Abstract
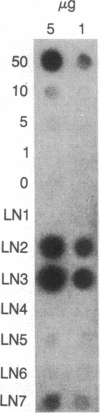
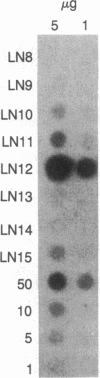
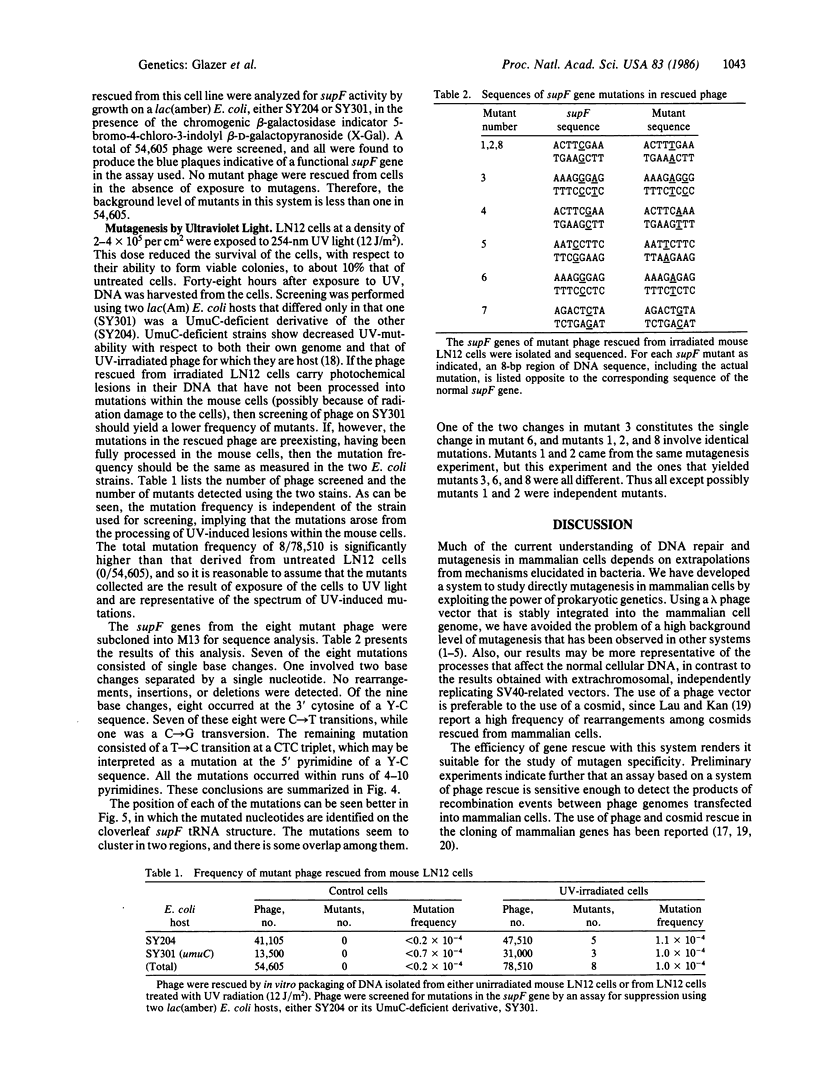
In order to study mutagenesis in mammalian cells, stable mouse L-cell lines were established with multiple copies of a lambda phage vector that contains the supF gene of Escherichia coli as a target for mutagenesis. Rescue of viable phage from high molecular weight mouse cell DNA using lambda in vitro packaging extracts was efficient (5 phage per microgram of cell DNA per copy) and yielded a negligible background of mutant phage (0 out of 54,605). From mouse cells exposed to 254-nm ultraviolet light (12J/m2), 78,510 phage were rescued, of which 8 were found to have mutant supF genes. DNA sequence analysis of the mutants suggests that the primary site of UV mutagenesis in mammalian cells is at pyrimidine-cytosine (Y-C) sequences, and that the most frequent mutation at this site is a C----T transition.
Full text
PDF
Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Biggin M. D., Gibson T. J., Hong G. F. Buffer gradient gels and 35S label as an aid to rapid DNA sequence determination. Proc Natl Acad Sci U S A. 1983 Jul;80(13):3963–3965. doi: 10.1073/pnas.80.13.3963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brash D. E., Haseltine W. A. UV-induced mutation hotspots occur at DNA damage hotspots. Nature. 1982 Jul 8;298(5870):189–192. doi: 10.1038/298189a0. [DOI] [PubMed] [Google Scholar]
- Brown E. L., Belagaje R., Ryan M. J., Khorana H. G. Chemical synthesis and cloning of a tyrosine tRNA gene. Methods Enzymol. 1979;68:109–151. doi: 10.1016/0076-6879(79)68010-2. [DOI] [PubMed] [Google Scholar]
- Calos M. P., Lebkowski J. S., Botchan M. R. High mutation frequency in DNA transfected into mammalian cells. Proc Natl Acad Sci U S A. 1983 May;80(10):3015–3019. doi: 10.1073/pnas.80.10.3015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Celis J. E., Piper P. W. Compilation of mutant suppressor tRNA sequences. Nucleic Acids Res. 1982 Jan 22;10(2):r83–r91. doi: 10.1093/nar/10.2.762-b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elledge S. J., Walker G. C. Proteins required for ultraviolet light and chemical mutagenesis. Identification of the products of the umuC locus of Escherichia coli. J Mol Biol. 1983 Feb 25;164(2):175–192. doi: 10.1016/0022-2836(83)90074-8. [DOI] [PubMed] [Google Scholar]
- Graham F. L., van der Eb A. J. A new technique for the assay of infectivity of human adenovirus 5 DNA. Virology. 1973 Apr;52(2):456–467. doi: 10.1016/0042-6822(73)90341-3. [DOI] [PubMed] [Google Scholar]
- Hohn B. In vitro packaging of lambda and cosmid DNA. Methods Enzymol. 1979;68:299–309. doi: 10.1016/0076-6879(79)68021-7. [DOI] [PubMed] [Google Scholar]
- KIT S., DUBBS D. R., PIEKARSKI L. J., HSU T. C. DELETION OF THYMIDINE KINASE ACTIVITY FROM L CELLS RESISTANT TO BROMODEOXYURIDINE. Exp Cell Res. 1963 Aug;31:297–312. doi: 10.1016/0014-4827(63)90007-7. [DOI] [PubMed] [Google Scholar]
- Kato T., Shinoura Y. Isolation and characterization of mutants of Escherichia coli deficient in induction of mutations by ultraviolet light. Mol Gen Genet. 1977 Nov 14;156(2):121–131. doi: 10.1007/BF00283484. [DOI] [PubMed] [Google Scholar]
- Lau Y. F., Kan Y. W. Direct isolation of the functional human thymidine kinase gene with a cosmid shuttle vector. Proc Natl Acad Sci U S A. 1984 Jan;81(2):414–418. doi: 10.1073/pnas.81.2.414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Law M. F., Byrne J. C., Howley P. M. A stable bovine papillomavirus hybrid plasmid that expresses a dominant selective trait. Mol Cell Biol. 1983 Nov;3(11):2110–2115. doi: 10.1128/mcb.3.11.2110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LeClerc J. E., Istock N. L. Specificity of UV mutagenesis in the lac promoter of M13lac hybrid phage DNA. Nature. 1982 Jun 17;297(5867):596–598. doi: 10.1038/297596a0. [DOI] [PubMed] [Google Scholar]
- Lebkowski J. S., DuBridge R. B., Antell E. A., Greisen K. S., Calos M. P. Transfected DNA is mutated in monkey, mouse, and human cells. Mol Cell Biol. 1984 Oct;4(10):1951–1960. doi: 10.1128/mcb.4.10.1951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindenmaier W., Hauser H., de Wilke I. G., Schütz G. Gene shuttling: moving of cloned DNA into and out of eukaryotic cells. Nucleic Acids Res. 1982 Feb 25;10(4):1243–1256. doi: 10.1093/nar/10.4.1243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liskay R. M., Stachelek J. L. Evidence for intrachromosomal gene conversion in cultured mouse cells. Cell. 1983 Nov;35(1):157–165. doi: 10.1016/0092-8674(83)90218-0. [DOI] [PubMed] [Google Scholar]
- Miller J. H. Mutational specificity in bacteria. Annu Rev Genet. 1983;17:215–238. doi: 10.1146/annurev.ge.17.120183.001243. [DOI] [PubMed] [Google Scholar]
- Panasenko S. M., Cameron J. R., Davis R. W., Lehman I. R. Five hundredfold overproduction of DNA ligase after induction of a hybrid lambda lysogen constructed in vitro. Science. 1977 Apr 8;196(4286):188–189. doi: 10.1126/science.322281. [DOI] [PubMed] [Google Scholar]
- Razzaque A., Chakrabarti S., Joffee S., Seidman M. Mutagenesis of a shuttle vector plasmid in mammalian cells. Mol Cell Biol. 1984 Mar;4(3):435–441. doi: 10.1128/mcb.4.3.435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Razzaque A., Mizusawa H., Seidman M. M. Rearrangement and mutagenesis of a shuttle vector plasmid after passage in mammalian cells. Proc Natl Acad Sci U S A. 1983 May;80(10):3010–3014. doi: 10.1073/pnas.80.10.3010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanger F., Nicklen S., Coulson A. R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sarasin A., Bourre F., Benoit A., Daya-Grosjean L., Gentil A. Molecular analysis of mutagenesis in mammalian cells. Int J Radiat Biol Relat Stud Phys Chem Med. 1985 May;47(5):479–488. doi: 10.1080/09553008514550711. [DOI] [PubMed] [Google Scholar]
- Sarkar S., Dasgupta U. B., Summers W. C. Error-prone mutagenesis detected in mammalian cells by a shuttle vector containing the supF gene of Escherichia coli. Mol Cell Biol. 1984 Oct;4(10):2227–2230. doi: 10.1128/mcb.4.10.2227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Southern E. M. Detection of specific sequences among DNA fragments separated by gel electrophoresis. J Mol Biol. 1975 Nov 5;98(3):503–517. doi: 10.1016/s0022-2836(75)80083-0. [DOI] [PubMed] [Google Scholar]
- Wood R. D., Skopek T. R., Hutchinson F. Changes in DNA base sequence induced by targeted mutagenesis of lambda phage by ultraviolet light. J Mol Biol. 1984 Mar 5;173(3):273–291. doi: 10.1016/0022-2836(84)90121-9. [DOI] [PubMed] [Google Scholar]