Abstract
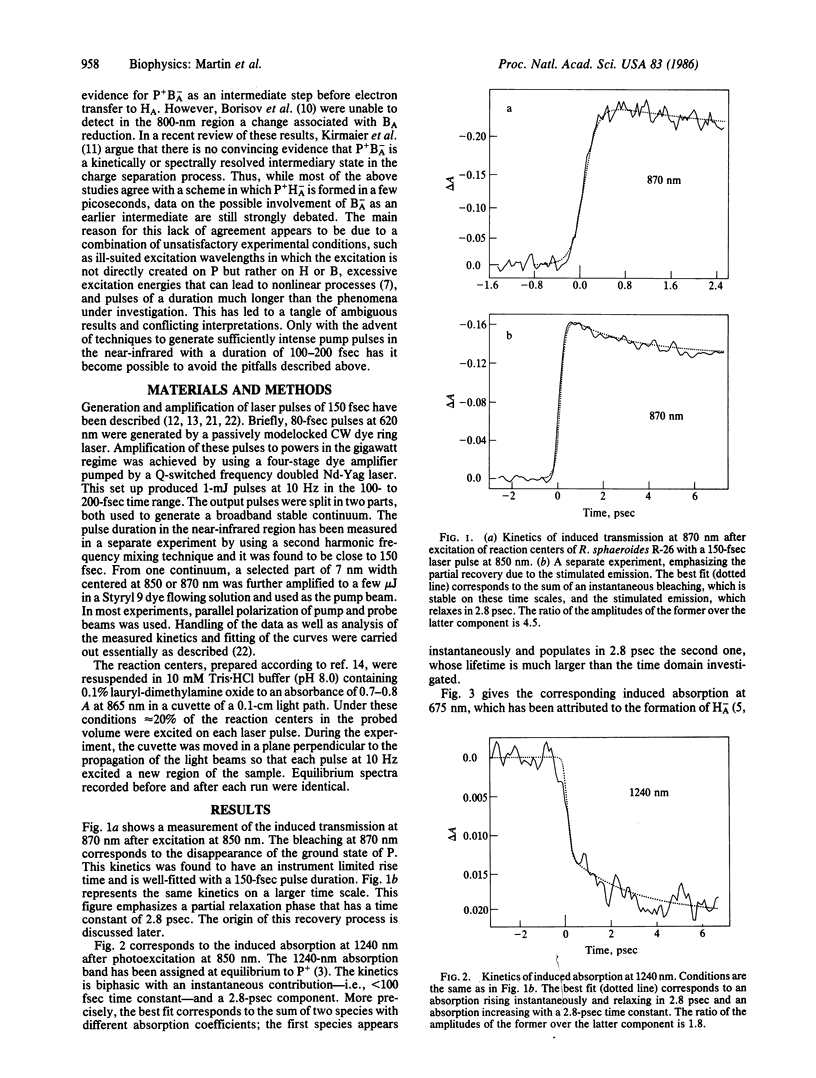
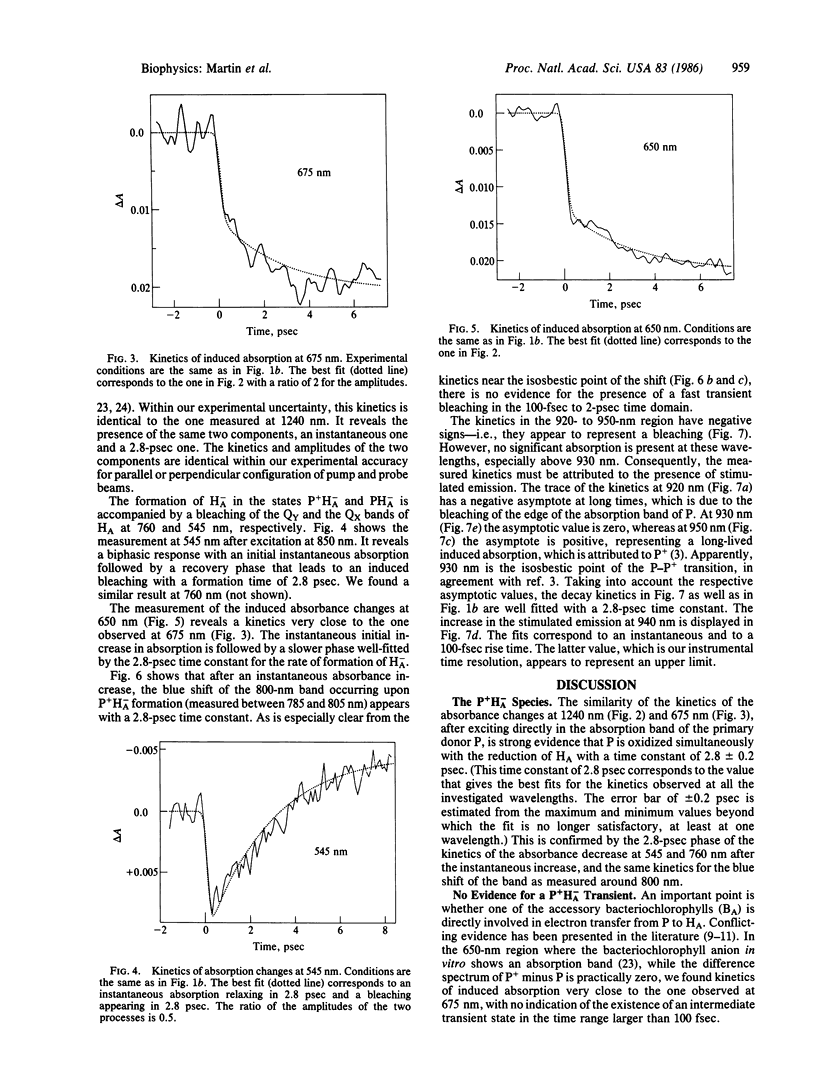
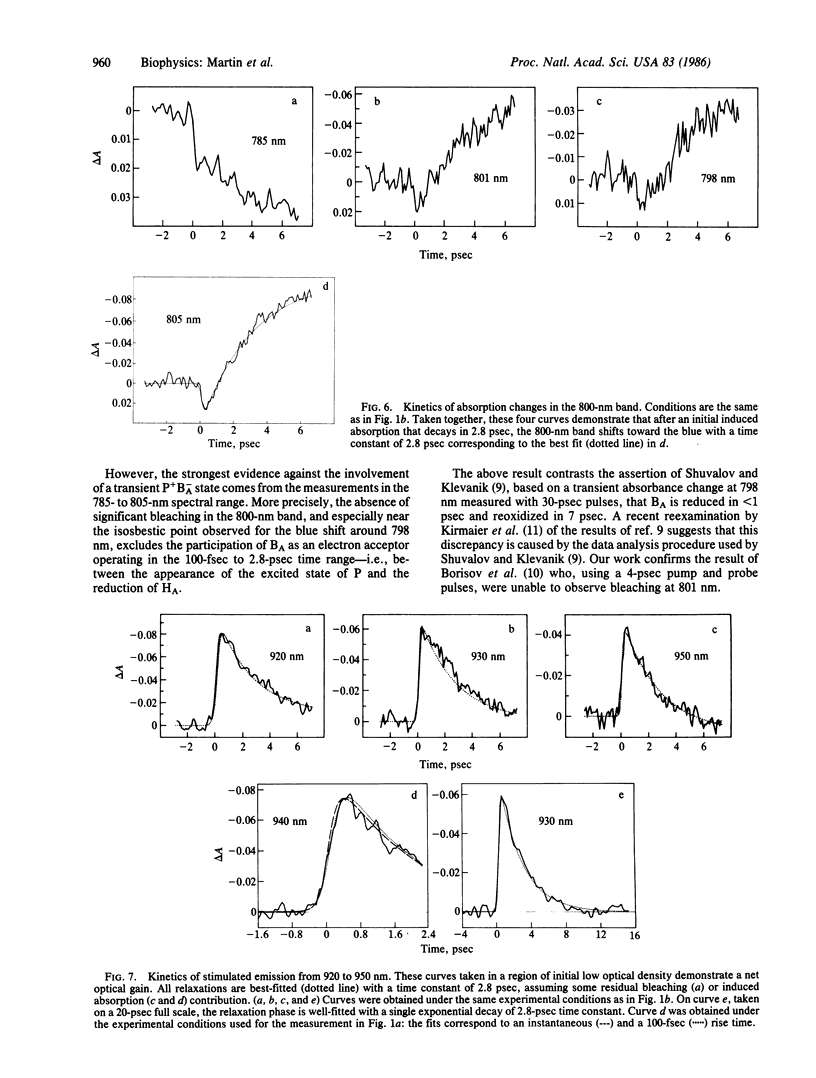
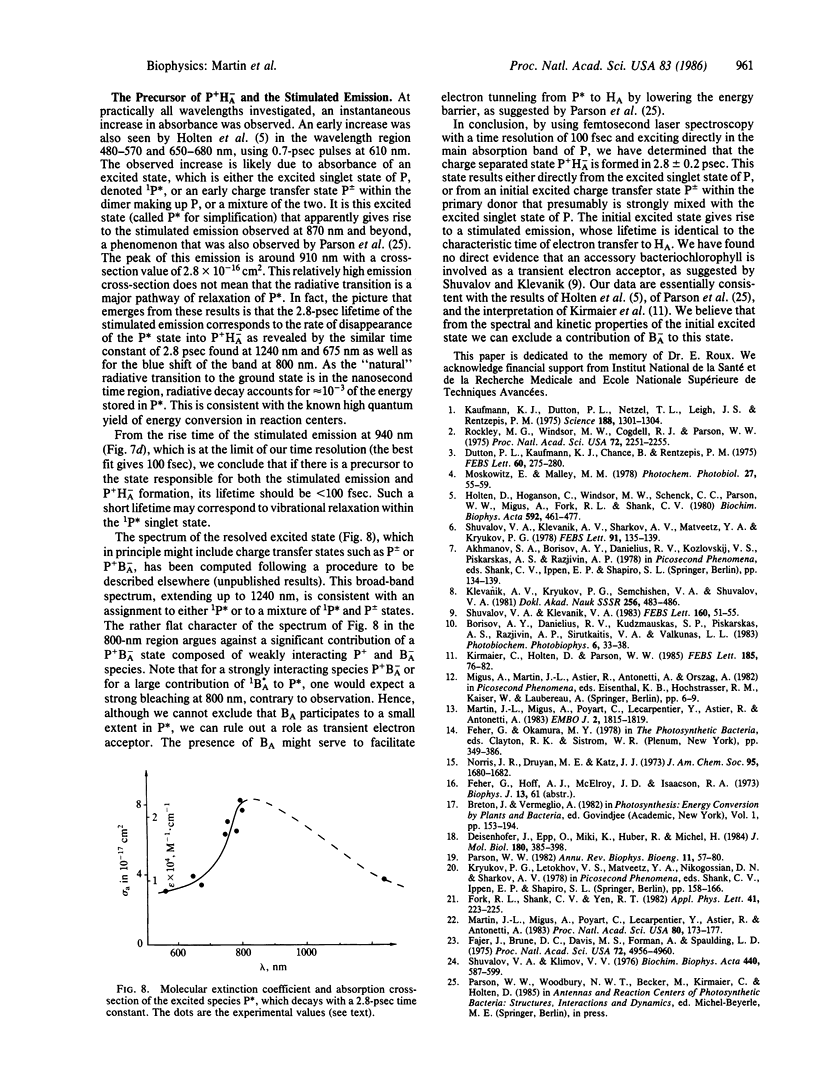
The primary light-induced charge separation in reaction centers from Rhodopseudomonas sphaeroides R-26 has been investigated after excitation with laser pulses of 150 fsec duration within the longwave absorption band of the primary donor at 850 nm. An excited state of the primary donor, characterized by a broad absorption spectrum extending over the whole spectral range investigated (545-1240 nm), appeared within 100 fsec and gave rise to stimulated emission in the 870- to 1000-nm region with a 2.8-psec lifetime. The photooxidation of the primary donor, as measured at 1240 nm, and the photoreduction of the bacteriopheophytin acceptor, monitored at 545 nm and 675 nm, have been found to proceed simultaneously with a time constant of 2.8 ± 0.2 psec. Kinetics of absorbance changes at other probe wavelengths gave no indication that an accessory bacteriochlorophyll is involved as a transient electron acceptor.
Keywords: photooxidation, charge separation, stimulated emission
Full text
PDF
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Deisenhofer J., Epp O., Miki K., Huber R., Michel H. X-ray structure analysis of a membrane protein complex. Electron density map at 3 A resolution and a model of the chromophores of the photosynthetic reaction center from Rhodopseudomonas viridis. J Mol Biol. 1984 Dec 5;180(2):385–398. doi: 10.1016/s0022-2836(84)80011-x. [DOI] [PubMed] [Google Scholar]
- Dutton P. L., Kaufmann K. J., Chance B., Rentzepis P. M. Picosecond kinetics of the 1250 nm band of the Rps. sphaeroides reaction center: the nature of the primary photochemical intermediary state. FEBS Lett. 1975 Dec 15;60(2):275–280. doi: 10.1016/0014-5793(75)80730-7. [DOI] [PubMed] [Google Scholar]
- Fajer J., Brune D. C., Davis M. S., Forman A., Spaulding L. D. Primary charge separation in bacterial photosynthesis: oxidized chlorophylls and reduced pheophytin. Proc Natl Acad Sci U S A. 1975 Dec;72(12):4956–4960. doi: 10.1073/pnas.72.12.4956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holten D., Hoganson C., Windsor M. W., Schenck G. C., Parson W. W., Migus A., Fork R. L., Shank C. V. Subpicosecond and picosecond studies of electron transfer intermediates in Rhodopseudomonas sphaeroides reaction centers. Biochim Biophys Acta. 1980 Oct 3;592(3):461–477. doi: 10.1016/0005-2728(80)90092-4. [DOI] [PubMed] [Google Scholar]
- Kaufmann K. J., Dutton P. L., Netzel T. L., Leigh J. S., Rentzepis P. M. Picosecond kinetics of events leading to reaction center bacteriochlorophyll oxidation. Science. 1975 Jun 27;188(4195):1301–1304. doi: 10.1126/science.188.4195.1301. [DOI] [PubMed] [Google Scholar]
- Martin J. L., Migus A., Poyart C., Lecarpentier Y., Astier R., Antonetti A. Femtosecond photolysis of CO-ligated protoheme and hemoproteins: appearance of deoxy species with a 350-fsec time constant. Proc Natl Acad Sci U S A. 1983 Jan;80(1):173–177. doi: 10.1073/pnas.80.1.173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin J. L., Migus A., Poyart C., Lecarpentier Y., Astier R., Antonetti A. Spectral evidence for sub-picosecond iron displacement after ligand detachment from hemoproteins by femtosecond light pulses. EMBO J. 1983;2(10):1815–1819. doi: 10.1002/j.1460-2075.1983.tb01663.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Norris J. R., Druyan M. E., Katz J. J. Electron nuclear double resonance of bacteriochlorophyll free radical in vitro and in vivo. J Am Chem Soc. 1973 Mar 7;95(5):1680–1682. doi: 10.1021/ja00786a066. [DOI] [PubMed] [Google Scholar]
- Parson W. W. Photosynthetic bacterial reaction centers: interactions among the bacteriochlorophylls and bacteriopheophytins. Annu Rev Biophys Bioeng. 1982;11:57–80. doi: 10.1146/annurev.bb.11.060182.000421. [DOI] [PubMed] [Google Scholar]
- Rockley M. G., Windsor M. W., Cogdell R. J., Parson W. W. Picosecond detection of an intermediate in the photochemical reaction of bacterial photosynthesis. Proc Natl Acad Sci U S A. 1975 Jun;72(6):2251–2255. doi: 10.1073/pnas.72.6.2251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shuvalov V. A., Klevanik A. V., Sharkov AV J. u., Matveetz A., Krukov P. G. Picosecond detection of BChl-800 as an intermediate electron carrier between selectively-excited p870 and bacteriopheophytin in Rhodospirillum rubrum relaction centers. FEBS Lett. 1978 Jul 1;91(1):135–139. doi: 10.1016/0014-5793(78)80034-9. [DOI] [PubMed] [Google Scholar]
- Shuvalov V. A., Klimov V. V. The primary photoreactions in the complex cytochrome-P-890-P-760 (bacteriopheophytin760) of Chromatium minutissimum at low redox potentials. Biochim Biophys Acta. 1976 Sep 13;440(3):587–599. doi: 10.1016/0005-2728(76)90044-x. [DOI] [PubMed] [Google Scholar]


