Abstract
Estrogens have pronounced effects on thermoregulation, as illustrated by the occurrence of hot flushes secondary to estrogen withdrawal in menopausal women. Because neurokinin B (NKB) gene expression is markedly increased in the infundibular (arcuate) nucleus of postmenopausal women, and is modulated by estrogen withdrawal and replacement in multiple species, we have hypothesized that NKB neurons could play a role in the generation of flushes. There is no information, however, on whether the primary NKB receptor [neurokinin 3 receptor (NK3R)] modulates body temperature in any species. Here, we determine the effects of microinfusion of a selective NK3R agonist (senktide) into the rat median preoptic nucleus (MnPO), an important site in the heat-defense pathway. Senktide microinfusion into the rat MnPO decreased core temperature in a dose-dependent manner. The hypothermia induced by senktide was similar in ovariectomized rats with and without 17β-estradiol replacement. The hypothermic effect of senktide was prolonged in rats exposed to an ambient temperature of 29.0 C, compared with 21.5 C. Senktide microinfusion also altered tail skin vasomotion in rats exposed to an ambient temperature of 29.0 but not 21.5 C. Comparisons of the effects of senktide at different ambient temperatures indicated that the hypothermia was not secondary to thermoregulatory failure or a reduction in cold-induced thermogenesis. Other than a very mild increase in drinking, senktide microinfusion did not affect behavior. Terminal fluorescent dextran microinfusion showed targeting of the MnPO and adjacent septum, and immunohistochemical studies revealed that senktide induced a marked increase in Fos-activation in the MnPO. Because MnPO neurons expressed NK3R-immunoreactivity, the induction of MnPO Fos by senktide is likely a direct effect. By demonstrating that NK3R activation in the MnPO modulates body temperature, these studies support the hypothesis that hypothalamic NKB neurons could be involved in the generation of menopausal flushes.
Thermoregulation is modulated by the reproductive axis across mammalian species. Body temperature changes with the menstrual cycle, pregnancy, and in response to oral contraceptive use. In menopausal women, instability and eventual loss of ovarian estrogen secretion leads to hot flushes, a disorder of hypothalamic thermoregulation (1, 2). Hot flushes are characterized by the activation of heat-defense effectors, including skin vasodilatation, sweating, and behavioral changes, which often results in a transient decrease in core temperature (3, 4). Although flushes coincide with pulses of LH in peripheral plasma (5, 6), neither LH nor GnRH is required for hot flush symptoms (3, 7). The close timing of flush episodes with LH pulses provides a clue that the onset of flushes is related to the hypothalamic circuitry controlling pulsatile GnRH secretion.
Body temperature and pulsatile GnRH secretion are regulated at the level of the hypothalamus. In postmenopausal women, ovarian failure markedly increases neurokinin B (NKB) gene expression in the hypothalamic infundibular (arcuate) nucleus, in a subpopulation of neurons coexpressing kisspeptin and estrogen receptor-α gene transcripts (8, 9). NKB is a tachykinin neuropeptide whose gene expression in the arcuate nucleus is modulated by ovariectomy and estrogen replacement in rats, mice, sheep and monkeys (10–14). Mutations in either the gene encoding NKB, or its primary receptor, neurokinin 3 receptor (NK3R), result in hypogonadotropic hypogonadism, demonstrating the essential nature of NKB signaling in reproductive regulation (15–17). Substantial evidence supports a role for these arcuate NKB/kisspeptin neurons in the hypothalamic circuitry regulating estrogen negative feedback on pulsatile GnRH secretion (18–21).
Because NKB gene expression is markedly altered in the hypothalamic infundibular nucleus of postmenopausal women, we hypothesized that these NKB neurons could mediate the effects of estrogen on thermoregulation and contribute to the generation of menopausal flushes (8). However, no information exists on whether the activation of NK3R modulates body temperature in any species. We have recently reported that the median preoptic nucleus (MnPO) is modulated by both ambient temperature and 17β-estradiol (E2), implicating this nucleus as a potential site for integration of the thermoregulatory and reproductive axes (22). Moreover, the rat MnPO receives projections from arcuate NKB neurons (23), expresses NK3R mRNA (24), and is an integral part of thermoregulatory heat-defense pathways (25). These observations suggest that the effects of estrogens on thermoregulation could occur via arcuate NKB projections to NK3R-expressing neurons in the MnPO. To determine whether NK3R signaling in the MnPO could be involved in the modulation of body temperature, here we evaluate the thermoregulatory effects of focal microinfusion of a NK3R agonist, senktide, into the rat MnPO. Senktide was chosen for these studies because it is a highly potent and selective NK3R agonist (26–28) and has been demonstrated to alter LH secretion in multiple mammalian species (29–33). Our goal was to provide the first information on whether NK3R signaling could participate in the thermoregulatory axis, in addition to its now established role in the reproductive axis.
Materials and Methods
Forty-nine female Sprague Dawley rats (150–200 g; Harlan Laboratories, Houston, TX) were housed in the Animal Care Facility at the University of Arizona on a 12-h light, 12-h dark cycle (lights on at 0700 h) with ad libitum food and water. Because standard rat chow contains high levels of phytoestrogens that can affect thermoregulation (34), rats were fed Teklad 2014 Global Maintenance Diet (Harlan Laboratories). Animal protocols were approved by the University of Arizona Animal Care and Use Committee and conformed to National Institutes of Health guidelines.
Animal surgery
Under general anesthesia, rats were ovariectomized (OVX) and implanted sc with 2 SILASTIC capsules (20-mm effective capsule length, 1.57-mm inner diameter, 3.18-mm outer diameter; Dow Corning, Midland, MI) containing either E2 (17β-estradiol) (360 μg/ml) or vehicle (sesame oil) to standardize serum estrogen levels. The E2 capsules produce low physiological levels of serum E2 (35). A Subcue Temperature Datalogger (Canadian Analytical Technologies, Inc., Calgary, Alberta, Canada) or a DSI telemetry probe (TA10-F20 or TA10-F40; Data Sciences International, St. Paul, MN) was inserted into the peritoneal cavity. Each rat was stereotaxically implanted with a 26-gauge guide cannula (C315GA; Plastics One, Roanoke, VA) targeting 1 mm above the MnPO (coordinates: 1 mm lateral, 0.0 mm posterior, and 6.7–6.9 mm ventral to Bregma, angled 9° toward the midline) capped with a dummy cannula (C315DC; Plastics One). After surgery, rats were given postoperative analgesia (0.03–0.05 mg/kg sc of buprenorphine) and allowed to recover undisturbed for 1 wk.
Microinfusion experiments
Table 1 summarizes the microinfusion experiments, which consisted of either senktide (Chemical Abstracts Service no. 106128-89-6; Tocris Bioscience, Ellisville, MI) in 300 nl of artificial cerebrospinal fluid (aCSF) (Harvard Apparatus, Holliston, MA) or vehicle (300 nl of aCSF). Each rat received both vehicle and senktide microinfusions (in random order) between d 15 and 27 after surgery, with not more than one microinfusion per day and at least 1 d between experiments. To reduce stress, rats were exposed to the experimental set-up for three training sessions. The rat was placed in a grid plastic cage (6″ × 6″ × 4″) with chow and water in an environmental chamber (Forma model 3940; Thermo Scientific, Asheville, NC) with controlled ambient temperature (either 21.5 or 29.0 C) and humidity set to 50%. Before each microinfusion, the rat was left in the chamber for at least 2 h. During the dose-response experiment, some rats were briefly removed from the chamber and restrained for cannula insertion. In all subsequent studies, rats were freely moving in the plastic cage within the environmental chamber for the entire experiment. The injection cannula (C315IA; Plastics One) extended 0.7–1.0 mm beyond the tip of the guide cannula and was attached via a connector assembly (C313C; Plastics One) to a 1-μl syringe (7101; Hamilton, Reno, NV) inserted into an UltraMicroPump with a Micro4 controller (UMP3; World Precision Instruments, Sarasota, FL). The infusion (2 nl/sec for 150 sec) was started 2 min after insertion of the injection cannula. After infusion, the injection cannula was left in place for 1 min for the dose-response experiment and 5 min for all other experiments.
Table 1.
Summary of microinfusion experiments
| Dose (300-nl solution) | Estrogen status | TAMBIENT | Thermoregulatory Evaluation | Evaluation of brain histology |
|---|---|---|---|---|
| 18 or 90 pmol senktide or vehicle | OVX + E2 | 21.5 C | TCORE | Nissl stain |
| 90 pmol senktide or vehicle | OVX + E2 | 21.5 C | TCORE, TSKIN, and behavior | Terminal fluorescent dextran, Nissl stain, and dual-label Fos-NK3R immunohistochemistry |
| 90 pmol senktide or vehicle | OVX + E2 | 29.0 C | TCORE and TSKIN | Terminal fluorescent dextran and Nissl stain |
| 90 pmol senktide or vehicle | OVX | 21.5 C | TCORE, TSKIN, and behavior | Terminal fluorescent dextran and Nissl stain |
n = 6–8 microinfusions/group. TCORE, Core temperature; TSKIN, tail skin temperature.
Temperature recordings and analysis
For the dose-response experiment, core temperature was recorded with an ip Subcue Datalogger. For all other experiments, core temperature was recorded by an ip DSI telemetry probe. Tail skin temperatures were recorded with a Subcue Datalogger attached 4–5 cm from the base of the tail by a 1-inch nylon cylinder as previously described (35). Ambient temperature was recorded either with a thermocouple (IT-18; Physitemp, Inc., Clifton, NJ) or a C10T probe (Data Sciences International).
The recorded temperatures (core, tail skin, and ambient) were sampled every 5 min starting 60 min before the start of microinfusion to 70–120 min after microinfusion. Heat loss index was calculated as a measure of tail skin vasomotion: (tail skin temperature − ambient temperature)/(core temperature − ambient temperature) (36). To transform the data from absolute temperature to a representation of treatment response from baseline, the average temperature during the 60 min before microinfusion was subtracted from the absolute temperature values for each rat. For statistical analysis, time 0 was based on values obtained 0–60 min before microinfusion. Two-way repeated measures ANOVA (time vs. treatment) followed by Tukey's post hoc analysis (α < 0.05) was used to assess significance for all temperature variables.
Behavioral recordings
Rats microinfused at 21.5 C were videotaped using a webcam and software (Logitech, Fremont, CA). A single investigator, blind to the experimental group, quantified grooming, eating, wet-dog shakes, locomotion (including turning or rearing), and drinking. A behavior exhibited for 10 sec or less resulted in a count of 1, with a maximum of six counts for each behavior in a given minute. The total number of counts from 30 to 5 min before microinfusion and 5 to 30 min after microinfusion was calculated for each rat to generate group means, and groups were compared using two-way ANOVA (time vs. treatment) with Tukey's post hoc analysis (α < 0.05).
Terminal microinfusion of fluorescent dextran to identify the injection site
Twenty minutes before killing, rats were anesthetized with isoflurane and microinfused with 300 nl of aCSF containing fluorescent dextran (3000 MW, TexasRed, lysine fixable; Invitrogen Corp., Carlsbad, CA). Rats were then injected with sodium pentobarbital (100 mg/kg ip) and perfused via the ascending aorta with heparinized saline followed by 4% paraformaldehyde in 0.1 m phosphate buffer (pH 7.4). Brains were postfixed in 4% paraformaldehyde for 1 h and cryoprotected with ascending concentrations of sucrose (10–30%) in PBS (pH 7.4). Brains were frozen with dry ice and sectioned at a thickness of 40 μm using a sliding microtome. Every third section was mounted and coverslipped with 90% glycerol in PBS. Remaining sections were stored at −20 C in cryoprotectant solution (37).
To examine TexasRed dextran diffusion, glycerol-mounted sections were inspected using a fluorescent Nikon E1000 microscope (Nikon, Tokyo, Japan) with an attached Photometrics Coolsnap FX camera (Roper Scientific, Trenton, NJ). The fluorescence was photographed with a filter cube optimized for TexasRed immunofluorescence (excitation, 540–580 nm; dichromatic mirror, 595 nm; emission filter, 600–660 nm). To visualize anatomical landmarks, a brightfield image was captured. These images were color combined in Adobe Photoshop (San Jose, CA) for evaluation of cannula tract and dextran diffusion radius.
Immunohistochemical studies
Antibodies
The rabbit polyclonal antibody against c-fos protein was raised against amino acids 4–17 of human c-fos (AB-5, lot no. D00007099; EMD Biosciences, La Jolla, CA) and has been extensively characterized (38, 39). The NK3R polyclonal rabbit antiserum (Ciofi, Code IS-7/7, bleed 040595) was generated against amino acids 443–452 of the C-terminal region of the rat NK3R protein (40). The labeling with this antibody agrees with the location of NK3R mRNA using in situ hybridization (24) and our previous descriptions of NK3R-immunoreactive (ir) neurons using a different antibody (41). Specific staining for NK3R using either fluorescent or chromogenic visualization was blocked both by omission of the primary antibody and by preadsorption controls (24-h incubation with 10 μm synthetic peptide; Biosynthesis, Lewisville, TX). In the diaminobenzidine (DAB) double-labeling procedure, omission of either primary antibody selectively blocked the DAB color associated with that antibody while preserving the labeling associated with the remaining antibody.
Dual-label DAB immunohistochemistry for Fos and NK3R in microinfused rats
Matched sections were selected using the Nissl stains and a rat brain atlas (42). Unless stated otherwise, between each step, there were multiple rinses of PBS for 5–10 min. Antigen retrieval was performed by incubation in 15 mm sodium citrate (pH 8.8; 30 min) in an 80 C water bath. Sections were cooled to room temperature. Sections were then incubated in 0.3% H2O2 in PBS for 30 min, blocked for 60 min (3% normal goat serum and 0.3% Triton-X in PBS), and incubated for 48 h at 4 C in the c-fos antibody diluted 1:25,000 in blocking solution. Sections were incubated for 2 h in biotinylated goat antirabbit IgG (lot no. V1011; Vector Laboratories, Inc.) diluted 1:600 in blocking solution and then exposed to the Vectastain Elite ABC kit solution for 60 min. A Nickel-intensified DAB reaction was performed by prerinses in 0.175 m sodium acetate, then 15 min in freshly prepared, filtered Ni-DAB solution (1.25 g of nickel sulfate, 10 mg of DAB in 50 ml of sodium acetate solution and 41.5 μl of 30% H2O2), followed by rinsing with sodium acetate solution. The labeling process beginning with the blocking step was then repeated with the anti-NK3R serum (1:8000 in blocking solution). The DAB reaction for NK3R immunolabeling was not intensified by nickel and involved rinsing sections in Tris buffer, incubating for 15 min with a freshly prepared, filtered DAB solution (0.4 mg/ml DAB, 0.05 m Tris buffer, and 0.83 μl/ml 30% H2O2) and rinsing in Tris buffer. Sections were mounted on gelatinized slides, dried, exposed to ascending concentrations of ethanol, cleared in xylene, and coverslipped (43).
Fos-ir cells and NK3R-ir cells were counted using an image-combining computer microscope outfitted with a motorized stage, Lucivid miniature CRT and Neurolucida Software (MicroBrightfield, Williston, VT). Slides were coded to prevent experimenter bias. The borders of specific brain areas were digitized using a ×4 Nikon objective, and labeled cells within the brain area borders were manually marked using a ×40 objective, as described previously (22). Cell counts were performed in sections corresponding to plates 33–34 for the MnPO, plate 34 for the septohypothalamic nucleus and plate 36 for the medial preoptic nucleus. Because of a dark brown background artifact adjacent to the cannula tract in both senktide and vehicle infused animals, the medial septum was not analyzed. For other regions, analysis was only performed on tissue unobscured by this artifact. For each brain area, the number of immunoreactive cells per section was averaged for each rat, and these numbers were used to generate group means and sem. The effect of senktide vs. vehicle in each area was compared with an unpaired Student's t test.
Single-label NK3R-immunofluorescence with tyramide signal amplification (TSA) in OVX + E2 rats
The dual-label procedure described above revealed fewer NK3R-ir neurons than described previously (41). Moreover, this procedure did not stain NK3R-ir elements in the MnPO, despite previous observations of MnPO NK3R mRNA (24) and NK3R-immunofluorescence using a different, but no longer commercially available, antibody (Krajewski, S. J., and N. E. Rance, unpublished observations). To further evaluate whether MnPO neurons express NK3R, a separate group of OVX + E2 rats (n = 3) was used for single-label NK3R-immunofluorescence with TSA amplification (44). These rats were OVX, implanted with E2 capsules, perfused 2 wk later, and the brains processed as described above. Sections were initially processed for immunohistochemistry as described above, with antigen retrieval (45), incubation with 0.3% H2O2, blocking, and then incubation with primary antibody (NK3R antibody, 1:1000 in blocking solution). Sections were incubated overnight at 4 C in biotinylated goat antirabbit IgG (1:5000 in blocking solution; Vector Laboratories, Inc.). The next day, the sections were exposed to avidin-biotin complex solution (Vectastain Elite ABC kit) for 30 min, incubated for 20 min with Biotinyl Tyramide (1:200, lot no. 560549; PerkinElmer, San Jose, CA) and 0.005% H2O2 in PBS then incubated with Streptavidin-Alexa Fluor 568 in PBS with 0.4% Triton-X (1:200, lot no. 3508208; Invitrogen Corp.) for 3 h in a 37 C water bath. Sections were mounted on gelatinized slides and coverslipped with the ProLong Antifade Reagent (Invitrogen Corp.).
Results
Senktide microinfusion into the MnPO decreased core temperature in a dose-dependent manner
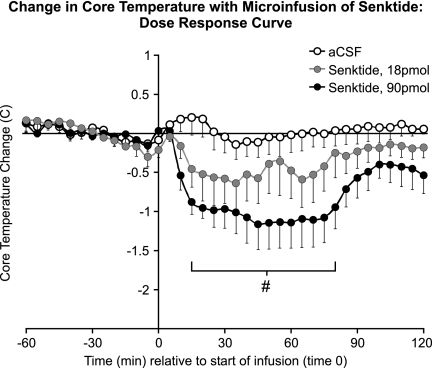
At 21.5 C, the average baseline core temperature of OVX + E2 rats (0–60 min before microinfusion) was 37.8 ± 0.1 C (mean ± sem). In rats microinfused with 90 pmol senktide, the average core temperature was significantly decreased from vehicle controls by approximately 1 C for 15–80 min after the start of microinfusion (Fig. 1). Although a similar trend was observed with infusion of 18 pmol senktide, this effect was not significantly different from vehicle. No behavioral effects (i.e. grooming, postural changes, activity, or wet-dog shakes) were qualitatively observed in response to senktide. Because 90 pmol senktide elicited the most robust effect on core temperature, this dose was selected for all subsequent studies.
Fig. 1.
Change from baseline in average core temperature in OVX + E2 rats after microinfusion of senktide (18 or 90 pmol) or vehicle (300 nl of aCSF) in the rat MnPO (ambient temperature of 21.5 C, n = 5–8 microinfusions/group). Senktide microinfusion reduced the average core temperature in a dose-dependent manner. The horizontal line represents the average baseline value of all groups. #, Significantly different, 90 pmol senktide compared with vehicle.
Senktide microinfusion into the MnPO resulted in hypothermia in both OVX + E2 and OVX rats
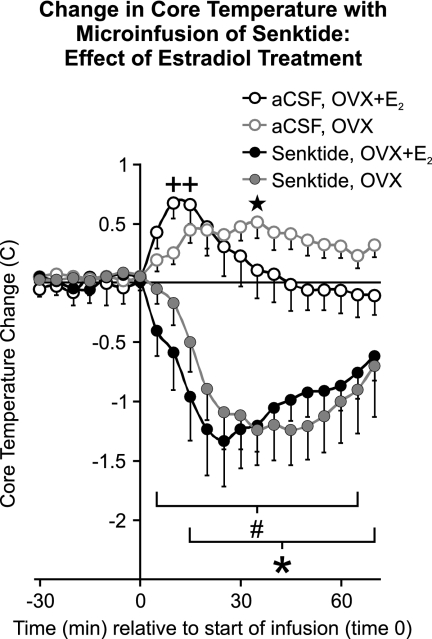
Average baseline core temperatures (0–60 min before microinfusion) were not significantly different between OVX + E2 and OVX rats (37.5 ± 0.08 vs. 37.6 ± 0.10 C, mean ± sem, respectively). As in the dose-response experiment, microinfusion of 90 pmol senktide significantly decreased core temperature compared with vehicle controls at the ambient temperature of 21.5 C (Fig. 2). The decrease in core temperature in response to senktide was nearly identical in OVX + E2 and OVX rats. Similarly, the core temperature response to vehicle microinfusion was not significantly different between OVX + E2 and OVX rats and included a mild hyperthermia. Transient elevations in core temperature have been previously described to occur as a result of animal handling (46).
Fig. 2.
Change from baseline in average core temperature after microinfusion of 90 pmol senktide or vehicle (aCSF) in the MnPO of OVX + E2 and OVX rats (ambient temperature of 21.5 C, n = 6–8 microinfusions/group). Senktide resulted in a significant decrease in core temperature that was similar in OVX + E2 and OVX rats. The horizontal line represents the average baseline value of all groups. #, Significantly different, senktide compared with vehicle, OVX + E2 rats; *, significantly different, senktide compared with vehicle, OVX rats; +, significantly different from time 0 in OVX + E2 vehicle-microinfused rats; five-pointed star, significantly different from time 0 in OVX vehicle-microinfused rats.
The drop in core temperature after senktide microinfusion into the MnPO was prolonged at the high neutral ambient temperature of 29 C
The experiments described above were performed in rats exposed to 21.5 C, an ambient temperature below the thermoneutral zone at which skin blood vessels are relatively constricted (47). Microinfusions were also performed in OVX + E2 rats exposed to 29.0 C, a high neutral ambient temperature. Ambient temperature had no effect on the average core temperature before infusion (at the ambient temperatures of 21.5 and 29.0 C, respectively, 37.5 ± 0.08 vs. 37.6 ± 0.09 C).
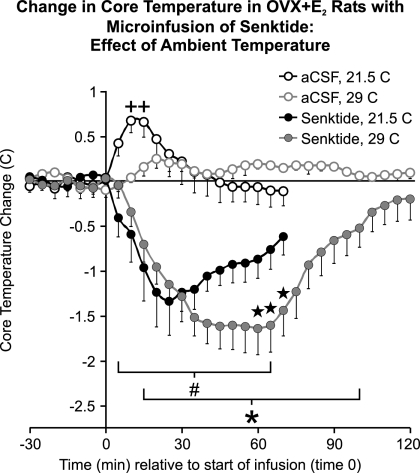
Senktide microinfusion into the MnPO resulted in hypothermia in OVX + E2 rats exposed to the ambient temperature of either 21.5 or 29.0 C. Within the first 30 min of senktide microinfusion, the reduction of core temperature was similar in rate and magnitude at both ambient temperatures (Fig. 3). The core temperature began returning toward baseline earlier in rats exposed to the subneutral ambient temperature of 21.5 C, compared with rats at the high neutral temperature 29.0 C. From 60 to 70 min after senktide microinfusion, the core temperature of rats at the ambient temperature of 29.0 C was significantly lower than that of rats exposed to 21.5 C ambient temperature. It was not possible to test whether this difference continued after 70 min, because most recordings at 21.5 C were stopped at this time for the Fos experiment. Unlike vehicle-infused OVX + E2 rats at 21.5 C, there was no transient, acute increase in core temperature in OVX + E2 vehicle controls at the ambient temperature of 29.0 C.
Fig. 3.
Change from baseline in average core temperature after microinfusion of 90 pmol senktide or vehicle in the MnPO of OVX + E2 rats at ambient temperatures of 21.5 or 29.0 C (n = 6–8 microinfusions/group). The data from OVX + E2 rats at the ambient temperature of 21.5 C is duplicated from Fig. 2 to facilitate comparisons. Senktide microinfusion resulted in a significant decrease in core temperature (compared with vehicle) in rats exposed to ambient temperatures of both 21.5 and 29.0 C. At the ambient temperature of 29.0 C, the hypothermia induced by senktide was prolonged. The horizontal line represents the average baseline value of all groups. #, Significantly different, senktide compared with vehicle, ambient temperature of 21.5 C; *, significantly different, senktide compared with vehicle, ambient temperature of 29.0 C; five-pointed star, significantly different, senktide at ambient temperature of 29.0 C, vs. senktide at ambient temperature of 21.5 C; +, significantly different from time 0 in vehicle-microinfused rats, ambient temperature of 21.5 C.
The microinfusion procedure caused acute tail skin vasoconstriction and this effect was prevented by senktide microinfusion at the ambient temperature of 29.0 C but not 21.5 C
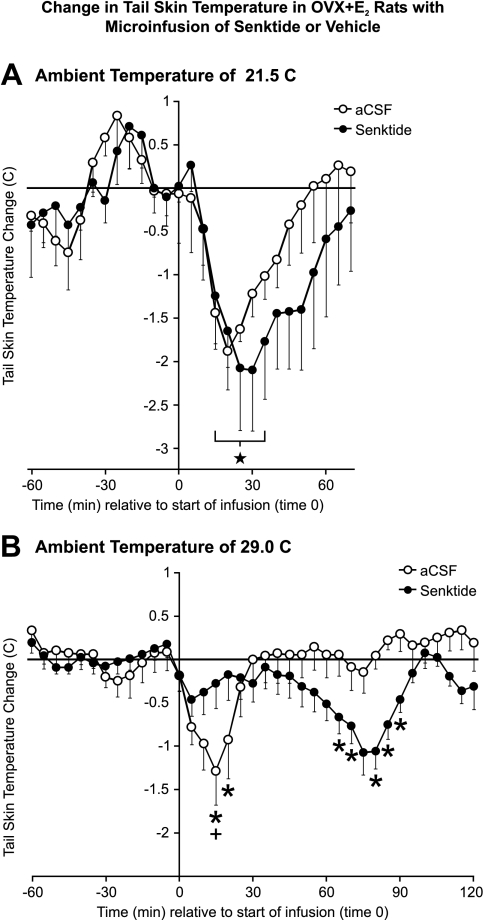
At the ambient temperature of 21.5 C, the average baseline tail skin temperature of the OVX + E2 rats was 27.8 ± 0.3 C. Fifteen to 35 min after microinfusion of either vehicle or senktide, the average tail skin temperature was significantly decreased by approximately 2 degrees (compared with time 0) (Fig. 4A). A similar transient decrease in tail skin temperature was seen in OVX rats after microinfusion of either senktide or vehicle (data not shown). There was no significant difference in average tail skin temperature between senktide and vehicle-microinfused rats in either the OVX + E2 or OVX experiments at 21.5 C. Because the acute drop in tail skin temperature occurred in vehicle controls, this change appears to be secondary to the microinfusion procedure and not a specific effect of senktide.
Fig. 4.
Change from baseline in average tail skin temperature in OVX + E2 rats after microinfusion of 90 pmol senktide or vehicle into MnPO at ambient temperatures of 21.5 C (n = 5–6 microinfusions/group) (A) or 29.0 C (n = 6–7 microinfusions/group) (B). At 21.5 C, both senktide and vehicle-microinfused rats exhibited an acute reduction in tail skin temperature, indicative of tail vasoconstriction secondary to the microinfusion procedure. Acute tail skin vasoconstriction was also observed in the vehicle-microinfused animals at the ambient temperature of 29.0 C (B). In contrast, within 30 min after microinfusion of senktide at 29.0 C, there was no significant change in tail skin temperature. These rats exhibited a delayed decrease in tail skin temperature (compared with vehicle controls) from 65 to 90 min after senktide microinfusion. The horizontal line represents the average baseline value of all groups. Five-pointed star, Significantly different from time 0 in senktide and vehicle-microinfused rats, ambient temperature of 21.5 C (A); +, significantly different from time 0 in vehicle-microinfused rats, ambient temperature of 29.0 C (B); *, significantly different, senktide compared with vehicle, ambient temperature of 29.0 C (B).
At the ambient temperature of 29.0 C, the average tail skin temperature of OVX + E2 rats before microinfusion was 32.7 ± 0.2 C. At this ambient temperature, vehicle microinfusion acutely decreased tail skin temperature, similar to the acute decrease in tail skin temperature elicited at the ambient temperature of 21.5 C (Fig. 4B). In contrast, the senktide-microinfused animals did not exhibit a significant acute decrease in tail skin temperature at the ambient temperature of 29.0 C (Fig. 4B). Sixty-five to 90 min after microinfusion, there was a delayed decrease in tail skin temperature in the senktide-microinfused animals (Fig. 4B). Although the reason for this delayed decrease in tail skin temperature in senktide-microinfused animals is not known, it may represent a compensatory vasoconstriction in response to the prolonged hypothermia induced by senktide (see Fig. 3).
Tail skin vasomotion was also evaluated using the heat loss index. Higher values of heat loss index indicate cutaneous vasodilatation and lower values indicate cutaneous vasoconstriction (36). At the ambient temperature of 21.5 C, microinfusion of vehicle or senktide transiently decreased the heat loss index in OVX + E2 rats (Table 2). The heat loss index also decreased acutely in vehicle-microinfused OVX + E2 rats exposed to the ambient temperature of 29.0 C. In contrast, at the ambient temperature of 29.0 C, the heat loss index did not significantly decrease within 30 min after senktide microinfusion. Fifteen minutes after microinfusion at the ambient temperature of 29.0 C, the heat loss index in senktide-microinfused rats was significantly higher than vehicle controls. Therefore, analysis of heat loss index confirmed that the microinfusion procedure induced a transient tail skin vasoconstriction that was inhibited by senktide in rats exposed to the ambient temperature of 29.0 C.
Table 2.
Transient changes in heat loss index after microinfusion of senktide or vehicle
| Ambient temperature | Baseline HLI | Maximal decrease from baseline after microinfusion |
|
|---|---|---|---|
| Vehicle | Senktide | ||
| 21.5 C | 0.35 ± 0.03 | 0.11 ± 0.02a | 0.11 ± 0.05a |
| 29.0 C | 0.42 ± 0.03 | 0.15 ± 0.05a | 0.06 ± 0.02 |
Baseline values represent the average heat loss index (HLI) ± sem during the hour before infusion in OVX + E2 rats (n = 11 at each ambient temperature). Vehicle and senktide values represent the maximal decrease from baseline during the first 30 min after microinfusion (n = 5–6 microinfusions/group).
Significantly different from baseline.
Other than a mild increase in drinking, senktide microinfusion elicited no behavioral response
The only significant change in behavior between treatment groups was a small increase in drinking in the senktide-microinfused rats (Table 3). Higher levels of grooming and locomotor activity (including turning and rearing) were observed after microinfusions of both senktide and vehicle. No differences in eating or posture were observed between treatment groups or as a result of the microinfusion procedure. Wet-dog shakes or tail whips were not observed in the videotaped animals or qualitatively in any of the experiments, unlike the previous behavioral descriptions in rats receiving iv or sc injections of senktide at higher doses (29, 48, 49).
Table 3.
Behavioral analysis before and after microinfusion of senktide or vehicle into the MnPO
| Behavioral activity | Before microinfusion (− 30 to − 5 min) |
After microinfusion (5 to 30 min) |
||
|---|---|---|---|---|
| Vehicle | Senktide | Vehicle | Senktide | |
| Locomotion, rearing, or turning | 17.6 ± 3.9 | 7.9 ± 1.8 | 29.1 ± 3.9b | 35.3 ± 4.2b |
| Eating | 9.6 ± 2.6 | 3.1 ± 2.0 | 6.7 ± 2.5 | 3.1 ± 1.6 |
| Drinking | 0.1 ± 0.1 | 0.4 ± 0.2 | 1.6 ± 0.8 | 5.5 ± 1.8a,b |
| Grooming | 5.4 ± 2.4 | 3.5 ± 1.5 | 26.5 ± 6.1b | 18.4 ± 2.2b |
Values represent the average total of behavior counts (mean ± sem) over a 25-min time period before (−30 to −5 min) or after (5 to 30 min) microinfusion at an ambient temperature of 21.5 C. No difference was detected between OVX and OVX + E2 rats so these data were pooled (n = 10–11 rats/group).
Significantly different, senktide vs. vehicle after the microinfusion.
Significantly different, before vs. after microinfusion within treatment group.
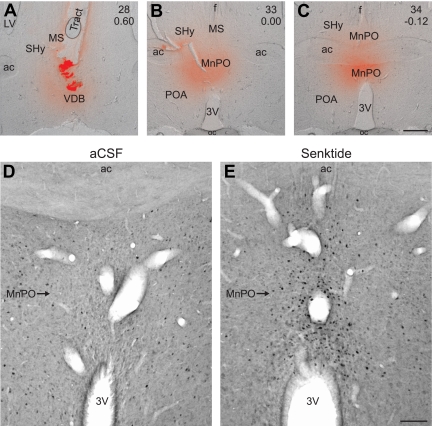
Microinfusion of fluorescent dextran showed targeting of the MnPO and adjacent septal region
Terminal microinfusion of fluorescent dextran showed a dense deposit at the site of injection with a diffusion radius ranging from 300 to 900 μm (Fig. 5, A–C). The infused dextran was clearly identified in the MnPO in nearly all of the rats and the medial septal and septohypothalamic nuclei in all animals. The fluorescent dextran occasionally diffused as far as the vertical limb of the diagonal band of Broca. Limited or no diffusion of fluorescent dextran was observed in other sites, such as the medial or lateral preoptic areas. In animals not terminally infused with dextran, there was minimal damage to brain parenchyma other than the guide cannula tract.
Fig. 5.
A–C, Representative photomicrographs from a rat receiving a terminal microinfusion of fluorescent dextran (red). These photomicrographs were created by combining brightfield images with fluorescent images of the red dextran dye. The numbers in the upper right corner of each photomicrograph indicate the plate number from the Paxinos rat brain atlas (42) and distance from Bregma. Diffusion of fluorescent dextran was identified in the vertical limb of the diagonal band of Broca (A), the medial septal nucleus (A and B), septohypothalamic nucleus (B and C), and the MnPO (B and C). The guide cannula tract and a dense deposit of fluorescent dextran is identified in A. D and E, Representative photomicrographs of Fos immunohistochemistry in OVX + E2 rats killed 90 min after microinfusion of either vehicle (D) or senktide (E) at the ambient temperature of 21.5 C. Microinfusion of senktide dramatically increased the number of Fos-ir cells in the MnPO (E). 3V, Third ventricle; ac, anterior commissure, LV, lateral ventricle; MS, medial septum, POA, preoptic area; SHy, septohypothalamic nucleus; oc, optic chiasm; VDB, vertical limb of the diagonal band of Broca. Scale bars, 500 μm (A–C) and 100 μm (D and E).
Microinfusion of senktide into the MnPO markedly increased the number of Fos-ir cells in the MnPO, with minimal numbers of Fos-ir neurons in adjacent nuclei
Senktide microinfusion significantly increased the number of Fos-ir cells in the MnPO of OVX + E2 rats by nearly 8-fold (aCSF, 21.0 ± 9.4 Fos-ir cells/section, n = 4 rats; senktide, 165.3 ± 24.2 Fos-ir cells/section, n = 3 rats) (Fig. 5, D and E). Although MnPO NK3R-ir staining was observed in the single-label procedure with TSA amplification (described below), lighter staining of NK3R-immunoreactivity in the dual-labeled procedure in the MnPO precluded identification of NK3R/Fos-ir cells in this nucleus.
In the septohypothalamic nucleus, senktide microinfusion produced a small increase in number of cells double-labeled for NK3R and Fos-ir (aCSF, 0.5 ± 0.3 NK3R-Fos-ir cells/section, n = 5 rats; senktide, 2.6 ± 0.7 NK3R-Fos-ir cells/section, n = 7 rats) but had no significant effect on the number of single-labeled Fos-ir cells (aCSF, 12.2 ± 4.2 Fos-ir cells/section, n = 5 rats; senktide, 13.2 ± 2.5 Fos-ir cells/section, n = 7 rats). In the medial preoptic nucleus, senktide had no significant effect on the number of Fos-ir cells (aCSF, 43.2 ± 9.0 Fos-ir cells/section, n = 5 rats; senktide, 43.1 ± 11.3 Fos-ir cells/section n = 7 rats). Numerous large NK3R-ir neurons were identified in the lateral preoptic nucleus, the horizontal and vertical limbs of the diagonal band of Broca, and the nucleus basalis of Meynert, but virtually no Fos-labeling was identified in these neurons regardless of treatment. Because of staining artifact adjacent to the cannula tract, we were unable to evaluate Fos, NK3R, or dual-labeled immunoreactive cells in the medial septal nucleus.
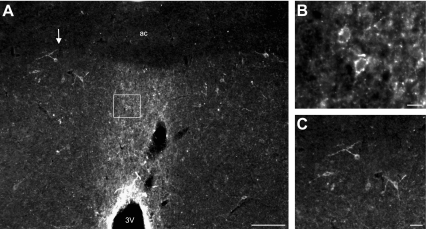
Single-label immunofluorescence with TSA amplification reveals NK3R-ir neurons in the MnPO, septum, and basal forebrain
The MnPO was highlighted by NK3R-immunofluoresence at low magnification (Fig. 6A). At higher magnification, punctate staining of neurons and fibers was observed within the boundaries of the MnPO (Fig. 6B). The NK3R antibody also labeled magnocellular neurons in the medial septal and septohypothalamic nuclei (Fig. 6C), vertical and horizontal limbs of the diagonal band of Broca, and the nucleus basalis of Meynert. A few large neurons were scattered in the dorsal-lateral preoptic area, and small NK3R-ir neurons were identified in the medial subdivision of the bed nucleus of the stria terminalis. For additional photomicrographs of NK3R-ir neurons in the rat hypothalamus, see Ref. 41.
Fig. 6.
Representative photomicrographs of single-label NK3R-immunofluorescence with TSA amplification in an OVX + E2 rat. At low magnification, the MnPO is highlighted by NK3R immunofluorescence (A). The box in A outlines the location of the small NK3R-ir cell bodies and puncta in the MnPO shown at higher magnification in B. The arrow in A points to the location of NK3R-ir cell bodies in the ventral septohypothalamic nucleus shown in higher magnification in C. ac, Anterior commissure; 3V, third ventricle. Scale bars, 100 μm (A), 10 μm (B), and 25 μm (C).
Discussion
The circuitry underlying hot flushes and other thermoregulatory responses to ovarian hormones is currently unknown. Recent studies have documented an essential role for NKB or its primary receptor, NK3R, for the regulation of reproduction (15–17). The present study provides the first evidence that NK3R signaling could also participate in the regulation of body temperature. Focal microinfusion of a highly selective and potent NK3R agonist, senktide, into the MnPO of the rat induced a rapid, dose-dependent drop in core temperature. The effect of senktide infusion was robust and consistent, occurring at both low and high ambient temperatures. Our immunohistochemical studies suggest that the thermoregulatory effect of senktide is due to direct activation of NK3R-expressing neurons in the MnPO.
We have previously shown that E2 treatment of OVX rats shifts the thermoneutral zone (47), lowers tail skin temperature (35), improves core temperature regulation during heat stress (47), and modifies Fos expression in MnPO neurons (22). In the present study, the hypothermia induced by senktide was virtually identical in OVX rats with and without E2 replacement. It is possible that a different dose of senktide, or a change in the ambient temperature, could have revealed an effect of E2 on the thermoregulatory response to senktide. On the other hand, it is well documented that estrogens markedly suppress the gene expression of NKB neurons in the arcuate nucleus (10–14), and these neurons project to the MnPO (23). The present data provide evidence that the marked influence of E2 on arcuate NKB neurons does not extend to changes in the sensitivity of NK3R at the level of the MnPO.
In our initial studies, the hypothermic effect of senktide was observed in rats exposed to an ambient temperature of 21.5 C. At this subneutral ambient temperature, hypothermia could result from a failure of thermoregulation, as with poikilothermic animals, in which core temperature varies with the ambient temperature (50, 51). Similarly, toxins can reduce body temperature when rodents are exposed to low environmental temperatures (52). However, in the poikilothermic state, and in response to toxins, the hypothermic effect is typically less pronounced when rats are exposed to warmer ambient temperatures (52). Therefore, a subsequent study was performed to determine the effects of senktide at the ambient temperature of 29.0 C. Interestingly, at the higher ambient temperature, the reduction in core temperature by senktide was similar in magnitude and even prolonged, compared with animals exposed to 21.5 C. Moreover, despite a 1 C drop in core temperature, senktide-treated rats did not exhibit behavioral effects to indicate that the animals were in shock or otherwise compromised. Thus, the senktide-induced hypothermia appears to represent a regulated decrease in core temperature, as opposed to a failure of thermoregulation.
It is not known if senktide microinfusion resulted in hypothermia because of increased heat loss or decreased heat production. In the rat, tail skin vasodilatation is an important heat-loss mechanism that can be monitored by changes in tail skin temperature. Because senktide did not induce an acute increase in tail skin temperature compared with baseline, the hypothermia cannot be explained by increased tail skin vasodilatation. Moreover, there was no effect of senktide on the behavioral component of evaporative cooling (grooming) or postural thermoregulatory behavior (53, 54). These observations do not exclude heat loss through other pathways, such as a redistribution of blood flow to the feet (53), proximal hairy skin (55), or other organs (52). In addition, a substantial contributor to heat loss in rodents is passive water loss (53). This form of heat loss could have been enhanced by an increase in respiratory rate, particularly if coupled with increased salivation. The slight increase in drinking in the senktide-treated animals may be an indication of increased water loss through this mechanism.
We also considered the possibility that the hypothermia induced by senktide could be secondary to decreased heat production. For example, at 21.5 C, a subneutral ambient temperature, senktide infusion could have decreased core temperature by impairing shivering or brown adipose tissue thermogenesis (53, 56). This explanation is unlikely, because senktide infusion also induced hypothermia in rats exposed to 29.0 C. At this high neutral ambient temperature, cold-induced thermogenesis would not be active and, thus, could not be reduced by senktide (36, 53). Alternatively, senktide infusion could have decreased other forms of metabolic heat production. For example, anesthesia is proposed to cause hypothermia by decreasing brain metabolism (57). Therefore, further studies will be necessary to determine the precise mechanism for the drop in core temperature after senktide infusion.
A transient decrease in tail skin temperature and heat loss index was observed within 15 min of vehicle microinfusion, indicative of sympathetic tail skin vasoconstriction secondary to the microinfusion procedure. Sympathetic tail skin vasoconstriction has been described as a response to a variety of stimuli, such as cold exposure, stress, or brief (nonnoxious) animal handling (58, 59). We have observed that even daily vaginal smearing for the evaluation of estrous cycles results in acute tail skin vasoconstriction (35). Remarkably, at the ambient temperature of 29.0 C, but not 21.5 C, the acute vasoconstriction observed in vehicle controls was prevented by senktide microinfusion. Because tail skin vasodilatation in the rat occurs via inhibition of vasoconstriction (60), these data indirectly demonstrate a vasodilator effect of NK3R activation. This vasomotor response is particularly relevant, because hot flushes involve transient skin vasodilatation (i.e. “flushing”) followed by a drop in core temperature (4). It is also very interesting that the effect of senktide on cutaneous vasomotion is only observed at the high ambient temperature of 29.0 C. Combined with the prolonged hypothermia after senktide microinfusion at 29.0 C, these data suggest that exposure to a higher ambient temperature increases the responsiveness of the thermoregulatory axis to NK3R signaling. These findings could provide a tentative explanation of why flushing is facilitated when symptomatic postmenopausal women are exposed to warm environments (4).
Microinfusion of senktide dramatically activated the MnPO, shown by the nearly 8-fold increase in the number of Fos-ir cells. Based on our identification of NK3R-ir neurons in the MnPO, and the previous description of NK3R mRNA expression in MnPO neurons (24), this induction of Fos is likely a direct effect of the NK3R agonist. Terminal fluorescent dextran infusions also showed targeting of the adjacent septum, but in the septohypothalamic nucleus, there was only a small increase in the number of double-labeled NK3R/Fos-ir cells and no increase in single-labeled Fos-ir cells. The diffusion pattern of fluorescent dextran indicated that it was unlikely that senktide reached other NK3R-expressing areas, such the horizontal limb of the diagonal band of Broca, the lateral preoptic area, paraventricular hypothalamus, and supraoptic nuclei. The target specificity of the microinfusion is also indicated by lack of dual-labeled NK3R/Fos-ir cells in the diagonal band of Broca and the lateral preoptic nucleus. Taken as a whole, the immunohistochemical studies indicate that senktide predominantly activated NK3R-ir neurons in the MnPO.
The MnPO is part of the neural circuitry triggering autonomic heat-loss mechanisms in response to warm environmental temperatures (25). Of note, our previous studies showed that both E2 and mild changes in ambient temperature modulated MnPO Fos and tail skin vasodilatation in a parallel manner, implicating the MnPO as a site for integration of the reproductive and thermoregulatory axes (22). The MnPO receives information from peripheral warm-sensitive thermoreceptors via the dorsal lateral parabrachial nucleus (25) and projects to thermoregulatory regions, including the rostral medullary raphe (46), dorsomedial nucleus (46), ventrolateral periaqueductal gray (61), and other preoptic regions (62). The MnPO also receives projections from estrogen-responsive NKB neurons in the arcuate nucleus (23). Although the septal area may be considered part of the thermoregulatory axis (63), lesions of the medial septum in rats decrease body temperature (64), and thus, stimulation of this area would not be expected to produce hypothermia. In contrast, lesions that include the MnPO increase body temperature (46, 65), and in previous studies, blocking of glutamate receptors in the MnPO prevented the heat-defense response to skin warming (25). These findings suggest that the hypothermia induced by senktide is due to activation of MnPO neurons.
In summary, the present study demonstrates that focal microinfusion of a selective NK3R agonist activates the MnPO, a site of NK3R-ir neurons, and reduces core temperature. The senktide-induced hypothermia does not appear to be due to a toxic effect or a global impairment in thermoregulation. Although the E2 status did not alter the thermoregulatory response to senktide, exposure of rats to a higher ambient temperature prolonged the hypothermia and revealed an effect of senktide on tail skin vasomotion. These data provide the first evidence that NKB signaling could participate in the thermoregulatory axis, in addition to the now established role of NKB neurons in reproduction. We hypothesize that NKB neurons in the infundibular (arcuate) nucleus could play a role in the generation of menopausal flushes (8). This postulate is based on the association between LH pulses and flushes (3, 6), the putative role of NKB neurons in pulsatile GnRH secretion (19–21), the changes in NKB gene expression in the hypothalamus of postmenopausal women (8), the responsiveness of both flushes and NKB neurons to estrogen withdrawal and replacement (2, 11, 13), and projections of arcuate NKB neurons to thermoregulatory centers, such as the MnPO (23). Demonstration of an effect of a NK3R receptor agonist on the thermoregulatory axis provides an important piece of information in support of this hypothesis.
Acknowledgments
We thank Dr. Christopher J. Gordon for advice on the experimental design and Dr. Gloria E. Hoffman for providing the immunohistochemistry protocols. Dr. Nathaniel T. McMullen provided invaluable surgical training, advice on experimental design, and critical reading of the manuscript. Dr. Phillipe Ciofi generously donated the NK3 receptor antibody. Jessica Brown and James Knitter provided valuable technical assistance. We also thank Dr. Andrew M. Dacks, Melinda Smith, Marina Cholanian, and Hemalini Williams for critically reading the manuscript.
This work was supported by National Institutes of Health (NIH) National Institute on Aging Grant R01 AG032315. P.A.D. was supported by the Achievement Rewards for College Scientists Foundation, the NIH National Institute on Aging Pre-doctoral Training Fellowship 1F31-AG030881, and the Evelyn F. McNight Brain Institute.
Disclosure Summary: The authors have nothing to disclose.
Footnotes
- aCSF
- Artificial cerebrospinal fluid
- DAB
- diaminobenzidine
- E2
- 17β-estradiol
- ir
- immunoreactive
- MnPO
- median preoptic nucleus
- NKB
- neurokinin B
- NK3R
- neurokinin 3 receptor
- OVX
- ovariectomized
- TSA
- tyramide signal amplification.
References
- 1. Stearns V, Ullmer L, López JF, Smith Y, Isaacs C, Hayes DF. 2002. Hot flushes. Lancet 360:1851–1861 [DOI] [PubMed] [Google Scholar]
- 2. Santoro N. 2008. Symptoms of menopause: hot flushes. Clin Obstet Gynecol 51:539–548 [DOI] [PubMed] [Google Scholar]
- 3. Casper RF, Yen SSC. 1985. Neuroendocrinology of menopausal flushes: an hypothesis of flush mechanism. Clin Endocrinol 22:293–312 [DOI] [PubMed] [Google Scholar]
- 4. Freedman RR. 2001. Physiology of hot flashes. Am J Human Biol 13:453–464 [DOI] [PubMed] [Google Scholar]
- 5. Casper RF, Yen SS, Wilkes MM. 1979. Menopausal flushes: a neuroendocrine link with pulsatile luteinizing hormone secretion. Science 205:823–825 [DOI] [PubMed] [Google Scholar]
- 6. Tataryn IV, Meldrum DR, Lu KH, Frumar AM, Judd HL. 1979. LH, FSH and skin temperature during the menopausal hot flash. J Clin Endocrinol Metab 49:152–154 [DOI] [PubMed] [Google Scholar]
- 7. Gambone J, Meldrum DR, Laufer L, Chang RJ, Lu JK, Judd HL. 1984. Further delineation of hypothalamic dysfunction responsible for menopausal hot flashes. J Clin Endocrinol Metab 59:1097–1102 [DOI] [PubMed] [Google Scholar]
- 8. Rance NE, Young WS., 3rd 1991. Hypertrophy and increased gene expression of neurons containing neurokinin-B and substance-P messenger ribonucleic acids in the hypothalami of postmenopausal women. Endocrinology 128:2239–2247 [DOI] [PubMed] [Google Scholar]
- 9. Rometo AM, Krajewski SJ, Voytko ML, Rance NE. 2007. Hypertrophy and increased kisspeptin gene expression in the hypothalamic infundibular nucleus of postmenopausal women and ovariectomized monkeys. J Clin Endocrinol Metab 92:2744–2750 [DOI] [PubMed] [Google Scholar]
- 10. Rance NE, Bruce TR. 1994. Neurokinin B gene expression is increased in the arcuate nucleus of ovariectomized rats. Neuroendocrinology 60:337–345 [DOI] [PubMed] [Google Scholar]
- 11. Abel TW, Voytko ML, Rance NE. 1999. The effects of hormone replacement therapy on hypothalamic neuropeptide gene expression in a primate model of menopause. J Clin Endocrinol Metab 84:2111–2118 [DOI] [PubMed] [Google Scholar]
- 12. Pillon D, Caraty A, Fabre-Nys C, Bruneau G. 2003. Short-term effect of oestradiol on neurokinin B mRNA expression in the infundibular nucleus of ewes. J Neuroendocrinol 15:749–753 [DOI] [PubMed] [Google Scholar]
- 13. Sandoval-Guzmán T, Stalcup ST, Krajewski SJ, Voytko ML, Rance NE. 2004. Effects of ovariectomy on the neuroendocrine axes regulating reproduction and energy balance in young cynomolgus macaques. J Neuroendocrinol 16:146–153 [DOI] [PubMed] [Google Scholar]
- 14. Dellovade TL, Merchenthaler I. 2004. Estrogen regulation of neurokinin B gene expression in the mouse arcuate nucleus is mediated by estrogen receptor α. Endocrinology 145:736–742 [DOI] [PubMed] [Google Scholar]
- 15. Topaloglu AK, Reimann F, Guclu M, Yalin AS, Kotan LD, Porter KM, Serin A, Mungan NO, Cook JR, Ozbek MN, Imamoglu S, Akalin NS, Yuksel B, O'Rahilly S, Semple RK. 2009. TAC3 and TACR3 mutations in familial hypogonadotropic hypogonadism reveal a key role for Neurokinin B in the central control of reproduction. Nat Genet 41:354–358 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Young J, Bouligand J, Francou B, Raffin-Sanson ML, Gaillez S, Jeanpierre M, Grynberg M, Kamenicky P, Chanson P, Brailly-Tabard S, Guiochon-Mantel A. 2010. TAC3 and TACR3 defects cause hypothalamic congenital hypogonadotropic hypogonadism in humans. J Clin Endocrinol Metab 95:2287–2295 [DOI] [PubMed] [Google Scholar]
- 17. Gianetti E, Tusset C, Noel SD, Au MG, Dwyer AA, Hughes VA, Abreu AP, Carroll J, Trarbach E, Silveira LF, Costa EM, de Mendonça BB, de Castro M, Lofrano A, Hall JE, Bolu E, Ozata M, Quinton R, Amory JK, Stewart SE, Arlt W, Cole TR, Crowley WF, Kaiser UB, Latronico AC, Seminara SB. 2010. TAC3/TACR3 mutations reveal preferential activation of gonadotropin-releasing hormone release by neurokinin B in neonatal life followed by reversal in adulthood. J Clin Endocrinol Metab 95:2857–2867 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Rance NE. 2009. Menopause and the human hypothalamus: evidence for the role of kisspeptin/neurokinin B neurons in the regulation of estrogen negative feedback. Peptides 30:111–122 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Lehman MN, Coolen LM, Goodman RL. 2010. Minireview: kisspeptin/neurokinin B/dynorphin (KNDy) cells of the arucate nucleus: a central node in the control of gonadotropin-releasing hormone secretion. Endocrinology 151:3479–3489 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Rance NE, Krajewski SJ, Smith MA, Cholanian M, Dacks PA. 2010. Neurokinin B and the hypothalamic regulation of reproduction. Brain Res 1364:116–128 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Wakabayashi Y, Nakada T, Murata K, Ohkura S, Mogi K, Navarro VM, Clifton DK, Mori Y, Tsukamura H, Maeda K, Steiner RA, Okamura H. 2010. Neurokinin B and dynorphin A in kisspeptin neurons of the arcuate nucleus participate in generation of periodic oscillation of neural activity driving pulsatile gonadotropin-releasing hormone secretion in the goat. J Neurosci 30:3124–3132 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Dacks PA, Krajewski SJ, Rance NE. 2011. Ambient temperature and 17β-estradiol modify fos immunoreactivity in the median preoptic nucleus, a putative regulator of skin vasomotion. Endocrinology 152:2750–2759 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Krajewski SJ, Burke MC, Anderson MJ, McMullen NT, Rance NE. 2010. Forebrain projections of arcuate neurokinin B neurons demonstrated by anterograde tract-tracing and monosodium glutamate lesions in the rat. Neuroscience 166:680–697 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Shughrue PJ, Lane MV, Merchenthaler I. 1996. In situ hybridization analysis of the distribution of neurokinin-3 mRNA in the rat central nervous system. J Comp Neurol 372:395–414 [DOI] [PubMed] [Google Scholar]
- 25. Nakamura K, Morrison SF. 2010. A thermosensory pathway mediating heat-defense responses. Proc Natl Acad Sci USA 107:8848–8853 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Laufer R, Gilon C, Chorev M, Selinger Z. 1986. Characterization of a neurokinin B receptor site in rat brain using a highly selective radioligand. J Biol Chem 261:10257–10263 [PubMed] [Google Scholar]
- 27. Wormser U, Laufer R, Hart Y, Chorev M, Gilon C, Selinger Z. 1986. Highly selective agonists for substance P receptor subtypes. EMBO J 5:2805–2808 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Dam TV, Escher E, Quirion R. 1990. Visualization of neurokinin-3 receptor sites in rat brain using the highly selective ligand [3H]senktide. Brain Res 506:175–179 [DOI] [PubMed] [Google Scholar]
- 29. Sandoval-Guzmán T, Rance NE. 2004. Central injection of senktide, an NK3 receptor agonist, or neuropeptide Y inhibits LH secretion and induces different patterns of Fos expression in the rat hypothalamus. Brain Res 1026:307–312 [DOI] [PubMed] [Google Scholar]
- 30. Navarro VM, Gottsch ML, Chavkin C, Okamura H, Clifton DK, Steiner RA. 2009. Regulation of gonadotropin-releasing hormone secretion by kisspeptin/dynorphin/neurokinin B neurons in the arcuate nucleus of the mouse. J Neurosci 29:11859–11866 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Billings HJ, Connors JM, Altman SN, Hileman SM, Holaskova I, Lehman MN, McManus CJ, Nestor CC, Jacobs BH, Goodman RL. 2010. Neurokinin B acts via the neurokinin-3 receptor in the retrochiasmatic area to stimulate luteinizing hormone secretion in sheep. Endocrinology 151:3836–3846 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Ramaswamy S, Seminara SB, Ali B, Ciofi P, Amin NA, Plant TM. 2010. Neurokinin B stimulates GnRH release in the male monkey (Macaca mulatta) and is colocalized with kisspeptin in the arcuate nucleus. Endocrinology 151:4494–4503 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Navarro VM, Castellano JM, McConkey SM, Pineda R, Ruiz-Pino F, Pinilla L, Clifton DK, Tena-Sempere M, Steiner RA. 2011. Interactions between kisspeptin and neurokinin B in the control of GnRH secretion in the female rat. Am J Physiol Endocrinol Metab 300:E202–E210 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Opas EE, Rutledge SJ, Vogel RL, Rodan GA, Schmidt A. 2004. Rat tail skin temperature regulation by estrogen, phytoestrogens and tamoxifen. Maturitas 48:463–471 [DOI] [PubMed] [Google Scholar]
- 35. Williams H, Dacks PA, Rance NE. 2010. An improved method for recording tail skin temperature in the rat reveals changes during the estrous cycle and effects of ovarian steroids. Endocrinology 151:5389–5394 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Romanovsky AA, Ivanov AI, Shimansky YP. 2002. Selected contribution: ambient temperature for experiments in rats: a new method for determining the zone of thermal neutrality. J Appl Physiol 92:2667–2679 [DOI] [PubMed] [Google Scholar]
- 37. Watson RE, Jr, Wiegand SJ, Clough RW, Hoffman GE. 1986. Use of cryoprotectant to maintain long-term peptide immunoreactivity and tissue morphology. Peptides 7:155–159 [DOI] [PubMed] [Google Scholar]
- 38. Elmquist JK, Ahima RS, Elias CF, Flier JS, Saper CB. 1998. Leptin activates distinct projections from the dorsomedial and ventromedial hypothalamic nuclei. Proc Natl Acad Sci USA 95:741–746 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Patronas P, Horowitz M, Simon E, Gerstberger R. 1998. Differential stimulation of c-fos expression in hypothalamic nuclei of the rat brain during short-term heat acclimation and mild dehydration. Brain Res 798:127–139 [DOI] [PubMed] [Google Scholar]
- 40. Griffond B, Ciofi P, Bayer L, Jacquemard C, Fellmann D. 1997. Immunocytochemical detection of the neurokinin B receptor (NK3) on melanin-concentrating hormone (MCH) neurons in rat brain. J Chem Neuroanat 12:183–189 [DOI] [PubMed] [Google Scholar]
- 41. Krajewski SJ, Anderson MJ, Iles-Shih L, Chen KJ, Urbanski HF, Rance NE. 2005. Morphologic evidence that neurokinin B modulates gonadotropin-releasing hormone secretion via neurokinin 3 receptors in the rat median eminence. J Comp Neurol 489:372–386 [DOI] [PubMed] [Google Scholar]
- 42. Paxinos G, Watson C. 2007. The rat brain in stereotaxic coordinates. Burlington, MA: Elsevier, Inc; [DOI] [PubMed] [Google Scholar]
- 43. Hoffman GE, Smith MS, Fitzsimmons MD. 1992. Detecting steroidal effects on immediate early gene expression in the hypothalamus. Neuroprotocols 1:52–66 [Google Scholar]
- 44. Hoffman GE, Le WW, Sita LV. 2008. The importance of titrating antibodies for immunohistochemical methods. Curr Protoc Neurosci Suppl 45:2.12.1–2.12.26 [DOI] [PubMed] [Google Scholar]
- 45. Jiao Y, Sun Z, Lee T, Fusco FR, Kimble TD, Meade CA, Cuthbertson S, Reiner A. 1999. A simple and sensitive antigen retrieval method for free-floating and slide-mounted tissue sections. J Neurosci Methods 93:149–162 [DOI] [PubMed] [Google Scholar]
- 46. Yoshida K, Li X, Cano G, Lazarus M, Saper CB. 2009. Parallel preoptic pathways for thermoregulation. J Neurosci 29:11954–11964 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Dacks PA, Rance NE. 2010. Effects of estradiol on the thermoneutral zone and core temperature in ovariectomized rats. Endocrinology 151:1187–1193 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Stoessl AJ, Dourish CT, Iversen SD. 1988. The NK-3 tachykinin receptor agonist senktide elicits 5-HT-mediated behaviour following central or peripheral administration in mice and rats. Br J Pharmacol 94:285–287 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Nordquist RE, Ballard TM, Algeyer B, Pauly-Evers M, Ozmen L, Spooren W. 2010. Pharmacological characterization of senktide-induced tail whips. Neuropharmacology 58:259–267 [DOI] [PubMed] [Google Scholar]
- 50. MacKenzie MA. 1997. Pathophysiology and clinical implications of human poikilothermia. Ann NY Acad Sci 813:738–740 [DOI] [PubMed] [Google Scholar]
- 51. Oerther S. 2000. Temperature set-point changes induced by DA D2/3 and 5-HT1A receptor agonists in the rat. Neuroreport 11:3949–3951 [DOI] [PubMed] [Google Scholar]
- 52. Gordon CJ. 2005. Temperature and toxicolgy: an integrative, comparative, and environmental approach. Boca Raton, FL: CRC Press [Google Scholar]
- 53. Gordon CJ. 1990. Thermal biology of the laboratory rat. Physiol Behav 47:963–991 [DOI] [PubMed] [Google Scholar]
- 54. Nagashima K, Nakai S, Tanaka M, Kanosue K. 2000. Neuronal circuitries involved in thermoregulation. Auton Neurosci 85:18–25 [DOI] [PubMed] [Google Scholar]
- 55. Tanaka M, Ootsuka Y, McKinley MJ, McAllen RM. 2007. Independent vasomotor control of rat tail and proximal hairy skin. J Physiol 582:421–433 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Morrison SF, Nakamura K, Madden CJ. 2008. Central control of thermogenesis in mammals. Exp Physiol 93:773–797 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Kiyatkin EA, Brown PL. 2005. Brain and body temperature homeostasis during sodium pentobarbital anesthesia with and without body warming in rats. Physiol Behav 84:563–570 [DOI] [PubMed] [Google Scholar]
- 58. Owens NC, Ootsuka Y, Kanosue K, McAllen RM. 2002. Thermoregulatory control of sympathetic fibres supplying the rat's tail. J Physiol 543:849–858 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. de Menezes RC, Ootsuka Y, Blessing WW. 2009. Sympathetic cutaneous vasomotor alerting responses (SCVARs) are associated with hippocampal theta rhythm in non-moving conscious rats. Brain Res 1298:123–130 [DOI] [PubMed] [Google Scholar]
- 60. O'Leary DS, Johnson JM, Taylor WF. 1985. Mode of neural control mediating rat tail vasodilation during heating. J Appl Physiol 59:1533–1538 [DOI] [PubMed] [Google Scholar]
- 61. Yoshida K, Konishi M, Nagashima K, Saper CB, Kanosue K. 2005. Fos activation in hypothalamic neurons during cold or warm exposure: projections to periaqueductal gray matter. Neuroscience 133:1039–1046 [DOI] [PubMed] [Google Scholar]
- 62. Romanovsky AA, Almeida MC, Garami A, Steiner AA, Norman MH, Morrison SF, Nakamura K, Burmeister JJ, Nucci TB. 2009. The transient receptor potential vanilloid-1 channel in thermoregulation: a thermosensor it is not. Pharmacol Rev 61:228–261 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Boulant JA. 2000. Role of the preoptic-anterior hypothalamus in thermoregulation and fever. Clin Infect Dis 31:S157–S161 [DOI] [PubMed] [Google Scholar]
- 64. Srividya R, Mallick HN, Kumar VM. 2005. Changes in brain temperature and thermoregulation produced by destruction of medial septal neurons in rats. Brain Res Bull 66:143–148 [DOI] [PubMed] [Google Scholar]
- 65. Romanovsky AA, Sugimoto N, Simons CT, Hunter WS. 2003. The organum vasculosum laminae terminalis in immune-to-brain febrigenic signaling: a reappraisal of lesion experiments. Am J Physiol Regul Integr Comp Physiol 285:R420–R428 [DOI] [PubMed] [Google Scholar]