Abstract
Cytochrome P450 (CYP)3A4 is the principal and most abundant human isoform of CYP responsible for the metabolism of more than 50% of all consumed drugs and innumerable endogenous compounds. Expression of CYP3A4 is sexually dimorphic and regulated by the combined actions of GH and glucocorticoids. In the case of the rat, nearly all of the CYPs are “intrinsically” or “inherently” sexually dimorphic, meaning that the expressed sex differences are permanent and irreversible. Using primary hepatocyte cultures derived from men and women exposed to physiologic-like levels of continuous GH (the feminine circulating profile) alone, dexamethasone alone, and the combined regimen, we observed a dramatic inherent CYP3A4 sexual dimorphism (women more than men) with all treatments. The molecular basis for this intrinsic sexually dimorphic expression of CYP3A4 appears to be due, at least in part, to a greater level of hormone-dependent activation and nuclear translocation of both hepatocyte nuclear factor-4α (HNF-4α) and pregnane X receptor in female hepatocytes. Furthermore, these transcription factors exhibited significantly higher DNA binding levels to their specific motifs on the CYP3A4 promoter in female hepatocytes, inferring a possible explanation for the elevated expression of CYP3A4 in women. Accordingly, experiments using HepG2 cells treated with small inhibitory RNA-induced knockdown of HNF-4α and/or transfected with luciferase reporter constructs containing a CYP3A4 promoter lacking HNF-4α-binding motifs demonstrated that GH, to a greater extent dexamethasone, and to the greatest extent the combine hormone regimen, stimulated HNF-4α and pregnane X receptor promoter transactivation, signifying enhanced transcription of CYP3A4 and, thus, identifying a molecular mechanism contributing to the intrinsic sexual dimorphic expression of human CYP3A4.
Cytochrome P450 (CYP)3A4 is the principal human isoform of CYP responsible for phase I metabolism of at least one-half of all consumed drugs and expressed at the highest concentration, i.e. 30–50% of the total pool of hepatic CYP (1, 2). Scores of chemicals (e.g. drugs, environmental, and endogenous compounds) are substrates for CYP3A4 (3). In vivo (4) and in vitro (5) studies have observed CYP3A4 to be female predominant with expression levels in women varying from approximately 25 to 200% above that in men. In fact, sex differences in CYP-dependent drug metabolism are quite common, existing in numerous diverse species from trout to humans (cf. 6).
The endogenous factor known to maintain sexually dimorphic expression of hepatic CYPs is GH (6, 7). Moreover, in all species examined, including humans (8–10), GH is secreted in a sexually dimorphic pattern; the masculine profile is deemed “episodic,” and the feminine is referred to as “continuous” (6, 11). In the case of the rat, the species that has received the most attention, males secrete GH in episodic bursts approximately every 3–4 h. Between the peaks, GH levels are undetectable. In female rats, the hormone pulses are more frequent and irregular and are of lower magnitude than males, whereas the interpeak concentration of GH is always measurable. Exposure to the continuous or “constant” feminine secretory profile of GH produces the characteristic pattern of CYP isoforms expressed in females. Conversely, the episodic or “pulsatile” rhythm of GH secretion characterized as masculine is responsible for the expression of CYPs observed in male rats (12, 13).
In humans, numerous reports, generally using GH-deficient individuals, have shown that GH replacement can restore drug-metabolizing enzymes to normal levels (14). More recently, in vivo studies have reported the inductive effects of GH therapy on CYP3A4 enzyme markers in GH-deficient individuals. In one case, the inductive effects of a daily sc GH injection on CYP3A4-dependent activity were assessed separately in GH-deficient young boys and girls (15). In another in vivo study, the differential effects of restored sex-dependent GH profiles (i.e. episodic and continuous) on CYP3A4, assessed by the erythromycin breath test, was reported on a combined cohort of GH-deficient men and women (16). In an in vitro study measuring CYP3A4 mRNA, protein, and specific catalytic activity in hepatocyte cultures, presumably from combined sexes, exposure to a constant pharmacologic GH dose was found to be clearly inductive (17). Extending these studies, we examined the effects of physiologic-like exposure doses of episodic or continuous human GH (hGH) on expression levels of several CYPs, including CYP3A4, in hepatocyte cultures derived from men and women donors (18). Whether in the presence or absence of dexamethasone (a positive regulator for all members of the CYP3A family) (17–19), and independent of sex, the masculine-like episodic GH profile suppressed CYP3A4 expression, whereas the feminine-like continuous GH profile was inductive.
In addition to observing the differential effects of the masculine and feminine GH profiles on CYP3A4 expression, we noted an apparent intrinsic sexually dimorphic response of several CYP isoforms, in that the episodic GH profile was more suppressive in hepatocytes from men than women, whereas the continuous GH profile was more inductive in hepatocytes from women than men. In this regard, the same once daily GH replacement regimen was significantly more suppressive of CYP3A4 enzymatic activity in boys than girls (15). This intrinsic sex difference in GH regulation of CYP also has been reported in rats in which renaturalization of the feminine circulating GH profile was considerably more effective, both in vivo and in vitro, in restoring expression levels of CYP isoforms in GH-depleted females than males (20, 21). Similar inherent sexually dimorphic responses in humans to the same episodic GH regimen have been reported for IGF-I, bone mineralization, lipid metabolism, growth rates, and growth hormone-binding protein; men more than women (22–26).
In the present study, we have compared the responsiveness of hepatocyte CYP3A4 from adult men and women exposed to the same regimen of either dexamethasone alone, GH alone, or the combined hormones. In addition, we have examined possible molecular mechanisms that could explain an intrinsic sexual dimorphic response.
Materials and Methods
Human hepatocyte culture
Male and female hepatocytes were isolated from human liver (27) and plated on rat tail collagen-coated flasks (T-25) in DMEM and were obtained through the Liver Tissue Procurement and Distribution System (Pittsburgh, PA). All of the samples were obtained with donors' consent and with approval of the appropriate hospital ethics committee. Male and female donors varied in age from 25 to 50 yr. About 80% was Caucasian, the remainder was African American and Hispanic. Alcohol consumption, smoking, and drug history as well as causes of death varied between donors. Approximately 50% of livers had some degree of steatosis (5–40%). Approximately 48 h after isolation and plating, the primary hepatocyte cultures arrived at our laboratory. The replacement medium and culture conditions were described previously (18, 21).
Hormonal conditions
We realized that because of radical differences in metabolism, it was not possible to translate normal circulating hormone levels into equivalent in vitro doses, but we did base the selected hormone concentrations in the hepatocyte cultures on physiologic levels. Dexamethasone is a highly potent, synthetic glucocorticoid. However, when comparing its biologic potency (e.g. gluconeogenic and glycogenolytic) with cortisol, the present levels (10 nm) would be comparable with resting plasma concentrations of the natural steroid in men and women (28). In addition, our hGH dose of 2 ng/ml is physiologic (29).
To replicate the more continuous feminine-like GH profile shown to be favored for human CYP3A4 expression (18), the primary hepatocytes were constantly exposed (2 ng/ml) to recombinant hGH, 2.2 IU/mg as determined in the hypophysectomized female rat body weight gain bioassay, purchased from the National Hormone and Peptide Program (Torrance, CA). Other cells were exposed to dexamethasone (4 ng/ml) alone or to both the glucocorticoid and recombinant hGH. Some hepatocytes were exposed to neither hormone. The medium was changed every 12 h. After 5 d in culture, cells were harvested 60 min after the final change of media as previously described (16, 21).
Preparation of whole-cell and nuclear extracts
To isolate protein for immunoblots, harvested hepatocytes were centrifuged (800 × g for 10 min), and the resulting cell pellets were resuspended in lysis buffer (30). The crude extract was passed through a 22-gauge needle 10 times. The solution was then gently mixed at 4 C for 20 min and centrifuged at 12,000 × g for 20 min. The supernatant (whole-cell extract) was then removed and stored at −80 C until analyses. Briefly, nuclei were isolated according to the method of Dignam et al. (31), by a series of centrifugations of resuspended, homogenized, and dialyzed crude nuclear extract originating from the low-speed pellet. Protein concentration was determined by the Bio-Rad protein assay (Bio-Rad, Hercules, CA).
TransSignal protein/DNA array I
To identify the relative binding of GH and dexamethasone-induced human liver transcription factors to their consensus sequences, we used the TransSignal protein/DNA array I (Panomics, Redwood City, CA). Array analysis was performed as per the manufacturer's instructions using nuclear extracts from cultured male and female human hepatocytes. Basically, 10 μg of nuclear extract from cultured male and female hepatocytes were incubated with 10 μl of TransSignal probe mix containing 56 biotin-labeled double-stranded DNA oligonucleotides. The biotin-labeled oligonucleotides specifically bound to the transcription factors were eluted and hybridized to the TransSignal array membrane containing their complementary oligonucleotides overnight at 42 C. The blots were then washed and incubated with a horseradish peroxidase-conjugated streptavidin according to the manufacturer's instructions. The resulting signals were visualized, captured as well as quantified on a FluorChem IS-8800 Imager (Alpha Innotech, San Leandro, CA) by using a movie mode. The spots were identified and normalized with references provided in the TransSignal protein/DNA array I kit.
Immunoblot analysis
Using standard protocol (18, 32), 25–50 μg of the 12,000 × g supernatant (i.e. whole-cell extract) and 50 μg of nuclear extract were resolved on 10% SDS-PAGE and transferred electrophoretically onto Immuno-Blot-polyvinylidene difluoride membranes with a Bio-Rad transfer unit. The membranes were then blocked with 5% nonfat dry milk and incubated with primary antibody raised against recombinant human CYP3A4 (kindly provided by F. Peter Guengerich), hepatocyte nuclear factor-4α (HNF-4α), or pregnane X receptor (PXR) (Sc-8987, Sc-25381; Santa Cruz Biotechnology, Inc., Santa Cruz, CA) antibodies. The primary antibody was located by using horseradish peroxidase conjugated to antirabbit IgG. The blots, incubated with SuperSignal West Femto (Pierce, Rockford, IL), were visualized, captured, and quantified by using an Alpha Innotech FluroChem 8800 Image system with a movie mode. Signals were normalized to a control sample, which was repeatedly run on each blot and exhibited a concentration variant between blots of 2.8–6.1% for the different proteins. Lastly, blots were stripped and reprobed with loading controls actin or p97 antibody and found to be comparable with those obtained with internal controls of the assayed samples.
Chromatin immunoprecipitation (ChIP) assay
After the hormonal regimen described above, ChIP assays were performed on primary human hepatocytes as well as HepG2 cells (HB-8065; American Type Culture Collections, Manassas, VA) according to our previously described methods (32, 33). Lysed, purified nuclei were sonicated to generate DNA fragments with an average length of 100-1000 bp. Equal concentrations of chromatin from all treatment groups were precleared with protein A agarose beads in the presence of 1 mg/ml BSA and 2 μg of sonicated salmon sperm DNA to reduce the nonspecific background. After removal of beads by centrifugation, 2 μg of HNF-4α- or PXR-specific antibody (Santa Cruz Biotechnology, Inc.) were added and kept at 4 C for overnight on a rotary platform. The immunoprecipitates were washed sequentially, eluted, and prepared as previously described (32). Immunoprecipitated DNA was purified using a PCR purification kit (QIAGEN, Valencia, CA) and resuspended in 50 μl of sterile water. The purified DNA from immunoprecipitation was subjected to semiquantitative PCR using HNF-4α forward, 5′-GTC GTT AGA ATC TGA ACT TCC-3′ and reverse, 5′-GGA TTC TAT GAG CTA AGT TCA-3′ and PXR forward primer, 5′-TAT GCC AAT GGC TCC ACT TGA G-3′ and reverse primer, 5′-TTG GAT TGT TTA TAT GCT AGA GAA GGA GGC-3′ binding sites of the CYP3A4 5′ flanking regions (34–36). No antibody (rabbit preimmune serum) and an HNF-4α, and PXR nonbinding region of the albumin gene with a forward 5′-CAG GGA TGG AAA GAA TCC TAT GCC-3′ and reverse 5′-CCA TGT TCC CAT TCC TGC TGT-3′ primers were used as negative controls. PCR products resolved on agarose gels were quantified using a FluroChem IS-8800 Imager.
Confirmation of HNF-4α binding to motifs I and II and PXR binding to its motif on the CYP3A4 promoter by Southern blotting
The PCR products (DNA) from the ChIP assays were denatured and transferred onto Nytran N filters from Schleicher and Schuell (Keene, NH). Southern blotting was carried out (32, 33) to confirm the HNF-4α-binding motifs in the PCR products by using γ-32P-labeled nucleotide sequence 5′-gtT CAT GTG CAA AGT TGA GTT a-3′ and 5′-gCT TTG AAC TTA GCT CAT AGa-3′ of the HNF-4α motifs I and II, respectively, binding sites of the human CYP3A4 promoter (35). The PXR-binding motif in the PCR product was confirmed by using a γ-32P-labeled nucleotide sequence 5′-ata TGAACT caaagg AGGTCA gtg-3′ of the PXR binding sites on the CYP3A4 promoter (34). The signals were scanned and quantitated by using a FluorChem IS-8800 Imager. The signals were normalized with a positive control, which was repeatedly run on each blot.
HNF-4α knockdown in HepG2 cells
HepG2 cells were cultured in DMEM/F-12 containing 10% fetal bovine serum, penicillin (100 U/ml), and streptomycin (100 μg/ml) under 5% CO2. Upon 50–70% confluency, small inhibitory RNA (siRNA)-mediated knockdown of HNF-4α was carried out as per manufacturer's instructions (Sc-35573; Santa Cruz Biotechnology, Inc.) as previously reported (37, 38). Twenty-four hours after transfection with the siRNA or scrambled (Scr) siRNA (controls), the HepG2 cells were exposed for 2 d to the hormonal treatments described above. Cells were harvested on the third day and assayed for nuclear HNF-4α protein, HNF-4α binding to its putative CYP3A4 promoter (ChIP), and for confirmation of the occupied binding motifs (Southern blotting) by procedures described above for primary hepatocytes.
Transient transfection and luciferase assay
Dual luciferase reporter assays were conducted to compare the emulative transcriptional activity between the CYP3A4 13-kb promoter containing HNF-4α binding sites I and II [i.e. the so-called constitutive liver enhancer module (CLEM) motif] and the CYP3A4 3-kb promoter lacking both HNF-4α binding sites. HepG2 cells were cultured to 50–70% confluency and transfected with either HNF-4α knockdown siRNA or the control Scr siRNA as we have described above. Twenty-four hours later, the cells were transiently transfected with either the 13- or 3-kb reporter plasmids (a generous gift from Garold S. Yost) using Lipofectamine LTX transfection reagent (Invitrogen Corp., Carlsbad, CA) as previously reported (36). The Renilla reniformis luciferase plasmid was cotransfected as an internal control. After 24 h, the cells were exposed for 2 d to the same hormonal treatments as described for the primary human hepatocytes. On the third day, cells were lysed, and the respective luciferase activities were determined (39) using the dual-luciferase assay system (Promega, Madison, WI). Firefly luciferase activities for the experimental constructs were normalized for transfection efficiency and cell loading using R. reniformis luciferase activity and total protein concentration, respectively.
Statistics
All data were subject to ANOVA. Significant differences were determined with t statistics and the Bonferroni procedure for multiple comparisons.
Results
Similar to observations in mice (6, 40), humans express large interperson variation in CYP levels. Depending upon the report, isoforms can vary up to 200-fold between individuals and usually more than 5- to 20-fold when determined in vivo or in freshly prepared cell extracts (41, 42). Accordingly, it becomes understandable why an estimated 30% of hospitalized patients experience an adverse drug reaction, 0.31% of which are fatal (male:female, 4:1) (43). To limit the effects of this variability, all determinations in the present study were performed on the same individuals. Nevertheless, for reasons previously discussed (18), there were always one or two donors whose hepatocytes' response to the hormone treatment was so incongruous (e.g. completely unresponsive) that these samples were eliminated from the study. Results of the Grubb's test for outliers as well as Dixon's test for extreme values agreed with our choice of the two outliers expunged from the data sets.
Sexually dimorphic response to hormonal regulation of CYP3A4 protein levels in primary hepatocytes from men and women
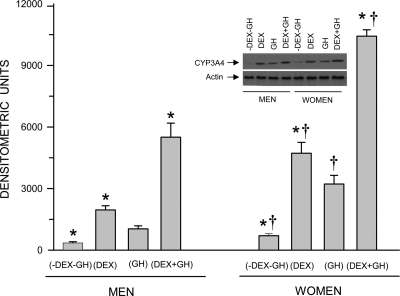
In agreement with our previous observations (18), hepatic CYP3A4 induction was greatest when the cells were exposed to the combined treatment of continuous dexamethasone and GH (Fig. 1). Alone, GH was moderately inductive and dexamethasone considerably more so. Sex differences in the induction of CYP3A4 were indicated by the significantly greater expression levels of the isoform in female hepatocytes after all hormonal treatments.
Fig. 1.
Sex-dependent hormonally regulated expression levels of CYP3A4 protein in hepatocytes derived from adult men and women. The cells were exposed to either continuous dexamethasone alone (DEX), continuous GH alone, both hormones (DEX+GH), or vehicle alone (-DEX-GH) for 5 d in culture, after which the cells were harvested and analyzed. Each data point is a mean ± sd for cells from six men, all treatments except DEX+GH, in which n = 5; six women, all treatments except -DEX-GH, in which n = 5. *, P < 0.01 compares with the GH alone treatment of the same sex; †, P < 0.01 compares women with men exposed to the same hormone treatment. A representative immunoblot of CYP3A4 and its respective loading control (actin) is presented in the figure.
Sex-dependent regulation of transcription factor binding to nuclear DNA from human hepatocytes
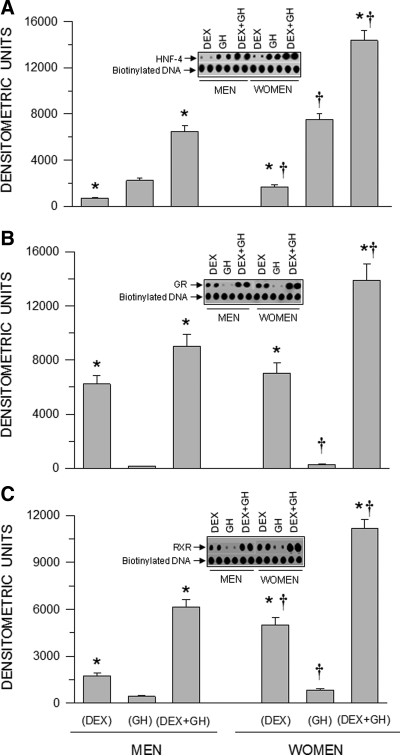
Employing a novel protein/DNA array I method, we detected 28 proteins that bound to the nuclear DNA of hepatocytes of both sexes (see Supplemental Table, published on The Endocrine Society's Journals Online web site at http://endo.endojournals.org). Irrespective of sex hormone treatments, binding of the remaining 28 proteins, including, were undetectable. Regarding our putative candidate proteins, we observed significant hormone-induced and sex-dependent differences in HNF-4, glucocorticoid receptor, and retinoid X receptor (RXR) binding to nuclear DNA in hepatocytes derived from men and women (Fig. 2). In contrast to the hormonal effects on CYP3A4 induction (Fig. 1), GH alone induced greater HNF-4/DNA binding than dexamethasone alone, whereas the combined hormonal treatment was significantly more effective than either treatment alone (Fig. 2A). Glucocorticoid receptor/DNA binding and RXR/DNA binding was more reflective of the hormonal effects on CYP3A4 induction. That is, GH alone moderately, at best, stimulated glucocorticoid receptor and RXR binding to DNA, whereas dexamethasone alone was more effective (considerably so in the case of glucocorticoid receptor/DNA binding), and the combined hormonal treatment was clearly the most stimulatory (Fig. 2, B and C). Sex differences in hormonal enhancement of transcription factor binding were observed with a greater effectiveness of every hormone regimen in female hepatocytes.
Fig. 2.
Sex-dependent hormonally regulated DNA bound HNF-4 (A), glucocorticoid receptor (GR) (B), and RXR (C) in nuclei from hepatocytes derived from adult men and woman. The cells were exposed to either continuous dexamethasone alone (DEX), continuous GH alone, or both hormones (DEX+GH) for 5 d in culture, after which the hepatocytes were harvested, and transcription factor bound DNA was determined by a TransSignal Protein/DNA Array I kit. The signals were normalized with references provided by the manufacturer. Each data point is a mean ± sd for cells from five individuals, all treatments. *, P < 0.01 compares with the GH alone treatment of the same sex; †, P < 0.01 compares women with men exposed to the same hormone treatment. Representative arrays of HNF-4/DNA, GR/DNA, and RXR/DNA complexes and their respective loading controls (biotinylated DNA) are presented in the figure.
Sex-dependent hormonal regulation of nuclear HNF-4α and PXR concentrations in human hepatocytes
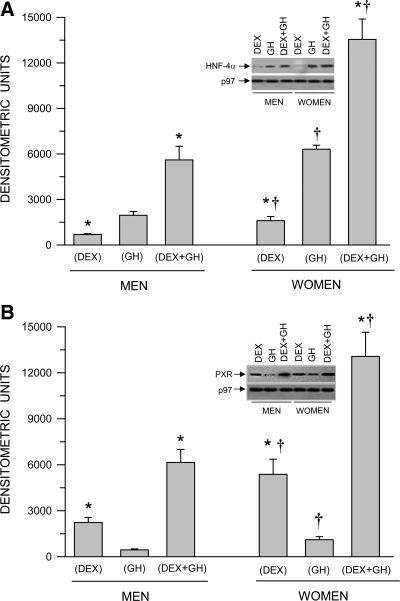
Regardless of sex, the apparent nuclear translocation of HNF-4α and PXR exhibited different responses to dexamethasone and GH when each hormone was administered separately. That is, nuclear concentrations of HNF-4α were two to three times greater after exposure to GH than dexamethasone (Fig. 3A). In contrast, nuclear levels of PXR were three times greater in hepatocytes exposed to dexamethasone than GH (Fig. 3B). Simultaneous exposure to both hormones increased nuclear concentrations of HNF-4α and PXR in both sexes to levels that exceeded the additive effects of individual hormone treatments. Again, nuclear translocation (as judged from nuclear concentrations) of both HNF-4α and PXR was dramatically greater, at all hormone treatments, in hepatocytes from women. Control hepatocytes (no hormone treatment) from both sexes exhibited, at best, trace nuclear concentrations of HNF-4α and PXR levels (data not shown). In addition, we observed no detectable levels of phospho-signal transducer and activator of transcription 5 in the nuclear hepatocytes of any of the treatment groups (data not shown).
Fig. 3.
Sex-dependent hormonal regulation of nuclear HNF-4α (A) and nuclear PXR (B) protein levels in hepatocytes derived from adult men and women. The cells were exposed to either continuous dexamethasone alone (DEX), continuous GH alone, or both hormones (DEX+GH) for 5 d in culture, after which the hepatocytes were harvested and analyzed. Each data point is a mean ± sd for cells from six men, all treatments except DEX+GH, in which n = 5; six women, all treatments. *, P < 0.01 compares with the GH alone treatment of the same sex; †, P < 0.01 compares women with men exposed to the same hormone treatment. Representative immunoblots of HNF-4α and PXR and their respective loading controls (p97) are presented in the figure. Positive controls (HNF-4α and PXR) were repeatedly run on all blots for procedural integrity (data not shown).
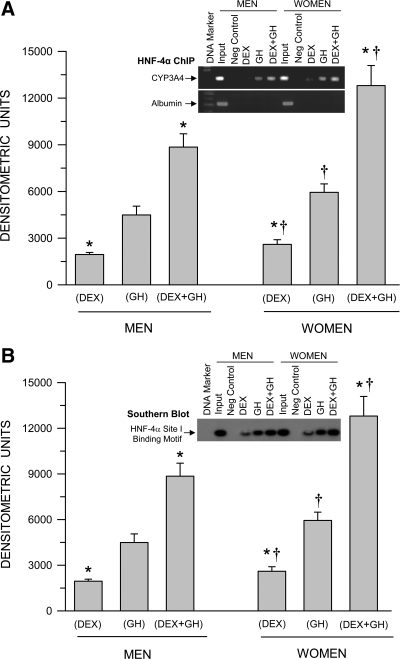
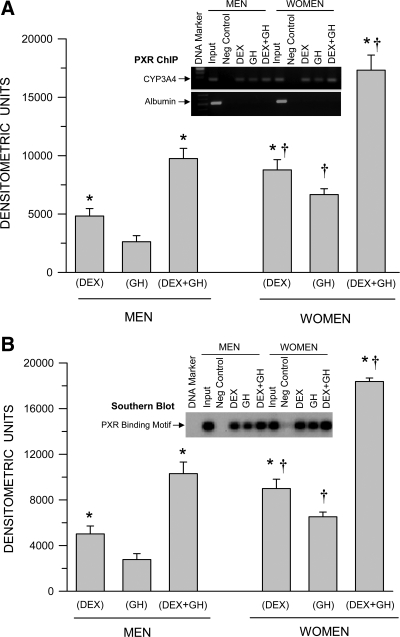
Sex-dependent hormonally regulated HNF-4α binding to the CYP3A4 promoter
Consistent with Figs. 2A and 3A, GH alone induced greater levels of HNF-4α binding to the CYP3A4 promoter than dexamethasone alone (Fig. 4A). The combined treatment of continuous GH and continuous dexamethasone was clearly the most effective, stimulating HNF-4α binding to the CYP3A4 promoter to levels greatly exceeding the sum of the individual effects of the two hormones. Once more, an intrinsic sexual dimorphism was observed in that HNF-4α binding to the CYP3A4 promoter was significantly greater with all hormone treatments in primary hepatocytes from women. In confirmation, using Southern blotting, we observed very much similar levels of the HNF-4α-binding motif of the CYP3A4 promoter bound to the activated transcription factor (Fig. 4B) as seen in the ChIP assay (Fig. 4A) Because of the very close proximity of the two putative binding sites for HNF-4α in the CYP3A4 promoter, the ChIP assay measures HNF-4α binding to both motifs. In contrast, the Southern blotting allowed us to measure either binding site. Accordingly, we analyzed both binding motifs I and II and have presented the former, whose results were near identical to the latter.
Fig. 4.
Sex-dependent hormonal regulation of HNF-4α binding to the CYP3A4 promoter (ChIP assay) (A) and confirmation of the occupied HNF-4α-binding motif in the CYP3A4 promoter (Southern blotting) (B) in hepatocytes derived from adult men and women. The cells were exposed to either continuous dexamethasone alone (DEX), continuous GH alone, or both hormones (DEX+GH) for 5 d in culture, after which the hepatocytes were harvested and analyzed. Each data point is a mean ± sd for cells from six men, all treatments except DEX+GH, in which n = 5; six women, all treatments. *, P < 0.01 compares with the GH alone treatment of the same sex; †, P < 0.01 compares women with men exposed to the same hormone treatment. A representative ChIP blot (A) with its input control (albumin) and a Southern blotting (B) are presented in the figure.
Sex-dependent hormonally regulated PXR binding to the CYP3A4 promoter
Consistent with Fig. 3B, dexamethasone alone induced greater levels of PXR binding to the CYP3A4 promoter than GH alone (Fig. 5A). The combined treatment of continuous dexamethasone and continuous GH was clearly the most effective, stimulating PXR binding to the CYP3A4 promoter to levels exceeding the additive effects of the two hormones administered separately. Again, an inherent sexual dimorphism was observed in that PXR binding to the CYP3A4 promoter was significantly greater with all hormone treatments in primary hepatocytes derived from women. In confirmation, using Southern blotting, we observed very much similar levels of the PXR-binding motif of the CYP3A4 promoter bound to the activated transcription factor (Fig. 5B), reflecting the results of the ChIP assay (Fig. 5A).
Fig. 5.
Sex-dependent hormonal regulation of PXR binding to the CYP3A4 promoter (ChIP assay) (A) and confirmation of the occupied PXR-binding motif in the CYP3A4 promoter (Southern blotting) (B) in hepatocytes derived from adult men and women. The cells were exposed to either continuous dexamethasone alone (DEX), continuous GH alone, or both hormones (DEX+GH) for 5 d, after which the hepatocytes were harvested and analyzed. Each data point is a mean ± sd for cells from six men, all treatments except DEX+GH, in which n = 5; six women, all treatments. *, P < 0.01 compares with the GH alone treatment of the same sex; †, P < 0.01 compares women with men exposed to the same hormone treatment. A representative ChIP blot (A) with its input control (albumin) and a Southern blotting (B) are presented in the figure.
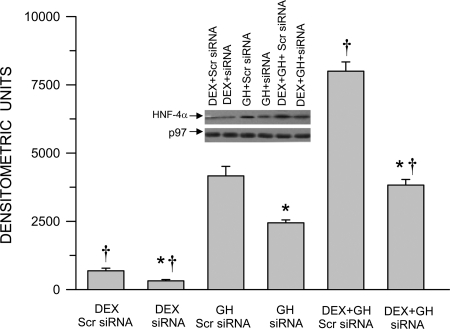
siRNA interference with hormonal regulation of nuclear HNF-4α accumulation in HepG2 cells
To identify the role of HNF-4α in the hormonal regulation of CYP3A4 expression for subsequent experiments, we examined the effectiveness of a siRNA designed to knock down HNF-4α in HepG2 cells (Fig. 6). The control HepG2 cells transfected with the nonspecific (Scr) siRNA exhibited the same hormonal responsiveness as the primary human hepatocytes (Fig. 3A). That is, dexamethasone alone induced the smallest increase in nuclear HNF-4α concentrations, whereas GH alone was several fold more effective, and the combined hormonal treatment was clearly the most inductive regimen. In general, exposure of the HepG2 cells to siRNA reduced the effectiveness of each hormone treatment to stimulate HNF-4α nuclear translocation by about 50%. Although this level of knockdown is somewhat modest when compared with the effects of other species of siRNA, it is in agreement with reports specifically using siRNA against HNF-4α (37, 38).
Fig. 6.
Hormonal regulation of nuclear HNF-4α protein levels in HNF-4α proficient and deficient HepG2 cells. After 50–70% confluency, HepG2 cells were transfected with either the control nonspecific Scr siRNA (proficient) or HNF-4α knockdown siRNA (deficient) and 24 h later exposed to either continuous dexamethasone alone (DEX), continuous GH alone, or both hormones (DEX+GH) for 2 d, after which the cells were harvested and analyzed. Each data point is a mean ± sd with an n = 7, except DEX siRNA, in which n = 6. *, P < 0.01 compares the effects of the knockdown siRNA with the control cells exposed to the same hormone treatment; †, P < 0.01 compares with GH alone in cells transfected with the same siRNA. A representative immunoblot of HNF-4α and its respective loading control (p97) are presented in the figure. A positive control (HNF-4α) was repeatedly run on all blots for procedural integrity (data not shown).
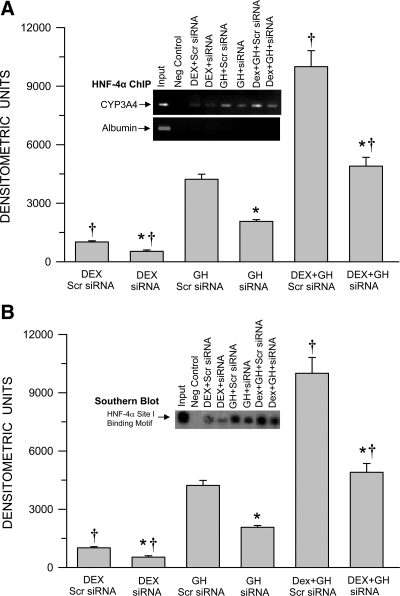
Hormonally regulated HNF-4α binding to the CYP3A4 promoter in HNF-4α knockdown HepG2 cells
Having established the effectiveness of the siRNA to inhibit nuclear accumulation of HNF-4α (Fig. 6), we proceeded to examine the role of the transcription factor in mediating hormonal induction of CYP3A4 expression. Consistent with our findings using primary human hepatocytes (Fig. 4A), in control (Scr siRNA) HepG2 cells, GH alone induced greater levels of HNF-4α binding to the CYP3A4 promoter than dexamethasone alone (Fig. 7A). The combined treatment of continuous GH and dexamethasone was clearly the most effective stimulating HNF-4α binding to the CYP3A4 promoter to levels greatly exceeding the sum of the individual effects of the two hormones. An approximately 50% reduction in HNF-4α nuclear translocation (Fig. 6) resulted in a similar percent reduction in the binding of the transcription factor to the CYP3A4 promoter at every hormone treatment, demonstrating the importance of HNF-4α recruitment by all the hormone regimens in their induction of CYP3A4 expression. In confirmation, using Southern blotting, we observed very much similar levels of the occupied HNF-4α-binding motif of the CYP3A4 promoter (Fig. 7B) as seen in the ChIP assay (Fig. 7A).
Fig. 7.
Hormonal regulation of HNF-4α binding to the CYP3A4 promoter (ChIP assay) (A) and confirmation of the occupied HNF-4α-binding motif in the CYP3A4 promoter (Southern blotting) (B) in HNF-4α proficient and deficient HepG2 cells. After 50–70% confluency, HepG2 cells were transfected with either the control nonspecific Scr siRNA (proficient) or HNF-4α knockdown siRNA (deficient) and 24 h later exposed to either continuous dexamethasone alone (DEX), continuous GH alone, or both hormones (DEX+GH) for 2 d, after which the cells were harvested and analyzed. Each data point is a mean ± sd with an n = 7, except DEX siRNA, in which n = 6. *, P < 0.01 compares the effects of the knockdown siRNA with the control cells exposed to the same hormone treatment; †, P < 0.01 compares with GH alone in cells transfected with the same siRNA. A representative ChIP blot (A) with its input control (albumin) and a Southern blotting (B) are presented in the figure.
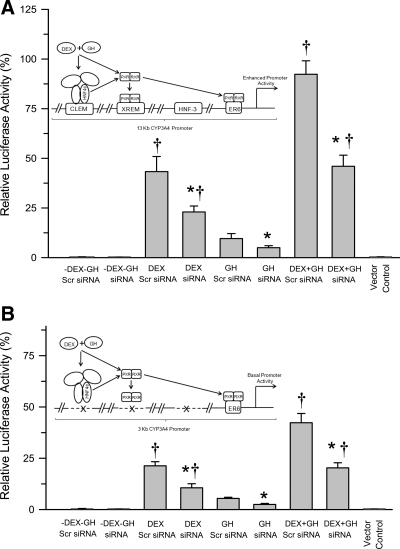
Hormonal regulation of CYP3A4 promoter activity in HNF-4α proficient and deficient HepG2 cells transfected with either HNF-4α-containing binding motifs (13-kb CYP3A4 promoter) or HNF-4α-devoid binding motifs (3-kb CYP3A4 promoter)
The aim of this experiment was to determine the requirement(s) for hormone-induced nuclear translocation and promoter binding of HNF-4α for transactivation of the CYP3A4 promoter as measured by luciferase activity. Accordingly, dexamethasone alone, GH alone, and the combined hormonal regimen require a CYP3A4 promoter with HNF-4α-binding motifs (i.e. CLEM element) for maximal activation of CYP3A4 expression (Fig. 8, A and B). Although GH was more effective than dexamethasone in elevating nuclear levels of HNF-4α (Fig. 6), as well as enhancing binding of the transcription factor to the CYP3A4 promoter (Fig. 7A), dexamethasone was considerably more effective than GH in activating the CYP3A4 promoter (Fig. 8A), perhaps indicating the greater importance of PXR-RXRα binding to the promoter (Fig. 5A). In agreement, dexamethasone alone was more effective than GH alone in inducing actual CYP3A4 protein levels in primary human hepatocytes (Fig. 1). After HNF-4α knockdown, nuclear levels of the transcription factor were reduced (Fig. 6), binding to the promoter was reduced (Fig. 7A), as was transactivation of the CYP3A4 promoter similarly reduced after all hormone treatments (Fig. 8A).
Fig. 8.
Hormonal regulation of CYP3A4 promoter activity in HepG2 cells transiently transfected with a full-length 13-kb CYP3A4 promoter construct (A) or truncated 3-kb CYP3A4 promoter construct (B), as well as a control nonspecific Scr siRNA or HNF-4α knockdown siRNA. Twenty-four hours later, the cells were exposed to either continuous dexamethasone alone (DEX), continuous GH alone, both hormones (DEX+GH), or vehicle alone (-DEX-GH) for 2 d, after which the cells were harvested and promoter activity measured using a dual luciferase reporter system. Luciferase activity was normalized with cotransfected Renilla luciferase activity. Each data point is a mean ± sd with an n = 5, all treatments. *, P < 0.01 compares the effects of knockdown siRNA with the control (Scr siRNA) cells transfected with the same promoter construct and exposed to the same hormone treatment; †, P < 0.01 compares with GH alone in cells transfected with the same promoter construct and same siRNA. Schematic explanations of the findings are presented in the figure. XREM, Xenobiotic-responsive enhancer module.
Transfection of the HepG2 cells with the truncated 3-kb CYP3A4 promoter lacking the HNF-4α-binding motifs (but containing one of the two PXR-RXRα binding elements) reduced by more than 50% CYP3A4 promoter activity after all hormone treatments (Fig. 8B). When these same cells were also transfected with siRNA against HNF-4α, CYP3A4 promoter activity was reduced an additional 50% with all hormone regimens. Briefly then, there appeared to be a requirement for HNF-4α binding to its element (CLEM) in the CYP3A4 promoter for maximal activation. Accordingly, an approximately 50% reduction in nuclear HNF-4α, resulting from siRNA-induced knockdown (Fig. 6), caused a similar percent decline in CYP3A4 promoter binding (Fig. 7A) and activation (Fig. 8, A and B). However, even in the absence of the HNF-4α-binding motifs in the CYP3A4 promoter (3-kb promoter), a minimal, perhaps baseline activity of the promoter, was observed as a possible consequence of PXR-RXRα binding to the everted repeat 6 (ER6) complex in the promoter (Fig. 8B).
Discussion
In addition to exhibiting a female predominance when determined in vivo (4, 16), in liver extracts (5), and in cultured hepatocytes (18), we now report an intrinsic sexual dimorphism in CYP3A4 expression, in which hepatocytes from women are the considerably more responsive sex to the normally inductive effects of continuous GH, alone or when administered with dexamethasone (the physiologic-like condition) or even to dexamethasone alone. In this regard, there is also an intrinsic sexually dimorphic response to the masculine circulating GH profile, which in contrast to the inductive effects of the feminine GH profile (17, 18) normally suppresses CYP3A4 expression (18) but is significantly more repressive in men both in vivo (15) and in primary hepatocyte cultures (18). Clearly, the sexually dimorphic secretory GH profiles can invariably explain the sex differences in constitutive levels of CYP3A4 as well as other isoforms in humans (cf. Ref. 18) and also in several other nonhuman mammalian CYPs (6). Curiously, however, the existence of intrinsic sexually dimorphic responses to the sex-dependent GH profiles seems an unwarranted redundancy, because it is highly unlikely that men would be exposed to the feminine GH circulating profile nor would women be exposed to the masculine profile. Nevertheless, intrinsic sexually dimorphic responses, both in vivo and in vitro, of CYPs to GH regulation have been found in other species, including a half-dozen constituent CYPs (20, 21, 32, 33, 44) and several inducible isoforms (45) in rats. Moreover, several inherent GH-dependent sexually dimorphic responses to non-CYP functions in humans have also been reported (22–26). Although any possible justification(s) for these intrinsic sexual dimorphisms is speculative, the present study has identified possible mechanisms responsible for their expression.
The human CYP3A4 gene is regulated by a notably promiscuous promoter capable of transactivation, directly or indirectly, by more than a dozen nuclear receptors/transcription factors. Various ligands (e.g. xenobiotics, environmental chemicals, hormones, and pathophysiologic damage) induce CYP3A4 expression, to different degrees, by activating different transcriptional networks, each containing novel as well as some overlapping components capable of transactivating a different assemblage of binding elements on the CYP3A4 promoter (35, 36, 46, 47). Because CYP3A4, the principal CYP isoform (1, 2), represents a major detoxification and metabolic pathway, it has been suggested that the functional redundancy and synergistic regulatory networks transactivate different promoter modules to always ensure some level of induction of CYP3A4 to meet various needs (47). Significantly, all of the identified transcriptional pathways appear to contain the obligatory nuclear receptor PXR, functioning as its heterodimer PXR·RXRα and invariably HNF-4α (35, 36, 46, 47), whose roles we have examined in the present study.
PXR and HNF-4α are themselves rather promiscuous transcription factors. It has been suggested (48, 49) that PXR has evolved to protect the body from toxic chemicals, because it can be activated by a structurally diverse collection of drugs, environmental chemicals, various endogenous lipophilic compounds (e.g. steroids, bile acids, and eicosanoids), as well as collaborative cell signaling pathways. HNF-4α, a zinc finger protein, is the most abundant transcription factor in the liver, having been reported to bind to the promoters of more than 1000 genes involved in most aspects of hepatocyte function (50, 51). It would appear that in addition to activating its own specific binding sites on the CYP3A4 promoter, HNF-4α induces CYP3A4 transcription by increasing PXR expression (52) and the subsequent transactivation of PXR·RXRα binding elements on the promoter (53–55). Accordingly, we have observed that continuous GH alone stimulates nuclear translocation of HNF-4α and significantly elevates binding levels of the transcription factor to its motifs on the CLEM module of the CYP3A4 promoter. Although dexamethasone can produce the same effect on HNF-4α, the magnitude of the effect is considerably less. Dexamethasone, however, is a known inducer of PXR expression (49, 52) and, as we have observed, stimulates nuclear translocation of PXR and the transactivation of the PXR·RXRα-specific xenobiotic-responsive enhancer module and, likely, the ER6 element in the CYP3A4 promoter. Continuous GH has similar actions on PXR but to a much lesser extent than dexamethasone. Understandably then, the combined treatment of continuous GH and dexamethasone, each acting through a different transcriptional network [see schematic representation in Fig. 8A, as proposed earlier (35, 36, 46, 48)] is a more effective inducer of CYP3A4 than the sum of the individual hormone treatments. As predicted earlier (49), we found that deletion of the HNF-4α binding sites on the CYP3A4 promoter permits only minimal activation, limited to PXR·RXRα binding to its ER6 element (see schematic representation, Fig. 8B), demonstrating the importance of the continuous secretory GH profile (via its actions on HNF-4α) in maintaining the elevated expression levels of CYP3A4 in women.
The present findings indicate that the feminine-like plasma GH profile along with glucocorticoids is responsible for the elevated sex-dependent expression levels of CYP3A4 by up-regulating PXR and HNF-4α transactivation of the CYP3A4 promoter. However, the same study has also shown that hepatocytes derived from men respond with strikingly lower induction levels of CYP3A4 than hepatocytes from women exposed to the same hormone regimens. This intrinsic sexual dimorphism can be explained, at least in part, by the suboptimal effectiveness of the hormone treatments to recruit PXR and HNF-4α as well as limiting both their nuclear translocation and subsequent transactivation of the CYP3A4 promoter in male hepatocytes. This observation is in agreement with our previous studies examining the molecular mechanisms responsible for the inherent sexually dimorphic responses of rat CYPs to sex-dependent GH profiles (32, 33, 44). In this regard, expression of CYP2C12, the primary female rat CYP, is dependent upon continuous GH activation of a complex network of signal transducers and transcription factors, including HNF-4α transactivation of the isoform's promoter (33, 56). Activation of this complex induction network and succeeding CYP2C12 expression is dramatically reduced in males, both in vivo (20) and in hepatocytes exposed to the feminine GH profile (33).
If activation of a particular CYP is solely due to the GH profile to which it is exposed, then irrespective of sex, the same sex-dependent GH profile should induce the same expressional levels of the isoform in both males and females. Clearly, this is not the case. Regarding CYP3A4, there are several structural polymorphisms in the CYP3A4 gene, including single nucleotide polymorphisms, that contribute to the variability of CYP3A4 expression in men and women (35, 57, 58). However, these polymorphisms are not necessarily sex linked and at best contribute to no more than 50% of the differences in CYP3A4 expression (57, 58) and thus are not likely to be the sole reason for the intrinsic sex differences in CYP expression. In contrast, perinatal hormones (e.g. testosterone and dihydrotestosterone) have been shown to imprint intrinsic, and thus irreversible, sex-dependent responses in adult neuroendocrine functions, e.g. gonadotropins secrectory profiles, sexual behavior, and food preferences (59). Accordingly, we propose that the intrinsic sexually dimorphic responses of CYP isoforms to sex-dependent GH profiles in humans as well as rats may result, at least in part, from permanent imprinting effects of perinatal hormones. Whether these intrinsic effects produce a selective advantage or are simply neutral phenotypes that may or may not be linked to beneficial sexual dimorphisms is unknown.
Supplementary Material
Acknowledgments
We thank Dr. F. Peter Guengerich (Vanderbilt University School of Medicine, Nashville, TN) for the generous gift of antibodies against the human isoform CYP3A4; Dr. Stephen C. Strom (University of Pittsburgh School of Medicine, Pittsburgh, PA) for supplying the human hepatocytes through the Liver Tissue Procurement Distribution system funded by National Institutes of Health Contract N01-DK-9-2310; and Dr. Garold S. Yost (University of Utah, Salt Lake City, UT) for providing the CYP3A4 13kb and 3kb luciferase-reporter constructs.
This work was supported by the National Institutes of Health Grant HD-061285 and the Chutzpah Foundation.
Disclosure Summary: The authors have nothing to disclose.
Footnotes
- ChIP
- Chromatin immunoprecipitation
- CLEM
- constitutive liver enhancer module
- CYP
- cytochrome P450
- ER6
- everted repeat 6
- hGH
- human GH
- HNF-4α
- hepatocyte nuclear factor-4α
- PXR
- pregnane X receptor
- RXR
- retinoid X receptor
- Scr
- scrambled
- siRNA
- small inhibitory RNA.
References
- 1. Shimada T, Yamazaki H, Mimura M, Inui Y, Guengerich FP. 1994. Interindividual variations in human liver cytochrome P450 enzymes involved in oxidation of drugs, carcinogens and toxic chemicals: studies with liver microsomes of 30 Japanese and 30 Caucasians. J Pharmacol Exp Ther 270:414–423 [PubMed] [Google Scholar]
- 2. Rendic S. 2002. Summary of information on human CYP enzymes: human P450 metabolism data. Drug Metab Rev 34:83–448 [DOI] [PubMed] [Google Scholar]
- 3. Li AP, Kaminski DL, Rasmussen A. 1995. Substrates of human hepatic cytochrome P450 3A4. Toxicology 104:1–8 [DOI] [PubMed] [Google Scholar]
- 4. Gleiter CH, Gundert-Remy U. 1996. Gender differences in pharmacokinetics. Eur J Drug Metab Pharmacokinet 21:123–128 [DOI] [PubMed] [Google Scholar]
- 5. Wolbold R, Klein K, Burk O, Nüssler AK, Neuhaus P, Eichelbaum M, Schwab M, Zanger UM. 2003. Sex is a major determinant of CYP3A4 expression in human liver. Hepatology 38:978–988 [DOI] [PubMed] [Google Scholar]
- 6. Shapiro BH, Agrawal AK, Pampori NA. 1995. Gender differences in drug metabolism regulated by growth hormone. Int J Biochem Cell Biol 27:9–20 [DOI] [PubMed] [Google Scholar]
- 7. Legraverend C, Mode A, Wells T, Robinson I, Gustafsson JA. 1992. Hepatic steroid hydroxylating enzymes are controlled by the sexually dimorphic pattern of growth hormone secretions in normal and dwarf rats. FASEB J 6:711–718 [DOI] [PubMed] [Google Scholar]
- 8. Hartman ML, Iranmanesh A, Thorner MO, Veldhuis JD. 1993. Evaluation of pulsatile patterns of growth hormone release in humans: a brief review. Am J Human Biol 5:603–614 [DOI] [PubMed] [Google Scholar]
- 9. van den Berg G, Veldhuis JD, Frölich M, Roelfsema F. 1996. An amplitude-specific divergence in the pulsatile mode of growth hormone (GH) secretion underlies the gender difference in mean GH concentrations in men and premenopausal women. J Clin Endocrinol Metab 81:2460–2467 [DOI] [PubMed] [Google Scholar]
- 10. Engström BE, Karlsson FA, Wide L. 1998. Marked gender differences in ambulatory morning growth hormone values in young adults. Clin Chem 44:1289–1295 [PubMed] [Google Scholar]
- 11. Jansson JO, Edén S, Isaksson O. 1985. Sexual dimorphism in the control of growth hormone secretion. Endocr Rev 6:128–150 [DOI] [PubMed] [Google Scholar]
- 12. Pampori NA, Shapiro BH. 1996. Feminization of hepatic cytochrome P450s by nominal levels of growth hormone in the feminine plasma profile. Mol Pharmacol 50:1148–1156 [PubMed] [Google Scholar]
- 13. Agrawal AK, Shapiro BH. 2000. Differential expression of gender-dependent hepatic isoforms of cytochrome P-450 by pulse signals in the circulating masculine episodic growth hormone profile of the rat. J Pharmacol Exp Ther 292:228–237 [PubMed] [Google Scholar]
- 14. Cheung NW, Liddle C, Coverdale S, Lou JC, Boyages SC. 1996. Growth hormone treatment increases cytochrome P450-mediated antipyrine clearance in man. J Clin Endocrinol Metab 81:1999–2001 [DOI] [PubMed] [Google Scholar]
- 15. Sinués B, Mayayo E, Fanlo A, Mayayo E, Jr, Bernal ML, Bocos P, Bello E, Labarta JI, Ferrández-Longás A. 2004. Effects of growth hormone deficiency and rhGH replacement therapy on the 6β-hydroxycortisol/free cortisol ratio, a marker of CYP3A activity, in growth hormone-deficient children. Eur J Clin Pharmacol 60:559–564 [DOI] [PubMed] [Google Scholar]
- 16. Jaffe CA, Turgeon DK, Lown K, Demott-Friberg R, Watkins APB. 2002. Growth hormone secretion pattern is an independent regulator of growth hormone actions in humans. Am J Physiol 283:E1008–E1015 [DOI] [PubMed] [Google Scholar]
- 17. Liddle C, Goodwin BJ, George J, Tapner M, Farrell GC. 1998. Separate and interactive regulation of cytochrome P450 3A4 by triiodothyronine, dexamethasone and growth hormone in cultured hepatocytes. J Clin Endocrinol Metab 83:2411–2416 [DOI] [PubMed] [Google Scholar]
- 18. Dhir RN, Dworakowski W, Thangavel C, Shapiro BH. 2006. Sexually dimorphic regulation of hepatic isoforms of human cytochrome P450 by growth hormone. J Pharmacol Exp Ther 316:87–94 [DOI] [PubMed] [Google Scholar]
- 19. Gonzalez FJ, Song BJ, Hardwick JP. 1986. Pregnenolone 16α-carbonitrile-inducible P450 gene family: gene conversion and differential regulation. Mol Cell Biol 6:2969–2976 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Pampori NA, Shapiro BH. 1999. Gender differences in the responsiveness of the sex-dependent isoforms of hepatic P450 to the feminine plasma growth hormones profile. Endocrinology 140:1245–1254 [DOI] [PubMed] [Google Scholar]
- 21. Thangavel C, Garcia MC, Shapiro BH. 2004. Intrinsic sex differences determine expression of growth hormone-regulated female cytochrome P450s. Mol Cell Endocrinol 220:31–39 [DOI] [PubMed] [Google Scholar]
- 22. Kuromaru R, Kohno H, Ueyama N, Hassan HM, Honda S, Hara T. 1998. Long-term prospective study of body composition and lipid profiles during and after growth hormone (GH) treatment in children with GH deficiency: gender-specific metabolic effects. J Clin Endocrinol Metab 83:3890–3896 [DOI] [PubMed] [Google Scholar]
- 23. Span JP, Pieters GF, Sweep FG, Hermus AR, Smals AG. 2001. Gender differences in rhGH-induced changes in body composition in GH-deficient adults. J Clin Endocrinol Metab 86:4161–4165 [DOI] [PubMed] [Google Scholar]
- 24. Burman P, Johansson AG, Siegbahn A, Vessby B, Karlsson FA. 1997. Growth hormone (GH)-deficient men are more responsive to GH replacement therapy than women. J Clin Endocrinol Metab 82:550–555 [DOI] [PubMed] [Google Scholar]
- 25. Soares DV, Conceição FL, Brasil RR, Spina LD, Lobo PM, Silva EM, Buescu A, Vaisman M. 2004. Insulin-like growth factor I levels during growth hormone (GH) replacement in GH-deficient adults: a gender difference. Growth Horm IGF Res 14:436–441 [DOI] [PubMed] [Google Scholar]
- 26. Johansson AG, Engström BE, Ljunghall S, Karlsson FA, Burman P. 1999. Gender differences in the effects of long term growth hormone (GH) treatment on bone in adults with GH deficiency. J Clin Endocrinol Metab 84:2002–2007 [DOI] [PubMed] [Google Scholar]
- 27. Strom SC, Pisarov LA, Dorko K, Thompson MT, Schuetz JD, Schuetz EG. 1996. Use of human hepatocytes to study P450 gene induction. Methods Enzymol 272:388–401 [DOI] [PubMed] [Google Scholar]
- 28. HaynesJr RC, Jr, Murad F. 1985. Adrenocorticotropic hormone; adrenocortical steroids and their synthetic analogs; inhibitors of adrenocortical steroid biosynthesis. In: Gilman AG, Goodman LS, Rall TW, Murad F. eds. Goodman and Gilman's the pharmacological basis of therapeutics. 7th ed New York: Macmillan; 1459–1489 [Google Scholar]
- 29. Murad F, Haynes RC., Jr 1985. Adenohypophyseal hormones and related substances. In: Gilman AG, Goodman LS, Rall TW, Murad F. eds. Goodman and Gilman's the pharmacological basis of therapeutics. 7th ed New York: Macmillan; 1362–1388 [Google Scholar]
- 30. Garcia MC, Thangavel C, Shapiro BH. 2001. Epidermal growth factor regulation of female-dependent CYP2A1 and CYP2C12 in primary rat hepatocyte culture. Drug Metab Dispos 29:111–120 [PubMed] [Google Scholar]
- 31. Dignam JD, Lebovitz RM, Roeder RG. 1983. Accurate transcription initiation by polymerase II in a soluble extract from isolated mammalian nuclei. Nucleic Acids Res 11:1475–1489 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Thangavel C, Shapiro BH. 2007. A molecular basis for the sexually dimorphic response to growth hormone. Endocrinology 148:2894–2903 [DOI] [PubMed] [Google Scholar]
- 33. Thangavel C, Shapiro BH. 2008. Inherent sexually dimorphic expression of hepatic CYP2C12 correlated with repressed activation of growth hormone-regulated signal transduction in male rats. Drug Metab Dispos 36:1884–1895 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Hashimoto H, Toide K, Kitamura R, Fujita M, Tagawa S, Itoh S, Kamataki T. 1993. Gene structure of CYP3A4, an adult-specific form of cytochrome P450 in human livers, and its transcriptional control. Eur J Biochem 218:585–595 [DOI] [PubMed] [Google Scholar]
- 35. Matsumura K, Saito T, Takahashi Y, Ozeki T, Kiyotani K, Fujieda M, Yamazaki H, Kunitoh H, Kamataki T. 2004. Identification of a novel polymorphic enhancer of the human CYP3A4 gene. Mol Pharmcol 65:326–334 [DOI] [PubMed] [Google Scholar]
- 36. Biggs JS, Wan J, Cutler NS, Hakkola J, Uusimäki P, Raunio H, Yost GS. 2007. Transcription factor binding to a putative double E-box motif represses CYP3A4 expression in human lung cells. Mol Pharmacol 72:514–525 [DOI] [PubMed] [Google Scholar]
- 37. Zhou Z, Kang X, Jiang Y, Song Z, Feng W, McClain CJ, Kang YJ. 2007. Preservation of hepatocyte nuclear factor-4α is associated with zinc protection against TNF-α hepatotoxicity in mice. Exp Biol Med 232:622–628 [PubMed] [Google Scholar]
- 38. Selva DM, Hammond GL. 2009. Thyroid hormones act indirectly to increase sex hormone-binding globulin production by liver via hepatocyte nuclear factor-4α. J Mol Endocrinol 43:19–27 [DOI] [PubMed] [Google Scholar]
- 39. Boopathi E, Lenka N, Prabu SK, Fang JK, Wilkinson F, Atchison M, Giallongo A, Avadhani NG. 2004. Regulation of murine cytochrome c oxidase Vb gene expression during myogenesis: YY-1 and heterogeneous nuclear ribonucleoprotein D-like protein (JKTBP1) reciprocally regulate transcription activity by physical interaction with the BERF-1/ZBP-89 factor. J Biol Chem 279:35242–35254 [DOI] [PubMed] [Google Scholar]
- 40. MacLeod JN, Sorensen MP, Shapiro BH. 1987. Strain independent elevation of hepatic mono-oxygenase enzymes in female mice. Xenobiotica 17:1095–1102 [DOI] [PubMed] [Google Scholar]
- 41. Wrighton SA, VandenBranden M, Ring BJ. 1996. The human drug metabolizing cytochromes P450. J Pharmacokinet Biopharm 24:461–473 [DOI] [PubMed] [Google Scholar]
- 42. Iyer KR, Sinz MW. 1999. Characterization of phase I and phase II hepatic drug metabolism activities in a panel of human liver preparations. Chem Biol Interact 118:151–169 [DOI] [PubMed] [Google Scholar]
- 43. Kando JC, Yonkers KA, Cole JO. 1995. Gender as a risk factor for adverse events to medications. Drugs 50:1–6 [DOI] [PubMed] [Google Scholar]
- 44. Dhir RN, Thangavel C, Shapiro BH. 2007. Attenuated expression of episodic growth hormone-induced CYP2C11 in female rats associated with suboptimal activation of the Jak/Stat5B and other modulating signaling pathways. Drug Metab Dispos 35:2102–2110 [DOI] [PubMed] [Google Scholar]
- 45. Agrawal AK, Shapiro BH. 1996. Phenobarbital induction of hepatic CYP2B1 and CYP2B2: pretranscriptional and post-translational effects of gender, adult age, and phenobarbital dose. Mol Pharmacol 49:523–531 [PubMed] [Google Scholar]
- 46. Martínez-Jiménez CP, Jover R, Donato MT, Castell JV, Gómez-Lechón MJ. 2007. Transcriptional regulation and expression of CYP3A4 in hepatocytes. Curr Drug Metab 8:185–194 [DOI] [PubMed] [Google Scholar]
- 47. Liu FJ, Song X, Yang D, Deng R, Yan B. 2008. The far and distal enhancers in the CYP3A4 gene co-ordinate the proximal promoter in responding similarly to the pregnane X receptor but differentially to hepatocyte nuclear factor-4α. Biochem J 409:243–250 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Lin W, Wu J, Dong H, Bouck D, Zeng FY, Chen T. 2008. Cyclin-dependent kinase 2 negatively regulates human pregnane X receptor-mediated CYP3A4 gene expression in HepG2 liver carcinoma cells. J Biol Chem 283:30650–30657 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Goodwin B, Redinbo MR, Kliewer SA. 2002. Regulation of CYP3A gene transcription by the pregnane X receptor. Annu Rev Pharmacol Toxicol 42:1–23 [DOI] [PubMed] [Google Scholar]
- 50. Schrem H, Klempnauer J, Borlak J. 2002. Liver-enriched transcription factors in liver function and development. Part I: the hepatocyte nuclear factor network and liver-specific gene expression. Pharmacol Rev 54:129–158 [DOI] [PubMed] [Google Scholar]
- 51. Odom DT, Zizlsperger N, Gordon DB, Bell GW, Rinaldi NJ, Murray HL, Volkert TL, Schreiber J, Rolfe PA, Gifford DK, Fraenkel E, Bell GI, Young RA. 2004. Control of pancreas and liver gene expression by HNF transcription factors. Science 303:1378–1381 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Pascussi JM, Drocourt L, Fabre JM, Maurel P, Vilarem MJ. 2000. Dexamethasone induces pregnane X receptor and retinoid X receptor-α expression in human hepatocytes: synergistic increase of CYP3A4 induction by pregnane X receptor activators. Mol Pharmacol 58:361–372 [DOI] [PubMed] [Google Scholar]
- 53. Iwahori T, Matsuura T, Maehashi H, Sugo K, Saito M, Hosokawa M, Chiba K, Masaki T, Aizaki H, Ohkawa K, Suzuki T. 2003. CYP3A4 inducible model for in vitro analysis of human drug metabolism using a bioartificial liver. Hepatology 37:665–673 [DOI] [PubMed] [Google Scholar]
- 54. Tirona RG, Lee W, Leake BF, Lan LB, Cline CB, Lamba V, Parviz F, Duncan SA, Inoue Y, Gonzalez FJ, Schuetz EG, Kim RB. 2003. The orphan nuclear receptor HNF4α determines PXR- and CAR-mediated xenobiotic induction of CYP3A4. Nat Med 9:220–224 [DOI] [PubMed] [Google Scholar]
- 55. Kamiyama Y, Matsubara T, Yoshinari K, Nagata K, Kamimura H, Yamazoe Y. 2007. Role of human hepatocyte nuclear factor 4α in the expression of drug-metabolizing enzymes and transporters in human hepatocytes assessed by use of small interfering RNA. Drug Metab Pharmacokinet 22:287–298 [DOI] [PubMed] [Google Scholar]
- 56. Waxman DJ, Holloway MG. 2009. Sex differences in the expression of hepatic drug metabolizing enzymes. Mol Pharmacol 76:215–228 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Westlind A, Löfberg L, Tindberg N, Andersson TB, Ingelman-Sundberg M. 1999. Interindividual differences in hepatic expression of CYP3A4: relationship to genetic polymorphism in the 5′ –upstream regulatory region. Biochem Biophys Res Commun 259:201–205 [DOI] [PubMed] [Google Scholar]
- 58. Schirmer M, Rosenberger A, Klein K, Kulle B, Toliat MR, Nürnberg P, Zanger UM, Wojnowski L. 2007. Sex-dependent genetic markers of CYP3A4 expression and activity in human liver microsomes. Pharmacogenomics 8:443–453 [DOI] [PubMed] [Google Scholar]
- 59. Feder HH. 1981. Perinatal hormones and their roles in the development of sexually dimorphic behaviors. In: Adler N. ed. Neuroendocrinology of reproduction, physiology and behavior. New York: Plenum Press; 127–158 [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.