Abstract
Thyroid hormone is essential for the development of the cochlea and auditory function. Cochlear response tissues, which express thyroid hormone receptor β (encoded by Thrb), include the greater epithelial ridge and sensory epithelium residing inside the bony labyrinth. However, these response tissues lack direct blood flow, implying that mechanisms exist to shuttle hormone from the circulation to target tissues. Therefore, we investigated expression of candidate thyroid hormone transporters L-type amino acid transporter 1 (Lat1), monocarboxylate transporter (Mct)8, Mct10, and organic anion transporting polypeptide 1c1 (Oatp1c1) in mouse cochlear development by in situ hybridization and immunofluorescence analysis. L-type amino acid transporter 1 localized to cochlear blood vessels and transiently to sensory hair cells. Mct8 localized to the greater epithelial ridge, tympanic border cells underlying the sensory epithelium, spiral ligament fibrocytes, and spiral ganglion neurons, partly overlapping with the Thrb expression pattern. Mct10 was detected in a highly restricted pattern in the outer sulcus epithelium and weakly in tympanic border cells and hair cells. Organic anion transporting polypeptide 1c1 localized primarily to fibrocytes in vascularized tissues of the spiral limbus and spiral ligament and to tympanic border cells. Investigation of hypothyroid Tshr−/− mice showed that transporter expression was delayed consistent with retardation of cochlear tissue maturation but not with compensatory responses to hypothyroidism. The results demonstrate specific expression of thyroid hormone transporters in the cochlea and suggest that a network of thyroid hormone transport underlies cochlear development.
A key function of thyroid hormone is to stimulate the development of hearing. Deafness is a known consequence of early developmental hypothyroidism in humans (1, 2). Studies in rodents identified the cochlea as a major site of thyroid hormone action and showed that thyroid hormone promotes the later stages of cochlear development and onset of auditory function by the end of the second postnatal week. Developmental hypothyroidism retards the remodeling of the greater epithelial ridge, deforms the tectorial membrane, impairs maturation of the sensory epithelium, delays myelination of the cochlear nerve, and results in permanent deafness (3–6). Thyroid hormone receptor (TR)β, encoded by Thrb, is essential for the development of hearing (7) and is expressed in the greater epithelial ridge, sensory epithelium, spiral ganglion, and at low levels in other cochlear regions (8–10). TRα1, encoded by Thra, also serves a contributory role in cochlear development (11, 12).
The intricate anatomy of the cochlea poses a puzzle concerning how thyroid hormone gains access to its target tissues. The sensory epithelium, including the hair cells that transduce sound into neural signals, lacks direct blood flow (13, 14). Blood enters the central axis of the cochlea through the spiral modiolar artery (see Fig. 1H). Short radiating arterioles loop through the spiral limbus and drain back to the modiolar vein, whereas longer branches extend laterally to form extensive capillary networks in the spiral ligament and stria vascularis. These vascular networks largely bypass the sensory epithelium. A need for hormonal transport is also implied by the evidence that type 2 deiodinase (Dio2), encoded by Dio2, amplifies levels of active thyroid hormone within the cochlea (9, 15). Dio2 converts T4, the main form of thyroid hormone in the blood, into T3, the primary ligand for the TR (16). In the cochlea, Dio2 is expressed in vascularized tissues in the modiolus and lateral wall but not in internal target tissues for thyroid hormone. This separation of T3-generating and T3-responsive tissues led us to propose a paracrine-like control of cochlear development necessitating local transport of T4 and T3 (15).
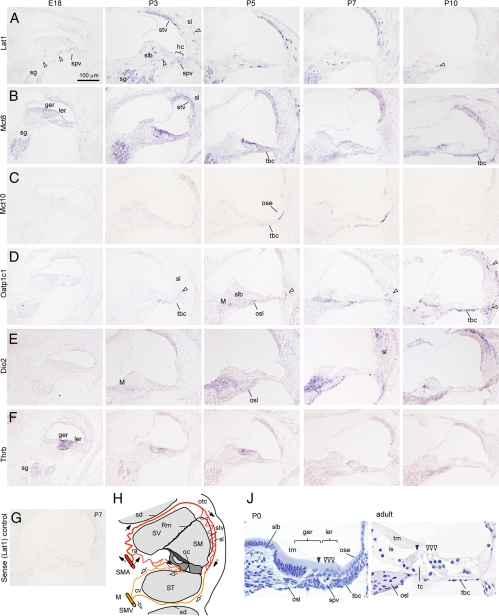
Fig. 1.
In situ hybridization analysis of thyroid hormone transporter mRNA expression in cochlear development. Lat1, Mct8, Mct10, and Oatp1c1 transporters (A–D) and for comparison, Dio2 (E) and Thrb (TRβ) (F). Signals, blue/purple staining. Scale bar in A also applies to B–G. A, Lat1 signal peaked at P1–P5 in the spiral ganglion (sg), spiral vessel (spv), spiral limbus (slb), spiral ligament (sl), and stria vascularis (stv). Fragmented signals (arrowheads) represent blood vessels. Weak signals were detected in hair cells (hc) at P3. B, Mct8 signal peaked at P1–P5 in the greater epithelial ridge (ger), lesser epithelial ridge (ler), sg, stv, and sl. After P5, signal increased in tympanic border cells (tbc). C, Mct10 signal appeared in the outer sulcus epithelium (ose) at P3–P7. By P7, Mct10 was also detected weakly in the inner sulcus epithelium (ise), hc, and tbc. D, Oatp1c1 signal appeared at P3–P5 and persisted at least until P15 in the modiolus (M), slb, and osseous spiral lamina (osl). Signal strengthened in tbc at P10. In the sl, signals were detected first in lower regions (one arrowhead at P5 and P7), then spreading to all regions of the sl (two arrowheads, P10). E, Dio2 signal detected in the M, osl, and sl. Dio2 signal peaked around P7. F, Thrb signal detected in the ger, sg, and weakly in the ler and lateral regions of the cochlear duct. Thrb signal peaked at E18–P1, then declined at P7–P10. G, A representative sense control probe (Lat1 shown) gave little or no background signal. H, Scheme of arterial (red, filled arrows) and venous (yellow, open arrows) blood flow in the cochlea. The greater epithelial ridge and organ of Corti (oc) shaded in dark gray receive little or no direct blood flow. J, Histology of immature (P0) and mature (adult) organ of Corti. Filled arrowhead, Inner hair cell; open arrowheads, outer hair cells. At mature stages, the ger has regressed to form the inner sulcus (is) cavity below the tectorial membrane (tm). Inner and outer hair cells are separated by the tunnel of Corti (tc). cv, Collecting venule; otc, otic capsule; ra, radiating arteriole; Rm, Reissner's membrane; sd, septal division between cochlear turns; SM, scala media; SMA, spiral modiolar artery; SMV, spiral modiolar vein; ST, scala tympani; SV, scala vestibuli.
Growing evidence indicates that T4 and T3 do not passively diffuse across cell membranes but require trans-membrane transporters for cellular uptake or efflux. Several proteins have been shown to transport thyroid hormones in vitro, including members of the monocarboxylate transporter (Mct), organic anion transporting polypeptide (Oatp), and L-type amino acid transporter (Lat) families (17). Human MCT8 mutations cause X-linked Allen-Herndon-Dudley syndrome, characterized by psychomotor retardation with abnormally high T3 and low T4 levels in serum (18, 19). Here, we investigated the expression of thyroid hormone transporters in mouse cochlear development. The results demonstrate cell- and developmental-specific expression patterns for Lat1 (Slc7a5), Mct8 (Slc16a2), Mct10 (Slc16a10), and Oatp1c1 (Slco1c1), suggesting a role for thyroid hormone transport in cochlear development.
Materials and Methods
Mouse strains
Timed pregnant C57BL/6J mice (The Jackson Laboratory, Bar Harbor, ME) were used to generate wild-type (+/+) progeny for analysis. TSH receptor knockout mice (Tshr+/−) on a mixed C57BL/6J × 129/Sv background (20) were crossed to generate wild-type (+/+) and Tshr−/− littermate pups for analysis. Genotypes were determined by PCR (20). Experiments were performed under approved institutional protocols at the National Institutes of Health/National Institute of Diabetes and Digestive and Kidney Diseases.
In situ hybridization
Cochleae were fixed overnight in 4% paraformaldehyde (PFA) at 4 C, washed three times in PBS, then cryoprotected overnight in 30% sucrose in PBS. For postnatal day (P)10 and P15, cochleae were cryoprotected overnight in 30% sucrose containing 0.1 m EDTA for decalcification. Samples were embedded in optimal cutting temperature compound (Tissue-Tek, Sakura Finetek USA, Torrance, CA) and stored at −80 C. Antisense and sense digoxigenin-labeled riboprobes were generated from plasmids carrying mouse cDNA sequences for Thrb and Dio2 as reported (10, 15) and for transporters as follows: Slc7a5 (Lat1; NM_011404.3) bp 470-1549, Slc16a2 (Mct8; BC080678.1) bp 599-1561, Slc16a10 (Mct10; NM_001114332.1) bp 596-1664, and Slco1c1 (Oatp1c1; NM_021471.2) bp 871-1969. Riboprobes were hybridized to 12-μm midmodiolar cochlear cryosections (21). For each gene, samples at all specified ages were processed in parallel in one experiment to allow comparative analysis. Groups were n ≥ 3 animals/age. The specificity of each probe was determined by parallel analysis with a corresponding sense probe (Supplemental Fig. 1, published on The Endocrine Society's Journals Online web site at http://endo.endojournals.org). Image brightness and contrast were adjusted using Adobe Photoshop CS4 applied equally to any given set of images.
Reverse transcription and quantitative PCR (qPCR)
Total RNA was isolated from whole cochlea at specified ages (n = 3 mice/age; both cochleae from single animals were pooled) using TRIzol Reagent (Invitrogen, Carlsbad, CA) according to the manufacturer's directions. Concentration and purity of RNA were determined spectroscopically using a Nanodrop-1000 (Thermo Scientific, Wilmington, DE). RNA (100-ng samples) was reverse transcribed using Superscript III (Invitrogen) and subjected to SYBR Green based real-time qPCR (Power Cyber Mastermix; Applied Biosystems, Carlsbad, CA) using a Step-One-Plus system (Applied Biosystems). Primer sets [forward (F) and reverse (R)]: Thrb (NM_001113417.1), F 5′-GCTGGTAGGAATGTCTGAAGC and R 5′-AGTCTGGAAAGTCTGGGCAC; Dio2 (NM_010050.2), F 5′-GATGCTCCCAATTCCAGTGTGG and R 5′-CCTCTTGGTTCCGGTGCTTCTT; Slc7a5 (NM_011404.3), F 5′-CTACTTCTTTGGTGTCTGGTGGAA and R 5′-GAGGTACCACCTGCATCAACTTC; Slc16a2 (NM_009197.2), F 5′-CCCTGGACTTAAGAAGATATACTTGCA and R 5′-CCCGAAGTCCCGGCATA; Slc16a10 (NM_138831.1), F 5′-AAGCTCCATCGAGCCTCTGTA and R 5′-GTCCCAAAATGACCAGTGACG; Slco1c1 (NM_021471.2), F 5′-GGGCCATCCTTTACAGTCGG and R 5′-CCTTCTCTCTATCTGAGTCACGG; and Actb (NM_007393.3), F 5′-TGCTGTCCCTGTATGCCTCTG and R 5′-TTGATGTCACGCACGATTTCC. No signal was observed for any gene with omission of cochlear cDNA template. Quantitative values were determined using the 2−ΔΔCT method by normalizing to β-actin (22). Values were calculated as an average of three separate samples and expressed relative to embryonic (E) 18 levels.
Immunofluorescence and confocal microscopy analyses
For immunofluorescence, cochlea were fixed at 4 C as follows: for Lat1, cochleae were fixed in 2% PFA for 2–4 h. For Mct8, cochleae were fixed in 4% PFA overnight. For Oatp1c1, cochleae were freshly frozen without fixation; cryosections were then immersion-fixed in 100% methanol for 10 min at −20 C, as described for brain (23). Specific Oatp1c1 signal could not be detected in PFA-fixed cochlea. For Lat1 and Mct8, fixed cochleae were washed three times in PBS, cryoprotected in 30% sucrose overnight at 4 C, embedded in optimal cutting temperature, and then stored at −80 C. For immunostaining, slides with 12-μm sections were equilibrated to room temperature, washed in PBS, then blocked and permeabilized with PBS containing 1% BSA, 2.5% normal serum, and 0.1% Triton X-100 for 1 h at room temperature. Primary antibodies in blocking buffer were applied for approximately 16 h at room temperature in a humidified chamber. Antibody dilution and source: goat antimouse Lat1 (1:250, SC54229; Santa Cruz Biotechnology, Inc., Santa Cruz, CA), rabbit antihuman Mct8 (1:2500, Ab1306; T. J. Visser), rabbit antimouse Oatp1c1 (1:3000, NP_067446 amino acids 696–715; gift from J. A. Battey), rat antimouse cluster of differentiation 31 (CD31) (1:250, 550274; BD Biosciences, San Diego, CA), rabbit antihuman myosin VIIa (1:1000, 25-6790; Proteus Biosciences, Ramona, CA), and mouse antirat neuron-specific class III beta-tubulin (Tuj1) (1:1000, MMS-435P; Covance, Emeryville, CA). Slides were washed three times 10 min in PBS and then incubated with secondary antibody-Alexa dye conjugates (Invitrogen) diluted 1:500 in blocking buffer, for 1 h at room temperature. Slides were washed three times 10 min in PBS before cover-slipping in Vector Mount containing 4′,6-diamidino-2-phenylindole (Vector Laboratories, Burlingame, CA).
Confocal imaging was performed on a Leica TCS SPE scanning confocal microscope (Leica, Wetzlar, Germany). Images represented collapsed stacks of two to four z-planes each of 0.5- to 1.0-μm thickness generated by imaging through the entire focal field of the specimen or as noted in the text. Brightness, contrast, orientation, and merging of images were adjusted using ImageJ (http://rsb.info.nih.gov/ij/).
Tests of antibody specificity (Supplemental Fig. 1)
Tests in the absence of primary antibodies were performed as negative controls for Lat1, Mct8, and Oatp1c1. For Lat1, preabsorption was performed using Lat1 polypeptide (SC54229-P; Santa Cruz Biotechnology, Inc.), which abolished all signals on P7 cochlear sections. Mct8 and Oatp1c1 antibodies were tested on samples from Mct8-deficient (24) and Oatp1c1-deficient (gift from H. Heuer) mice, respectively. For Oatp1c1−/− mice at P7 or P15, no Oatp1c1 signal was detected in any cochlear region. For Mct8−/y mice at P7 or P15, signal was abrogated in tympanic border cells, stria vascularis/spiral ligament, and the greater epithelial ridge. However, Mct8 antibody produced nonspecific nuclear signal in inner hair cells and spiral ganglion cells in Mct8−/y mice. In contrast to this nonspecific nuclear signal, specific Mct8 signals that were abolished in Mct8−/y mice were localized to the cell membrane.
Bioinformatic analysis of transporter expression
The presence of transporter expressed sequence tag (EST) was investigated in the National Center for Biotechnology Information (NCBI) UniGene EST profile viewer (http://www.ncbi.nlm.nih.gov/unigene) and the Morton Fetal Human EST database [http://brighamandwomens.org/Research/labs/BWH_Hearing/Cochlear_ESTs.aspx (25)].
Results
In situ hybridization analysis of thyroid hormone transporter expression in cochlear development
In situ hybridization analysis was performed from E18 to P15, the period during which hypothyroidism retards cochlear maturation in mice (Fig. 1, A–G). As an anatomical guide, Fig. 1H shows a diagram of a midturn section of the cochlea revealing the sensory tissues of the organ of Corti and the three fluid-filled chambers, the scala vestibuli, scala media, and scala tympani. The modiolus contains blood vessels and spiral ganglion neurons that project to the brain through the cochlear nerve. Figure 1H also indicates direction of blood flow in the cochlea with the spiral modiolar artery ascending and spiral modiolar vein descending in the modiolus. The organ of Corti receives little or no direct blood flow. The immature organ of Corti is remodeled postnatally before hearing begins (Fig. 1J). The in situ hybridization images shown represent a single midapical turn of the cochlea.
Lat1
Lat1 mRNA was detected in the spiral ganglion, spiral limbus, spiral ligament, and stria vascularis in a pattern indicative of sectioned blood vessels (Fig. 1A). Lat1 signal was also detected in the spiral vessel, a blood vessel below the organ of Corti that is prominent at neonatal stages but that diminishes in size after about one postnatal week of age. Weaker signals were detected in nonvascular cell types in the spiral ganglion and the greater and lesser epithelial ridges of the organ of Corti at P3 (see detail later, Fig. 3). Temporally, Lat1 was first detected weakly at E18, then with increasing strength from P1 to P5. Signals decreased after P7. A control sense probe gave little or no signal at any age (Fig. 1G).
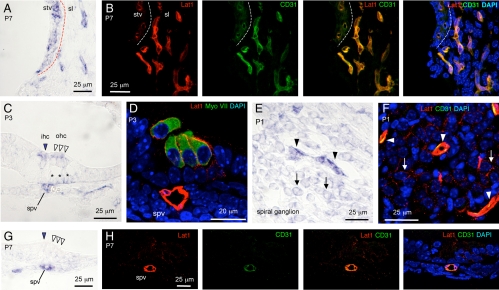
Fig. 3.
Localization of Lat1 in the cochlea. In situ hybridization for Lat1 mRNA in stria vascularis (stv)/spiral ligament (sl) at P7 (A), sensory epithelium at P3 (C) and P7 (G), and spiral ganglion (sg) at P1 (E). Similar regions under immunofluorescence analysis for Lat1 protein (red) and endothelial cell (CD31, green; B, F, and H) or hair cell (MyoVII, green; D) markers. A, Lat1 mRNA signal in stv and sl. Dashed line, Boundary between stv and sl (same in B). B, Lat1 signal in CD31-positive blood vessels in stv and sl (merged, orange/yellow). C, Organ of Corti region at P3 showing Lat1 mRNA signal in spiral vessel (spv), inner (ihc, arrow), and outer hair cells (ohc, arrowheads) and Deiters' cells (asterisks). D, In hair cells, Lat1 protein signal was in the cell membrane external to the cytosolic hair cell marker myosin VIIa. The spv was Lat1-positive. E, Lat1 mRNA signal in blood vessels (arrowheads) and neurons (arrows) of the sg. F, Lat1 immunofluorescence in CD31-positive vessels (arrowheads) and in neuronal soma (arrows) of the sg. G, At P7, Lat1 mRNA was detected in the spv but not hair cells (compare with P3 in C). H, The spv was both Lat1- and CD31-positive. Note, in B, to illustrate blood vessels better, a collapsed, full-stack z-projection is shown through the entire tissue section. DAPI, 4′,6-diamidino-2-phenylindole.
Mct8
Mct8 mRNA was detected in the spiral ganglion, greater epithelial ridge, stria vascularis, and at lower levels in the spiral ligament, spiral limbus, and lesser epithelial ridge (Fig. 1B). In the spiral ganglion and greater epithelial ridge, the Mct8 expression pattern partly overlapped with that of Thrb (Fig. 1F). Mct8 mRNA signals were weak at E18, increased during the first postnatal week, then decreased after P5. Developmentally, the greater epithelial ridge regresses between P3 and P15, and this was accompanied by decreasing Mct8 mRNA signal as the tissue was lost. However, the spiral ganglion, stria vascularis, and spiral ligament maintained some level of expression at least until P15. Mct8 signal strengthened in tympanic border cells at P7–P15 (see detail later, Fig. 4). An Mct8 sense probe gave little or no signal (Supplemental Fig. 1 shows results for sense probes for all transporter genes studied).
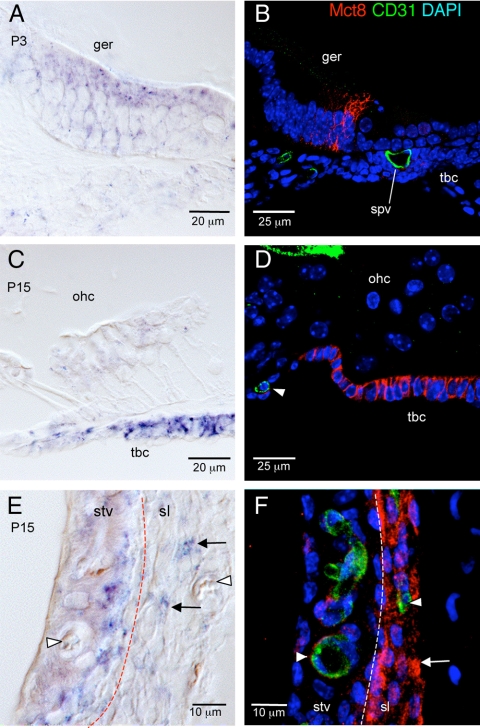
Fig. 4.
Localization of Mct8 in the cochlea. Left panels, In situ hybridization of Lat1 mRNA in the greater epithelial ridge (ger) (A), sensory epithelium region (C), and stria vascularis (stv)/spiral ligament (sl) (E). Right panels, Similar regions showing immunofluorescence for Mct8 protein (red) and CD31 endothelial marker (green). A, Mct8 mRNA signal in ger at P3. B, Mct8 protein localized to ger but not to CD31-positive blood vessels. Mct8 protein localized to the cell membrane of columnar cells of the ger. C, Mct8 mRNA signal in tympanic border cells (tbc) at P15. ohc, Outer hair cells. D, Mct8 protein localized to tbc and not to CD31-positive vessels (arrowhead). Mct8 protein localized to the cell membrane in tbc. E, Mct8 mRNA signal in stv and sl at P15 (dashed line, boundary between stv and sl). Arrowheads, Blood vessels; arrows, sl fibrocytes. F, Mct8 immunofluorescence (red) although weak, localized to the abluminal side of CD31-positive blood capillaries (arrowhead, green) in stv. In the sl, Mct8 protein was detected widely in fibrocytes (red) and not in CD31-positive vessels (green). spv, Spiral vessel; DAPI, 4′,6-diamidino-2-phenylindole.
Mct10
Mct10 mRNA exhibited the most restricted pattern of the transporters investigated (Fig. 1C). Mct10 mRNA was not detected until P3 when signal appeared weakly in the outer sulcus epithelium. At P5–P7, Mct10 signal increased markedly in the outer sulcus epithelium and appeared weakly in the tympanic border cells. By P7, weak Mct10 signal also appeared in the hair cell region of the organ of Corti and in the inner sulcus epithelium, which was beginning to form by this stage. By P10, Mct10 signal fell below detection in all areas. An Mct10 sense control probe gave little or no signal.
Oatp1c1
Oatp1c1 mRNA signal was first detected weakly at P3 in the tympanic border cells and in fibrocytes in the spiral ligament, where it adjoins the basilar membrane (Fig. 1D). By P5, Oatp1c1 signal was detected in fibrocytes in the spiral limbus and modiolus. From P7 to P15, Oatp1c1 expression spread widely throughout most regions of the spiral ligament. Oatp1c1 signal in the tympanic border cells increased substantially between P7 and P15 (see detail later, Fig. 5). No signal was detected with an Oatp1c1 sense probe.
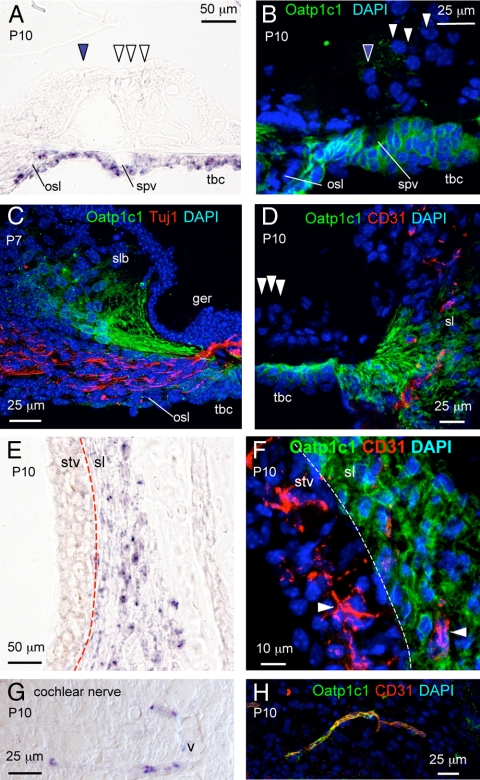
Fig. 5.
Localization of Oatp1c1 in the cochlea. In situ hybridization analysis of Oatp1c1 mRNA in sensory epithelium region (A), stria vascularis (stv)/spiral ligament (sl) (E), and cochlear nerve (G). Immunofluorescence for Oatp1c1 protein in sensory epithelium region (B), spiral limbus (slb) region (C), outer sulcus/sl (D), stv/sl (F), and cochlear nerve (H). A, Oatp1c1 mRNA signal in tympanic border cells (tbc) and osseous spiral lamina (osl) at P10. Inner and outer hair cells, filled and open arrowheads, respectively. B, Oatp1c1 immunofluorescence (green) in tbc and osl at P10. Oatp1c1 signal localized to the cell membrane in the tbc. C, Oatp1c1 protein (green) in slb fibrocytes that project toward the sensory epithelium and tbc. The fibers did not costain with Tuj1 marker (red) for neuronal fibers that innervate hair cells. D, Oatp1c1 protein (green) in tbc and in sl fibrocytes that project toward tbc below the sensory epithelium. Oatp1c1 did not colocalize with CD31-positive vessels (red) in the sl. E, Oatp1c1 mRNA detected in sl but not stv. F, Oatp1c1 protein localized to sl but not stv. Oatp1c1 did not colocalize with CD31-positive vessels in the stv or sl. G, Oatp1c1 mRNA signal in vessel-like structures in the cochlear nerve. H, Oatp1c1 immunofluorescence colocalized in CD31-positive blood vessels (yellow in merged image) in cochlear nerve. spv, Spiral vessel; ger, greater epithelial ridge; DAPI, 4′,6-diamidino-2-phenylindole.
The Oatp1c1 expression pattern partly overlapped with that of Dio2 (Fig. 1E). Both genes were expressed in the modiolus, osseous spiral lamina, and spiral ligament. Also, expression of both genes began at early postnatal ages and rose in parallel until P7. However, beyond P10, Dio2 signal decreased, as reported (15), whereas Oatp1c1 signal was sustained at least until P15.
Quantitative and bioinformatic analysis of transporter mRNA
Expression of Lat1, Mct8, Mct10, and Oatp1c1 mRNA in the cochlea as revealed by in situ hybridization analysis was corroborated by real-time qPCR analysis (Fig. 2A). Moreover, qPCR analysis provided an independent means of monitoring the trend of expression levels of a given transporter over development. Oatp1c1 mRNA levels increased markedly between E18 and P15, whereas Lat1, Mct8, and Mct10 mRNA levels increased only moderately. In general, qPCR results were consistent with in situ hybridization data with the exception of Mct10 mRNA, which was not detectable by in situ hybridization after P10. This difference may be due to different assay sensitivities with qPCR detecting weak global signal in whole cochlea RNA samples, even though specific, focal signal is no longer detected by in situ hybridization. For comparison, Dio2 mRNA levels increased sharply during the first postnatal week to peak at P7, then declined, as reported previously (Fig. 2B) (15). Thrb (TRβ1) mRNA levels remained relatively constant during the first postnatal week but thereafter decreased (Fig. 2B).
Fig. 2.
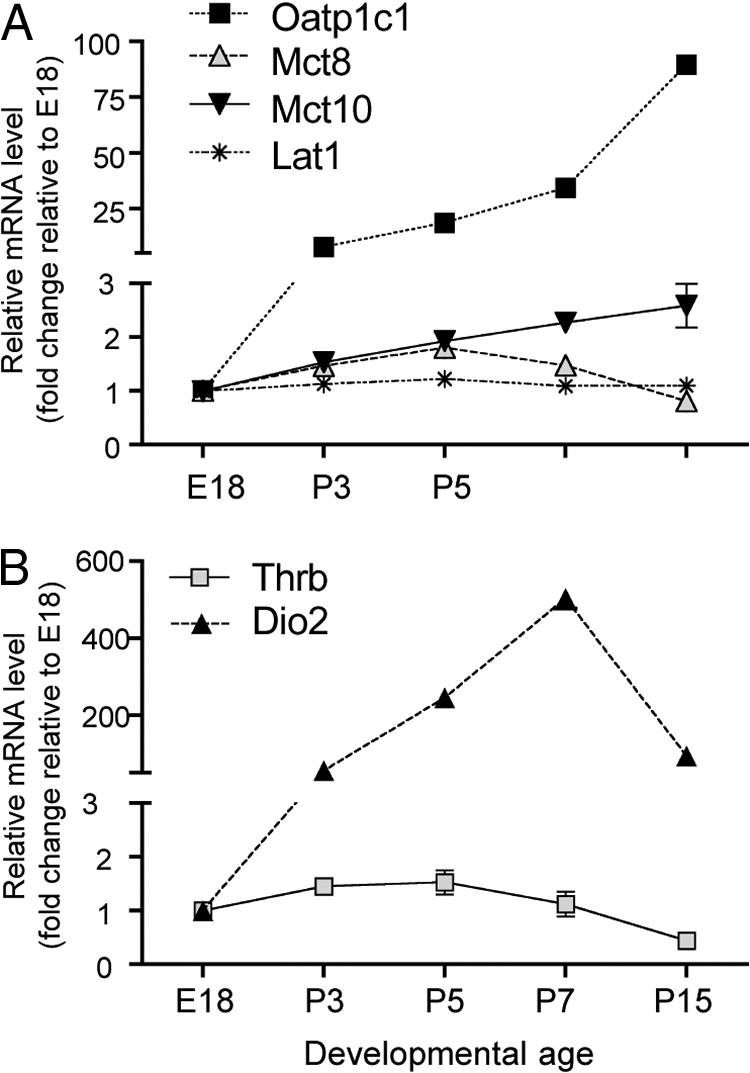
Relative levels of Lat1, Mct8, Mct10, and Oatp1c1 mRNA in cochlear development. A, Real-time qPCR analysis of transporter mRNA levels normalized to β-actin and plotted relative to E18 values, given an arbitrary value of 1.0. Each point represents mean ± sem for three individual cochleae. The analysis reveals trends in expression levels over developmental time for a given gene but does not allow direct comparison of absolute expression levels between different genes. B, Parallel analysis of Thrb (TRβ1 isoform) and Dio2 mRNA levels.
Expression of Lat1, Mct8, Mct10, and Oatp1c1 was further corroborated by identification of expressed sequence tags for these same transporters in a mouse cochlea database using the NCBI UniGene EST Profile Viewer. MCT8 and OATP1C1 mRNA sequences were also identified in human cochlea using the NCBI UniGene EST Profile Viewer and the Morton Fetal Cochlea cDNA Library databases (25).
Cellular localization of thyroid hormone transporters in the cochlea
To define cochlear cell types that expressed Lat1, Mct8, and Oatp1c1, high-power magnification of in situ hybridization patterns and immunofluorescence analyses were performed at selected ages.
Lat1 protein localization
In the cochlear lateral wall (Fig. 3A), in situ hybridization analysis detected Lat1 mRNA in the intermediate region of stria vascularis and in blood vessel-like structures in the spiral ligament. Dual immunofluorescence analysis for Lat1 and CD31, an endothelial cell marker, localized Lat1 protein almost exclusively to CD31-positive blood vessels in the spiral ligament and stria vascularis (Fig. 3B and Supplemental Video 1).
In the organ of Corti at P3 (Fig. 3C), in situ hybridization detected Lat1 mRNA transiently in inner and outer hair cells and more weakly in Deiters support cells residing below the outer hair cells. By P7, these signals were no longer detectable (Fig. 3G). Immunofluorescence also detected Lat1 protein in hair cells and Deiters cells and, moreover, localized Lat1 signal to the cell membrane of these cells at P3 (Fig. 3D). Lat1 immunofluorescent signal in the cell membrane was external to the cytosolic signal of hair cell-specific myosin VIIa (Fig. 3D).
In the spiral ganglion (Fig. 3E), in situ hybridization detected Lat1 mRNA in vessel-like structures (Fig. 3E, arrowheads) and weakly in cell bodies of neurons (Fig. 3E, arrows). Immunofluorescence similarly identified Lat1 signals in CD31-positive blood vessels and weakly in neuronal soma in the spiral ganglion (Fig. 3F).
In situ hybridization detected Lat1 mRNA in the spiral vessel below the sensory epithelium at P3 and P7 (Fig. 3, C and G, and Supplemental Video 2). Immunofluorescence demonstrated that the spiral vessel was both Lat1- and CD31-positive (Fig. 3H).
Mct8 protein localization
In the organ of Corti at P3 (Fig. 4A), in situ hybridization detected Mct8 mRNA in the columnar cells of the greater epithelial ridge. Immunofluorescence localized Mct8 protein signal to the membrane of these cells as expected for a membrane transporter (Fig. 4B). No localization was observed in CD31-positive capillaries. In situ hybridization at P15 detected Mct8 mRNA in the tympanic border cells beneath the sensory epithelium (Fig. 4C). Immunofluorescence also localized Mct8 signal in the cell membrane of these cells (Fig. 4D).
In the lateral wall (Fig. 4E), in situ hybridization identified Mct8 mRNA in the intermediate/basal region of stria vascularis and in spiral ligament fibrocytes at P15. Immunofluoresence (Fig. 4F) detected Mct8 signal in spiral ligament fibrocytes [type I and IV fibrocytes, based on zonal location in the spiral ligament (26)] but not in CD31-positive capillaries. In the stria vascularis, weak Mct8 signal was detected in some CD31-positive capillaries (Fig. 4F).
In the spiral ganglion, in situ hybridization detected specific Mct8 mRNA signal in a neuron-like pattern up to P5, with signal declining after P7 (see Fig. 1B). However, Mct8 antibody produced nonspecific signal in nuclei of spiral ganglion cells, precluding localization of Mct8 protein in the spiral ganglion (see Materials and Methods, tests of specificity).
Oatp1c1 protein localization
In the organ of Corti region at P10, in situ hybridization detected strong Oatp1c1 mRNA signal in tympanic border cells and in fibrocytes at the tympanic lip of the osseous spiral lamina (Fig. 5A). Immunofluoresence detected Oatp1c1 protein in these same cells and, moreover, localized Oatp1c1 protein to the cell membrane as expected for a transporter (Fig. 5B).
In accord with in situ hybridization data (see earlier Fig. 1D), immunofluorescence identified Oatp1c1-positive fibrocytes in both the spiral limbus and spiral ligament. These fibrocytes projected elongations toward the sensory epithelium and tympanic border cells (Fig. 5, C and D). Immunofluorescence for the neuronal marker Tuj1 (Fig. 5C, red) excluded the possibility that Oatp1c1 signal in the fibrous projections was in neuronal dendrites that innervate hair cells. Immunofluorescence demonstrated that Oatp1c1 protein did not colocalize with CD31-positive capillaries in the spiral limbus and spiral ligament nor in the spiral vessel (Fig. 5D and data not shown).
In the lateral wall, Oatp1c1 mRNA localized to the spiral ligament (Fig. 5E). Immunofluorescence localized Oatp1c1 protein in fibrocytes rather than CD31-positive blood vessels in the spiral ligament (Fig. 5F and Supplemental Video 3).
In the cochlear nerve, near the cochlear base, in situ hybridization identified Oatp1c1 mRNA in vessel-like structures at P10 (Fig. 5G). Immunofluorescence identified these structures as Oatp1c1/CD31-positive blood vessels (Fig. 5H).
Transporter gene expression in hypothyroid mice
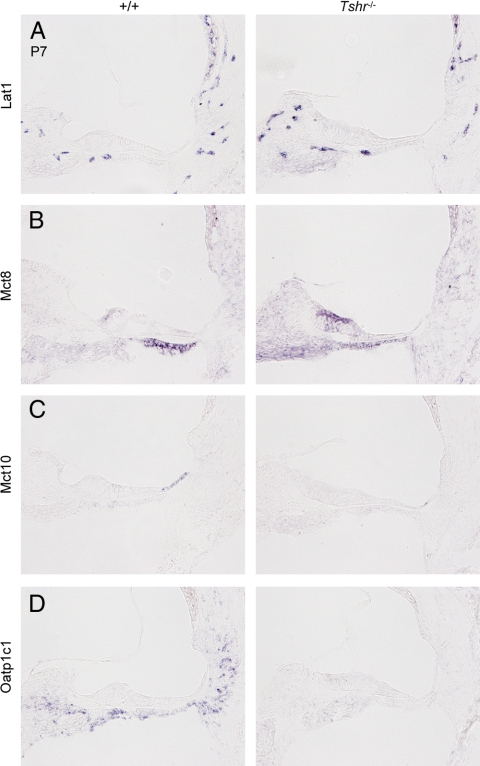
To determine whether transporter expression in the immature cochlea responded to a lack of thyroid hormone in a manner consistent with a compensatory mechanism, we analyzed transporter mRNA by in situ hybridization in congenitally hypothyroid Tshr−/− mice at P3 (data not shown) and P7 (Fig. 6). Expression of Lat1 mRNA was unchanged in Tshr−/− mice compared with +/+ pups. Mct8 mRNA signal was elevated in the greater epithelial ridge and reduced in tympanic border cells in Tshr−/− mice at P7. This pattern resembled that in +/+ mice at P3, consistent with tissue development being delayed by several days in hypothyroid mice rather than indicating a specific adaptive response. Similarly, both Mct10 and Oatp1c1 mRNA signals were reduced in Tshr−/− mice at P7, consistent with a general delay in tissue development. The unchanged (Lat1) or retarded (Mct8, Mct10, and Oatp1c1) patterns of transporter gene expression in Tshr−/− mice did not indicate obvious compensatory responses to hypothyroidism in development.
Fig. 6.
Transporter expression in the cochlea of hypothyroid Tshr−/− mice. In situ hybridization analysis of Lat1 (A), Mct8 (B), Mct10 (C), and Oatp1c1 (D) mRNA in the cochlea at P7. A, Lat1 mRNA expression patterns were similar in Tshr−/− and +/+ pups. B, Mct8 mRNA expression was retarded in Tshr−/− mice. The changed pattern was accounted for by the delayed stage of tissue differentiation in Tshr−/− pups compared with +/+ pups. The pattern in Tshr−/− mice at P7 resembled that of +/+ pups at P3 (compare with +/+ at P3 in Fig. 1B). C and D, Expression of Mct10 (C) and Oatp1c1 mRNA (D) was reduced at P7 in Tshr−/− compared with +/+ mice and was consistent with a delay in tissue development in Tshr−/− mice.
Discussion
Our study identifies thyroid hormone transporter expression in the mouse cochlea and suggests how thyroid hormone may gain access to cochlear target cells, many of which lack direct blood flow. We discuss the results with respect to three questions. 1) How is thyroid hormone taken up from the circulation? 2) Which transporters may operate in cochlear tissues that express Dio2, an enzyme that can amplify local T3 levels by conversion from T4 (9)? 3) How is T3 transferred to internal target cells? The transporter expression patterns detected (summary in Table 1) suggest a network of hormonal communication in the cochlea, in accord with the hypothesis that thyroid hormone promotes cochlear development through a combination of endocrine and paracrine-like signaling (15). Somewhat analogous questions have been discussed regarding thyroid hormone access to target cells in the brain (27–30).
Table 1.
Summary of thyroid hormone transporter expression in cochlear development
| Cochlear region postnatal age | Transporter gene |
|||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
Lat1 |
Mct8 |
Mct10 |
Oatp1c1 |
|||||||||
| P1 | P7 | P15 | P1 | P7 | P15 | P1 | P7 | P15 | P1 | P7 | P15 | |
| Blood vessels | ||||||||||||
| Spiral limbus | + | ++ | + | − | − | − | − | − | − | − | − | − |
| Spiral vessel | + | ++ | + | − | − | − | − | − | − | − | − | − |
| Stria vascularis | + | ++ | + | − | +/− | +/− | − | − | − | − | − | − |
| Spiral ligament | + | ++ | + | − | +/− | +/− | − | − | − | − | − | − |
| Spiral ganglion | + | ++ | + | − | − | +/− | − | − | − | − | − | − |
| Organ of Corti | ||||||||||||
| Hair cells | + | − | − | − | − | − | − | + | − | − | − | − |
| Greater epitheliial ridge | − | − | n.a. | ++ | + | n.a. | − | − | n.a. | − | − | n.a. |
| Inner sulcus epithelium | n.a. | − | − | n.a. | − | − | n.a. | + | − | n.a. | − | − |
| Outer sulcus epithelium | − | − | − | − | − | − | − | +++ | − | − | − | − |
| Deiters/support cells | + | − | − | − | − | − | − | +/− | − | − | − | − |
| Connective tissues | ||||||||||||
| Sp. ligament fibrocytes | − | − | − | ++ | + | + | − | − | − | − | ++ | +++ |
| Sp. limbus fibrocytes | − | − | − | ++ | + | + | − | + | − | − | ++ | +++ |
| Tympanic border cells | − | − | − | − | + | +++ | − | + | − | − | + | +++ |
| Spiral ganglion neurons | + | + | − | + | ++ | + | − | − | − | − | − | − |
n.a., Not applicable, tissue does not exist at this stage; Sp., spiral. Approximate signal level from weakest to strongest: +/−, +, ++, +++; −, no signal detected.
Transporters in the cochlear blood vessels
The widespread expression of Lat1 in the cochlear vasculature suggests a role for Lat1 in the initial uptake of thyroid hormone from the blood into endothelial cells. Lat1 transports T4 and T3, as well as large neutral amino acids, such as tyrosine and phenylalanine, with the affinity for T3 being the greatest for any known substrate (31, 32). Lat1 protein localized to both luminal and abluminal membranes of cochlear blood vessels, suggesting that Lat1 can mediate uptake of T4 and T3 from the vessel lumen and efflux into the surrounding extracellular space. Although the affinity of Lat1 for T4 is lower than for T3, the higher concentration of T4 in serum would help T4 compete for Lat1-mediated transport under physiological conditions. The subsequent release of T4 from endothelial cells by Lat1 may provide nearby Dio2-expressing cells in the modiolus and spiral ligament with T4 for conversion to T3. Although in the brain Lat1, Mct8, and Oatp1c1 localize to blood microvessels (23, 33, 34), in the cochlea, only Lat1 and, very weakly, Mct8 were detected in blood vessels (Table 1), suggesting that endothelial transport mechanisms vary in different tissues.
Transporters in Dio2-expressing and adjacent tissues
Oatp1c1 is a high-affinity T4 transporter that in the brain localizes to microvessels (23, 35, 36). However, in the cochlea, Oatp1c1 localized to fibrocytes in proximity to blood vessels. The Oatp1c1 mRNA expression pattern overlapped with that of Dio2, and the mRNA levels of both Oatp1c1 and Dio2 increased in parallel until P7, when Dio2 expression peaks. Potentially, Oatp1c1 allows Dio2-positive cells to take up T4 for conversion to T3. Currently available reagents, however, are unable to detect Dio2 protein in the cochlea, thus precluding direct analysis of coexpression of Oatp1c1 and Dio2. We also observed that Oatp1c1-positive fibrocytes in the central spiral limbus and lateral spiral ligament extend projections toward the greater epithelial ridge and tympanic border cells (Fig. 5, C and D), thus, conceivably providing one route of shuttling locally generated T3 into the vicinity of target tissues, where presumably another transport step would transfer T3 into target cells.
Mct10 transports aromatic amino acids, T3, and with lower affinity, T4 (37, 38). In the cochlea, Mct10 was expressed in specialized epithelial cells of the outer sulcus. The outer sulcus epithelium contributes to endolymph homeostasis by absorption of K+ and Na+ through cation channels on the apical membrane facing the scala media (see Fig. 1H) (39, 40). It is possible that these cells perform a relay task in transferring T3 from Dio2-positive fibrocytes in the spiral ligament toward the sensory epithelium and tympanic border cells. In the small intestine and liver, Mct10 is expressed in basolateral membranes of epithelial cells, where it may participate in recycling amino acids (41).
Transporters in cochlear target cells
The uptake of T3 by target tissues may involve a variety of transporters, because multiple cochlear cell types depend upon T3 for their differentiation and maturation (3, 11, 12, 42). Morphologically, the most obvious role for T3 in cochlear development is to remodel the greater epithelial ridge and sensory epithelium before the onset of hearing. Of the transporter genes investigated, only Mct8 was expressed in the greater epithelial ridge, where it overlapped with Thrb expression. Thus, Mct8 may facilitate uptake of T3 to promote regression of the tissue. Mutations in human MCT8 cause Allen-Herndon-Dudley syndrome, which is associated with mental retardation and speech defects but not overt deafness (43). Therefore, it is unclear what role Mct8 has in the cochlea, and its function may be substituted by other transporters as has been suggested in other tissues (44, 45). We note that Mct8 mediates both uptake and efflux of T4 and T3 (37, 46), suggesting an alternative possibility that Mct8 at neonatal stages mediates T3 efflux to protect the greater epithelial ridge from premature differentiation. A related protective role has also been proposed for type 3 deiodinase in the greater eipthelial ridge (10).
In the sensory epithelium, Lat1 was transiently expressed in hair cells and the underlying Deiters' cells, which provide structural and functional support for outer hair cells (47). The maturation of hair cells requires T3 signaling (11, 12, 48), which may reflect a direct action of T3 if Lat1 mediates uptake of T3 by hair cells.
We note that transporter-expressing cells in more external regions of the cochlea, such as endothelial tissues or the stria vascularis, may not only serve a role in shuttling thyroid hormone to internal target tissues but may also themselves respond in more subtle ways to T3 (42).
Possible developmental and other functions of transporters in the cochlea
In summary, our results suggest that thyroid hormone transporters are a component of the thyroid hormone-signaling mechanism that promotes cochlear development. The expression patterns of Lat1, Mct8, Mct10, and Oatp1c1 suggest functions that may be tested in future studies in transporter-deficient mice. It is of interest that the human OATP1C1 gene is located within a large 15-Mb interval of the DFNB62 deafness locus on chromosome 12 (49). We note that other transporters, known or unknown, may contribute to thyroid hormone signaling in the cochlea.
In some adult tissues, thyroid hormone transporters have been suggested to mediate homeostatic responses that compensate for reduced thyroid hormone availability. For example, in brain capillaries, Oatp1c1 mRNA levels change in response to thyroid hormone fluctuations, reflecting a possible adaptive mechanism (35). Additionally, the amphibian Lat1 homolog IU12 is induced in T3-mediated intestinal remodeling during metamorphosis (50, 51). However, transporters are unlikely to mediate such responses in the immature cochlea, because no transporter showed obvious compensatory changes in congenitally hypothyroid Tshr−/− pups. Instead, the changes in Mct8, Mct10, and Oatp1c1 expression patterns were consistent with a general delay of the tissue developmental stage (Fig. 6).
Lastly, it is important to note that some of the transporters investigated may transport other natural substrates or xenobiotic compounds in addition to thyroid hormones. Lat1 is an obligate amino acid exchanger and may contribute to amino acid equilibration across membranes (52). Mct10 can transport tyrosine, phenylalanine, and tryptophan as well as amino acid-related compounds, such as l-DOPA (L-3,4-dihydroxyphenylalanine) (37, 38). Oatp1c1 is a low-affinity transporter of amphipathic organic anions, such as 17β estradiol-D-17β-glucuronide, the statin-type of cholesterol lowering drug cerivastatin, the antidiabetic drug troglitazone sulfate (35, 53), and the nonsteroidal antiinflammatory fenamate drug diclofenac (54). Regarding the cochlea, it is of interest that the melanin precursor, l-DOPA, an Mct10 substrate, can protect against age- and noise-induced hearing loss in albino mice (55). Furthermore, in clinical trials, tinnitus and hearing loss were reported as side-effects of taking diclofenac, an Oatp1c1 substrate (56).
Supplementary Material
Acknowledgments
We thank Lily Ng for advice, Terry Davies for Tshr−/− mice, Heike Heuer for samples from Mct8 and Oatp1c1-deficient mice, and Jim F. Battey and Maria Claudia Lattig Matiz for Oatp1c1 antibody.
This work was supported by the National Institutes of Health Intramural Research Program at the National Institute of Diabetes and Digestive and Kidney Diseases.
Disclosure Summary: The authors have nothing to disclose.
For editorial see page 4478
- CD 31
- Cluster of differentiation 31
- Dio2
- type 2 deiodinase
- E
- embryonic
- EST
- expressed sequence tag
- F
- forward
- Lat
- L-type amino acid transporter
- Mct
- monocarboxylate transporter
- NCBI
- National Center for Biotechnology Information
- Oatp
- organic anion transporting polypeptide
- P
- postnatal day
- PFA
- paraformaldehyde
- qPCR
- quantitative PCR
- R
- reverse
- TR
- thyroid hormone receptor
- Tuj1
- neuron-specific class III beta-tubulin.
References
- 1. DeLong GR, Stanbury JB, Fierro-Benitez R. 1985. Neurological signs in congenital iodine-deficiency disorder (endemic cretinism). Dev Med Child Neurol 27:317–324 [DOI] [PubMed] [Google Scholar]
- 2. Rovet J, Walker W, Bliss B, Buchanan L, Ehrlich R. 1996. Long-term sequelae of hearing impairment in congenital hypothyroidism. J Pediatr 128:776–783 [DOI] [PubMed] [Google Scholar]
- 3. Deol MS. 1976. The role of thyroxine in the differentiation of the organ of Corti. Acta Otolaryngol 81:429–435 [DOI] [PubMed] [Google Scholar]
- 4. Hébert R, Dussault JH. 1984. Permanent peripheral hearing system alteration following transient neonatal hyperthyroidism in rats. Brain Res 316:159–164 [DOI] [PubMed] [Google Scholar]
- 5. Knipper M, Bandtlow C, Gestwa L, Köpschall I, Rohbock K, Wiechers B, Zenner HP, Zimmermann U. 1998. Thyroid hormone affects Schwann cell and oligodendrocyte gene expression at the glial transition zone of the VIIIth nerve prior to cochlea function. Development 125:3709–3718 [DOI] [PubMed] [Google Scholar]
- 6. Uziel A, Rabie A, Marot M. 1980. The effect of hypothyroidism on the onset of cochlear potentials in developing rats. Brain Res 182:172–175 [DOI] [PubMed] [Google Scholar]
- 7. Forrest D, Erway LC, Ng L, Altschuler R, Curran T. 1996. Thyroid hormone receptor β is essential for development of auditory function. Nat Genet 13:354–357 [DOI] [PubMed] [Google Scholar]
- 8. Bradley DJ, Towle HC, Young WS., 3rd 1992. Spatial and temporal expression of α- and β-thyroid hormone receptor mRNAs, including the β 2-subtype, in the developing mammalian nervous system. J Neurosci 12:2288–2302 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Ng L, Goodyear RJ, Woods CA, Schneider MJ, Diamond E, Richardson GP, Kelley MW, Germain DL, Galton VA, Forrest D. 2004. Hearing loss and retarded cochlear development in mice lacking type 2 iodothyronine deiodinase. Proc Natl Acad Sci USA 101:3474–3479 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Ng L, Hernandez A, He W, Ren T, Srinivas M, Ma M, Galton VA, St Germain DL, Forrest D. 2009. A protective role for type 3 deiodinase, a thyroid hormone-inactivating enzyme, in cochlear development and auditory function. Endocrinology 150:1952–1960 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Rusch A, Ng L, Goodyear R, Oliver D, Lisoukov I, Vennstrom B, Richardson G, Kelley MW, Forrest D. 2001. Retardation of cochlear maturation and impaired hair cell function caused by deletion of all known thyroid hormone receptors. J Neurosci 21:9792–9800 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Winter H, Braig C, Zimmermann U, Geisler HS, Fränzer JT, Weber T, Ley M, Engel J, Knirsch M, Bauer K, Christ S, Walsh EJ, McGee J, Köpschall I, Rohbock K, Knipper M. 2006. Thyroid hormone receptors TRα1 and TRβ differentially regulate gene expression of Kcnq4 and prestin during final differentiation of outer hair cells. J Cell Sci 119:2975–2984 [DOI] [PubMed] [Google Scholar]
- 13. Axelsson A. 1988. Comparative anatomy of cochlear blood vessels. Am J Otolaryngol 9:278–290 [DOI] [PubMed] [Google Scholar]
- 14. Nakashima T, Naganawa S, Sone M, Tominaga M, Hayashi H, Yamamoto H, Liu X, Nuttall AL. 2003. Disorders of cochlear blood flow. Brain Res Brain Res Rev 43:17–28 [DOI] [PubMed] [Google Scholar]
- 15. Campos-Barros A, Amma LL, Faris JS, Shailam R, Kelley MW, Forrest D. 2000. Type 2 iodothyronine deiodinase expression in the cochlea before the onset of hearing. Proc Natl Acad Sci USA 97:1287–1292 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Bianco AC, Salvatore D, Gereben B, Berry MJ, Larsen PR. 2002. Biochemistry, cellular and molecular biology, and physiological roles of the iodothyronine selenodeiodinases. Endocr Rev 23:38–89 [DOI] [PubMed] [Google Scholar]
- 17. Friesema EC, Jansen J, Milici C, Visser TJ. 2005. Thyroid hormone transporters. Vitam Horm 70:137–167 [DOI] [PubMed] [Google Scholar]
- 18. Dumitrescu AM, Liao XH, Best TB, Brockmann K, Refetoff S. 2004. A novel syndrome combining thyroid and neurological abnormalities is associated with mutations in a monocarboxylate transporter gene. Am J Hum Genet 74:168–175 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Friesema EC, Grueters A, Biebermann H, Krude H, von Moers A, Reeser M, Barrett TG, Mancilla EE, Svensson J, Kester MH, Kuiper GG, Balkassmi S, Uitterlinden AG, Koehrle J, Rodien P, Halestrap AP, Visser TJ. 2004. Association between mutations in a thyroid hormone transporter and severe X-linked psychomotor retardation. Lancet 364:1435–1437 [DOI] [PubMed] [Google Scholar]
- 20. Marians RC, Ng L, Blair HC, Unger P, Graves PN, Davies TF. 2002. Defining thyrotropin-dependent and -independent steps of thyroid hormone synthesis by using thyrotropin receptor-null mice. Proc Natl Acad Sci USA 99:15776–15781 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Wangemann P, Kim HM, Billings S, Nakaya K, Li X, Singh R, Sharlin DS, Forrest D, Marcus DC, Fong P. 2009. Developmental delays consistent with cochlear hypothyroidism contribute to failure to develop hearing in mice lacking Slc26a4/pendrin expression. Am J Physiol Renal Physiol 297:F1435–F1447 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Livak KJ, Schmittgen TD. 2001. Analysis of relative gene expression data using real-time quantitative PCR and the 2(−ΔΔC(T)) method. Methods 25:402–408 [DOI] [PubMed] [Google Scholar]
- 23. Roberts LM, Woodford K, Zhou M, Black DS, Haggerty JE, Tate EH, Grindstaff KK, Mengesha W, Raman C, Zerangue N. 2008. Expression of the thyroid hormone transporters monocarboxylate transporter-8 (SLC16A2) and organic ion transporter-14 (SLCO1C1) at the blood-brain barrier. Endocrinology 149:6251–6261 [DOI] [PubMed] [Google Scholar]
- 24. Trajkovic M, Visser TJ, Mittag J, Horn S, Lukas J, Darras VM, Raivich G, Bauer K, Heuer H. 2007. Abnormal thyroid hormone metabolism in mice lacking the monocarboxylate transporter 8. J Clin Invest 117:627–635 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Skvorak AB, Weng Z, Yee AJ, Robertson NG, Morton CC. 1999. Human cochlear expressed sequence tags provide insight into cochlear gene expression and identify candidate genes for deafness. Hum Mol Genet 8:439–452 [DOI] [PubMed] [Google Scholar]
- 26. Spicer SS, Schulte BA. 1996. The fine structure of spiral ligament cells relates to ion return to the stria and varies with place-frequency. Hear Res 100:80–100 [DOI] [PubMed] [Google Scholar]
- 27. Bernal J. 2005. The significance of thyroid hormone transporters in the brain. Endocrinology 146:1698–1700 [DOI] [PubMed] [Google Scholar]
- 28. Freitas BC, Gereben B, Castillo M, Kalló I, Zeöld A, Egri P, Liposits Z, Zavacki AM, Maciel RM, Jo S, Singru P, Sanchez E, Lechan RM, Bianco AC. 2010. Paracrine signaling by glial cell-derived triiodothyronine activates neuronal gene expression in the rodent brain and human cells. J Clin Invest 120:2206–2217 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Guadaño-Ferraz A, Obregón MJ, St Germain DL, Bernal J. 1997. The type 2 iodothyronine deiodinase is expressed primarily in glial cells in the neonatal rat brain. Proc Natl Acad Sci USA 94:10391–10396 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Robbins J, Goncalves E, Lakshmanan M, Foti D. 1989. Thyroid hormone transport from blood into brain cells. In: DeLong GR, Robbins J, Condliffe PG. eds. Iodine and the brain. New York: Plenum Press; 39–50 [Google Scholar]
- 31. Verrey F. 2003. System L: heteromeric exchangers of large, neutral amino acids involved in directional transport. Pflugers Arch 445:529–533 [DOI] [PubMed] [Google Scholar]
- 32. Friesema EC, Docter R, Moerings EP, Verrey F, Krenning EP, Hennemann G, Visser TJ. 2001. Thyroid hormone transport by the heterodimeric human system L amino acid transporter. Endocrinology 142:4339–4348 [DOI] [PubMed] [Google Scholar]
- 33. Kageyama T, Imura T, Matsuo A, Minato N, Shimohama S. 2000. Distribution of the 4F2 light chain, LAT1, in the mouse brain. Neuroreport 11:3663–3666 [DOI] [PubMed] [Google Scholar]
- 34. Matsuo H, Tsukada S, Nakata T, Chairoungdua A, Kim DK, Cha SH, Inatomi J, Yorifuji H, Fukuda J, Endou H, Kanai Y. 2000. Expression of a system L neutral amino acid transporter at the blood-brain barrier. Neuroreport 11:3507–3511 [DOI] [PubMed] [Google Scholar]
- 35. Sugiyama D, Kusuhara H, Taniguchi H, Ishikawa S, Nozaki Y, Aburatani H, Sugiyama Y. 2003. Functional characterization of rat brain-specific organic anion transporter (Oatp14) at the blood-brain barrier: high affinity transporter for thyroxine. J Biol Chem 278:43489–43495 [DOI] [PubMed] [Google Scholar]
- 36. Tohyama K, Kusuhara H, Sugiyama Y. 2004. Involvement of multispecific organic anion transporter, Oatp14 (Slc21a14), in the transport of thyroxine across the blood-brain barrier. Endocrinology 145:4384–4391 [DOI] [PubMed] [Google Scholar]
- 37. Friesema EC, Jansen J, Jachtenberg JW, Visser WE, Kester MH, Visser TJ. 2008. Effective cellular uptake and efflux of thyroid hormone by human monocarboxylate transporter 10. Mol Endocrinol 22:1357–1369 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Kim DK, Kanai Y, Chairoungdua A, Matsuo H, Cha SH, Endou H. 2001. Expression cloning of a Na+-independent aromatic amino acid transporter with structural similarity to H+/monocarboxylate transporters. J Biol Chem 276:17221–17228 [DOI] [PubMed] [Google Scholar]
- 39. Chiba T, Marcus DC. 2000. Nonselective cation and BK channels in apical membrane of outer sulcus epithelial cells. J Membr Biol 174:167–179 [DOI] [PubMed] [Google Scholar]
- 40. Marcus DC, Chiba T. 1999. K+ and Na+ absorption by outer sulcus epithelial cells. Hear Res 134:48–56 [DOI] [PubMed] [Google Scholar]
- 41. Ramadan T, Camargo SM, Summa V, Hunziker P, Chesnov S, Pos KM, Verrey F. 2006. Basolateral aromatic amino acid transporter TAT1 (Slc16a10) functions as an efflux pathway. J Cell Physiol 206:771–779 [DOI] [PubMed] [Google Scholar]
- 42. Mustapha M, Fang Q, Gong TW, Dolan DF, Raphael Y, Camper SA, Duncan RK. 2009. Deafness and permanently reduced potassium channel gene expression and function in hypothyroid Pit1dw mutants. J Neurosci 29:1212–1223 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Schwartz CE, Stevenson RE. 2007. The MCT8 thyroid hormone transporter and Allan-Herndon-Dudley syndrome. Best Pract Res Clin Endocrinol Metab 21:307–321 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Wirth EK, Roth S, Blechschmidt C, Hölter SM, Becker L, Racz I, Zimmer A, Klopstock T, Gailus-Durner V, Fuchs H, Wurst W, Naumann T, Bräuer A, de Angelis MH, Köhrle J, Grüters A, Schweizer U. 2009. Neuronal 3′,3,5-triiodothyronine (T3) uptake and behavioral phenotype of mice deficient in Mct8, the neuronal T3 transporter mutated in Allan-Herndon-Dudley syndrome. J Neurosci 29:9439–9449 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Braun D, Kinne A, Bräuer AU, Sapin R, Klein MO, Köhrle J, Wirth EK, Schweizer U. 2011. Developmental and cell type-specific expression of thyroid hormone transporters in the mouse brain and in primary brain cells. Glia 59:463–471 [DOI] [PubMed] [Google Scholar]
- 46. Friesema EC, Ganguly S, Abdalla A, Manning Fox JE, Halestrap AP, Visser TJ. 2003. Identification of monocarboxylate transporter 8 as a specific thyroid hormone transporter. J Biol Chem 278:40128–40135 [DOI] [PubMed] [Google Scholar]
- 47. Flock A, Flock B, Fridberger A, Scarfone E, Ulfendahl M. 1999. Supporting cells contribute to control of hearing sensitivity. J Neurosci 19:4498–4507 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Brandt N, Kuhn S, Münkner S, Braig C, Winter H, Blin N, Vonthein R, Knipper M, Engel J. 2007. Thyroid hormone deficiency affects postnatal spiking activity and expression of Ca2+ and K+ channels in rodent inner hair cells. J Neurosci 27:3174–3186 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Ali G, Santos RL, John P, Wambangco MA, Lee K, Ahmad W, Leal S. 2006. The mapping of DFNB62, a new locus for autosomal recessive non-syndromic hearing impairment, to chromosome 12p13.2-p11.23. Clin Genet 69:429–433 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Liang VC, Sedgwick T, Shi YB. 1997. Characterization of the Xenopus homolog of an immediate early gene associated with cell activation: sequence analysis and regulation of its expression by thyroid hormone during amphibian metamorphosis. Cell Res 7:179–193 [DOI] [PubMed] [Google Scholar]
- 51. Ritchie JW, Peter GJ, Shi YB, Taylor PM. 1999. Thyroid hormone transport by 4F2hc-IU12 heterodimers expressed in Xenopus oocytes. J Endocrinol 163:R5–R9 [DOI] [PubMed] [Google Scholar]
- 52. Meier C, Ristic Z, Klauser S, Verrey F. 2002. Activation of system L heterodimeric amino acid exchangers by intracellular substrates. EMBO J 21:580–589 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Westholm DE, Salo DR, Viken KJ, Rumbley JN, Anderson GW. 2009. The blood-brain barrier thyroxine transporter organic anion-transporting polypeptide 1c1 displays atypical transport kinetics. Endocrinology 150:5153–5162 [DOI] [PubMed] [Google Scholar]
- 54. Westholm DE, Stenehjem DD, Rumbley JN, Drewes LR, Anderson GW. 2009. Competitive inhibition of organic anion transporting polypeptide 1c1-mediated thyroxine transport by the fenamate class of nonsteroidal antiinflammatory drugs. Endocrinology 150:1025–1032 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Murillo-Cuesta S, Contreras J, Zurita E, Cediel R, Cantero M, Varela-Nieto I, Montoliu L. 2010. Melanin precursors prevent premature age-related and noise-induced hearing loss in albino mice. Pigment Cell Melanoma Res 23:72–83 [DOI] [PubMed] [Google Scholar]
- 56. Bombardier C, Peloso PM, Goldsmith CH. 1995. Salsalate, a nonacetylated salicylate, is as efficacious as diclofenac in patients with rheumatoid arthritis. Salsalate-Diclofenac study group. J Rheumatol 22:617–624 [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.