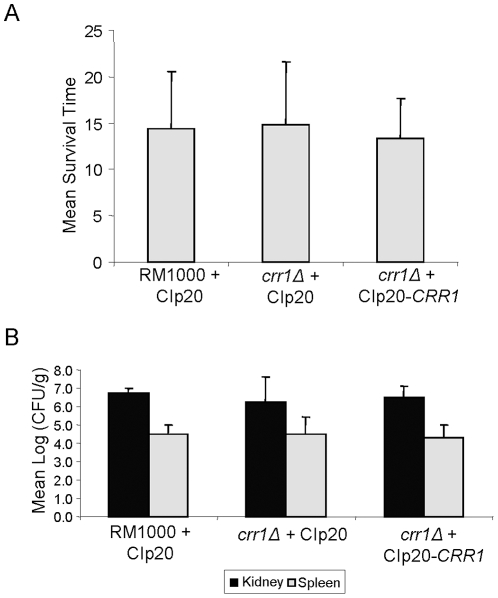
As shown above, Crr1 is required for wild-type levels of oxidative stress resistance in C. albicans. Interestingly, loss of Ssk1, but not Skn7, influences the virulence of C. albicans despite the observations that both are implicated in the oxidative stress response [24], [42]. Hence, we examined the potential role of Crr1 in virulence using the standard 28 day murine model of systemic candidiasis. Isogenic wild-type (JC806), crr1Δ (JC566), and reintegrant crr1Δ+CRR1 (JC803) strains, which all express URA3 from the RPS10 locus, were tested in the murine model of systemic candidiasis. Such controls are necessary as it is well-established that the genomic location of the URA3 disruption marker can influence expression levels which significantly impacts on the virulence of C. albicans [43]. However, deletion of CRR1 was found to have no detectable impact on the virulence of C. albicans (Fig. 5A). Mice infected with the crr1Δ mutant had a mean survival time of 14.8±6.7 days compared with 14.3±6.2 and 13.3±4.3 days for mice infected with wild-type or reintegrant crr1Δ+CRR1 cells, respectively. Kaplan-Meier and log rank tests showed no difference in virulence between the strains (P = 0.877). Consistent with these conclusions, no statistically significant difference in either kidney (P = 0.314) or spleen (P = 0.782) fungal burdens from mice infected with wild-type, crr1Δ or crr1Δ+CRR1 reintegrant cells was detected (Fig. 5B). Hence, in contrast to the C. albicans Ssk1 response regulator [42], we find no evidence that Crr1 is involved in the virulence of this fungal pathogen using the mouse model of systemic candidiasis.
Figure 5. Crr1 is dispensable for the virulence of C. albicans.
(A) Mean survival times, and (B) organ fungal burdens, for BALB/c mice infected with either WT (RM1000+Clp20, JC806), crr1Δ (crr1Δ+Clp20, JC566) or crr1Δ reintegrant (crr1Δ+Clp20-CRR1, JC803) cells following the standard 28-day survival murine model of systemic candidiasis.
Discussion
Here we have identified and characterised a novel response regulator in C. albicans which we have named Crr1. Two component signal transduction pathways are utilised to respond to environmental conditions in fungi and Crr1 was found to be important for the response of C. albicans to hydrogen peroxide. Furthermore, mutant cells expressing Crr1 in which the predicted phospho-aspartate was mutated to asparagine also displayed increased sensitivity to hydrogen peroxide, indicating two component-mediated phosphorelay is important for Crr1 function. Notably, extensive phylogenetic analyses revealed that this previously uncharacterised response regulator is solely found in fungi belonging to the Candida CTG clade [38]. Thus, these data suggest that the Crr1 family is involved in the response of cells to oxidative stress within a specific subgroup of fungal species.
C. albicans also contains members of the ubiquitous Ssk1 and Skn7 families of response regulator proteins which are present in diverse fungal species in addition to the Candida CTG clade [44]. It is intriguing that all three response regulators, Crr1, Skn7 and Ssk1, are required for oxidative stress resistance in C. albicans [24], [25]. Whilst Skn7 likely directly mediates the expression of antioxidant encoding genes, Ssk1 has been shown to be required for the hydrogen peroxide-induced activation of the Hog1 SAPK [25]. Here, our analysis of cells lacking CRR1 revealed that Hog1 activation is not impaired in crr1Δ cells. However, as ssk1Δcrr1Δ cells were no more sensitive to hydrogen peroxide than either single mutant, this suggests that Crr1 and Ssk1 may act in the same pathway. Thus, whilst Ssk1 functions upstream of Hog1, Crr1 may function downstream of this SAPK. Clearly, the nature of the relationship between Ssk1 and Crr1 function in the response of cells to hydrogen peroxide requires further investigation.
Although the receiver domain located in the C-terminal region of Crr1 contains all of the key residues required for the function of this domain, sequence analysis of the N-terminal region of Crr1 provided no insight into the potential function of this response regulator protein. Furthermore, this analysis was not facilitated by defining the localisation of Crr1, which is found throughout the cell. However, the open reading frame encoding Crr1 (orf19.5843) was previously identified in transcript profiling studies as a gene whose expression was up-regulated in the absence of the adenylyl cyclase Cdc35 [45], or in a conditional phospholipase C mutant at elevated temperatures [46]. Nonetheless, an extensive analysis of crr1Δ cells failed to establish a link between Crr1 and any cAMP- [45], [47] or phospholipase C- [46] dependent processes in C. albicans (Fig. 2). Interestingly, a recent report linked cAMP-mediated signalling to oxidative stress resistance in C. albicans, as the quorum sensing molecule farnesol stimulates resistance to hydrogen peroxide by inhibiting the Ras-cAMP pathway [48]. However, similar increases in farnesol-induced hydrogen peroxide resistance were observed in both crr1Δ (6.9%) and crr1Δ+CRR1 (8.9%) reconstituted cells to those reported previously [48]. Thus, these data indicate that Crr1 mediates the resistance of C. albicans to hydrogen peroxide independently of both Hog1 activation and farnesol-mediated inhibition of cAMP signalling.
In this paper we describe the identification of a response regulator protein that appears to be confined to the Candida CTG clade [38]. All of the Crr1-related proteins in the haploid and diploid members of the clade share extensive homology between their receiver domains suggesting that they had a common ancestor (Figs. 1B; S1). In addition, we have identified at least two conserved regions within the N-terminal regions of the diploid members of the Candida clade (Fig. 1B) although the potential function(s) of these regions awaits further investigation. Given that two component signal transduction pathways are utilised to respond to the environment by regulating appropriate cellular responses it is tempting to speculate that the diploid members of the Candida CTG clade respond to the oxidising agent hydrogen peroxide in a similar manner through the function of these Crr1 homologues. It is important to note, however, that whilst all members of the Candida CTG clade can cause disease in humans [49], we can find no evidence that Crr1 affects the virulence of C. albicans in a standard mouse model of systemic candidiasis. Thus, it is possible that Crr1 functions to allow adaptation to an environmental niche outside the human host. Alternatively, Crr1 function may be required for C. albicans to exist as a commensal organism within specific host niches that are not replicated in a systemic model of disease. In this regard it is noteworthy that a recent study revealed that the expression of CRR1 in C. albicans is induced during the late stages of biofilm formation [50]. Clearly, much is still to be learnt about the biological roles of the novel Crr1 response regulator, which is only present in the Candida CTG clade of fungal species.
Supporting Information
Sequence analysis of the closest homologues of CaCrr1 in the haploid members of the Candida CTG clade. (A) Clustal alignment of CaCrr1 with the closest homologues of CaCrr1 present in Debaromyces hansenii (DEHA2G23386g), Candida guilliermondii (PGUG_04093) and Candida lusitaniae (CLUG_02461). The main shared region of homology is limited to the potential receiver domain located in all of these proteins. Residues that are identical between all four proteins are indicated by bold, the aspartate and lysine residues conserved in all receiver domains are shown in red bold, and the aspartate residue which is predicted to be phosphorylated by two component signal transduction by a bold red “P”. Note that the homology between the receiver domains extends to the replacement of amino acids with others with similar chemical properties. A colon indicates a highly similar substitution and a full stop a similar substitution. (B) Clustal alignment of the closest homologues of CaCrr1 present in D. hansenii (DEHA2G23386g), C. guilliermondii (PGUG_04093) and C. lusitaniae (CLUG_02461) revealed that the main region of homology shared between proteins in the haploid group in the Candida clade is limited to the potential receiver domain located in all three proteins. Residues shared by all three proteins are highlighted as described in (A) above. The predicted protein sequences of the Crr1 homologues in the haploid members of the Candida clade were obtained by BLAST analyses at the C. albicans genome web site (http://candidagenome.org/).
(TIFF)
Phenotypic analysis of Crr1 function in the SN148 C. albicans background, replicates that in RM1000 cells. (A) SN148 cells lacking CRR1 are sensitive to hydrogen peroxide but not other compounds. Approximately 103 cells, and 10-fold dilutions thereof, from exponentially-growing WT (SN148+CIp30; JC747), crr1Δ (JC1572) and crr1Δ+CRR1 (JC1574) strains were spotted onto YPD plates containing the indicated agents. Plates were incubated at 30°C for 24 h. (B) Ssk1 but not Crr1 is required for Hog1 activation in response to hydrogen peroxide in SN148 cells. Western blot analysis of whole cell extracts isolated from wild-type (WT, JC747), ssk1Δ (JC1552), crr1Δ (JC1572), and crr1Δ+CRR1 (JC1574) cells after treatment with 5 mM hydrogen peroxide or 1 M NaCl for the specified times. Western blots were probed with an anti-phospho-p38 antibody, which specifically recognises the phosphorylated, active form of C. albicans Hog1 (Hog1-P). Total levels of Hog1 protein were determined by stripping the blot and reprobing with an anti-Hog1 antibody which recognises both phosphorylated and unphosphorylated forms of Hog1 (Hog1). (C) Mutation of the putative phospho-aspartate of Crr1 impairs hydrogen peroxide resistance in SN148 cells. 103 cells, and 10-fold dilutions thereof, of exponentially-growing crr1Δ cells expressing either CRR1-GFP (JC1576) or CRR1D/N-GFP (JC1578) were spotted onto YPD plates containing the indicated concentrations of hydrogen peroxide and incubated at 30°C for 24 h.
(TIFF)
Acknowledgments
We are grateful to Iswarya Dantuluru for her technical assistance during the early stages of this project.
Footnotes
Competing Interests: The authors have declared that no competing interests exist.
Funding: This work was supported by the Wellcome Trust (grant numbers 078779 and 086048, www.wellcome.ac.uk) and Nuffield Foundation (grant number URB/BH081624, www.nuffieldfoundation.org). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Egger LA, Park H, Inouye M. Signal Transduction via the Histidyl-Aspartyl Phosphorelay. Genes To Cells. 1997;2:167–184. doi: 10.1046/j.1365-2443.1997.d01-311.x. [DOI] [PubMed] [Google Scholar]
- 2.Santos JL, Shiozaki K. Fungal histidine kinases. Sci STKE. 2001;2001:RE1. doi: 10.1126/stke.2001.98.re1. [DOI] [PubMed] [Google Scholar]
- 3.Smith DA, Morgan BA, Quinn J. Stress signalling to fungal stress-activated protein kinase pathways. FEMS Microbiology Letters. 2010;306:1–8. doi: 10.1111/j.1574-6968.2010.01937.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hohmann S. Osmotic stress signaling and osmoadaptation in yeasts. Microbiol Mol Biol Rev. 2002;66:300–372. doi: 10.1128/MMBR.66.2.300-372.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Reiser V, Raitt DC, Saito H. Yeast osmosensor Sln1 and plant cytokinin receptor Cre1 respond to changes in turgor pressure. J Cell Biol. 2003;161:1035–1040. doi: 10.1083/jcb.200301099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Posas F, Wurgler-Murphy SM, Maeda T, Witten EA, Thai TC, et al. Yeast HOG1 MAP kinase cascade is regulated by a multistep phosphorelay mechanism in the SLN1-YPD1-SSK1 “two-component” osmosensor. Cell. 1996;86:865–875. doi: 10.1016/s0092-8674(00)80162-2. [DOI] [PubMed] [Google Scholar]
- 7.Posas F, Saito H. Activation of the yeast SSK2 MAP kinase kinase kinase by the SSK1 two-component response regulator. Embo J. 1998;17:1385–1394. doi: 10.1093/emboj/17.5.1385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Buck V, Quinn J, Soto Pino T, Martin H, Saldanha J, et al. Peroxide sensors for the fission yeast stress-activated mitogen-activated protein kinase pathway. Mol Biol Cell. 2001;12:407–419. doi: 10.1091/mbc.12.2.407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Quinn J, Malakasi P, Smith DA, Cheetham J, Buck V, et al. Two-component mediated peroxide sensing and signal transduction in fission yeast. Antioxid Redox Signal. 2011;15:153–165. doi: 10.1089/ars.2010.3345. [DOI] [PubMed] [Google Scholar]
- 10.Nguyen AN, Lee A, Place W, Shiozaki K. Multistep phosphorelay proteins transmit oxidative stress signals to the fission yeast stress-activated protein kinase. Mol Biol Cell. 2000;11:1169–1181. doi: 10.1091/mbc.11.4.1169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Brown JL, Bussey H, Stewart RC. Yeast Skn7p functions in a eukaryotic two-component regulatory pathway. Embo J. 1994;13:5186–5194. doi: 10.1002/j.1460-2075.1994.tb06849.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ohmiya R, Kato C, Yamada H, Aiba H, Mizuno T. A fission yeast gene (prr1+) that encodes a response regulator implicated in oxidative stress response. J Biochem (Tokyo) 1999;125:1061–1066. doi: 10.1093/oxfordjournals.jbchem.a022387. [DOI] [PubMed] [Google Scholar]
- 13.Li S, Dean S, Li Z, Horecka J, Deschenes RJ, et al. The eukaryotic two-component histidine kinase Sln1p regulates OCH1 via the transcription factor, Skn7p. Mol Biol Cell. 2002;13:412–424. doi: 10.1091/mbc.01-09-0434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Morgan BA, Banks GR, Toone WM, Raitt D, Kuge S, et al. The Skn7 response regulator controls gene expression in the oxidative stress response of the budding yeast Saccharomyces cerevisiae. Embo J. 1997;16:1035–1044. doi: 10.1093/emboj/16.5.1035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chen D, Wilkinson CR, Watt S, Penkett CJ, Toone WM, et al. Multiple pathways differentially regulate global oxidative stress responses in fission yeast. Mol Biol Cell. 2008;19:308–317. doi: 10.1091/mbc.E07-08-0735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kruppa M, Calderone R. Two-component signal transduction in human fungal pathogens. FEMS Yeast Res. 2006;6:149–159. doi: 10.1111/j.1567-1364.2006.00024.x. [DOI] [PubMed] [Google Scholar]
- 17.Brown AJ, Haynes K, Quinn J. Nitrosative and oxidative stress responses in fungal pathogenicity. Curr Opin Microbiol. 2009;12:384–391. doi: 10.1016/j.mib.2009.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Nagahashi S, Mio T, Ono N, Yamada-Okabe T, Arisawa M, et al. Isolation of CaSLN1 and CaNIK1, the genes for osmosensing histidine kinase homologues, from the pathogenic fungus Candida albicans. Microbiology. 1998;144:425–432. doi: 10.1099/00221287-144-2-425. [DOI] [PubMed] [Google Scholar]
- 19.Calera JA, Choi GH, Calderone RA. Identification of a putative histidine kinase two-component phosphorelay gene (CaHK1) in Candida albicans. Yeast. 1998;14:665–674. doi: 10.1002/(SICI)1097-0061(199805)14:7<665::AID-YEA246>3.0.CO;2-#. [DOI] [PubMed] [Google Scholar]
- 20.Alex LA, Korch C, Selitrennikoff CP, Simon MI. COS1, a two-component histidine kinase that is involved in hyphal development in the opportunistic pathogen Candida albicans. Proc Natl Acad Sci U S A. 1998;95:7069–7073. doi: 10.1073/pnas.95.12.7069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Srikantha T, Tsai L, Daniels K, Enger L, Highley K, et al. The two-component hybrid kinase regulator CaNIK1 of Candida albicans. Microbiology. 1998;144:2715–2729. doi: 10.1099/00221287-144-10-2715. [DOI] [PubMed] [Google Scholar]
- 22.Calera JA, Herman D, Calderone R. Identification of YPD1, a gene of Candida albicans which encodes a two-component phosphohistidine intermediate protein. Yeast. 2000;16:1053–1059. doi: 10.1002/1097-0061(200008)16:11<1053::AID-YEA598>3.0.CO;2-H. [DOI] [PubMed] [Google Scholar]
- 23.Calera JA, Calderone RA. Identification of a putative response regulator two-component phosphorelay gene (CaSSK1) from Candida albicans. Yeast. 1999;15:1243–1254. doi: 10.1002/(SICI)1097-0061(19990915)15:12<1243::AID-YEA449>3.0.CO;2-5. [DOI] [PubMed] [Google Scholar]
- 24.Singh P, Chauhan N, Ghosh A, Dixon F, Calderone R. SKN7 of Candida albicans: mutant construction and phenotype analysis. Infect Immun. 2004;72:2390–2394. doi: 10.1128/IAI.72.4.2390-2394.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Chauhan N, Inglis D, Roman E, Pla J, Li D, et al. Candida albicans response regulator gene SSK1 regulates a subset of genes whose functions are associated with cell wall biosynthesis and adaptation to oxidative stress. Eukaryot Cell. 2003;2:1018–1024. doi: 10.1128/EC.2.5.1018-1024.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Menon V, Li D, Chauhan N, Rajnarayanan R, Dubrovska A, et al. Functional studies of the Ssk1p response regulator protein of Candida albicans as determined by phenotypic analysis of receiver domain point mutants. Mol Microbiol. 2006;62:997–1013. doi: 10.1111/j.1365-2958.2006.05438.x. [DOI] [PubMed] [Google Scholar]
- 27.Li D, Gurkovska V, Sheridan M, Calderone R, Chauhan N. Studies on the regulation of the two-component histidine kinase gene CHK1 in Candida albicans using the heterologous lacZ reporter gene. Microbiology. 2004;150:3305–3313. doi: 10.1099/mic.0.27237-0. [DOI] [PubMed] [Google Scholar]
- 28.Roman E, Nombela C, Pla J. The Sho1 adaptor protein links oxidative stress to morphogenesis and cell wall biosynthesis in the fungal pathogen Candida albicans. Mol Cell Biol. 2005;25:10611–10627. doi: 10.1128/MCB.25.23.10611-10627.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sherman F. Getting started with yeast. Methods Enzymol. 1991;194:3–21. doi: 10.1016/0076-6879(91)94004-v. [DOI] [PubMed] [Google Scholar]
- 30.Fonzi WA, Irwin MY. Isogenic strain construction and gene mapping in Candida albicans. Genetics. 1993;134:717–728. doi: 10.1093/genetics/134.3.717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Murad AM, Lee PR, Broadbent ID, Barelle CJ, Brown AJ. CIp10, an efficient and convenient integrating vector for Candida albicans. Yeast. 2000;16:325–327. doi: 10.1002/1097-0061(20000315)16:4<325::AID-YEA538>3.0.CO;2-#. [DOI] [PubMed] [Google Scholar]
- 32.Noble SM, Johnson AD. Strains and strategies for large-scale gene deletion studies of the diploid human fungal pathogen Candida albicans. Eukaryot Cell. 2005;4:298–309. doi: 10.1128/EC.4.2.298-309.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Dennison PMJ, Ramsdale M, Manson CL, Brown AJP. Gene disruption in Candida albicans using a synthetic, codon-optimised Cre loxP system. Fungal Genet Biol. 2005;42:737–748. doi: 10.1016/j.fgb.2005.05.006. [DOI] [PubMed] [Google Scholar]
- 34.Barelle CJ, Manson CL, MacCallum DM, Odds FC, Gow NA, et al. GFP as a quantitative reporter of gene regulation in Candida albicans. Yeast. 2004;21:333–340. doi: 10.1002/yea.1099. [DOI] [PubMed] [Google Scholar]
- 35.Blackwell C, Russell CL, Argimon S, Brown AJ, Brown JD. Protein A-tagging for purification of native macromolecular complexes from Candida albicans. Yeast. 2003;20:1235–1241. doi: 10.1002/yea.1036. [DOI] [PubMed] [Google Scholar]
- 36.Smith DA, Nicholls S, Morgan BA, Brown AJ, Quinn J. A conserved stress-activated protein kinase regulates a core stress response in the human pathogen Candida albicans. Mol Biol Cell. 2004;15:4179–4190. doi: 10.1091/mbc.E04-03-0181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Stock JB, Ninfa AJ, Stock AM. Protein phosphorylation and regulation of adaptive responses in bacteria. Microbiol Rev. 1989;53:450–490. doi: 10.1128/mr.53.4.450-490.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Butler G, Rasmussen MD, Lin MF, Santos MA, Sakthikumar S, et al. Evolution of pathogenicity and sexual reproduction in eight Candida genomes. Nature. 2009;459:657–662. doi: 10.1038/nature08064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Roman E, Cottier F, Ernst JF, Pla J. Msb2 signaling mucin controls activation of Cek1 mitogen-activated protein kinase in Candida albicans. Eukaryot Cell. 2009;8:1235–1249. doi: 10.1128/EC.00081-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Lu JM, Deschenes RJ, Fassler JS. Saccharomyces cerevisiae histidine phosphotransferase Ypd1p shuttles between the nucleus and cytoplasm for SLN1-dependent phosphorylation of Ssk1p and Skn7p. Eukaryot Cell. 2003;2:1304–1314. doi: 10.1128/EC.2.6.1304-1314.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Klose KE, Weiss DS, Kustu S. Glutamate at the site of phosphorylation of nitrogen-regulatory protein NTRC mimics aspartyl-phosphate and activates the protein. J Mol Biol. 1993;232:67–78. doi: 10.1006/jmbi.1993.1370. [DOI] [PubMed] [Google Scholar]
- 42.Calera JA, Zhao XJ, Calderone R. Defective hyphal development and avirulence caused by a deletion of the SSK1 response regulator gene in Candida albicans. Infect Immun. 2000;68:518–525. doi: 10.1128/iai.68.2.518-525.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Brand A, MacCallum DM, Brown AJ, Gow NA, Odds FC. Ectopic expression of URA3 can influence the virulence phenotypes and proteome of Candida albicans but can be overcome by targeted reintegration of URA3 at the RPS10 locus. Eukaryot Cell. 2004;3:900–909. doi: 10.1128/EC.3.4.900-909.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Nikolaou E, Agrafioti I, Stumpf M, Quinn J, Stansfield I, et al. Phylogenetic diversity of stress signalling pathways in fungi. BMC Evol Biol. 2009;9:44. doi: 10.1186/1471-2148-9-44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Harcus D, Nantel A, Marcil A, Rigby T, Whiteway M. Transcription profiling of cyclic AMP signaling in Candida albicans. Mol Biol Cell. 2004;15:4490–4499. doi: 10.1091/mbc.E04-02-0144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Kunze D, Melzer I, Bennett D, Sanglard D, MacCallum D, et al. Functional analysis of the phospholipase C gene CaPLC1 and two unusual phospholipase C genes, CaPLC2 and CaPLC3, of Candida albicans. Microbiology. 2005;151:3381–3394. doi: 10.1099/mic.0.28353-0. [DOI] [PubMed] [Google Scholar]
- 47.Rocha CR, Schroppel K, Harcus D, Marcil A, Dignard D, et al. Signaling through adenylyl cyclase is essential for hyphal growth and virulence in the pathogenic fungus Candida albicans. Mol Biol Cell. 2001;12:3631–3643. doi: 10.1091/mbc.12.11.3631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Deveau A, Piispanen AE, Jackson AA, Hogan DA. Farnesol induces hydrogen peroxide resistance in Candida albicans yeast by inhibiting the Ras-cyclic AMP signaling pathway. Eukaryot Cell. 2010;9:569–577. doi: 10.1128/EC.00321-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Pfaller MA, Diekema DJ. Epidemiology of invasive candidiasis: a persistent public health problem. Clin Microbiol Rev. 2007;20:133–163. doi: 10.1128/CMR.00029-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Bonhomme J, Chauvel M, Goyard S, Roux P, Rossignol T, et al. Contribution of the glycolytic flux and hypoxia adaptation to efficient biofilm formation by Candida albicans. Mol Microbiol. 2011;80:995–1013. doi: 10.1111/j.1365-2958.2011.07626.x. [DOI] [PubMed] [Google Scholar]
- 51.Negredo A, Monteoliva L, Gil C, Pla J, Nombela C. Cloning, analysis and one-step disruption of the ARG5,6 gene of Candida albicans. Microbiology. 1997;143:297–302. doi: 10.1099/00221287-143-2-297. [DOI] [PubMed] [Google Scholar]
- 52.da Silva Dantas A, Patterson MJ, Smith DA, Maccallum DM, Erwig LP, et al. Thioredoxin regulates multiple hydrogen peroxide-induced signaling pathways in Candida albicans. Mol Cell Biol. 2010;30:4550–4563. doi: 10.1128/MCB.00313-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Sequence analysis of the closest homologues of CaCrr1 in the haploid members of the Candida CTG clade. (A) Clustal alignment of CaCrr1 with the closest homologues of CaCrr1 present in Debaromyces hansenii (DEHA2G23386g), Candida guilliermondii (PGUG_04093) and Candida lusitaniae (CLUG_02461). The main shared region of homology is limited to the potential receiver domain located in all of these proteins. Residues that are identical between all four proteins are indicated by bold, the aspartate and lysine residues conserved in all receiver domains are shown in red bold, and the aspartate residue which is predicted to be phosphorylated by two component signal transduction by a bold red “P”. Note that the homology between the receiver domains extends to the replacement of amino acids with others with similar chemical properties. A colon indicates a highly similar substitution and a full stop a similar substitution. (B) Clustal alignment of the closest homologues of CaCrr1 present in D. hansenii (DEHA2G23386g), C. guilliermondii (PGUG_04093) and C. lusitaniae (CLUG_02461) revealed that the main region of homology shared between proteins in the haploid group in the Candida clade is limited to the potential receiver domain located in all three proteins. Residues shared by all three proteins are highlighted as described in (A) above. The predicted protein sequences of the Crr1 homologues in the haploid members of the Candida clade were obtained by BLAST analyses at the C. albicans genome web site (http://candidagenome.org/).
(TIFF)
Phenotypic analysis of Crr1 function in the SN148 C. albicans background, replicates that in RM1000 cells. (A) SN148 cells lacking CRR1 are sensitive to hydrogen peroxide but not other compounds. Approximately 103 cells, and 10-fold dilutions thereof, from exponentially-growing WT (SN148+CIp30; JC747), crr1Δ (JC1572) and crr1Δ+CRR1 (JC1574) strains were spotted onto YPD plates containing the indicated agents. Plates were incubated at 30°C for 24 h. (B) Ssk1 but not Crr1 is required for Hog1 activation in response to hydrogen peroxide in SN148 cells. Western blot analysis of whole cell extracts isolated from wild-type (WT, JC747), ssk1Δ (JC1552), crr1Δ (JC1572), and crr1Δ+CRR1 (JC1574) cells after treatment with 5 mM hydrogen peroxide or 1 M NaCl for the specified times. Western blots were probed with an anti-phospho-p38 antibody, which specifically recognises the phosphorylated, active form of C. albicans Hog1 (Hog1-P). Total levels of Hog1 protein were determined by stripping the blot and reprobing with an anti-Hog1 antibody which recognises both phosphorylated and unphosphorylated forms of Hog1 (Hog1). (C) Mutation of the putative phospho-aspartate of Crr1 impairs hydrogen peroxide resistance in SN148 cells. 103 cells, and 10-fold dilutions thereof, of exponentially-growing crr1Δ cells expressing either CRR1-GFP (JC1576) or CRR1D/N-GFP (JC1578) were spotted onto YPD plates containing the indicated concentrations of hydrogen peroxide and incubated at 30°C for 24 h.
(TIFF)