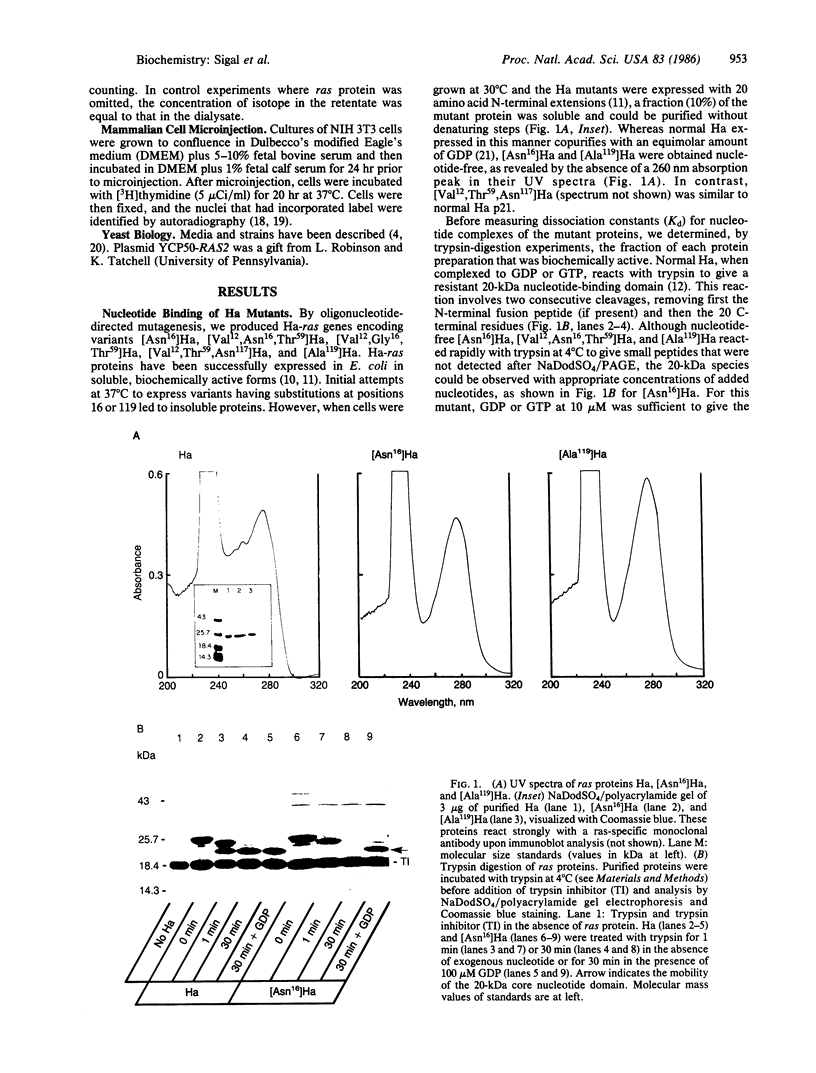
Abstract
We report that residues Lys-16 and Asp-119 play critical roles in the guanine nucleotide binding and, consequently, the biological function of the Ha-ras-encoded protein (Ha). Substitution of an asparagine residue for Lys-16 reduces the affinity of Ha for GDP and GTP by a factor of 100 but does not alter the specificity of nucleotide binding. The replacement of Asp-119 with an alanine residue reduces the affinity of Ha for GDP and GTP by a factor of 20 and reduces the relative affinity of Ha for GDP over IDP from 200-500 to 10. Based on these observations, a structural model for the GDP/GTP-binding site of Ha is proposed. By microinjecting purified proteins into NIH 3T3 cells, we observed that the ability of [Ala119]Ha to induce changes characteristic of cellular transformation was much greater than that of normal Ha and similar to that of the oncogenic [Val12, Thr59]Ha. In this assay, [Asn16]Ha and [Val12, Asn16, Thr59]Ha were similar in potency to normal Ha. In yeast cells, Ha proteins with reduced nucleotide affinity exert a dominant temperature-dependent lethality that is avoided by the coexpression of the activated yeast ras gene [Ala18, Val19]RAS2. We interpret the biological consequences of reducing the nucleotide affinity of ras proteins in terms of two opposing factors: a growth-promoting effect, resulting from an increase in the GDP-GTP exchange rate, and a growth-limiting effect, resulting from an increase in the nucleotide-free ras protein species.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Broek D., Samiy N., Fasano O., Fujiyama A., Tamanoi F., Northup J., Wigler M. Differential activation of yeast adenylate cyclase by wild-type and mutant RAS proteins. Cell. 1985 Jul;41(3):763–769. doi: 10.1016/s0092-8674(85)80057-x. [DOI] [PubMed] [Google Scholar]
- DeFeo-Jones D., Scolnick E. M., Koller R., Dhar R. ras-Related gene sequences identified and isolated from Saccharomyces cerevisiae. Nature. 1983 Dec 15;306(5944):707–709. doi: 10.1038/306707a0. [DOI] [PubMed] [Google Scholar]
- DeFeo-Jones D., Tatchell K., Robinson L. C., Sigal I. S., Vass W. C., Lowy D. R., Scolnick E. M. Mammalian and yeast ras gene products: biological function in their heterologous systems. Science. 1985 Apr 12;228(4696):179–184. doi: 10.1126/science.3883495. [DOI] [PubMed] [Google Scholar]
- Feramisco J. R., Gross M., Kamata T., Rosenberg M., Sweet R. W. Microinjection of the oncogene form of the human H-ras (T-24) protein results in rapid proliferation of quiescent cells. Cell. 1984 Aug;38(1):109–117. doi: 10.1016/0092-8674(84)90531-2. [DOI] [PubMed] [Google Scholar]
- Finkel T., Der C. J., Cooper G. M. Activation of ras genes in human tumors does not affect localization, modification, or nucleotide binding properties of p21. Cell. 1984 May;37(1):151–158. doi: 10.1016/0092-8674(84)90310-6. [DOI] [PubMed] [Google Scholar]
- Gay N. J., Walker J. E. Homology between human bladder carcinoma oncogene product and mitochondrial ATP-synthase. Nature. 1983 Jan 20;301(5897):262–264. doi: 10.1038/301262a0. [DOI] [PubMed] [Google Scholar]
- Gibbs J. B., Sigal I. S., Poe M., Scolnick E. M. Intrinsic GTPase activity distinguishes normal and oncogenic ras p21 molecules. Proc Natl Acad Sci U S A. 1984 Sep;81(18):5704–5708. doi: 10.1073/pnas.81.18.5704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilman A. G. G proteins and dual control of adenylate cyclase. Cell. 1984 Mar;36(3):577–579. doi: 10.1016/0092-8674(84)90336-2. [DOI] [PubMed] [Google Scholar]
- Hattori S., Ulsh L. S., Halliday K., Shih T. Y. Biochemical properties of a highly purified v-rasH p21 protein overproduced in Escherichia coli and inhibition of its activities by a monoclonal antibody. Mol Cell Biol. 1985 Jun;5(6):1449–1455. doi: 10.1128/mcb.5.6.1449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hughes S. M. Are guanine nucleotide binding proteins a distinct class of regulatory proteins? FEBS Lett. 1983 Nov 28;164(1):1–8. doi: 10.1016/0014-5793(83)80006-4. [DOI] [PubMed] [Google Scholar]
- Johnston M., Davis R. W. Sequences that regulate the divergent GAL1-GAL10 promoter in Saccharomyces cerevisiae. Mol Cell Biol. 1984 Aug;4(8):1440–1448. doi: 10.1128/mcb.4.8.1440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jurnak F. Structure of the GDP domain of EF-Tu and location of the amino acids homologous to ras oncogene proteins. Science. 1985 Oct 4;230(4721):32–36. doi: 10.1126/science.3898365. [DOI] [PubMed] [Google Scholar]
- Kataoka T., Powers S., Cameron S., Fasano O., Goldfarb M., Broach J., Wigler M. Functional homology of mammalian and yeast RAS genes. Cell. 1985 Jan;40(1):19–26. doi: 10.1016/0092-8674(85)90304-6. [DOI] [PubMed] [Google Scholar]
- Kataoka T., Powers S., McGill C., Fasano O., Strathern J., Broach J., Wigler M. Genetic analysis of yeast RAS1 and RAS2 genes. Cell. 1984 Jun;37(2):437–445. doi: 10.1016/0092-8674(84)90374-x. [DOI] [PubMed] [Google Scholar]
- Leberman R., Egner U. Homologies in the primary structure of GTP-binding proteins: the nucleotide-binding site of EF-Tu and p21. EMBO J. 1984 Feb;3(2):339–341. doi: 10.1002/j.1460-2075.1984.tb01808.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manne V., Yamazaki S., Kung H. F. Guanosine nucleotide binding by highly purified Ha-ras-encoded p21 protein produced in Escherichia coli. Proc Natl Acad Sci U S A. 1984 Nov;81(22):6953–6957. doi: 10.1073/pnas.81.22.6953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGrath J. P., Capon D. J., Goeddel D. V., Levinson A. D. Comparative biochemical properties of normal and activated human ras p21 protein. Nature. 1984 Aug 23;310(5979):644–649. doi: 10.1038/310644a0. [DOI] [PubMed] [Google Scholar]
- Pai E. F., Sachsenheimer W., Schirmer R. H., Schulz G. E. Substrate positions and induced-fit in crystalline adenylate kinase. J Mol Biol. 1977 Jul;114(1):37–45. doi: 10.1016/0022-2836(77)90281-9. [DOI] [PubMed] [Google Scholar]
- Papageorge A., Lowy D., Scolnick E. M. Comparative biochemical properties of p21 ras molecules coded for by viral and cellular ras genes. J Virol. 1982 Nov;44(2):509–519. doi: 10.1128/jvi.44.2.509-519.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poe M., Scolnick E. M., Stein R. B. Viral Harvey ras p21 expressed in Escherichia coli purifies as a binary one-to-one complex with GDP. J Biol Chem. 1985 Apr 10;260(7):3906–3909. [PubMed] [Google Scholar]
- Powers S., Kataoka T., Fasano O., Goldfarb M., Strathern J., Broach J., Wigler M. Genes in S. cerevisiae encoding proteins with domains homologous to the mammalian ras proteins. Cell. 1984 Mar;36(3):607–612. doi: 10.1016/0092-8674(84)90340-4. [DOI] [PubMed] [Google Scholar]
- Scolnick E. M., Papageorge A. G., Shih T. Y. Guanine nucleotide-binding activity as an assay for src protein of rat-derived murine sarcoma viruses. Proc Natl Acad Sci U S A. 1979 Oct;76(10):5355–5359. doi: 10.1073/pnas.76.10.5355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stacey D. W., Kung H. F. Transformation of NIH 3T3 cells by microinjection of Ha-ras p21 protein. Nature. 1984 Aug 9;310(5977):508–511. doi: 10.1038/310508a0. [DOI] [PubMed] [Google Scholar]
- Sweet R. W., Yokoyama S., Kamata T., Feramisco J. R., Rosenberg M., Gross M. The product of ras is a GTPase and the T24 oncogenic mutant is deficient in this activity. Nature. 1984 Sep 20;311(5983):273–275. doi: 10.1038/311273a0. [DOI] [PubMed] [Google Scholar]
- Tatchell K., Chaleff D. T., DeFeo-Jones D., Scolnick E. M. Requirement of either of a pair of ras-related genes of Saccharomyces cerevisiae for spore viability. Nature. 1984 Jun 7;309(5968):523–527. doi: 10.1038/309523a0. [DOI] [PubMed] [Google Scholar]
- Temeles G. L., Gibbs J. B., D'Alonzo J. S., Sigal I. S., Scolnick E. M. Yeast and mammalian ras proteins have conserved biochemical properties. Nature. 1985 Feb 21;313(6004):700–703. doi: 10.1038/313700a0. [DOI] [PubMed] [Google Scholar]
- Willumsen B. M., Norris K., Papageorge A. G., Hubbert N. L., Lowy D. R. Harvey murine sarcoma virus p21 ras protein: biological and biochemical significance of the cysteine nearest the carboxy terminus. EMBO J. 1984 Nov;3(11):2581–2585. doi: 10.1002/j.1460-2075.1984.tb02177.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yatsunami K., Khorana H. G. GTPase of bovine rod outer segments: the amino acid sequence of the alpha subunit as derived from the cDNA sequence. Proc Natl Acad Sci U S A. 1985 Jul;82(13):4316–4320. doi: 10.1073/pnas.82.13.4316. [DOI] [PMC free article] [PubMed] [Google Scholar]