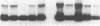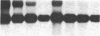Abstract
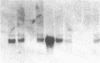
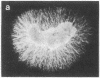
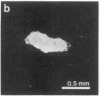
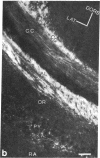
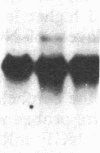
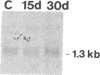
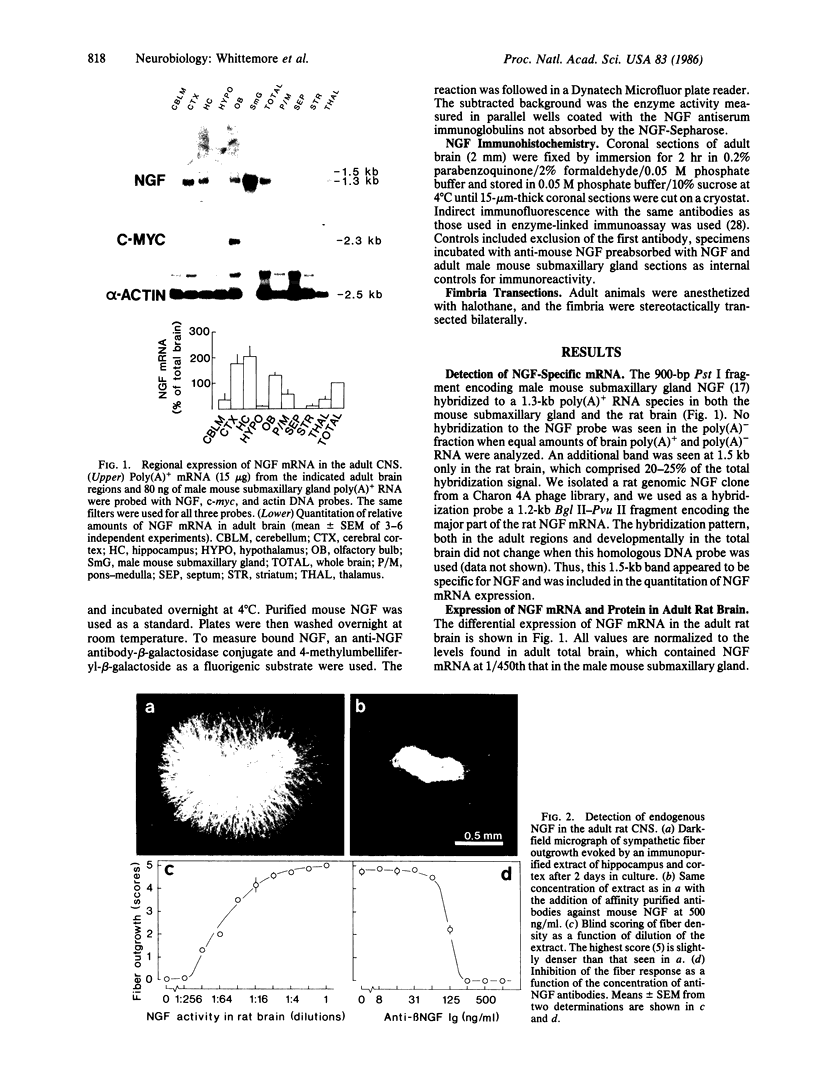
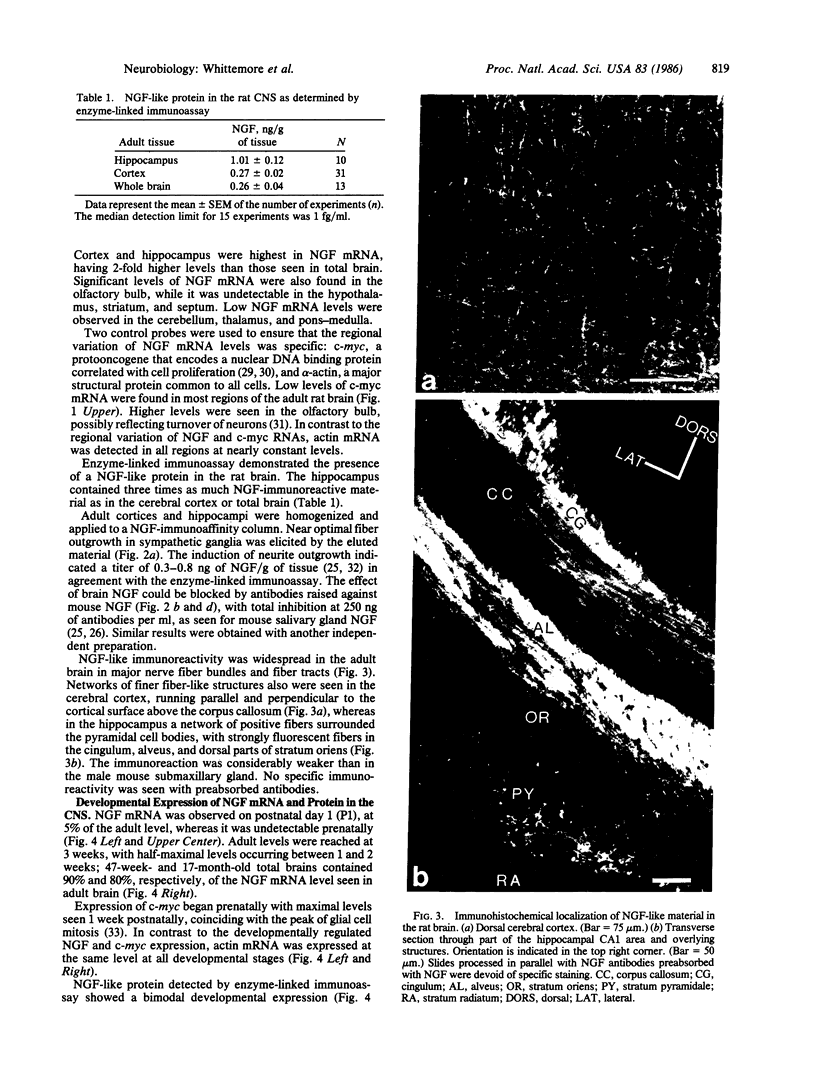
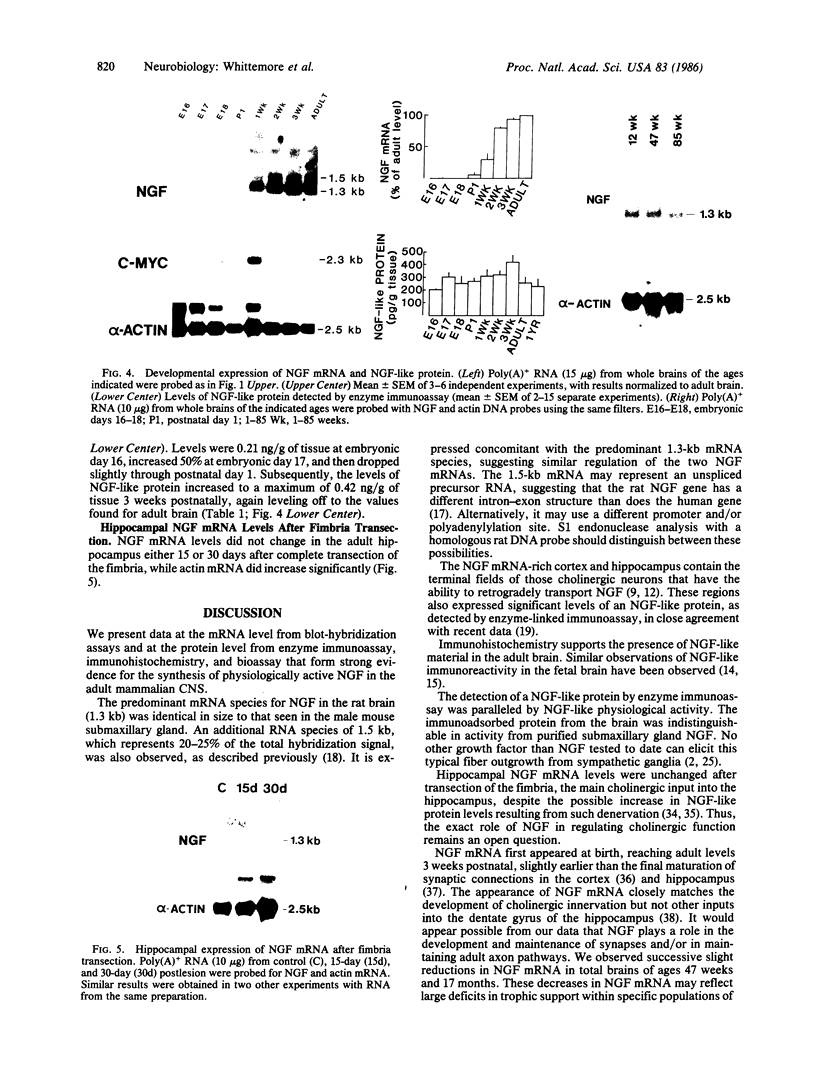
The presence of nerve growth factor (NGF) mRNA and protein in the rat central nervous system is documented. Blot-hybridization analysis showed an abundance of NGF mRNA in the hippocampus, cerebral cortex, and olfactory bulb. Enzyme immunoassay confirmed significant levels of a NGF-like protein in the hippocampus and cerebral cortex. Bioassay of a NGF-like immunoaffinity-purified protein from these regions was physiologically indistinguishable from NGF. Immunohistochemistry revealed a widespread distribution of NGF-like reactivity in the adult brain, preferentially in fiber tracts. NGF mRNA accumulation began at birth, with adult levels reached 3 weeks postnatally. Enzyme immunoassay detected the presence of a NGF-like protein in the embryonic rat brain. Postnatally, the level of NGF-like protein reached a maximum at 3 weeks. Additionally, a distinct fetal form of NGF may exist.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aghajanian G. K., Bloom F. E. The formation of synaptic junctions in developing rat brain: a quantitative electron microscopic study. Brain Res. 1967 Dec;6(4):716–727. doi: 10.1016/0006-8993(67)90128-x. [DOI] [PubMed] [Google Scholar]
- Aviv H., Leder P. Purification of biologically active globin messenger RNA by chromatography on oligothymidylic acid-cellulose. Proc Natl Acad Sci U S A. 1972 Jun;69(6):1408–1412. doi: 10.1073/pnas.69.6.1408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ayer-Lelievre C. S., Ebendal T., Olson L., Seiger A. Localization of nerve growth factor-like immunoreactivity in rat nervous tissue. Med Biol. 1983;61(6):296–304. [PubMed] [Google Scholar]
- Battey J., Moulding C., Taub R., Murphy W., Stewart T., Potter H., Lenoir G., Leder P. The human c-myc oncogene: structural consequences of translocation into the IgH locus in Burkitt lymphoma. Cell. 1983 Oct;34(3):779–787. doi: 10.1016/0092-8674(83)90534-2. [DOI] [PubMed] [Google Scholar]
- COONS A. H. Fluorescent antibody methods. Gen Cytochem Methods. 1958;1:399–422. [PubMed] [Google Scholar]
- Campisi J., Gray H. E., Pardee A. B., Dean M., Sonenshein G. E. Cell-cycle control of c-myc but not c-ras expression is lost following chemical transformation. Cell. 1984 Feb;36(2):241–247. doi: 10.1016/0092-8674(84)90217-4. [DOI] [PubMed] [Google Scholar]
- Chirgwin J. M., Przybyla A. E., MacDonald R. J., Rutter W. J. Isolation of biologically active ribonucleic acid from sources enriched in ribonuclease. Biochemistry. 1979 Nov 27;18(24):5294–5299. doi: 10.1021/bi00591a005. [DOI] [PubMed] [Google Scholar]
- Cotman C. W., Nieto-Sampedro M., Harris E. W. Synapse replacement in the nervous system of adult vertebrates. Physiol Rev. 1981 Jul;61(3):684–784. doi: 10.1152/physrev.1981.61.3.684. [DOI] [PubMed] [Google Scholar]
- Crain B., Cotman C., Taylor D., Lynch G. A quantitative electron microscopic study of synaptogenesis in the dentate gyrus of the rat. Brain Res. 1973 Dec 7;63:195–204. doi: 10.1016/0006-8993(73)90088-7. [DOI] [PubMed] [Google Scholar]
- Crutcher K. A., Brothers L., Davis J. N. Sympathetic noradrenergic sprouting in response to central cholinergic denervation; a histochemical study of neuronal sprouting in the rat hippocampal formation. Brain Res. 1981 Apr 6;210(1-2):115–128. doi: 10.1016/0006-8993(81)90889-1. [DOI] [PubMed] [Google Scholar]
- Crutcher K. A., Chandler J. P. Evidence for sprouting specificity following medial septal lesions in the rat. J Comp Neurol. 1985 Jul 1;237(1):116–126. doi: 10.1002/cne.902370109. [DOI] [PubMed] [Google Scholar]
- Dreyfus C. F., Peterson E. R., Crain S. M. Failure of nerve growth factor to affect fetal mouse brain stem catecholaminergic neurons in culture. Brain Res. 1980 Aug 4;194(2):540–547. doi: 10.1016/0006-8993(80)91239-1. [DOI] [PubMed] [Google Scholar]
- Ebendal T., Olson L., Seiger A., Hedlund K. O. Nerve growth factors in the rat iris. Nature. 1980 Jul 3;286(5768):25–28. doi: 10.1038/286025a0. [DOI] [PubMed] [Google Scholar]
- Ebendal T., Olson L., Seiger A. The level of nerve growth factor (NGF) as a function of innervation. A correlation radio-immunoassay and bioassay study of the rat iris. Exp Cell Res. 1983 Oct 15;148(2):311–317. doi: 10.1016/0014-4827(83)90155-6. [DOI] [PubMed] [Google Scholar]
- Freed W. J. The role of nerve-growth factor (NGF) in the central nervous system. Brain Res Bull. 1976 Jul-Aug;1(4):393–412. doi: 10.1016/0361-9230(76)90033-2. [DOI] [PubMed] [Google Scholar]
- Gnahn H., Hefti F., Heumann R., Schwab M. E., Thoenen H. NGF-mediated increase of choline acetyltransferase (ChAT) in the neonatal rat forebrain: evidence for a physiological role of NGF in the brain? Brain Res. 1983 Jul;285(1):45–52. doi: 10.1016/0165-3806(83)90107-4. [DOI] [PubMed] [Google Scholar]
- Hefti F., Dravid A., Hartikka J. Chronic intraventricular injections of nerve growth factor elevate hippocampal choline acetyltransferase activity in adult rats with partial septo-hippocampal lesions. Brain Res. 1984 Feb 20;293(2):305–311. doi: 10.1016/0006-8993(84)91237-x. [DOI] [PubMed] [Google Scholar]
- Kelly K., Cochran B. H., Stiles C. D., Leder P. Cell-specific regulation of the c-myc gene by lymphocyte mitogens and platelet-derived growth factor. Cell. 1983 Dec;35(3 Pt 2):603–610. doi: 10.1016/0092-8674(83)90092-2. [DOI] [PubMed] [Google Scholar]
- Konkol R. J., Mailman R. B., Bendeich E. G., Garrison A. M., Mueller R. A., Breese G. R. Evaluation of the effects of nerve growth factor and anti-nerve growth factor on the development of central catecholamine-containing neurons. Brain Res. 1978 Apr 14;144(2):277–285. doi: 10.1016/0006-8993(78)90154-3. [DOI] [PubMed] [Google Scholar]
- Korsching S., Auburger G., Heumann R., Scott J., Thoenen H. Levels of nerve growth factor and its mRNA in the central nervous system of the rat correlate with cholinergic innervation. EMBO J. 1985 Jun;4(6):1389–1393. doi: 10.1002/j.1460-2075.1985.tb03791.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levi-Montalcini R., Angeletti P. U. Nerve growth factor. Physiol Rev. 1968 Jul;48(3):534–569. doi: 10.1152/physrev.1968.48.3.534. [DOI] [PubMed] [Google Scholar]
- Levi-Montalcini R., Booker B. EXCESSIVE GROWTH OF THE SYMPATHETIC GANGLIA EVOKED BY A PROTEIN ISOLATED FROM MOUSE SALIVARY GLANDS. Proc Natl Acad Sci U S A. 1960 Mar;46(3):373–384. doi: 10.1073/pnas.46.3.373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matthews D. A., Nadler J. V., Lynch G. S., Cotman C. W. Development of cholinergic innervation in the hippocampal formation of the rat. I. Histochemical demonstration of acetylcholinesterase activity. Dev Biol. 1974 Jan;36(1):130–141. doi: 10.1016/0012-1606(74)90196-1. [DOI] [PubMed] [Google Scholar]
- Menesini Chen M. G., Chen J. S., Levi-Montalcini R. Sympathetic nerve fibers ingrowth in the central nervous system of neonatal rodent upon intracerebral NGF injections. Arch Ital Biol. 1978 Jan;116(1):53–84. [PubMed] [Google Scholar]
- Minty A. J., Caravatti M., Robert B., Cohen A., Daubas P., Weydert A., Gros F., Buckingham M. E. Mouse actin messenger RNAs. Construction and characterization of a recombinant plasmid molecule containing a complementary DNA transcript of mouse alpha-actin mRNA. J Biol Chem. 1981 Jan 25;256(2):1008–1014. [PubMed] [Google Scholar]
- Mobley W. C., Rutkowski J. L., Tennekoon G. I., Buchanan K., Johnston M. V. Choline acetyltransferase activity in striatum of neonatal rats increased by nerve growth factor. Science. 1985 Jul 19;229(4710):284–287. doi: 10.1126/science.2861660. [DOI] [PubMed] [Google Scholar]
- Olson L., Ebendal T., Seiger A. NGF and anti-NGF: evidence against effects on fiber growth in locus coeruleus from cultures of perinatal CNS tissues. Dev Neurosci. 1979;2(4):160–176. doi: 10.1159/000112451. [DOI] [PubMed] [Google Scholar]
- Schwab M. E., Otten U., Agid Y., Thoenen H. Nerve growth factor (NGF) in the rat CNS: absence of specific retrograde axonal transport and tyrosine hydroxylase induction in locus coeruleus and substantia nigra. Brain Res. 1979 Jun 8;168(3):473–483. doi: 10.1016/0006-8993(79)90303-2. [DOI] [PubMed] [Google Scholar]
- Scott J., Selby M., Urdea M., Quiroga M., Bell G. I., Rutter W. J. Isolation and nucleotide sequence of a cDNA encoding the precursor of mouse nerve growth factor. Nature. 1983 Apr 7;302(5908):538–540. doi: 10.1038/302538a0. [DOI] [PubMed] [Google Scholar]
- Seiler M., Schwab M. E. Specific retrograde transport of nerve growth factor (NGF) from neocortex to nucleus basalis in the rat. Brain Res. 1984 May 21;300(1):33–39. doi: 10.1016/0006-8993(84)91338-6. [DOI] [PubMed] [Google Scholar]
- Shelton D. L., Reichardt L. F. Expression of the beta-nerve growth factor gene correlates with the density of sympathetic innervation in effector organs. Proc Natl Acad Sci U S A. 1984 Dec;81(24):7951–7955. doi: 10.1073/pnas.81.24.7951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stoeckel K., Gagnon C., Guroff G., Thoenen H. Purification of nerve growth factor antibodies by affinity chromatography. J Neurochem. 1976 Jun;26(6):1207–1211. doi: 10.1111/j.1471-4159.1976.tb07008.x. [DOI] [PubMed] [Google Scholar]
- Thoenen H., Barde Y. A. Physiology of nerve growth factor. Physiol Rev. 1980 Oct;60(4):1284–1335. doi: 10.1152/physrev.1980.60.4.1284. [DOI] [PubMed] [Google Scholar]
- Ullrich A., Gray A., Berman C., Dull T. J. Human beta-nerve growth factor gene sequence highly homologous to that of mouse. Nature. 1983 Jun 30;303(5920):821–825. doi: 10.1038/303821a0. [DOI] [PubMed] [Google Scholar]