Abstract
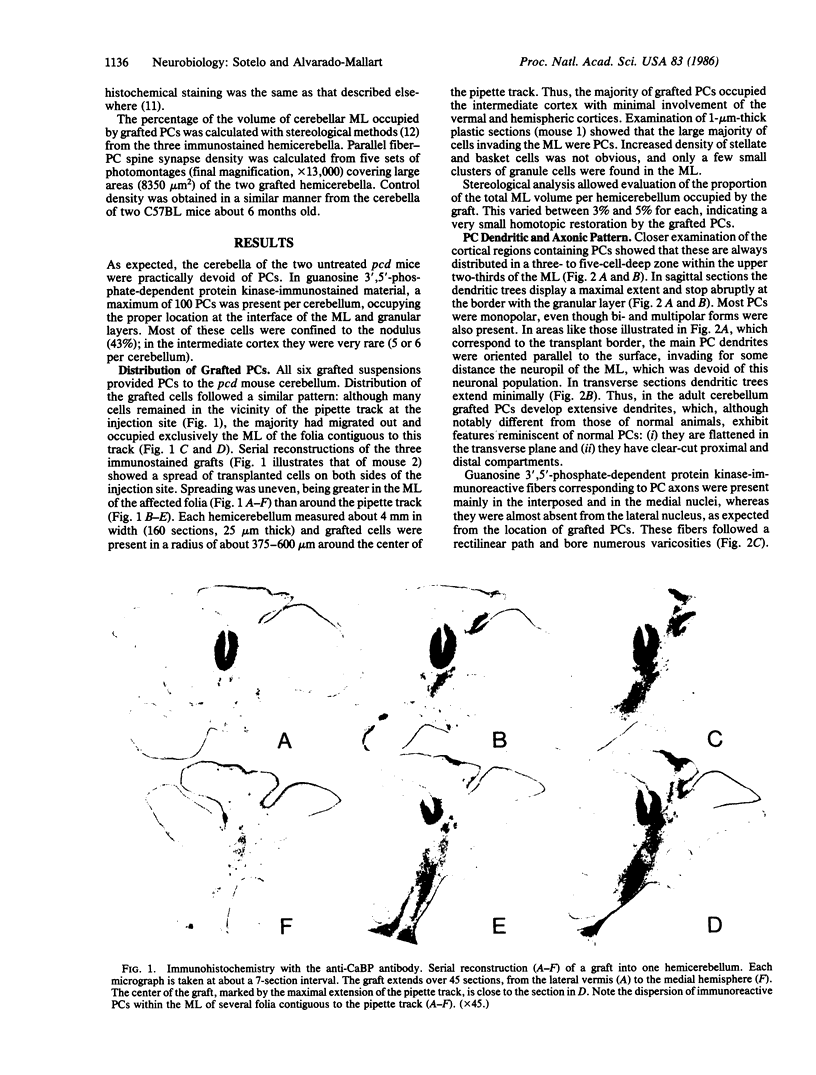
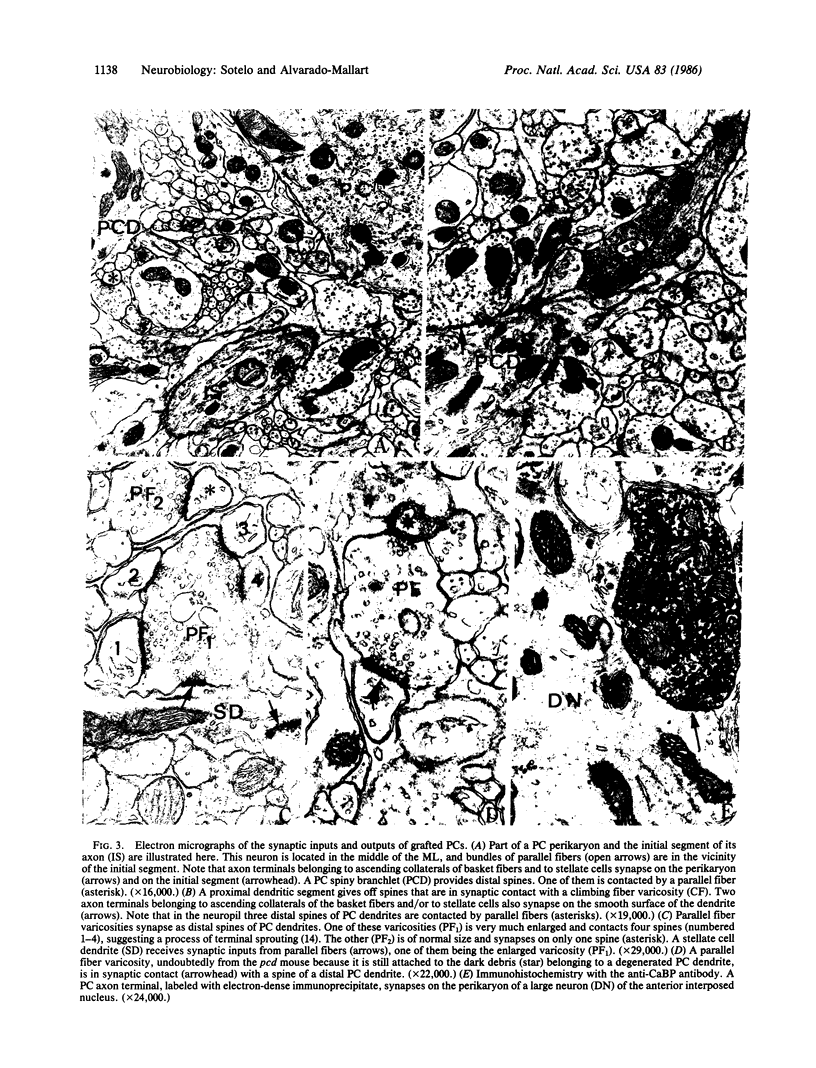
Cell suspensions from cerebellar primordia of 12-day mouse embryos were grafted into the cerebellum of 4-month-old Purkinje cell degeneration (pcd) mutant mice and examined 2-3 months later. In contrast to those of nontreated mutants, all of the grafted cerebella exhibited Purkinje cells that had migrated into the molecular layer, where they were clustered over its superficial two-thirds. These Purkinje cells develop flattened dendritic trees perpendicular to bundles of parallel fibers. Ultrastructural examination of their synaptic inputs and outputs disclosed that (i) as in normal cerebella, climbing fibers and axons from basket and stellate cells synapse on thick dendrites, whereas parallel fibers almost exclusively contact the distal spiny branchlets, and (ii) a substantial number of Purkinje cell axons reach their appropriate targets in the deep cerebellar nuclei, where they establish synaptic connections on large and small neurons. These results indicate that embryonic Purkinje cells grafted into the cerebellum of adult mice with heredodegenerative ataxia integrate themselves very specifically into the cerebellar circuitry of the recipient mouse, where they can replace the missing Purkinje cells. They also provide a morphological basis favoring the notion of functional restorative capabilities of neural grafts in systems in which neurons are connected in an almost point-to-point manner.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Björklund A., Gage F. H., Schmidt R. H., Stenevi U., Dunnett S. B. Intracerebral grafting of neuronal cell suspensions. VII. Recovery of choline acetyltransferase activity and acetylcholine synthesis in the denervated hippocampus reinnervated by septal suspension implants. Acta Physiol Scand Suppl. 1983;522:59–66. [PubMed] [Google Scholar]
- Björklund A., Stenevi U., Dunnett S. B., Iversen S. D. Functional reactivation of the deafferented neostriatum by nigral transplants. Nature. 1981 Feb 5;289(5797):497–499. doi: 10.1038/289497a0. [DOI] [PubMed] [Google Scholar]
- Björklund A., Stenevi U., Svendgaard N. Growth of transplanted monoaminergic neurones into the adult hippocampus along the perforant path. Nature. 1976 Aug 26;262(5571):787–790. doi: 10.1038/262787a0. [DOI] [PubMed] [Google Scholar]
- Low W. C., Lewis P. R., Bunch S. T., Dunnett S. B., Thomas S. R., Iversen S. D., Björklund A., Stenevi U. Function recovery following neural transplantation of embryonic septal nuclei in adult rats with septohippocampal lesions. Nature. 1982 Nov 18;300(5889):260–262. doi: 10.1038/300260a0. [DOI] [PubMed] [Google Scholar]
- Mariani J., Crepel F., Mikoshiba K., Changeux J. P., Sotelo C. Anatomical, physiological and biochemical studies of the cerebellum from Reeler mutant mouse. Philos Trans R Soc Lond B Biol Sci. 1977 Nov 2;281(978):1–28. doi: 10.1098/rstb.1977.0121. [DOI] [PubMed] [Google Scholar]
- Mobley P., Greengard P. Evidence for widespread effects of noradrenaline on axon terminals in the rat frontal cortex. Proc Natl Acad Sci U S A. 1985 Feb;82(3):945–947. doi: 10.1073/pnas.82.3.945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mullen R. J., Eicher E. M., Sidman R. L. Purkinje cell degeneration, a new neurological mutation in the mouse. Proc Natl Acad Sci U S A. 1976 Jan;73(1):208–212. doi: 10.1073/pnas.73.1.208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mullen R. J. Site of pcd gene action and Purkinje cell mosaicism in cerebella of chimaeric mice. Nature. 1977 Nov 17;270(5634):245–247. doi: 10.1038/270245a0. [DOI] [PubMed] [Google Scholar]
- Prochiantz A., di Porzio U., Kato A., Berger B., Glowinski J. In vitro maturation of mesencephalic dopaminergic neurons from mouse embryos is enhanced in presence of their striatal target cells. Proc Natl Acad Sci U S A. 1979 Oct;76(10):5387–5391. doi: 10.1073/pnas.76.10.5387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sotelo C. Purkinje cell ontogeny: formation and maintenance of spines. Prog Brain Res. 1978;48:149–170. doi: 10.1016/S0079-6123(08)61021-3. [DOI] [PubMed] [Google Scholar]
- Sotelo C., Triller A. Fate of presynaptic afferents to Purkinje cells in the adult nervous mutant mouse: a model to study presynaptic stabilization. Brain Res. 1979 Oct 12;175(1):11–36. doi: 10.1016/0006-8993(79)90511-0. [DOI] [PubMed] [Google Scholar]
- Sternberger L. A., Hardy P. H., Jr, Cuculis J. J., Meyer H. G. The unlabeled antibody enzyme method of immunohistochemistry: preparation and properties of soluble antigen-antibody complex (horseradish peroxidase-antihorseradish peroxidase) and its use in identification of spirochetes. J Histochem Cytochem. 1970 May;18(5):315–333. doi: 10.1177/18.5.315. [DOI] [PubMed] [Google Scholar]
- Wassef M., Zanetta J. P., Brehier A., Sotelo C. Transient biochemical compartmentalization of Purkinje cells during early cerebellar development. Dev Biol. 1985 Sep;111(1):129–137. doi: 10.1016/0012-1606(85)90441-5. [DOI] [PubMed] [Google Scholar]





