Abstract
A precise inference of past demographic histories including dating of demographic events using Bayesian methods can only be achieved with the use of appropriate molecular rates and evolutionary models. Using a set of 596 mitochondrial cytochrome c oxidase I (COI) sequences of two sister species of European green crabs of the genus Carcinus (C. maenas and C. aestuarii), our study shows how chronologies of past evolutionary events change significantly with the application of revised molecular rates that incorporate biogeographic events for calibration and appropriate demographic priors. A clear signal of demographic expansion was found for both species, dated between 10,000 and 20,000 years ago, which places the expansions events in a time frame following the Last Glacial Maximum (LGM). In the case of C. aestuarii, a population expansion was only inferred for the Adriatic-Ionian, suggestive of a colonization event following the flooding of the Adriatic Sea (18,000 years ago). For C. maenas, the demographic expansion inferred for the continental populations of West and North Europe might result from a northward recolonization from a southern refugium when the ice sheet retreated after the LGM. Collectively, our results highlight the importance of using adequate calibrations and demographic priors in order to avoid considerable overestimates of evolutionary time scales.
Introduction
Molecular data are the sole font of information to infer the demographic history of a species when a complete fossil record is lacking. The molecular clock has become a fundamental tool within evolutionary biology, facilitating independent time-scales to be placed on evolutionary events, such as glaciations and sea level changes [1]–[3]. Yet molecular chronologies crucially depend on accurate estimates of substitution rates and on the use of an appropriate evolutionary model. The initial assumption of a constant rate of evolution among lineages was challenged by results from datasets showing considerable departures from clockwise evolution [4]–[6]. Such problems with the molecular clock hypothesis have been partially addressed by the introduction in the past two decades of a number of relaxed-clock models, which allow for rate variation between and within lineages in a phylogeny [7]–[11]. On this subject, the advent of Bayesian methods provides the means to explore the demographic history of a species/genus, to use flexible models of population size changes through time (i.e. Bayesian skyline plots) and to take into account the effects of demography on the overall pattern of genetic variation [12], [13]. Moreover, the use of calibration points makes it possible to directly incorporate into the analysis information from fossils, biogeographic events and/or radio-carbon dated sequences, as well as to take into account uncertainty in calibration dates [9], [14].
Although it has long been recognized that different genes and taxa experience different rates of DNA change, recent studies have yielded a remarkable disparity in molecular rates estimated from population-level and pedigree data (mutation rate) compared to phylogenetic studies (substitution rate) [15], which can differ by up to an order of magnitude [16]. The time dependency hypothesis argues for an inconstant molecular clock over time to explain such rate differences [15], [17], [18]. A direct consequence of the time dependency of molecular rate estimates is that recent divergence times could be biased when using deep fossil calibration, resulting in divergence estimates older than they actually are. The extent of this bias is still under debate [19]–[24].
Ho et al. [25] emphasized the need for appropriate calibration, especially when deep fossil calibrations or universal substitution rates (e.g., 1% per million years) inferred from inter-specific comparisons are used on studies at the intraspecific level. The extrapolation of rates across the population-species boundary can yield invalid estimates of molecular time-scales, with significant impacts on subsequent evolutionary and ecological inferences [25]–[27]. When assessing the effect of inappropriate calibration using several case studies (avian speciation in the Late Pleistocene, the demographic history of bowhead whales, and the Pleistocene biogeography of brown bears), Ho et al. [25] showed that confoclusions changed significantly when applying revised internally-calibrated substitution rates.
Here we illustrate the calibration problem using the case study of the evolutionary history of the green crabs of the genus Carcinus, which consists of a sibling species pair with an allopatric native distribution, C. maenas in the North-West Atlantic Ocean and C. aestuarii in the Mediterranean Sea (Fig. 1). Some particular life-history traits of the species (wide environmental tolerance including euryhalinity, high fecundities, long larval stages) make them successful invasive species, particularly C. maenas, which has colonized littoral habitats around the world [28], [29]. The taxonomic status of the Atlantic and Mediterranean forms was initially debated based on a lack of distinction in the larval stages [30] and in early allozyme data [31]. However, despite substantial overlap in morphometric and ecological characteristics, the adult stages show morphological diagnostic characters that indicate a species split between the forms of the Atlantic and Mediterranean basins [32]. The species status was proposed by Demeusy [33], who described the Mediterranean Carcinus as a Tethys remnant and proposed that the complete isolation of Atlantic and Mediterranean during the Messinian Salinity Crisis (5.59–5.33 MY ago; [34]) promoted allopatric speciation in the genus. This view is supported by the current understanding that estuaries functioned during the whole range of the Messinian Salinity Crisis, when shallow marginal water bodies with brackish, marine to hypersaline environments existed [35] and allowed survival of an euryhaline-adapted fauna [36]. Recently, genetic studies using 16s rRNA [37] and cytochrome c oxidase I (COI) [38] sequences further confirmed the distinction of Atlantic and Mediterranean green crabs by showing reciprocal monophyly and a clear genetic break with a 2.5% and a 11% sequence divergence, respectively.
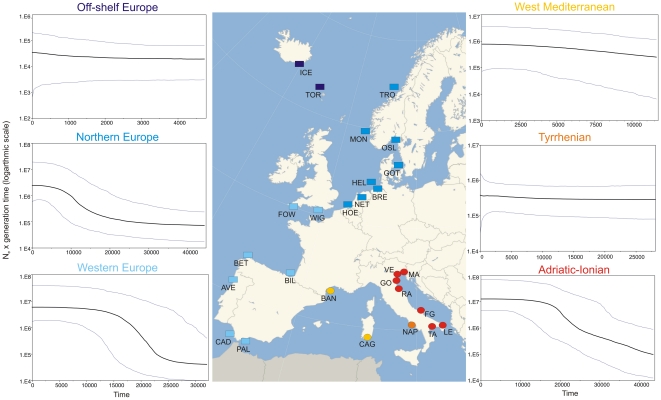
Figure 1. Distribution of collection sites and Bayesian skyline plot.
Sampling locations of Carcinus genus in Europe and demographic histories of all C. aestuarii and C. maenas groups using Bayesian skyline plots are shown. Reported are the plots of N e (effective population size multiplied per generation time) against time in years from present. Mean N e is shown as a solid black line and light blue lines enclose the 95% highest posterior density interval. Samples are labelled as in Table 1. The map shows the groups identified by AMOVA and MDS analyses for C. aestuarii (circles) and C. maenas (squares), coded in different colours.
Using a data set of 596 mitochondrial COI sequences of C. aestuarii and C. maenas, corresponding to 27 sampling locations, we inferred the demographic histories for all differentiated groups within species. Our approach consisted in obtaining an accurate rate calibration by incorporating deep calibration points derived from biogeographic events together with appropriate demographic priors. Results are contrasted with those obtained by a simplified approach using phylogenetic priors that ignores the inter-specific nature of the data and the effect of demographic processes [39]–[41]. Chronologies of past events change significantly with the application of revised molecular rates that, following Ho et al. [42], consider a separate demographic model of exponential growth for each individual species. Collectively, our results stress the importance of using adequate calibrations and demographic priors in order to infer more accurately recent evolutionary events, and importantly, to avoid considerable overestimates of evolutionary time scales.
Results
Diversity indices are summarized in Table 1. A total of 172 haplotypes were recovered among the 596 individuals analyzed, 105 haplotypes in C. aestuarii and 67 haplotypes in C. maenas. A 10.35% sequence divergence was observed between C. aestuarii and C. maenas, separated by at least 39 substitutions, all of which were synonymous. Similar values of haplotype (C. aestuarii: 0.883; C. maenas: 0.858) and nucleotide (C. aestuarii: 0.007; C. maenas: 0.006) diversity were found across species. Within C. aestuarii, West Mediterranean locations showed lower haplotype diversity (0.51–0.64) in comparison with the rest of locations (0.83–0.99), although sample size in one of the two western Mediterranean localities (Banyuls-sur-Mer) was only 17 individuals. All locations showed similar nucleotide diversity (0.002–0.007) except Naples (0.021). Within C. maenas, diversities were similar across locations with N>5 individuals sampled (Table 1).
Table 1. Diversity indices within C. aestuarii and C. maenas.
| Species | Region | Location | Code | N | H | h | π |
| C. aestuarii | Adriatic-Ionian | Marano, Italy | MA | 32 | 18(11) | 0.897 | 0.005 |
| Venice, Italy | VE | 32 | 16(5) | 0.909 | 0.004 | ||
| Goro, Italy | GO | 32 | 27(18) | 0.988 | 0.007 | ||
| Ravenna, Italy | RA | 31 | 23(12) | 0.938 | 0.005 | ||
| Lesina, Italy | FG | 32 | 19(12) | 0.911 | 0.005 | ||
| Aquatina, Italy | LE | 32 | 15(6) | 0.869 | 0.004 | ||
| Taranto, Italy | TA | 32 | 14(7) | 0.825 | 0.004 | ||
| Tyrrhenian | Naples, Italy | NAP | 29 | 13(8) | 0.892 | 0.021 | |
| West Mediterranean | Cagliari, Italy | CAG | 32 | 11(6) | 0.643 | 0.006 | |
| Banyuls-sur-Mer, France | BAN | 17 | 5(1) | 0.507 | 0.002 | ||
| C. maenas | Off-shelf Europe | Seltjarnarnes, Iceland | ICE | 18 | 1(0) | 0.000 | 0.000 |
| Torshavn, Faeroe Islands | TOR | 19 | 2(1) | 0.515 | 0.003 | ||
| Northern Europe | Trondheim, Norway | TRO | 3 | 3(0) | 1.000 | 0.007 | |
| Mongstadt, Norway | MON | 22 | 12(5) | 0.853 | 0.004 | ||
| Oslo, Norway | OSL | 9 | 5(1) | 0.806 | 0.007 | ||
| Goteborg, Sweden | GOT | 15 | 10(3) | 0.933 | 0.007 | ||
| Helgoland, Germany | HEL | 5 | 2(0) | 0.400 | 0.001 | ||
| Bremerhaven, Germany | BRE | 15 | 7(2) | 0.827 | 0.005 | ||
| Hoek van Holland, The Netherlands | HOE | 19 | 7(3) | 0.813 | 0.004 | ||
| Den Helder, The Netherlands | NET | 45 | 17(8) | 0.784 | 0.004 | ||
| Western Europe | Fowey, England | FOW | 14 | 8(3) | 0.890 | 0.005 | |
| Isle of Wight, England | WIG | 3 | 3(3) | 1.000 | 0.011 | ||
| Bilbao, Spain | BIL | 15 | 6(3) | 0.648 | 0.003 | ||
| Betanzos, Spain | BET | 14 | 7(4) | 0.758 | 0.003 | ||
| Aveiro, Portugal | AVE | 23 | 9(5) | 0.795 | 0.004 | ||
| Cadiz, Spain | CAD | 47 | 21(14) | 0.864 | 0.004 | ||
| Palmones, Spain | PAL | 9 | 8(2) | 0.972 | 0.005 |
Reported are: sampling locations, number of individuals (N), number of haplotypes (H, in brackets are exclusive haplotypes), haplotype (h) and nucleotide (π) diversity.
The McDonald-Kreitman test revealed no signatures of positive selection (p = 1.000) after observing all fixed differences between species being synonymous (10 out of 10) and most polymorphic mutations being synonymous as well (111 out of 113). Only 2 out of 113 polymorphic mutations were replacements (non-synonymous). In addition, a codon-based Z-test of selection showed no excess of amino acid replacements between any pair of sequences (p = 1.000 for all pairwise comparisons). Using the program HyPhy, no codons were suggested to be under positive selection as all codons showed higher or equal number of synonymous than non-synonymous substitutions. Collectively, all tests agreed in showing no excess of amino acid replacements, with >98% of changes being silent (synonymous), which is suggestive of the action of purifying selection but not positive selection.
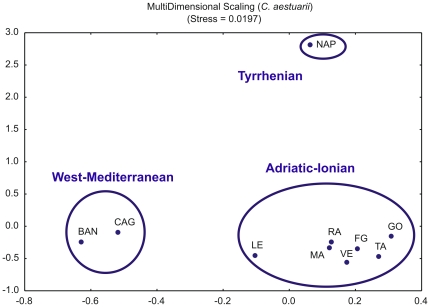
Overall genetic differentiation among C. aestuarii locations was significant (F ST = 0.285, p<0.001). A MultiDimensional Scaling analysis for all C. aestuarii locations suggested three different groupings within the Mediterranean Sea: Adriatic-Ionian, Tyrrhenian (Naples) and West Mediterranean (Cagliari, Banyuls-sur-Mer) (Fig. 2). Accordingly, a hierarchical AMOVA partitioned significantly among groups when considering 3 groups (Adriatic-Ionian, Tyrrhenian, West Mediterranean; F CT = 0.292, p = 0.002), while no significant differentiation was found among locations within groups (Table 2). Similarly, a significant genetic differentiation was found among C. maenas locations (F ST = 0.492, p<0.001). Genetic differentiation partitioned significantly when considering the three groups previously proposed by Roman and Palumbi [38], Off-shelf, Northern Europe and Western Europe (F CT = 0.470, p<0.001) and was about 10 times larger than the differentiation observed among locations within groups (Table 2).
Figure 2. MultiDimensional Scaling analysis (MDS).
Plots from MDS analysis for all C. aestuarii locations based on pairwise linearized genetic distance (F ST/(1- F ST)) values are shown. Samples are labelled as in Table 1. Groupings have been circled for clarity.
Table 2. Analysis of Molecular Variance (AMOVA).
| AMOVA | Sum of squares | Variance | F statistic |
| C. aestuarii | |||
| Among groups | 77.77 | 0.61 | F CT = 0.292 (p = 0.002)* |
| Among locations within groups | 7.51 | −0.01 | F SC = −0.009 (p = 0.553) |
| Within locations | 431.48 | 1.48 | |
| (Total) | 516.75 | 2.07 | F ST = 0.285 (p<0.001)* |
| C. maenas | |||
| Among groups | 161.46 | 0.89 | F CT = 0.470 (p<0.001)* |
| Among locations within groups | 22.91 | 0.04 | F SC = 0.042 (p = 0.004)* |
| Within locations | 267.16 | 0.96 | |
| (Total) | 451.54 | 1.89 | F ST = 0.492 (p<0.001)* |
Results from partitioning genetic differentiation among groups (C. aestuarii: Adriatic/Ionian, Tyrrhenian, Western Mediterranean; C. maenas: Off-shelf, Northern Europe, Western Europe) and among populations within groups.
*p<0.005.
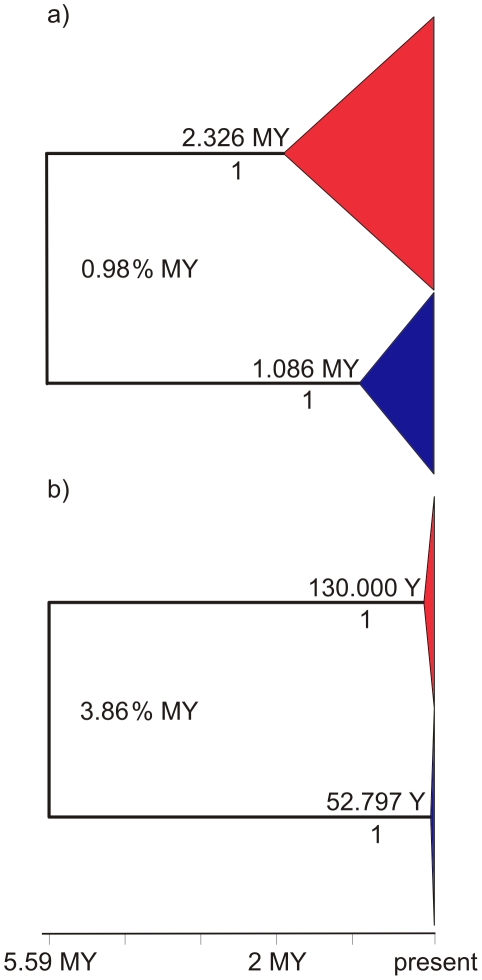
The evolutionary histories of C. aestuarii and C. maenas were explored by Bayesian inference. Prior to the analysis, the most appropriate model of sequence evolution was selected using MODELTEST, which suggested an HKY+G substitution model. In a first analysis including only unique haplotypes, using a 5.59 MY split between C. aestuarii and C. maenas [34] and assuming a Birth-Death prior and a strict clock, two highly supported clades corresponding to the two species were evidenced, with an estimated substitution rate of 1.06% per MY. When repeating the analysis using a relaxed clock, the standard deviation of the uncorrelated lognormal relaxed clock was significantly different than zero (ucld.stdev = 0.26, 95% HPD: 1.06×10−4–0.49), indicative of variation in rates among branches and suggesting that a strict clock is not appropriate for our data. Using a relaxed clock, we estimated a substitution rate of 0.98% per MY (Fig. 3), which is congruent with the substitution rate of 0.70–1.15% per MY estimated for crustaceans [43], [44]. The tMRCA was estimated to 2.33 MY (95% HPD: 1.32–3.54 MY) for C. aestuarii and 1.09 MY (95% HPD: 0.58–1.67 MY) for C. maenas.
Figure 3. Consensus Trees of the Carcinus genus.
Trees showing inferred rates and times of the most recent common ancestor (tMRCA) of C. aestuarii (red) and C. maenas (blue) assuming a total tree height of 5.59 MY. tMRCAs are reported above the branches, and posterior support for the corresponding node, below. (a) Estimates obtained using phylogenetic priors and only unique haplotypes, and (b) Using demographic priors and all sequences.
When demographic priors were incorporated in the Bayesian analysis by imposing a different exponential prior for each species and including all sequences, the rate of exponential growth was positive for both species, with an associated 95% HPD interval excluding zero (C. aestuarii: g = 1.32×10−4 years−1; 95% HPD: 7.23×10−6−4.20x10−4 years−1; C. maenas: g = 3.93×10−4 years−1; 95% HPD: 1.78×10−5−1.28×10−3 years−1). Bayes factors indicated a much better fit for the model that incorporated demographic priors and allowed exponential growth (ln = −11,345.4) than for a model in which no growth was assumed (ln = −12,457.6), providing a very strong support for the use of exponential demographic priors (2 ln(B10) = 2224.4; [45]). The application of exponential demographic priors resulted in a different tree shape, compressed near the tips and a four-fold higher substitution rate of 3.86% per MY was obtained (Fig. 3). Accordingly, estimated times of the MRCA were younger than before, 130,000 years (95% HPD: 4,595–350,120 years) for C. aestuarii and 52,797 years (95% HPD: 2,119–146,500 years) for C. maenas. At the intraspecific level, Iceland and Faeroe Islands were characterized exclusively by private haplotypes that clustered in a single group with high posterior support, with an estimate of the time of split between the Off-shelf and continental haplotypes of 45,414 years (95% HPD: 1,764–128,050 years).
Finally, demographic analyses were conducted separately for all groups identified by AMOVA. A pattern of demographic expansion was suggested for the Adriatic-Ionian group in C. aestuarii and for the Northern Europe and Western Europe groups in C. maenas, all of which were characterized by growth values significantly higher than zero (Table 3). The remaining groups, Tyrrhenian and West Mediterranean in C. aestuarii and Off-shelf Europe in C. maenas, showed evidence of demographic stability, with HPD values centred around zero. Figure 1 depicts the estimated demographic history of all groups using Bayesian skyline plots. The expansion suggested for the Adriatic-Ionian group in C. aestuarii and for the Northern Europe and Western Europe groups in C. maenas occurred roughly between 10,000 and 20,000 years ago. The observation of significant negative values of Tajima's D and Fu's FS tests together with Ramos-Onsis and Rozas' R2 values close to zero supports the expansion pattern in the Adriatic-Ionian region in the Mediterranean and along the Atlantic coast (Table 3).
Table 3. Demographic analyses.
| Location | g | 95% HPD of g | T-D | F-FS | R2 | |
| C. aestuarii | ||||||
| Adriatic-Ionian | 1.64×10−4 | 9.14×10−5 | 2.46×10−4 | −2.33*** | −26.89*** | 0.02*** |
| Tyrrhenian | 1.93×10−6 | −4.82×10−6 | 8.67×10−6 | 1.02 | 0.89 | 0.16 |
| West Mediterranean | −7.22×10−7 | −8.04×10−6 | 6.17×10−6 | −1.98*** | −8.48*** | 0.04* |
| C. maenas | ||||||
| Off-shelf | −8.49×10−6 | −4.82×10−4 | 5.76×10−4 | 1.01 | 3.73 | 0.17 |
| Northern Europe | 1.58×10−4 | 3.63×10−5 | 3.22×10−4 | −1.77* | −27.02*** | 0.04* |
| Western Europe | 3.20×10−4 | 1.28×10−4 | 5.55×10−4 | −2.20*** | −27.52*** | 0.002** |
Rate of exponential growth g using demographic analyses in BEAST for all C. aestuarii and C. maenas groups, including 95% highest posterior density interval (HPD), plus Tajima's D statistic (T-D), Fu's FS statistic (F-FS) and Ramos-Onsis and Rozas' R2.
*p<0.05;
**<0.01;
***<0.005.
Discussion
Inferring the Demographic Histories of C. aestuarii and C. maenas
Our molecular analysis using COI sequences clearly confirms C. aestuarii and C. maenas as two separate species in accordance with previous studies [28], [29], [37], [38], with completely supported reciprocal monophyly between haplotypes of the two forms achieved in this extended dataset. An overall sequence divergence of 10.35% has been found, closely matching previous estimates based on a much smaller sampling effort for C. aestuarii [38] and also fitting the range described for other crustacean species (9–25%; [46]).
Although the deepest split occurs between the Mediterranean and Atlantic forms, regional genetic substructuring was observed among coastal populations of both C. aestuarii and C. maenas. MultiDimensional Scaling and AMOVA analyses suggested the existence of three groupings for C. aestuarii (Adriatic-Ionian, Tyrrhenian and West Mediterranean Sea). The level of subdivision found might be a consequence of the physical oceanography of the Mediterranean Sea, including (i) circulation being forced by a particularly complex topography limiting water exchange through the various straits, (ii) internal counterclockwise circulation pattern favoring larval retention and (iii) locally deep convection formation acting as barrier between water basins [47]. Recently, Borrero-Pérez et al. [48] argued that the major genetic breaks within the Mediterranean are related to the hydrographic isolation of the Adriatic, Aegean and/or Black Sea, after reporting a significant genetic differentiation between Eastern and Western Mediterranean populations of the sea cucumber Holothuria mammata using mitochondrial 16S and COI gene sequences. In the case of C. maenas, three groupings were also observed (Off-shelf, Northern and Western Europe) in agreement with the previous studies of Roman and Palumbi [38] and Darling et al. [28]. The slight but significant break between Western and Northern Europe could reflect regional current patterns, in particular, larval retention could be associated with seasonal circulation in the North Sea [49], [50]. The highest genetic differentiation for C. maenas was observed between the off-shelf (Iceland and Faeroe Islands) and the continental-shelf populations. The deep genetic division found is due to the presence of unique haplotypes in the off-shelf region, and might be attributable to the deep water barrier to dispersal in the open sea between the off-shelf and the continental shelf, a pattern suggested for other marine organisms in the region including Atlantic halibut Hippoglossus hippoglossus [51], Atlantic herring Clupea harengus [52] and plaice Pleuronectes platessa [53].
A clear signal of expansion was found for both C. aestuarii and C. maenas. However, re-analysis taking into account the groupings identified by MultiDimensional Scaling and AMOVA inferred demographic expansion for the Adriatic-Ionian (but not Tyrrhenian nor West Mediterranean) in C. aestuarii, and for the Northern and Western Europe groups (but not Off-shore Europe) in C. maenas. All expansions were dated between 10,000 and 20,000 years ago, which places the expansion events in a time frame following the Last Glacial Maximum (LGM, between 26,500–20,000 years ago; [54]).
During the LGM, the Mediterranean Sea experienced a dramatic sea-level fluctuation and cold (but ice-free) conditions [47], [55]–[58]. Immediately after the LGM, drastic alterations took place within the Mediterranean Sea, including the rising of the Adriatic Sea, which flooded 18,000 years ago [59]. We hypothesize that the Adriatic Sea could have been colonized by populations of C. aestuarii sometime during the last 18,000 years by Mediterranean populations that probably originated from the adjacent Ionian Sea. A group of individuals colonizing a new geographic region and establishing a new population would explain the signature of demographic expansion suggested for the Adriatic group using Bayesian inference. Similarly, a recent study on the demographic history of the marine bivalve Flexopecten glaber, suggested the colonization of the Adriatic Sea following the flooding of the Adriatic Sea [60]. The lack of barriers to gene flow between Adriatic and Ionian populations has been demonstrated in many marine species including the sand smelt Atherina boyeri [61], [62], the European sprat Sprattus sprattus [63] and the sea urchin Paracentrotus lividus [64]. The existence of gene exchange between Adriatic-Ionian Sea is evident in our dataset as the Taranto sample from the Ionian Sea clusters together with all the Adriatic samples in the MultiDimensional Scaling analysis. The fact that a demographic expansion in the Mediterranean was only suggested for the Adriatic Sea but not for the Tyrrhenian and West Mediterranean might be due to the lack of new geographic regions to be colonized following the LGM in the western Mediterranean basin. While the flooding of the Adriatic Sea permitted adjacent populations to expand by occupying new habitats, populations from the western Mediterranean did not encounter a similar scenario, which would explain the lack of a demographic expansion signal in those populations.
During the LGM the ice sheet covered most of the North Atlantic from the Barents Sea and the Scandinavian Peninsula to the British Isles [65], [66], with the southern limit of the permafrost located North off the Iberian Peninsula, Italy and the Balkans, which could have acted as refugia [67]. Previous studies have shown that the Quaternary in Europe is characterized by many cycles of contraction of geographic ranges to southern regions during cold periods and expansion northward during subsequent warmings [68]. We propose that during the LGM, C. maenas remained in a southern glacial refugium located along the Atlantic coast of the Iberian Peninsula. When the ice sheet retreated after the LGM, C. maenas carried out an extensive northward colonization following the European Atlantic coast, recolonizing the newly opened coastal habitats off northern Europe including the Irish Sea, the North Sea and the Baltic Sea, which would explain the signatures of demographic expansion for the Atlantic continental-shelf populations of C. maenas in our study. A similar pattern of post-glacial expansion has been suggested for the common goby Pomatoschistus microps [69] and the Atlantic cod Gadus morhua [70]. Alternatively, additional refugia might have existed in northern Europe (i.e. a glacial lake potentially served as refugium in the southern North Sea; [71]). This could explain the similar genetic diversities found across continental regions in our study, as opposed to the expected high genetic diversity in regions close to the potential refugia (Spain-Portugal) and low genetic diversity in more distant areas.
In contrast with the continental-shelf populations, Bayesian inference suggested a stationary population for the off-shelf region (Faeroe Islands and Iceland), which is congruent with the low genetic diversity found: a single haplotype out of 18 individuals in Iceland and two haplotypes out of 19 individuals in Faeroe Islands, one shared with Iceland. Roman and Palumbi [38] attributed the reduced diversity found in Iceland to a relatively recent colonization event, by means of a stepping-stone range expansion from the northern UK to the Shetland Islands, Faeroe Islands and Iceland after deglaciation. However, our analysis estimates the age of the most recent common ancestor of the off-shore and continental groups to be around 45,000 years, which places it before the LGM [54]. Although the confidence intervals of our dates still incorporate the time period post LGM, we propose that a separate refugium area existed in the off-shelf during the LGM that was the origin of the current Faeroe Islands and Iceland populations, as alternative to a stepping-stone range expansion after the LGM. A potential glacial refugium in Iceland seems plausible on the basis of a suggested limited glaciation, with large areas of northern Iceland remaining ice-free during the LGM [72]. The low genetic diversity observed in this island group might be consequence of a small founder population size, which has remained of constant low size over time (as suggested by the Bayesian analysis) and has not expanded because of the impossibility of finding new habitats due to its isolation. The geographic position of the Faeroe Islands and Iceland at the northern limit of the geographical range of C. maenas might also account for the low genetic variation, which is expected in marginal populations at the edges of species distribution range [73]. The existence of two separate refugia is supported by a Bayes factor analysis in which a model with one refugium only (with no constrain on the topology of the tree) was compared with a two refugia model (imposing the monophyly of the two clades, Iceland and the rest of Europe). Twice the difference in logarithm of harmonic means of likelihoods was compared using the approach of Kass and Raftery [45]. The two refugia model fits the data better, with a 2 ln(B10) of 6.412, suggesting a strong evidence against the one refugium model.
Appropriate Calibration using Demographic Information
By demonstrating that estimated divergence times within species change significantly when including prior information on demographic processes, our findings reiterate the importance of using appropriate calibrations in analyses of recent evolutionary events.
Ho et al. [25] advocated the use of internal, intraspecific calibration points as opposed to using deep calibration points, particularly those based on the fossil record. Alternatively, we demonstrate that a more accurate rate calibration can be obtained by using appropriate demographic priors together with deep calibration points obtained from biogeography. Our study shows that the application of revised molecular rates that consider separate demographic models of exponential growth for each species allows the proper inference of chronologies within species. Our study also denotes a strong underestimation of substitution rates when ignoring evidence for recent population expansion. In the case of C. aestuarii and C. maenas, a four-fold higher rate (3.86% per MY) was obtained when demographic information was incorporated into the analysis by means of imposing an exponential prior for each species. Bayes factors provided a very strong evidence against a model with no growth, supporting the use of exponential demographic priors. This rate strongly differs from the commonly used crustacean rate of 0.70–1.15% per MY [43], [44] and from the rate inferred by the simplified phylogenetic approach in our study (0.98% per MY). The latter differs only slightly from a rate of 0.93% MY that can be inferred considering the uncorrected divergence between species (10.35%) and our calibration point (5.59 MY), which indicates that in our case the inclusion of a relaxed clock and the consideration of best fit model of sequence evolution has only a minor impact on rate estimates.
Under the time dependency of molecular rate estimates hypothesis, the rate heterogeneity at different scales is associated with the fact that slightly deleterious mutations contribute to measured genetic differences at the shallow time scale but are progressively removed by purifying selection during further evolutionary divergence [15], [17], [19], [26], [74]. In our dataset, tests of selection showed >98% of all changes to be synonymous, with no excess of amino acid replacements, which is suggestive of the action of purifying selection. Importantly, our study provides evidence that recent demographic history can play a role in producing a much higher level of genetic polymorphism at the intraspecific level that results in higher rates. In this sense, demography and purifying selection might be acting in concert considering that in recently expanded populations slightly deleterious mutations can accumulate in the gene pool due to the large population sizes [75].
Typically, many published studies on historical demography are based on the identification of population expansions using neutrality tests (Tajima's D, Fu's FS, Ramos-Onsis and Rozas' R2) or the estimation of time of sudden expansion by mismatch distribution and application of universal clock rates [76]–[79]. Those studies ignore the effects of rate calibration, model of sequence evolution and population history, which might result in an overestimation of evolutionary time scales. Furthermore, the advent of Bayesian methods to reconstruct historical demographies leaves little space for the use of simplified methods such as neutrality tests or mismatch distribution that cannot account for rate heterogeneity and are based on expectations of simple models of sudden population expansion. As an example, our study provides insights on the use of neutrality tests to pick up a signal of population expansion. All neutrality tests congruently identified a demographic expansion in the Adriatic, Northern and Western Europe and identified the Tyrrhenian and Atlantic Off-shore as stationary populations. However, all neutrality tests suggested a strong expansion for the West Mediterranean, which was not suggested by BEAST nor supported by the paleoclimatic history of this region. In view of our results, neutrality tests should be complemented by approaches based on more complex demographic models, in spite of increased computational time.
In summary, our findings point out the difficulties in producing accurate date estimates due to rate heterogeneity when recent calibration points are not available. The approach proposed in our study can be summarized in the following steps: (i) use calibration points based on biogeographic events or fossil records if the former are not available; (ii) use all individuals for the analysis, not only unique haplotypes; (iii) incorporate accurate demographic priors, allowing for exponential growth when suggested; (iv) estimate dates in a relaxed clock framework that allows for rate variation among branches; and (v) perform analyses for all data and separately for all differentiated groups within species as identified by AMOVA analyses. Our data clearly show that analyses performed within this framework represent an improved method for calibrating estimates of divergence times and evolutionary histories from molecular data. Finally, it should be stressed that one limitation of our study is the use of a single mitochondrial gene (reflecting only the maternal line and possibly insensitive to population size [80]) without confirmation from nuclear genes. Complementary data based on other mitochondrial or nuclear data could be useful to compare maternally-inherited and biparental markers and to detect how male-mediated gene flow might have influenced the global evolutionary history of the species.
Materials and Methods
Samples of C. aestuarii (N = 255) were collected during 2005–2008 in 8 locations in the Mediterranean Sea (Table 1, Figure 1). Specimens were frozen at −80°C after collection. Total genomic DNA was extracted from chelipeds using a salting out protocol [81]. We amplified a 569 base-pair (bp) fragment of the mitochondrial COI (cytochrome oxidase subunit I) gene using primers developed by Roman and Palumbi [38] for C. maenas. PCR products were obtained in a GeneAmp PCR System 2700 Thermocycler (Applied Biosystems). PCR reactions and conditions followed Roman and Palumbi (2004) and PCR products were sequenced using an ABI 3130 AVANT automatic capillary sequencer (Applied Biosystems) on both strands. All sequences have been deposited on GenBank (full details are given in Table S1). Additionally, we analyzed 46 COI sequences from two Mediterranean locations of C. aestuarii, Banyuls-sur-Mer and Naples, and 295 COI sequences of C. maenas obtained from GenBank (accession numbers FJ159008 - FJ159102; [28]). In total, the data set comprised 596 individuals corresponding to 27 sampling locations, 10 Mediterranean and 17 Atlantic (Table 1).
Mitochondrial DNA sequences were aligned using Clustal_X [82] and 480 bp of overlapping sequences were used for all subsequent analyses. We used DnaSP v.5 [83] to calculate haplotype (h) and nucleotide (π) diversity, McDonald-Kreitman's [84] test of selection, Tajima's [85] D statistic test, Fu's [86] F statistic and Ramos-Onsis and Rozas' [87] R2 statistic, together with the uncorrected sequence divergence between species. We used MEGA 5 [88] to conduct the codon-based Z-test of selection and test for selection across codons using HyPhy.
Genetic differentiation was explored by calculating pairwise F ST values between all locations. Significance tests were assessed with 10,000 permutations. A multivariate ordination was conducted by MultiDimensional Scaling analysis using STATISTICA (StatSoft). Non-hierarchical and hierarchical analysis of molecular variance (AMOVA; [89]) using ARLEQUIN [90] was conducted to explore the relative contribution of molecular variance at different geographical scales (localities included in each group are reported in Table 1). Uncorrected pairwise differences were used to partition molecular variance among regions and among sampling locations within regions.
The demographic histories of C. aestuarii and C. maenas were estimated using a Bayesian Markov-Chain Monte Carlo (MCMC) coalescent approach implemented in BEAST 1.4.8 [13]. Prior to the analysis, the most appropriate model of sequence evolution for the data set was selected using MODELTEST v3.06 [91] based on Akaike Information Criterion scores. The closure of the Gibraltar strait at the start of the Messinian Salinity Crisis (5.59 MY ago; [34]) was used as calibration point for rate estimation. During the Messinian Salinity Crisis, the contact between the Mediterranean and the Atlantic Ocean was interrupted altogether, thus providing the geographic barrier necessary to the speciation of C. aestuarii and C. maenas [33]. Uncertainty on the divergence time was modeled using a normal prior with a standard deviation of 55,000 years.
In the first step, a simplified phylogenetic approach was used, ignoring the intraspecific nature of our data, using a Birth-Death prior [92], [93] and including only unique haplotype sequences. Analyses were repeated with a strict clock and an uncorrelated lognormal relaxed clock [9]. For each model, the MCMC was run for 100 million steps and sampled every 1,000 steps. The first 10% steps of each run were discarded as burn-in, and after checking convergence by examining Effective Sample Sizes (ESS) of all parameters using Tracer v1.5 [94], molecular rate and time of the most recent common ancestor (tMRCA) for the two species were determined and reported as mean value with 95% highest posterior density interval (HPD).
In the second step, we followed the approach of Ho et al. [42], which consisted of applying a separate demographic model of exponential growth to each species in the form of a coalescent prior. All sequences were used, not merely unique haplotypes. Considering the size of the dataset (N = 596), exponential growth was chosen to avoid over parameterization of the model, maintaining the possibility to estimate population growth, decline or constant size. The exponential growth prior was restricted to ingroups and a normal prior on the age of the root was placed as before. Following previous analyses, an uncorrelated relaxed clock was used to accommodate rate variation among lineages. The MCMC was run as described above but the sampling was performed each 100,000 steps and the analysis was repeated (N = 8) until acceptable mixing (ESS>100) and convergence to the stationary distribution were recovered. Samples from different runs were combined and model parameters estimated. In addition to overall substitution rate and tMRCAs, growth rate was also estimated. Bayes factors, calculated as the harmonic mean of likelihoods [95] were used to evaluate the adequacy of the exponential growth model, following Kass & Raftery [45].
In the final step, demographic Bayesian analyses were performed separately for all differentiated groups within species (as identified by AMOVA analyses), including all sequences of a given group and importing the rate estimated in the previous steps as normal prior with appropriate mean and standard deviation. Demographic histories were estimated using both an exponential prior and a Bayesian skyline plot setting the number of groups to m = 5 and the parameterization described above. MCMC was run for 50 million steps with sampling performed every 10,000 steps.
Supporting Information
GenBank accession numbers and distribution in the populations for C. aestuarii COI haplotypes obtained in this study.
(DOC)
Acknowledgments
We thank SYW Ho for advice on the implementation of demographic priors.
Footnotes
Competing Interests: The authors have declared that no competing interests exist.
Funding: This work was supported by a CORILA (Consortium for Coordination of Research Activities Concerning the Venice Lagoon System) grant to LZ and a PhD Fellowship in “Biologia Evoluzionistica” from the University of Padova to IAMM. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Lovette IJ. Glacial cycles and the tempo of avian speciation. Trends Ecol Evol. 2005;20:57–59. doi: 10.1016/j.tree.2004.11.011. [DOI] [PubMed] [Google Scholar]
- 2.Hofreiter M, Rabader G, Jaenicke-Despres V, Withalm G, Nagel D, et al. Evidence for reproductive isolation between cave bear populations. Curr Biol. 2004;14:40–43. doi: 10.1016/j.cub.2003.12.035. [DOI] [PubMed] [Google Scholar]
- 3.Saarma U, Ho SYW, Pybus OG, Kaljuste M, Tumanov IL, et al. Mitogenetic structure of brown bears (Ursus arctos) in northeastern Europe and a new time frame for the formation of brown bear lineages. Mol Ecol. 2007;16:401–413. doi: 10.1111/j.1365-294X.2006.03130.x. [DOI] [PubMed] [Google Scholar]
- 4.Ritten RJ. Rates of DNA sequence evolution differ between taxonomic groups Science. 1986;231:1393–1398. doi: 10.1126/science.3082006. [DOI] [PubMed] [Google Scholar]
- 5.Hasegawa M, Kishino H. Heterogeneity of tempo and mode of mitochondrial DNA evolution among mammalian orders. Jpn J Genet. 1989;64:243–258. doi: 10.1266/jjg.64.243. [DOI] [PubMed] [Google Scholar]
- 6.Ayala FJ. Vagaries of the molecular clock. P Natl Acad Sci USA. 1997;94:7776–7783. doi: 10.1073/pnas.94.15.7776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Huelsenbeck JP, Larget B, Swofford D. A compound Poisson process for relaxing the molecular clock. Genetics. 2000;154:1879–1892. doi: 10.1093/genetics/154.4.1879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kishino H, Thorne JL, Bruno WJ. Performance of a divergence time estimation method under a probabilistic model of rate evolution. Mol Biol Evol. 2001;18:352–361. doi: 10.1093/oxfordjournals.molbev.a003811. [DOI] [PubMed] [Google Scholar]
- 9.Drummond AJ, Ho SYW, Phillips MJ, Rambaut A. Relaxed phylogenetics and dating with confidence. PloS Biol. 2006;4:e88. doi: 10.1371/journal.pbio.0040088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lepage T, Lawi S, Tupper P, Bryant D. Continuous and tractable models for the variation of evolutionary rates. Math Biosci. 2006;199:216–233. doi: 10.1016/j.mbs.2005.11.002. [DOI] [PubMed] [Google Scholar]
- 11.Rannala B, Yang Z. Inferring speciation times under an episodic molecular clock. Syst Biol. 2007;56:453–466. doi: 10.1080/10635150701420643. [DOI] [PubMed] [Google Scholar]
- 12.Drummond AJ, Rambaut A, Shapiro B, Pybus OG. Bayesian coalescent inference of past population dynamics from molecular sequences. Mol Biol Evol. 2005;22:1185–1192. doi: 10.1093/molbev/msi103. [DOI] [PubMed] [Google Scholar]
- 13.Drummond AJ, Rambaut A. BEAST: Bayesian evolutionary analysis by sampling trees. BMC Evol Biol. 2007;7:214. doi: 10.1186/1471-2148-7-214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ho SYW. Calibrating molecular estimates of substitution rates and divergence times in birds. J Avian Biol. 2007;38:409–414. [Google Scholar]
- 15.Howell N, Smejkal CB, Mackey DA, Chinnery PF, Turnbull DM, et al. The pedigree rate of sequence divergence in the human mitochondrial genome: there is a difference between phylogenetic and pedigree rates. Am J Hum Genet. 2003;72:659–670. doi: 10.1086/368264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Pereira SL, Baker AJ. A mitogenomic timescale for birds detects variable phylogenetic rates of molecular evolution and refutes the standard molecular clock. Mol Biol Evol. 2006;23:1731–1740. doi: 10.1093/molbev/msl038. [DOI] [PubMed] [Google Scholar]
- 17.Ho SYW, Phillips MJ, Cooper A, Drummond AJ. Time dependency of molecular rate estimates and systematic overestimation of recent divergence times. Mol Biol Evol. 2005;22:1561–1568. doi: 10.1093/molbev/msi145. [DOI] [PubMed] [Google Scholar]
- 18.Ho SYW, Lanfear R, Bromham L, Phillips MJ, Soubrier J, et al. Time-dependent rates of molecular evolution. Mol Ecol. 2011;20:3087–3101. doi: 10.1111/j.1365-294X.2011.05178.x. [DOI] [PubMed] [Google Scholar]
- 19.Burridge CP, Craw D, Fletcher D, Waters JM. Geological dates and molecular rates: Fish DNA sheds light on time dependency. Mol Biol Evol. 2008;25:624–633. doi: 10.1093/molbev/msm271. [DOI] [PubMed] [Google Scholar]
- 20.Howell N, Howell C, Elson JL. Time dependency of molecular rate estimates for mtDNA: this is not the time for wishful thinking. Heredity. 2008;101:107–108. doi: 10.1038/hdy.2008.52. [DOI] [PubMed] [Google Scholar]
- 21.Millar CD, Dodd A, Anderson J, Gibb GC, Ritchie PA, et al. Mutation and evolutionary rates in Adélie Penguins from the Antarctic. PLoS Genet. 2008;4:e1000209. doi: 10.1371/journal.pgen.1000209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Debruyne R, Poinar HN. Time dependency of molecular rates in ancient DNA data sets, a sampling artifact? Syst Biol. 2009;58:348–360. doi: 10.1093/sysbio/syp028. [DOI] [PubMed] [Google Scholar]
- 24.Ramakrishnan U, Hadly EA. Do complex population histories drive estimates of substitution rate in phylogenetic reconstructions? Mol Ecol. 2009;18:4341–4343. doi: 10.1111/j.1365-294X.2009.04334.x. [DOI] [PubMed] [Google Scholar]
- 25.Ho SYW, Saarma U, Barnett R, Haile J, Shapiro B. The effect of inappropriate calibration: three case studies in molecular ecology. PLoS One. 2008;3:e1615. doi: 10.1371/journal.pone.0001615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Penny D. Evolutionary biology: Relativity for molecular clocks. Nature. 2005;436:183–184. doi: 10.1038/436183a. [DOI] [PubMed] [Google Scholar]
- 27.Ho SYW, Larson G. Molecular clocks: when times are a-changin'. Trends Genet. 2006;22:79–83. doi: 10.1016/j.tig.2005.11.006. [DOI] [PubMed] [Google Scholar]
- 28.Darling JA, Bagley MJ, Roman J, Tepolt CK, Geller JB. Genetic patterns across multiple introductions of the globally invasive crab genus Carcinus. Mol Ecol. 2008;17:4992–5007. doi: 10.1111/j.1365-294X.2008.03978.x. [DOI] [PubMed] [Google Scholar]
- 29.Darling JA. Interspecific hybridization and mitochondrial introgression in invasive Carcinus shore crabs. PLoS ONE. 2011;6:e17828. doi: 10.1371/journal.pone.0017828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Rice AL, Ingle RW. The larval development of Carcinus maenas and C. mediterraneus Czerniavsky (Crustacea, Brachyura, Portunidae) reared in the laboratory. Bull Br Mus Nat Hist. 1975;28:101–119. [Google Scholar]
- 31.Bulnheim HP, Bahns S. Genetic variation and divergence in the genus Carcinus (Crustacea, Decapoda). Int Rev Gesamten Hydrobiol. 1996;81:611–619. [Google Scholar]
- 32.Yamada SB. Corvalis OR: Oregon State University; 2001. Global invader: the European green crab.123 [Google Scholar]
- 33.Demeusy N. Recherches sur la mue de puberte du decapode brachyure Carcinus maenas Linne. Arch Zool Exp Gen. 1958;95:253–491. [Google Scholar]
- 34.Krijgsman W, Hilgen FJ, Raffi I, Sierro FJ, Wilson DS. Chronology, causes and progression of the Messinian salinity crisis. Nature. 1999;400:652–655. [Google Scholar]
- 35.Por FD. The new Tethyan ichthyofauna of the Mediterranean- historical background and prospect. In: Golani D, Appelbaum-Golani B, editors. Fish Invasion of the Mediterranean Sea: Change and Renewal. Sofia-Moscow: Pensoft; 2010. pp. 13–33. [Google Scholar]
- 36.Por FD, Dimentman C. Sofia-Moscow: Pensoft; 2006. Mare Nostrum: Neogene and anthropic natural history of the Mediterranean basin with emphasis on the Levant.350 [Google Scholar]
- 37.Geller JB, Walton ED, Grosholz AD, Ruiz GM. Cryptic invasions of the crab Carcinus detected by molecular plylogeography. Mol Ecol. 1997;6:901–906. doi: 10.1046/j.1365-294x.1997.00256.x. [DOI] [PubMed] [Google Scholar]
- 38.Roman J, Palumbi SR. A global invader at home: population structure of the green crab, Carcinus maenas, in Europe. Mol Ecol. 2004;13:2891–2898. doi: 10.1111/j.1365-294X.2004.02255.x. [DOI] [PubMed] [Google Scholar]
- 39.Mona S, Randi E, Tommaseo-Ponzetta M. Evolutionary history of the genus Sus inferred from cytochrome b sequences. Mol Phylogenet Evol. 2007;45:757–762. doi: 10.1016/j.ympev.2007.05.025. [DOI] [PubMed] [Google Scholar]
- 40.Villacorta C, Jaume D, Oromi P, Juan C. Under the volcano: phylogeography and evolution of the cave-dwelling Palmorchestia hypogaea (Amphipoda, Crustacea) at La Palma (Canary Islands). BMC Biol. 2008;6:7. doi: 10.1186/1741-7007-6-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Zinner D, Groeneveld LF, Keller C, Roos C. Mitochondrial phylogeography of baboons (Papio spp.) - Indication for introgressive hybridization? BMC Evol Biol. 2009;9:83. doi: 10.1186/1471-2148-9-83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ho SYW, Larson G, Edwards CJ, Heupink TH, Lakin KE, et al. Correlating Bayesian date estimates with climatic events and domestication using a bovine case study. Biol Letters. 2008;4:370–374. doi: 10.1098/rsbl.2008.0073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Knowlton N, Weigt LA. New dates and new rates for divergence across the Isthmus of Panama. P Roy Soc B-Biol Sci. 1998;265:2257–2263. [Google Scholar]
- 44.Schubart CD, Diesel R, Hedges SB. Rapid evolution to terrestrial life in Jamaican crabs. Nature. 1998;393:363–365. [Google Scholar]
- 45.Kass RE, Raftery AE. Bayes Factors. J Am Stat Assoc. 1995;90:773–795. [Google Scholar]
- 46.Bucklin A, Frost BW, Bradford-Grieve J, Allen LD, Copley NJ. Molecular systematics and phylogenetic assessment of 34 calanoid copepod species of the Calanidae and Clausocalanidae. Mar Biol. 2003;142:333–343. [Google Scholar]
- 47.Patarnello T, Volckaert FAM, Castilho R. Pillars of Hercules: is the Atlantic-Mediterranean transition a phylogeographical break? Mol Ecol. 2007;16:4426–4444. doi: 10.1111/j.1365-294X.2007.03477.x. [DOI] [PubMed] [Google Scholar]
- 48.Borrero-Pérez GH, González-Wangüemert M, Marcos C, Pérez-Ruzafa A. Phylogeography of the Atlanto-Mediterranean sea cucumber Holothuria (Holothuria) mammata: the combined effects of historical processes and current oceanographical pattern. Mol Ecol. 2011;20:1964–1975. doi: 10.1111/j.1365-294X.2011.05068.x. [DOI] [PubMed] [Google Scholar]
- 49.Brown J, Hill AE, Fernand L, Horsburgh KJ. Observation of a seasonal jet-like circulation at the central North Sea cold pool margin. Estuar Coast Shelf S. 1999;48:343–355. [Google Scholar]
- 50.Domingues CP, Creer S, Taylor MI, Queiroga H, Carvalho GH. Genetic structure of Carcinus maenas within its native range: larval dispersal and oceanographic variability. Mar Ecol Prog Ser. 2010;410:111–123. [Google Scholar]
- 51.Foss A, Imsland AK, Naevdal G. Population genetic studies of the Atlantic halibut in the North Atlantic Ocean. J Fish Biol. 1998;53:901–905. [Google Scholar]
- 52.Shaw PW, Turan C, Wright JM, O'Connell M, Carvalho GR. Microsatellite DNA analysis of population structure in Atlantic herring (Clupea harengus), with direct comparison to allozyme and mtDNA RFLP analyses. Heredity. 1999;83:490–499. doi: 10.1038/sj.hdy.6885860. [DOI] [PubMed] [Google Scholar]
- 53.Hoarau G, Piquet AMT, Van der Veer HW, Rijnsdorp AD, Stam WT, et al. Population structure of plaice (Pleuronectes platessa) in northern Europe: a comparison of resolving power between microsatellites and mitochondrial DNA data. J Sea Res. 2004;51:183–190. [Google Scholar]
- 54.Clark PU, Dyke AS, Shakun JD, Carlson AE, Clark J, et al. The last glacial maximum. Science. 2009;325:710–714. doi: 10.1126/science.1172873. [DOI] [PubMed] [Google Scholar]
- 55.Rögl F, Steininger F. Vom Zerfall der Tethys zu Mediterran und Paratethys. Ann Nat Hist Mus Wien. 1983;85A:135–163. [Google Scholar]
- 56.Dawson AG. London: Routledge; 1992. Ice Age Earth: Late Quaternary Geology and Climate.293 [Google Scholar]
- 57.Rögl F. Mediterranean and Paratethys. Facts and hypotheses of an Oligocene to Miocene paleogeography (short overview). Geol Carpath. 1999;50:339–349. [Google Scholar]
- 58.Waelbroeck C, Labeyrie L, Michel E, Duplessy JC, McManus JF, et al. Sea-level and deep water temperature changes derived from benthic foraminifera isotopic records. Quaternary Sci Rev. 2002;21:295–305. [Google Scholar]
- 59.Thiede J. A glacial Mediterranean. Nature. 1978;276:680–683. [Google Scholar]
- 60.Pujolar JM, Marčeta T, Saavedra C, Bressan M, Zane L. Inferring the demographic history of the Adriatic Flexopecten complex. Mol Phylogenet Evol. 2010;57:942–947. doi: 10.1016/j.ympev.2010.08.002. [DOI] [PubMed] [Google Scholar]
- 61.Congiu L, Rossi R, Colombo G. Population analysis of the sand smelt Atherina boyeri (Teleostei Atherinidae), from Italian coastal lagoons by random amplified polymorphic DNA. Mar Ecol Prog Ser. 2002;229:279–289. [Google Scholar]
- 62.Astolfi L, Dupanloup I, Rossi R, Bisol PM, Faure E, et al. Mitochondrial variability of sand smelt Atherina boyeri populations from north Mediterranean coastal lagoons. Mar Ecol Prog Ser. 2005;297:233–243. [Google Scholar]
- 63.Debes PV, Zachos FE, Hanel R. Mitochondrial phylogeography of the European sprat (Sprattus sprattus, Clupeidae) reveals isolated climatically vulnerable populations in the Mediterranean Sea and range expansion in the northeast Atlantic. Mol Ecol. 2008;17:3873–3888. doi: 10.1111/j.1365-294X.2008.03872.x. [DOI] [PubMed] [Google Scholar]
- 64.Maltagliati F, Di Giuseppe G, Barbieri M, Castelli A, Dini F. Phylogeography and genetic structure of the edible sea urchin Paracentrotus lividus (Echinodermata: Echinoidea) inferred from the mitochondrial cytochrome b gene. Biol J Linn Soc. 2010;100:910–923. [Google Scholar]
- 65.Siegert MJ, Dowdeswell JA. Numerical reconstruction of the Eurasian Ice Sheet and climate during the late Weichselian. Quaternary Sci Rev. 2004;23:1273–1283. [Google Scholar]
- 66.Svendsen JIS, Alexanderson H, Astakhov VI, Deminov I, Dowdswell JA, et al. Late Quaternary ice sheet history of northern Eurasia. Quaternary Sci Rev. 2004;23:1229–1271. [Google Scholar]
- 67.Taberlet P, Fumagalli L, Wust-Saucy AG, Cosson JF. Comparative phylogeography and postglacial colonization routes in Europe. Mol Ecol. 1998;7:453–464. doi: 10.1046/j.1365-294x.1998.00289.x. [DOI] [PubMed] [Google Scholar]
- 68.Hewitt GM. Some genetic consequences of ice ages, and their role in divergence and speciation. Biol J Linn Soc. 1996;58:247–276. [Google Scholar]
- 69.Gysels ES, Hellemans B, Pampoulie C, Volckaert FAM. Phylogeography of the common goby, Pomatoschistus microps, with particular emphasis on the colonization of the Mediterranean and the North Sea. Mol Ecol. 2004;13:403–417. doi: 10.1046/j.1365-294x.2003.02087.x. [DOI] [PubMed] [Google Scholar]
- 70.Pampoulie C, Stefansson MO, Jorundsdottir TD, Danilowicz BS, Danielsdottir AK. Recolonization history and large-scale dispersal in the open sea: the case study of the North Atlantic cod, Gadus morhua. Biol J Linn Soc. 2008;94:315–329. [Google Scholar]
- 71.Balson PS, Laban C, Frantsen PJ, Parker N, Henriet JP, et al. Southampton: Geological Survey of The Netherlands and Belgian Geological Survey; 1991. Ostend: Sheet 52°N/02°E. Solid Geology, 1∶250 000 Series British Geological Survey. [Google Scholar]
- 72.Van Vliet-Lanoë B, Guðmundsson Å, Guillou H, Duncan RA, Genty D, et al. Limited glaciation and very early deglaciation in central Iceland. Implications for climate change. C R Geosci. 2007;339:1–12. [Google Scholar]
- 73.Eckert CG, Samis KE, Lougheed SC. Genetic variation across species' geographical ranges: the central-marginal hypothesis and beyond. Mol Ecol. 2008;17:1170–1188. doi: 10.1111/j.1365-294X.2007.03659.x. [DOI] [PubMed] [Google Scholar]
- 74.Santos C, Montiel R, Sierra B, Bettencourt C, Fernandez E, et al. Understanding differences between phylogenetic and pedigree-derived mtDNA mutation rate: a model using families from the Azores islands (Portugal). Mol Biol Evol. 2005;22:1490–1505. doi: 10.1093/molbev/msi141. [DOI] [PubMed] [Google Scholar]
- 75.Ohta T. The nearly neutral theory of molecular evolution. Annu Rev Ecol Syst. 1992;23:263–286. [Google Scholar]
- 76.Sušnik S, Snoj A, Wilson IF, Mrdak D, Weiss S. Historical demography of brown trout (Salmo trutta) in the Adriatic drainage including the putative S. letnica endemic to Lake Ohrid. Mol Phylogenet Evol. 2007;44:63–76. doi: 10.1016/j.ympev.2006.08.021. [DOI] [PubMed] [Google Scholar]
- 77.Marko PB, Hoffman JM, Emme SA, McGovern TM, Keever CC, et al. The ‘Expansion–Contraction’ model of Pleistocene biogeography: rocky shores suffer a sea change? Mol Ecol. 2010;19:146–169. doi: 10.1111/j.1365-294x.2009.04417.x. [DOI] [PubMed] [Google Scholar]
- 78.Lecomte F, Grant WS, Dodson JJ, Rodríguez-Sánchez R, Bowen BW. Living with uncertainty: genetic imprints of climate shifts in East Pacific anchovy (Engraulis mordax) and sardine (Sardinops sagax). Mol Ecol. 2004;13:2169–2182. doi: 10.1111/j.1365-294X.2004.02229.x. [DOI] [PubMed] [Google Scholar]
- 79.Zane L, Marcato S, Bargelloni L, Bortolotto E, Papetti C, et al. Demographic history and population structure of the Antarctic silverfish Pleuragramma antarcticum. Mol Ecol. 2006;15:4499–4511. doi: 10.1111/j.1365-294X.2006.03105.x. [DOI] [PubMed] [Google Scholar]
- 80.Bazin E, Glémin S, Galtier N. Population size does not influence mitochondrial genetic diversity in animals. Science. 2006;312:570–572. doi: 10.1126/science.1122033. [DOI] [PubMed] [Google Scholar]
- 81.Patwary MU, Kenchington EL, Bird CJ, Zouros E. The use of random amplified polymorphic DNA (RAPD) markers in genetic studies of the sea scallop Plactopecten magellanicus. J Shellfish Res. 1994;13:547–553. [Google Scholar]
- 82.Thompson JD, Gibson TJ, Plewniak F, Jeanmougin F, Higgins DG. The Clustal_X windows interface: flexible strategies for multiple sequence alignment aided by quality analysis tools. Nucleic Acids Res. 1997;25:4876–4882. doi: 10.1093/nar/25.24.4876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Librado P, Rozas J. DnaSP v5: A software for comprehensive analysis of DNA polymorphism data. Bioinformatics. 2009;25:1451–1452. doi: 10.1093/bioinformatics/btp187. [DOI] [PubMed] [Google Scholar]
- 84.McDonald JH, Kreitman M. Adaptive protein evolution at the Adh locus in Drosophila. Nature. 1991;351:652–654. doi: 10.1038/351652a0. [DOI] [PubMed] [Google Scholar]
- 85.Tajima F. Statistical method for testing the neutral mutation hypothesis by DNA polymorphism. Genetics. 1989;123:585–595. doi: 10.1093/genetics/123.3.585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Fu YX. Statistical tests of neutrality of mutations against population growth, hitchhiking and background selection. Genetics. 1997;147:915–925. doi: 10.1093/genetics/147.2.915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Ramos-Onsins SE, Rozas J. Statistical properties of new neutrality tests against population growth. Mol Biol Evol. 2002;19:2092–2100. doi: 10.1093/oxfordjournals.molbev.a004034. [DOI] [PubMed] [Google Scholar]
- 88.Tamura K, Peterson D, Peterson N, Stecher G, Nei M, et al. MEGA5: Molecular evolutionary genetics analysis using Maximum Likelihood, evolutionary distance and Maximum Parsimony methods. Mol Biol Evol. 2011;28:2731–2739. doi: 10.1093/molbev/msr121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Excoffier L, Smouse PE, Quattro JM. Analysis of molecular variance inferred from metric distances among DNA haplotypes: application to mitochondrial DNA restriction data. Genetics. 1992;131:479–491. doi: 10.1093/genetics/131.2.479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Excoffier L, Laval G, Schneider S. Arlequin ver. 3.0: An integrated software package for population genetics data analysis. Evol Bioinform Online. 2005;1:47–50. [PMC free article] [PubMed] [Google Scholar]
- 91.Posada D, Crandall KA. MODELTEST: testing the model of DNA substitution. Bioinformatics. 1998;14:817–818. doi: 10.1093/bioinformatics/14.9.817. [DOI] [PubMed] [Google Scholar]
- 92.Rannala B, Yang Z. Probability distribution of molecular evolutionary trees: a new method of phylogenetic inference. J Mol Evol. 1996;43:304–311. doi: 10.1007/BF02338839. [DOI] [PubMed] [Google Scholar]
- 93.Yang Z, Rannala B. Bayesian phylogenetic inference using DNA sequences: a Markov chain Monte Carlo method. Mol Biol Evol. 1997;14:717–724. doi: 10.1093/oxfordjournals.molbev.a025811. [DOI] [PubMed] [Google Scholar]
- 94.Rambaut A, Drummond AJ. Tracer version 1.5. Molecular Evolution, Phylogenetics and Epidemiology website. 2007. Available: http://tree.bio.ed.ac.uk/software/tracer. Accessed 2011 Nov 14.
- 95.Nylander JAA, Ronquist F, Huelsenbeck JP, Nieves-Aldrey JL. Bayesian phylogenetic analysis of combined data. Syst Biol. 2004;53:47–67. doi: 10.1080/10635150490264699. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
GenBank accession numbers and distribution in the populations for C. aestuarii COI haplotypes obtained in this study.
(DOC)