Abstract
Increases in extracellular proton concentrations, which takes place in physiological conditions such as synaptic signaling and pathological conditions such as tissue inflammation, ischemic stroke, traumatic brain injury, and epileptic seizure, activates a unique family of membrane ion channels; the acid-sensing ion channels (ASICs). All ASICs belong to amiloride-sensitive degenerin/epithelial Na+ channel superfamily. Four genes encoded at seven sub-units have been identified. ASICs are expressed primarily in neurons and have been shown to play critical roles in synaptic plasticity, learning/memory, fear conditioning, sensory transduction, pain perception, ischemic brain injury, seizure, and other neurological as well as psychological disorders. Although protons are the primary activator for ASICs, the properties and/or level of expression of these channels are modulated dramatically by neuropeptides, di-and polyvalent cations, inflammatory mediators, associated proteins, and protein phosphorylations, etc. Modulation of ASICs can result in profound changes in the activities and functions of these channels in both physiological and pathological processes. In this article, we provide an up to date review on the modulations of ASICs by exogenous agents and endogenous signaling molecules. A better understanding of how ASICs can be modulated should help define new strategies to counteract the deleterious effects of dysregulated ASIC activity.
Keywords: Acid-sensing ion channel, acidosis, neuron, modulation
1. Introduction
Neuronal function is highly sensitive to variations in proton concentrations. Similar to non-neuronal cells, neurons maintain differences in extracellular vs. intracellular pH levels through various H+ transporting mechanisms such as Na+/H+ and Cl-/HCO3- systems (for review see [1]). In normal brain tissues, for example, extracellular pH (pHo) is maintained at ∼7.3 while intracellular pH (pHi) is at ∼7.0 [1-3]. A marked reduction of tissue pH, a condition termed acidosis, accompanies a variety of neuropathological conditions including tissue inflammation, ischemic stroke, traumatic brain injury, and epileptic seizure [2,4-11].
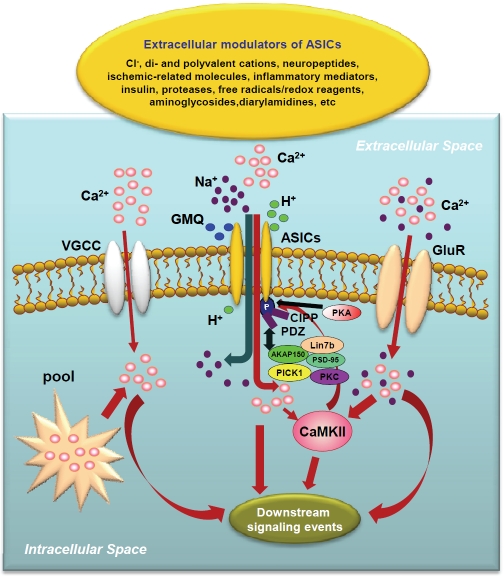
Changes in proton concentrations affect a variety of membrane receptors and ion channels. For example, the activity of a majority of voltage-gated and ligand-gated ion channels is inhibited by reduced pHo, and potentiated by increased pHo, from a baseline of 7.3 - 7.4. For example, N-methyl-D-aspartate (NMDA) receptor-gated cation channels are strongly inhibited by decreases in pHo [12,13]. Similarly, glycine receptor-gated chloride channels are inhibited by acidic pH [14]. In addition to its modulating effect on other channels, recent studies have demonstrated that lowering pHo can, by itself, activate a distinct family of ligand-gated cation channels, the acid-sensing ion channels (ASICs), in both peripheral sensory neurons and neurons of the central nervous system (CNS) [15-26]. This finding has shed light on acidosis-associated changes of neuronal activity and function and provided new targets for therapeutic inventions [27-33]. Although protons are the most widely studied agonist for the activation of ASICs, a recent study has identified a non-proton activator for ASIC3 [34]. Moreover, a variety of extracellular and intracellular signaling molecules can modulate the activities of ASICs (Figure 1). The present review will focus on recent advances in our understanding of the modulations of ASICs.
Figure 1.
Modulation of ASICs by extracellular and intracellular signaling molecules. ASICs are activated by protons and non-proton molecules such as GMQ (for ASIC3 only). Activation of ASIC1a, voltage-gated calcium channels (VGCCs), glutamate receptors (GluRs) and the releases of intracellular Ca2+ pools increases intracellular [Ca2+]i. The resulting increase in [Ca2+]i triggers downstream signaling events, including CaMKII activation. ASIC activity is also regulated by interactions with CaMKII, PKA, PKC, AKAP150, calcineurin, PICK1 and possibly other PDZ-domain proteins such as CIPP.
2. Molecular profiles of ASICs
ASICs are voltage-independent, amiloride-sensitive, cation-selective, channels which belong to the degenerin/epithelial Na+ channel (DEG/ENaC) superfamily [35]. To date, seven subunits of ASICs (1a, 1b1, 1b2, 2a, 2b, 3, and 4) encoded by four genes have been identified [15,36]. High-level expression of 1a, 2a, 2b, and 4 has been demonstrated in CNS neurons, while all others ASICs, except ASIC4, are expressed in peripheral sensory neurons. ASIC genes are also expressed in non-neuronal tissue such as vascular smooth muscle cells [37] and bone [38].
Among all ASICs, the homomeric ASIC1a channel is of particular interest, because of its permeability to Ca2+, whereas other homomeric or heteromeric ASICs are largely impermeable to Ca2+ [16,30,33,39]. Each ASIC subunit consists of two transmembrane domains (TM1 and TM2) and a large, cysteine rich extracellular loop, with the pre-TM2 region essential for ion permeability and the gating of these channels [16,36,40]. The crystal structure of chicken ASIC1a channels has revealed a trimeric assembly [41].
The main functions of ASICs in peripheral sensory neurons include nociception [42-48] and mechanosensation [49-51]. ASIC1a and ASIC3, for example, contribute to pain sensation. It has also been suggested that activation of ASICs is involved in taste transduction [52-54]. In CNS, ASIC1a is involved in synaptic plasticity, learning/memory, fear conditioning [19,55], and retinal physiology [56] (also see [57]). ASIC2a is required for the maintenance of retinal integrity [58], baroreceptor sensitivity [59], and survival of neurons following global ischemia [60]. It has also been demonstrated that activation or sensitization of Ca2+-permeable ASIC1a channels is involved in acidosis-mediated neuronal injury [30,61-64]. Furthermore, ASICs, like ENaC, are implicated in the pathology of tumor cells such as malignant glioma [65,66].
The exact mechanisms by which ASICs provide these functions remain unclear. Under experimental conditions (e.g. in patch-clamp recordings), ASICs are activated only by rapid pH drops and the currents of most ASIC subtypes desensitize rapidly in the continuous presence of acidic pH. The questions of whether tissue pH is subject to quick fluctuations of a magnitude sufficient to activate ASICs, and whether the effects of ASIC activation could be long-lasting in vivo, would be crucial in determining the functional significance of these channels. One explanation for this latter concept, of how a transient opening of ASICs can generate a long-lasting effect, is the capacity of endogenous signaling molecules and biochemical changes associated with various pathological conditions to modulate the properties of ASICs. For example, the expression and/or activities of ASICs are dramatically enhanced after peripheral inflammation or global ischemia [61,67,68], exposure to psychological stimulants [69,70], or ischemia related signaling molecules (e.g. arachidonic acid and lactate) [71,72]. In addition, the desensitization of ASICs can be dramatically reduced by FMRFamide and related mammalian peptides (e.g. NPFF and NPSF) [73,74], dynorphin opioid peptides [75], and other endogenous signaling molecules (e.g. spermine) [76]. A detailed understanding of how ASICs are regulated by endogenous modulators is critical to our comprehension of the precise role of these channels in various physiological and pathological conditions.
3. Pharmacology of ASICs
3.1. Nonselective ASIC inhibitors
Amiloride, a previously widely used K+-sparing diuretic agent, is a nonselective blocker of ENaC. As the member of ENaC superfamily [36], all ASICs (excluding the sustained component of ASIC3 current) are inhibited by amiloride. In general, micromolar concentrations of amiloride inhibit ASIC currents in a concentration-dependent manner [16,30]. Amiloride has also been shown to decrease ASIC-mediated increases in intracellular Ca2+, and attenuate acid-induced membrane depolarization [30,33,47,77]. When combined with other pharmacological or molecular biological approaches, amiloride has been used as a pharmacological tool to identify the involvement of ASICs in physiological and pathological processes. For ASIC1a, ASIC1b, and ASIC2a sub-units, the IC50 for amiloride inhibition is ∼20 μM, whereas slightly higher concentrations of amiloride (IC50 of ∼60 μM) are needed for the inhibition of transient ASIC3 currents [78-81]. Interestingly, the sustained component of the ASIC3 currents is completely resistant to amiloride blockade. Although the exact site(s) involved in amiloride's effect on individual ASICs remain to be determined, the pre-M2 region is known to be involved in amiloride blockade of epithelial Na+ channels and ASICs. Mutation of Gly-430 on ASIC2a subunit, for example, dramatically alter the amiloride sensitivity [81]. Amiloride has been shown to have therapeutic effects against acid-induced pain in human [44], and protect CNS neurons against acidosis-induced neuronal damage both in vitro and in vivo [30].
A-317567, a small molecule ASIC blocker unrelated to amiloride, has recently been described [82]. A-317567 concentration-dependently inhibits ASIC1a-like, ASIC2a-like, and ASIC3-like currents in rat dorsal root ganglion (DRG) neurons. The IC50 values for blocking ASIC1a-like, ASIC2a-like, and ASIC3-like currents are 2.0, 29.1, and 9.5 μM, respectively. Unlike amiloride, A-317567 blocks both the fast and sustained phases of the ASIC3-like current with equal potency. In both in vitro studies and in vivo pain models, A-317567 appears to be more potent than amiloride [82].
3.2. Subunit-specific inhibitors of ASICs
Two peptides, one derived from the venom of spiders and the other from sea anemones, have been characterized as subunit-selective ASIC inhibitors. These inhibitors are important tools for exploring the functional roles of distinct ASIC subunits in native neurons in vitro and in vivo.
Psalmotoxin 1 (PcTx1), isolated from the venom of South American tarantulas (Psalmopoeus cambridgei), is a specific inhibitor for ASIC1a channels [83]. It contains 40 amino acids cross -linked by three disulfide bridges. PcTx1 potently inhibits the homomeric ASIC1a current with an IC50 of 0.9 nM, without affecting other configurations of ASICs. Thus PcTx1 has been used to determine the presence, and function, of homomeric ASIC1a in native neurons [30,56,83,84]. PcTx1 inhibits ASIC1a channels by increasing their apparent affinity for H+[85], and the interaction between PcTx1 and ASIC1a depends on the state of the channel [86]. It binds tightly to the channel in open and desensitized states, thus promoting channel inactivation. The binding site for PcTx1, recently analyzed using radio-labeled tools, involves cysteine -rich domains I and II (CRDI and CRDII) of the extracellular loop [87]. Although the post-transmembrane I (M1) and pre-transmembrane II (M2) domains are not directly involved in the binding, they are crucial to the ability of PcTx1 to inhibit channel. The linker domain between CRDI and CRDII also appears to be important by contributing to the correct spatial positioning to form the PcTx1 binding site [87]. In addition to ASIC1a, PcTx1 also interacts with the ASIC1b subunit, a splice variant of ASIC1a. However, it enhances rather than inhibits the activity of ASIC1b. PcTx1 exerts its potentiation of ASIC1b at much higher concentration (>10 nM) than the concentration that inhibits ASIC1a. It binds to the ASIC1b in open state, promoting channel opening [86].
APETx2, a 42-amino-acid peptide toxin isolated from sea anemones (Anthopleura elegantissima), is a selective inhibitor for ASIC3 and ASIC3 containing channels [88]. Similar to PcTx1, APETx2 is cross-linked by three disulfide bonds. It belongs to the disulfide-rich all-beta structural family of peptide toxins commonly seen in animal venoms [89]. APETx2 inhibits transient ASIC3 currents with an IC50 of 63 nM, without affecting sustained ASIC3 currents. The affinity of this ASIC3 inhibitor, however, is decreased when ASIC3 is associated with other ASIC subunits. For example, the IC50 for ASIC3/ ASIC2b is 117 nM, while the IC50 for ASIC3/ ASIC1a is 2 μM. APETX2 directly inhibits the ASIC3 channel by acting at its external side [88].
4. Modulators of ASICs
Although protons were the only known activator for ASICs when they were discovered, the existence of other ASIC agonist(s) has recently been reported. Yu et al., demonstrated that 2-guanidine-4-methylquinazoline (GMQ), a small molecule containing a guanidinium group and a heterocyclic ring, causes persistent activation of ASIC3 channels at normal pH [34]. They also demonstrated that residues around E423 and E79 of the extracellular “palm” domain of the ASIC3 channels are crucial for the activation by GMQ. Consistent with ASIC3 activation, GMQ activates sensory neurons and induces pain-related behaviors in an ASIC3-dependent manner. Similarly, ASIC1a channels appear to be gated by ammonium independent of changes in proton concentration [90]. Pidoplichko and Dani reported that, in midbrain dopamine neurons and in HEK 293 cells endogenously expressing ASIC1 subunits, ASIC currents are activated by NH4Cl at millimolar concentrations. Thus, ligands, other than protons, may activate ASICs under physiological and/or pathological conditions through nonproton ligand sensors, resulting in channel activation independent of marked acidosis.
The sensitivity of ASICs to pHo, and their activation/inactivation kinetics, vary according to the ASIC subtypes and subunit composition of the channel complex, with pH50's ranging from 4.0 to 6.5, and activation thresholds for most ASICs (excluding ASIC2a) close to pH7.0 [16,36]. However, various factors, discussed below, can modulate ASICs and dramatically change the properties and dynamics of these channels (Figure 1). These include Cl-, di- and polyvalent cations [91-97], free radicals and redox reagents [98,99], neuropeptides [73,100,101], proteases and ischemia-related signaling molecules [71,72,102,103], intracellular accessory proteins [104-107], and protein kinases [61,84,108,109].
4.1. Extracellular Modulators
4.1.1 Cl-
Recent crystallization of chicken ASIC1a resulted in the identification of three potential binding sites for CI- ions in the extracellular domain [41]. These sites are coordinated by two nearby residues (Arg-310 and Glu-314) on an α-helix of one subunit, and a third residue (Lys-212) from an adjacent subunit, all three of which are conserved among H+-gated ASIC iso-forms. The functional significance of Cl- binding to ASIC channels, however, was not clear. Recently, Kusama and coworkers investigated the effect of Cl- substitution on heterologously expressed ASIC1a currents and native ASIC currents in hippocampal neurons [110]. They showed that, replacement of the extracellular Cl- ions with impermeable and inert anion methanesulfonate (MeSO3-) accelerates the rapid desensitization of the ASIC1a current but attenuates tachyphylaxis, a phenomenon of slow and gradual decrease of the current amplitude. Replacement of extracellular Cl- ions with other anions, including Br-, I-, and thiocyanate, also alters the kinetics of ASIC1a desensitization and tachyphylaxis. Mutations affecting the amino acid residues that form the Cl--binding site in ASIC1a abolish the modulatory effects associated with substituting other anions for Cl-. The results of anion substitution on native ASIC channels in hippocampal neurons mirror those in heterologously expressed ASIC1a, and alter acid-induced neuronal death. Anion modulation of ASICs provides new insight into channel gating, and may prove important in pathological conditions associated with changes in pH and Cl-.
4.1.2 Di-and polyvalent cations
Ca2+
Divalent cations such as Ca2+ are important modulators of various voltage-gated and ligand-gated ion channels including ASICs. The effect of changing extracellular Ca2+ on ASICs depends on whether Ca2+ is co-applied with acidic solution or pre-applied prior to channel activation. Co-application of Ca2+ with acidic solution reduces ASIC currents [16,47,93,96,111]. Similarly, pretreatment followed by continuous presence of the extracellular Ca2+ inhibits the ASIC currents [112,113]. However, pretreatment with Ca2+, prior to ASIC activation in the absence of Ca2+, enhances ASIC responses [94,113]. These experiments must be performed and interpreted with caution, since a drop in Ca2+ itself may activate separate non-selective cation currents (i.e. non ASIC) in neurons and other cells [114,115]. Investigation into the mechanisms underlying Ca2+ modulation of ASICs has led to the finding that Ca2+ decreases the affinity of ASICs (e.g. ASIC3) for H+ [94,96]. It has been proposed that, at a pH of 7.4, ASIC3 channels are closed because of the Ca2+ blockade. As the pHo is decreased, binding of H+ to the channel displaces Ca2+ from its binding site, leading to opening of the channel [96]. For ASIC1a channels, different models have been proposed [97,111]. Paukert and colleagues showed that two negatively charged residues near the entrance of the channel pore, E425 and D432, are crucial for Ca2+ blockade of the ASIC1a channel [111]. They proposed that more than one Ca2+ binding site, one that mediates blocking and one mediates modulation, exist on the channel. Based on the data from the single-channel recordings in heterologous expression systems, Zhang et al. suggest that Ca2+ modulates the ASIC1 channel in an allosteric manner [97].
Zn2+
A number of studies have demonstrated that extracellular Zn2+, an endogenous trace element released during neuronal activity [116,117], bi-directionally modulates ASIC activities. Chu et al. showed that, at nanomolar concentrations, Zn2+ dose-dependently inhibits ASIC currents in cultured mouse cortical neurons [92]. In Chinese hamster ovary (CHO) cells expressing various combinations of ASIC sub-units, Zn2+ inhibits the currents mediated by homomeric ASIC1a and heteromeric ASIC1a/ ASIC2a channels, without affecting currents mediated by homomeric ASIC1b, ASIC2a, or ASIC3 channels. In addition to a reduction in the current amplitude, Zn2+ reduces the affinity of ASIC1a for protons. Mutation of lysine-133 in the extracellular domain of the ASIC1a subunit abolishes the high-affinity Zn2+ inhibition [92].
At high micromolar concentrations (>100 μM), however, Zn2+ also binds to low-affinity site(s) on the ASIC2a subunit, resulting in increased affinity for proton binding and a potentiation of the activity of ASIC2a containing channels [91]. In addition to increasing the current amplitude, higher concentrations of Zn2+ also slow down the inactivation kinetics of the ASIC currents in hippocampal neurons [93]. Similar to the effect on the ASIC current, micromolar concentrations of Zn2+ facilitate acid-induced membrane depolarization in cultured [91] and acutely dissociated hippocampal neurons [93]. Thus, in regions of the brain that contain large amount of Zn2+ (e.g. the hippocampus) where homomeric ASIC1a and heteromeric ASIC1a/ASIC2a channels are the most common configurations of ASICs [16,19,26,84], ASICs might be a physiological target of Zn2+. Site-directed mutagenesis studies demonstrated that two histidine residues, His-162 and His-339 in the extracellular loop of the ASIC2a subunit, are involved in low affinity Zn2+ potentiation of the ASIC2a containing channels [91].
In contrast to ASIC2a containing channels, high concentrations of zinc inhibits ASIC3 channels [118,119]. In CHO cells expressing ASIC3 channels, ASIC3 currents are inhibited by pretreatment with zinc in a concentration-dependent manner with an IC50 of 61 μM. Zinc inhibits ASIC3 in a pH- and Ca2+-independent manner and the inhibition of ASIC3 currents is dependent upon the interaction of zinc with binding sites in the extracellular domain(s) of ASIC3. Thus, at physiological concentrations, zinc is an important negative regulator of ASIC3 channels [119]. Like ASIC3, ASIC1b is inhibited by zinc with an IC50 of 26 μM. Cysteine 149 in the extracellular finger domain of the ASIC1b subunit is involved in this inhibition [120].
Mg2+
The effect of Mg2+ on ASICs is comparable to that of Ca2+. In a heterologous expression system, sequential application of Mg2+ and pH drop produces concentration-dependent and voltage-independent potentiation of ASIC1 [94]. For ASIC3, Mg2+ concentration-dependently reduces the channel activity when co-applied with acidic solutions, comparable to the effect of Ca2+ [96].
Cu2+
Cu2+ is the third most abundant trace element
in human body. A recent study has shown that this divalent cation has a modulatory effect on ASICs in cultured hypothalamic, hippocampal and cortical neurons [121]. It concentration-dependently reduces the amplitude of ASIC currents, but slows down the current desensitization. Consistent with the inhibition of ASIC current, micromolar concentrations of Cu2+ attenuate acid-induced membrane depolarization. Thus Cu2+ may represent another endogenous modulator of ASIC channels in the CNS that could negatively modulate increased neuronal excitability caused by ASIC activation.
Pb2+
Pb2+ is a toxic metal ion that has well documented detrimental effects on the CNS. A variety of membrane receptors and ion channels, including ASICs, have been proposed to mediate the toxicity of Pb2+. In acutely dissociated CNS neurons, Pb2+ inhibits ASIC currents in a concentration-dependent manner [113]. Pb2+ also decreases ASIC-mediated increases in intracellular Ca2+, and attenuates acid-induced membrane depolarization. In CHO cells transfected with various ASIC subunits, currents mediated by ASIC1a, ASIC1b, and ASIC3 subunits were inhibited by Pb2+. Since ASIC activation contributes to normal synaptic function, Pb2+ induced neurotoxicity may be partially explained by its inhibition of ASIC function.
Ni2+ and Cd2+
Ni2+ and Cd2+, like Pb2+, are heavy metal ions that affect various membrane ion channels and receptors, and are neurotoxic. Both Ni2+ and Cd2+ reversibly inhibit the ASIC activity, in a concentration-dependent manner [122]. Ni2+ and Cd2+also decrease the pH-sensitivity of ASICs. In CHO cells transfected with multiple ASIC sub-units, 1 mM Ni2+ selectively inhibits homomeric ASIC1a and heteromeric ASIC1a/ASIC2a channels, whereas Cd2+ inhibits homomeric ASIC2a, ASIC3, and heteromeric ASIC1a/ASIC2a, ASIC1a/ASIC3, and ASIC2a/ASIC3 channels. Both the fast and the sustained phases of the ASIC3 current are sensitive to Cd2+ inhibition [122].
Gd3+
Gd3+, a trivalent metal ion, has been reported to inhibit both the fast phase and the sustained component of the currents mediated by homomeric ASIC3 and heteromeric ASIC2a/ASIC3 channels [95]. Since Gd3+ has previously been used to block stretch activated responses in neurons [123], the inhibition of ASIC3 and ASIC2a/ASIC3 currents by Gd3+ might suggest an involvement of ASIC3 containing channels in the transduction of mechanosensory stimuli [95].
Spermine
Spermine is a polyvalent cation whose extracellular concentration fluctuates significantly within the nervous system. Studies by Babini and colleagues showed that spermine potentiates the activities of ASIC1a and ASIC1b channels through a shift in the steady-state inactivation toward more acidic pH and stabilization of the resting state [94]. Consistent with the potentiation of ASIC1a by spemine, Duan et al., recently showed that extracellular spermine exacerbated ischemic neuronal injury through sensitization of ASIC1a channels to extracellular acidosis [76]. Pharmacological blockade of ASIC1a or deletion of the ASIC1 gene greatly reduced the enhancing effect of spermine on ischemic neuronal damage, both in cultures of dissociated neurons and in a mouse model of focal ischemia. Spermine also reduced desensitization of ASIC1a in the open state and accelerating recovery from desensitization in response to repeated acid stimulation. Functionally, enhanced channel activity was accompanied by increased acid-induced neuronal depolarization and cytoplasmic Ca2+ overload, which may partially explain the exacerbation of neuronal damage caused by spermine. Thus, extracellular spermine contributes to ischemic neuronal injury, at least in part, by enhancing ASIC1a activity [76].
In summary, endogenous and exogenous di- and polyvalent cations represent the largest categories of ASIC modulators. Understanding the mechanisms underlying the modulation of ASICs by these cations may provide valuable insights that can facilitate the design of novel therapeutic agents for protecting neurons against acidotoxic damage.
4.1.3 Redox reagents and free radicals
Redox status is known to affect the function of various voltage-gated and ligand-gated ion channels [124-128]. This oxidation/reductiondependent modulation of ion channel function is important because redox status can change dramatically in physiological/pathological conditions [129-131]. Recent studies demonstrated that ASIC currents in both central and peripheral neurons are potentiated by reducing agents, and inhibited by oxidizing agents [98,99,132]. Consistent with their effects on ASIC currents, reducing agents increased, while oxidizing agents decreased, acid-induced membrane depolarization and intracellular Ca2+ accumulation [99]. In CHO cells expressing various ASICs, redox modulation was found to be ASIC1a selective [99]. Site-directed mutagenesis studies showed that cysteine 61 and lysine 133, located in the extracellular domain of the ASIC1a subunit, are involved in the modulation of ASICs by oxidizing and reducing agents, respectively. These data suggest that the redox status of ASIC1a subunits is important in determining the overall physiological function and pathological role of ASICs within the CNS. Given that excessive activation of ASIC1a is associated with acidosis-induced neuronal injury [30,33,61], targeting redox modulating sites of ASIC1a subunits is a tempting strategy for developing novel therapeutic agents.
The mechanism underlying the modulation of ASIC activity by redox reagents is not fully understood. Recently, Zha et al demonstrated that ASIC1a forms inter-subunit disulfide bonds and that H2O2 increases this link between subunits [133]. They found that Cys-495 near the C terminus of ASIC1a is particularly important for inter-subunit disulfide bond formation. Inter-subunit disulfide bond formation reduced the proportion of ASIC1a located on the cell surface, contributing to H2O2-induced decreases in H+-gated currents. Thus, H2O2 and likely other oxidants, decrease ASIC activity by increasing formation of disulfide bonds between intracellular ASIC1a subunits, which in turn suppress their expression on the cell surface.
Nitric oxide (NO) is a short-lived mediator whose release is strongly enhanced by inflammation. NO regulates the protein function by two main pathways. An indirect mechanism involves the production of cGMP and the activation of protein kinase G. NO can also directly modify the tertiary structure of proteins by S-nitrosylating the thiol side-chains of cysteine residues, leading to the formation of disulfide bonds between neighboring cysteine residues [134]. Due to the large number of cysteine residues on their extracellular loop, ASICs are a potential target by NO. Consistent with this idea, Cadiou et al., found that proton-gated currents of both sensory neurons and ASIC isoforms are potentiated by NO. Intracellular pathways modulated by NO are not involved in the enhancement of acid-gated currents, and the mechanism of the effect is instead via a direct action on the extracellular surface of the ion channel [135].
4.1.4. Neuropeptides
Dynorphin opioid peptides are abundantly expressed in the CNS. Recently, Sherwood and Askwith reported that dynorphin A and big dynorphin potentiate acid-activated currents in cortical neurons and in CHO cells expressing homomeric ASIC1a subunits [75]. The potentiation of the ASIC1a activity was mediated through a limitation of steady-state desensitization of the channel. The potentiation of ASIC1a activity by dynorphin was not mediated by opioid or bradykinin receptor activation but through a direct interaction with ASIC1a. Alteration of steady-state desensitization by dynorphins enhanced ASIC1a-triggered neuronal injury during prolonged acidosis. Thus, ASIC1a is a new non-opioid receptor target for dynorphins, and that dynorphins can enhance ischemic brain injury by preventing steady-state desensitization of ASIC1a channels.
FMRFamide and structurally related peptides are abundant in invertebrate nervous systems where they function as neurotransmitters and neuromodulators. Although FMRFamide itself has not been isolated in mammals, several FMRFamide-related peptides exist in the mammalian nervous system. FMRFamide and related peptides are generally thought to exert their physiological roles through G-protein coupled receptors [136]. However, two ionotropic receptors involved in the function of these peptides have recently been identified. FMRFamide -gated Na+ channel (FaNaC), which is a neuronal Na+ channel in invertebrates, is directly activated by micromolar concentrations of FMRFamide and RFamide-related peptides (RFRPs) [136]. In addition, ASICs, which share significant structure and sequence homology with FaNaC in the mammalian nervous system, can be modulated by FMRFamide and RFRPs. FMRFamide, and RFRPs such as neuropeptide FF were incapable of generating any ASIC currents on their own, but significantly potentiated ASIC currents in sensory neurons and in het-erologous expression systems [73,100,101]. In addition to their effects on the amplitude of ASIC currents, FMRFamide and RFRPs also reduced the rate of current desensitization [73,100,101]. Studies using knockout mice have suggested that ASIC3 plays a major role in RFamide modulation of proton-gated currents in sensory neurons [100, 137]. In contrast, the capacity of these peptides to modulate ASIC currents appears to be only modestly dependent on the ASIC1a, and essentially independent of ASIC2a [100]. These data are consistent with the effects of FMRFamide and related peptides on homomeric ASIC1a and ASIC3 channels expressed in a heterologous system [73]. Though ASIC2a is not directly modulated by these peptides, the presence of ASIC2a sub-units has been shown to enhance the capacity of these peptides to modulate both ASIC1a-containing and ASIC3-containing channels [101,138].
As a further complication, different ASICs show distinct responses to different FMRFamide related peptides. For example, FMRFamide and NPFF have a different effect on the inactivation of ASIC3 currents than neuropeptide SF [139]. Similarly, FRRFamide and FMRFamide have comparable effects on ASIC1a, but ASIC1b currents are inactivated much more rapidly by FRRFamide than by FMRFamide [73]. FMRFamide also drastically slows down the desensitization of ASIC1b/3 heteromers [140]. The potentiation of ASIC activity by endogenous FMRFamide neuropeptides likely contributes to the response of sensory and central neurons to noxious acidosis [73,136]. An extensive review of the modulation of ASIC by FMRFamide and related peptides is presently available [136].
4.1.5. Non-steroid anti-inflammatory drugs and inflammatory mediators
Decreases in tissue pH to levels well below the threshold for ASIC activation is a common feature of inflammation [10,11]. It is therefore expected that ASICs, particularly the ASIC1a and ASIC3 channels which are highly sensitive to moderate pH changes, are activated during tissue inflammation and participate in pain sensation. This notion was supported by the finding that local injection of acid activates nociceptors and produces pain in human subjects [141-144]. Moreover, acid-induced pain could be inhibited by amiloride, a non-selective ASIC blocker [44,141,144]. Furthermore, chronic hyperalgesia induced by repeated acid injections into muscle is abolished by the loss of ASIC3 [45]. Together, these findings strongly suggest that ASIC activation contributes to the transduction of pain sensation.
Non-steroid anti-inflammatory drugs (NSAIDs) constitute a diverse group of chemicals that are commonly used to treat a variety of inflammatory conditions [27]. Although inhibition of cyclooxygenase enzymes (COXs) and consequent reductions in prostaglandin synthesis constitutes the main mechanism by which most NSAIDs inhibit inflammation, abundant evidence suggests that mechanisms other than COX inhibition contribute to their analgesic effect. For example, COX knockout mice still showed sensitivity to analgesic action of NSAIDs [145]. It is also known that both S- and R-enantimeres of flurbiprofen are effective in pain relief. Unlike the S-form, which inhibits COX and prostaglandin synthesis, the R-form inhibits prostaglandin synthesis with much lower efficiency, but retains its analgesic properties [146]. It has also been shown that diclofenac has an analgesic effect independent of COX inhibition [147], and that NSAIDs can reduce acid induced cutaneous pain in the absence of inflammation [148].
This collective evidence, in conjunction with earlier findings that protons activate depolarizing inward currents in nociceptors [42,43] and the recent discovery of ASICs as proton receptors in sensory neurons, suggests a strong link between NSAIDs and ASICs. Indeed, studies by Voilley et al demonstrated that NSAIDs directly inhibit the activity of ASICs in DRG neurons and in CHO cells at concentrations relevant to their analgesic effects [68]. Ibuprofen and flurbiprofen, for example, inhibit ASIC1a containing channels with an IC50 of 350 μM. Aspirin and salicylate inhibit ASIC3 containing channels with an IC50 of 260 μM, whereas diclofenac inhibits the same channels with an IC50 of 92 μM. In addition to a direct inhibition of the ASIC activity, NSAIDs largely prevent inflammation induced increases of ASIC expression in sensory neurons [68]. Thus, ASICs represent novel targets in the search for new analgesic agents.
Proinflammatory mediators such as nerve growth factor (NGF), TNF, histamine, bradykinin, serotonin, interleukin-1, substance P, and PGE2 are pro-nociceptive agents, as they sensitize nociceptive fibers [149]. Though details regarding the mechanisms underlying this sensitization remain unclear, earlier studies have demonstrated that the excitatory effects of protons on sensory neurons are potentiated by these pro-inflammatory mediators [150,151]. In addition, NGF is required for long-term up-regulation of proton-induced excitation of DRG neurons [152]. These findings suggest that the pro-inflammatory mediators may have an effect on the expression and/or activity of ASICs in nociceptors. Indeed, studies by Mamet and colleagues demonstrated that the majority of pro-inflammatory mediators stimulate the expression, and increase the activity, of ASICs [67]. At concentrations relevant to tissue inflammation, NGF, serotonin, IL-1, and bradykinin induced a dramatic (up to 10 fold) increase in ASIC1a, ASIC1b, ASIC2b, and ASIC3 expression in cultured DRG neurons [67]. Electrophysiological recordings confirmed the increase of ASIC current density in DRG neurons following the treatment with pro-inflammatory mediators [67].
4.1.6. Aminoglycosides
Aminoglycosides (AGs) are a group of antibiotics that have been shown to block Ca2+ channels, excitatory amino acid receptors, and transient-receptor-potential V1 channels. Recently, Garza et al demonstrated that aminoglycosides (AGs) (streptomycin, neomycin and gentamicin) have modulating effects on ASIC currents [153]. In rat DRG neurons, streptomycin and neomycin produced a reversible reduction in the amplitude of proton-gated currents in a concentration -dependent manner. In addition, streptomycin and neomycin slowed down the desensitization rates of ASIC currents. In HEK-293 cells endogenously expressing hASIC1a channels, streptomycin produced a significant reduction in the amplitude of the proton-gated current, whereas neomycin and gentamicin had no effect. Reduction of extracellular Ca2+ concentrations enhanced the action of streptomycin and neomycin on the desensitization of ASIC currents. These results indicate that ASICs are molecular targets for AGs, which may explain, in part, their effects on excitable cells.
4.1.7. Diarylamidines
Diarylamidines have been widely used for the treatment of protozoan diseases such as try-panosomiasis and leishmaniasis since 1930s. Recently, Chen and colleges found that four members of the diarylamidines, 4', 6-diamidino-2-phenylindole, diminazene, hydroxystil-bamidine and pentamidine strongly inhibit ASIC currents in hippocampal neurons with apparent affinities of 2.8 μM, 0.3 μM, 1.5 μM and 38 μM, respectively. Sub-maximal concentrations of diminazene also accelerate the desensitization of ASIC currents. In CHO cells expressing different ASIC subunits, diminazene blocks ASIC1a, 1b, 2a, and 3 currents with a rank order of potency 1b > 3 > 2a > 1a. This study indicates that diarylamidines constitute a novel class of non-amiloride ASIC blockers and suggests that diarylamidines as small molecules may be developed as therapeutic agents in the treatment of ASIC-involved diseases [154].
4.1.8. Proteases and ischemia-associated molecules
Neurological diseases, such as brain ischemia and neurotrauma, are associated with a variety of biochemical changes in addition to decreases in pH. Alterations in ion homeostasis (Ca2+, K+ for example), increased protein and lipid metabolism and generation of lipid metabolites (e.g. arachidonic acid), changes in the level/ activity of proteases, kinases, and phos-phatates, are among the common biochemical changes associated with brain ischemia. Many of these changes have been shown to modulate the activities of various voltage-gated and ligand-gated channels. For example, the activities of NMDA receptor-gated channels are modulated by protease [155], arachidonic acid [156], and protein phosphorylation [157,158]. Similarly, the activities of ASICs are modulated by ischemia-related signaling molecules.
Proteases
Brain injury is accompanied by increased protease activity [159]. Blood-derived proteases such as thrombin, tissue plasminogen activator, and plasmin can gain access to CNS interstitial spaces due to a compromised blood-brain barrier [159,160]. Previous studies have demonstrated that proteases modulate the activities of various ion channels, including ENaC, which belongs to the same family as ASICs [155,161,162]. Similarly, recent studies by Poirot and colleagues demonstrated modulation of the ASIC1a function by serine proteases [102]. The exposure of CHO cells stably expressing ASIC1a channels to trypsin or other serine proteases (e.g. proteinase K and chy-motrypsin) shifted the pH dependence of activation and steady-state inactivation of the ASIC1a channels to more acidic pH values. As a consequence, protease exposure leads to a decrease in ASIC1a activity when the current is activated by a pH drop from 7.4. Interestingly, if the channel is activated from a basal pH of 7, protease exposure increases, rather than decreases, the ASIC1a activity. In addition, protease treatment dramatically accelerates the recovery rate of ASIC1a channels from desensitization [102]. The effects of proteases on ASICs appear to involve proteolysis of the channel protein, as the capacity of trypsin to modulate ASIC1a was diminished with soybean trypsin inhibitor or modification of trypsin's catalytic site with TLCK. TLCK is a small reagent that modifies irreversibly a histidine residue in the catalytic site of trypsin. Cleavage of the channel protein was confirmed by Western blot analysis showing reduction of a 64-kDa ASIC protein band to a lower molecular weight band of 49 kDa [102]. Further studies demonstrated that trypsin cleaves ASIC1a subunits at Arg-145 in the N-terminal part of the extracellular loop. The cleavage site is between a highly conserved sequence and a sequence that is critical for ASIC1a inhibition by PcTx1 [103]. Since activation of ASIC1a is involved in acidosis-mediated ischemic brain injury [30,33], modulation of ASIC1a by proteases could be relevant to its pathophysiology role in brain ischemia.
Matriptase is an 80-90-kDa type II transmembrane protease of epithelial cells that belongs to the S1 family of trypsin-like serine proteases. It is crucial for epidermal barrier formation and is involved in hair follicle growth and thymocyte development. In addition, matriptase has been implicated in many epithelial cancers. Due to the expression of ASICs in malignant tumor cells, Clark et al tested whether matriptase can modulate the activity of ASIC1 channels [163]. They showed that Matriptase decreases ASIC1 currents recorded in Xenopus oocytes. This effect is mediated by cleavage of ASIC1 by Matriptase. Inactivated matriptase, due to an S805A mutation, does not cleave ASIC1 and has no effect on ASIC1 currents. The effect of matriptase on ASIC1 is specific, as it does not affect ASIC2 currents. Three matriptase recognition sites have been identified in ASIC1 (Arg-145, Lys-185, and Lys-384); site-directed mutagenesis of these sites prevents cleavage of ASIC1 by matriptase.
Arachidonic acid
Arachidonic acid (AA) is a major metabolite of membrane phospholipids, which is involved in a variety of physiological processes [164,165] and pathophysiology of several neurological disorders [165-167]. During brain ischemia, for example, the rise of [Ca2+]i leads to the activation of phospholipase A2 which results in increased production of AA [165,166,168]. Earlier studies have shown that AA has effects on a variety of voltage-gated and ligand-gated ion channels [169-175]. For example, it potentiates the opening of NMDA-gated channels [156, 169,175]. Recent studies have shown that AA also enhances ASIC currents in rat cerebellar Purkinje and DRG neurons [72]. The potentiation of the ASIC currents appears to be produced by AA itself and not by its derivatives, since an agent known to block the breakdown of AA did not affect its capacity to potentiate ASIC currents [72]. The molecular mechanism for AA potentiation of ASICs is controversial. One potential explanation, similar to that proposed for NMDA channels, is that insertion of AA into the membrane induces membrane stretch and that the ASICs are stretch-sensitive [156]. This explanation is supported by the finding that perfusion of neurons with hypotonic saline, which causes cell swelling and membrane stretch, mimicked the potentiation of ASIC currents by AA [72]. Studies by Smith et al, however, suggested that AA potentiates ASIC activation by a direct mechanism [176]. They showed that inhibition of AA metabolism had no effect on the potentiation of ASIC1a, and that potentiation of single ASIC2a channels by AA could be observed in cell-free patches.
Lactate
Anaerobic metabolism of glucose during ischemia leads to increased production of lactate. Following ischemia, concentrations of lactate between 12-20 mM have been reported in the extracellular space, dramatically higher than the ∼ 1 mM lactate in the normal conditions [177,178]. In sensory neurons that innervate the heart, Immke & McCleskey demonstrated that addition of 15 mM lactate dramatically increased the amplitude the ASIC current activated by a moderate pH drop to ∼7.0 [71]. Applications of the same concentration of lactate at pH values that do not activate ASICs (8.0 or 7.4) caused no response. Thus, lactate acts by potentiating but not activating the ASICs. In COS -7 transfected with different subunit of ASICs, both ASIC3 and ASIC1a currents were potentiated by lactate [71]. The effect of lactate does not require second messenger or signaling cascade because the potentiation persists in excised membrane patches. Since lactate has the ability to chelate the divalent cations including Ca2+ and Mg2+, and the concentration of extracellular divalent cations, particularly Ca2+, has a modulatory role on various membrane receptors and ion channels [179-181], the authors hypothesized that potentiation of the ASICs may be due to the chelation of divalent cations. Indeed, adjusting the concentrations of Ca2+ and Mg2+eliminated the effect of lactate, whereas reducing the divalent concentrations mimicked the effect of lactate [71]. Other monocarboxylic acids which have the divalent cation chelation ability also potentiated the ASIC current. Similar to the cardiac sensory neurons, potentiation of the ASIC current by lactate has been reported in other neurons such as cerebellar Purkinje neurons [72].
4.1.9. Insulin
Insulin is present throughout the brain. Although insulin generally functions in glucose uptake, brain insulin is involved in neuronal growth and maturation and modulation of surface expression of various ion channels and neurotransmitter receptors. Recent studies by Chai et al demonstrated that insulin also regulates membrane trafficking of ASIC1a channels [182]. In CHO cells expressing ASIC1a subunit, serum depletion induces a significant increase in ASIC1a surface expression that results in the potentiation of ASIC1a activity. Among the components of serum, insulin is identified as the key factor that maintains a low level of ASIC1a on the plasma membrane. Similarly, neurons subjected to insulin depletion increase the surface expression of ASIC1a with resultant potentiation of ASIC1a currents. In the normal condition, ASIC1a is predominantly localized to the endo-plasmic reticulum. Under conditions of serum or insulin depletion, the intracellular ASIC1a is translocated to the cell surface, increasing the surface expression level [182]. These results reveal an important function of insulin in regulating the trafficking of ASIC1a subunit.
4.1.10. Psychoactive drugs
The striatum is a key structure in reward circuits involved in biological actions of addictive drugs. Recent studies indicated that ASIC1a is the predominant subtype of ASICs in the medium spiny neurons of striatum [183]. Since homomeric ASIC1a channel is involved in synaptic plasticity [19], it is likely that changes in the expression and/or activity of ASIC1a channels may contribute to the synaptic modification during drug addiction. To test this possibility, Zhang et al investigated the possible effect of psychostimulatant administration on basal ASIC gene expression in the striatum and other forebrain regions in vivo using cocaine sensitization model [69]. They found that ASIC1, but not ASIC2, is a target of cocaine as its protein expression can be readily regulated by the drug. Chronic cocaine exposure induces a profound increase in ASIC1, but not ASIC2, protein level in the striatum (CPu and NAc), indicating a sub-unit-dependent modulation by cocaine. In contrast to striatum, ASIC1 and ASIC2 protein levels in other forebrain regions such as median pre-frontal cortex (mPFC) and hippocampus remaine relatively stable after chronic cocaine injections, suggesting a region-specific modulation by chronic cocaine. Similar to chronic cocaine exposure, Suman et al., showed that ASIC1 protein levels is increased in the defined surface and intracellular pools in the striatum (both CPu and NAc) in chronic amphetamine treated, but not saline-treated rats [70]. ASIC2 proteins, however, remain stable in the striatum. In mPFC, repeated amphetamine administration has no effect on ASIC1 expression in either surface or the intracellular compartment. However, amphetamine selectively reduces the surface expression of ASIC2 in this region. The mechanism underlying the alternation of ASIC protein expression in response to chronic drug exposure is unclear and requires further investigation.
4.2. INTRACELLULAR MODULATORS
4.2.1. Protein kinases and phosphatases
Besides direct modulations by the extracellular factors discussed above, ASICs can be regulated by a variety of intracellular modulators (Figure 1). Baron et al. first showed that protein kinase C (PKC) activator OAG enhances the ASIC2a current in the presence of protein interacting with C kinase 1 (PICK1). The PICK1-dependent and PKC-induced phosphorylation does not change the kinetics, pH dependence, and the unitary conductance of ASIC2a channels but induces an increase of the channel open probability [84]. Deval et al., demonstrated that ASIC3-like currents in cultured DRG neurons are increased by PKC activator phorbol ester PDBu and pain mediators, such as serotonin, which are also known to activate PKC through their binding to G protein-coupled receptors [109]. Interestingly, PKC regulation of ASIC3 activity involves the ASIC2b subunit, since heteromultimeric ASIC3/ASIC2b channels, but not homomeric ASIC3 channels, are potentiated by PKC. The increase of ASIC3/ASIC2b current is accompanied by a shift in H+ dose-response toward more physiological pH values. The up-regulation by PKC requires the presence of PICK1, a PDZ domain-containing protein, which interacts with C-terminus of ASIC1a, ASIC2a, and ASIC2b, but not ASIC3 [105,106, 109]. Point mutations identified involvement of Thr-40 and Ser-523 on ASIC3 subunit in PICK1-dependent PKC modulation of the ASIC3/ ASIC2b channels [109]. Association with PICK1 likely provides a mechanism for selective targeting of PKCα to ASIC3 phosphorylation sites. In this process, ASIC2b subunits appear to play an essential regulatory role as a link between the PICK-1/PKC complex and the ASIC3 subunits [109].
In contrast to other subunits of ASICs, ASIC1a is not phosphorylated by PKC but by cAMP-dependent protein kinase A (PKA). Leonard et al showed that PKA phosphorylates Ser-479 of ASIC1a. Phosphorylation of ASIC1a by PKA reduces PICK1 binding and attenuates the cluster of ASIC1a in hippocampal neurons. These results suggest that the delivery of ASIC1 subunits is regulated by a combination of protein interactions and posttranslational modifications. Regulation of this interaction by phosphorylation may provide a mechanism to control the cellular localization of ASIC1 [108].
Under pathological conditions such as brain ischemia, a novel form of regulation of ASIC1a by calcium/calmodulin-dependent protein kinase II (CaMKII) has been reported [61]. Studies by Gao et al., demonstrated that global brain ischemia in rats results in an increased phosphorylation of ASIC1a by CaMKII at Ser-478 and Ser-479 of its C-terminal. This phosphorylation sensitizes the channel to low pH, exacerbating cell death by allowing increased calcium conductance [61]. The phosphorylation is a result of activation of NR2B-containing N-methyl-D-aspartate subtype of glutamate receptors (NMDARs) and increase of intracellular Ca2+ during ischemia. In addition to serine/threonine phosphorylation, tyrosine kinase may also be involved in the modulation of ASIC expression/ function, though not directly [184]. It has been shown that the level of basal expression of ASIC3 depends largely on the constitutive activation of trkA, the NGF-specific receptor, followed by activation of the PLC/PKC pathway [184].
4.2.2. Postsynaptic scaffolding proteins
Similar to most membrane receptors and ion channels, ASICs reside in macromolecular complexes. Several ASIC-interacting proteins have been recently identified which regulate the localization and the function of ASICs. For example, the PDZ domain of PICK1 interacts directly with the C-terminal of ASIC1a, ASIC2a and ASIC2b, but not ASIC3 [84,105,106]. Interaction with PICK1 facilitates phosphorylation of the ASIC2a subunit and the enhancement of the ASIC2a current by PKC [84], whereas the interaction between ASIC1a and PICK1 affects the cellular localization of this subunit. The synaptic localization of PICK1 and its interaction with ASICs raised the question of whether ASICs are also localized at the synapse. Indeed, colocalization of ASIC1a with PICK1 has been demonstrated at the synapses of cultured hippocampal neurons [106]. A recent study by Zha and coworkers, performed in hippocampal slices, further confirmed that ASIC1a subunit localizes at dendritic spines, the postsynaptic site of most excitatory synapses [185]. It was demonstrated that, the expression of ASIC1a affects the density of spines. Suppressing the ASIC1a expression reduces the number of spines, whereas over-expressing ASIC1a increases the number of spines. ASIC1a-dependent elevation of intracellular Ca2+ concentration at dendritic spines and an increased activation of Ca2+/calmuduling dependent protein kinase II signaling pathway is likely responsible for the increase of the spine density [185]. Recent studies by the same authors also identified the localization of ASIC2a in dendrites, dendritic spines, and brain synaptosomes. This localization of ASIC2a at dendritic spines largely relies on ASIC2a binding to PSD-95. Through its interaction with PSD-95, ASIC2 increased the localization of ASIC1a, which does not coimmunoprecipitate with PSD-95, in dendritic spines. Thus, it appears that ASIC2a and ASIC1a subunits work in concert to regulate neuronal function [186].
The C-terminus of ASIC3 shares homology with type I PDZ-binding motifs. Channel-interacting PDZ domain protein (CIPP), which contains four PDZ domains, is reported to interact with the ASIC3 C-terminus and increase H+-gated current [187]. The interaction with CIPP enhances the surface expression of ASIC3, and may bring together ASIC3 and functionally related proteins at the membrane of sensory neurons. Additionally, the PDZ-binding motif at the ASIC3 C-terminus also interacts with four other proteins that contain PDZ domains: PSD-95, Lin-7b, MAGI-1b, and PIST [107]. Like ASIC3, these interacting proteins are expressed in DRG and spinal cord. However, the outcome of interactions varies with different PDZ-containing proteins. For instance, co-expression with PSD-95 reduces the amplitude of ASIC3 currents, whereas expression of Lin-7b increases the current. PSD-95 and Lin-7b alter the current density by decreasing or increasing, respectively, the amount of ASIC3 expressed on the cell surface [107].
A kinase-anchoring protein 150 (AKAP150), the neuron specific rodent ortholog of human AKAP79, is present in the postsynaptic density in association with cAMP-dependent PKA, PKC, and calcineurin. These enzymes are associated with AKAP79/150 in inactive states, and the interaction with the anchoring protein has different consequences for the activity of these enzymes. Using pulldown assays and mass spectrometry, Chai and coworkers have identified AKAP150 and the protein phosphatase calcineurin as binding proteins to ASIC2a [188]. Extended pulldown and co-immunoprecipitation assays showed that these regulatory proteins also interact with ASIC1a. Transfection of rat cortical neurons with constructs encoding green fluorescent protein- or hemagglutinin-tagged channels show expression of ASIC1a and ASIC2a in punctate and clustering patterns in dendrites that co-localized with AKAP150. Inhibition of PKA binding to AKAPs by Ht-31 peptide reduces ASIC currents in cortical neurons and CHO cells, suggesting a role for AKAP150 in association with PKA in ASIC function. They also demonstrate a regulatory function for calcineurin in ASIC1a and ASIC2a activity. Cyclosporin A, an inhibitor of calcineurin, increases ASIC currents in CHO cells and in cortical neurons, suggesting that activity of ASICs is inhibited by calcineurin-dependent dephosphorylation. These data imply that ASIC down-regulation by calcineurin could play an important role under pathological conditions accompanying intracellular Ca2+ overload and tissue acidosis to circumvent harmful activities mediated by these channels.
4.2.3. Intracellular pH
Along with pHo, changes in pHi takes place in both physiological and pathological conditions [2,6]. Although pHi drops during ischemia in general, the degree of pHi changes is not homogeneous across all brain regions, and an alkalization of pHi has been demonstrated in cortical penumbra regions following focal ischemia [3]. Changing the level of pHi has been shown previously to modulate the activities of several ion channels [189-191]. Similarly, changes in pHi also affect the activities of ASICs. In cultured mouse cortical neurons, Wang et al., demonstrated that the overall activities of ASICs, including channel activation, inactivation, and recovery from desensitization, are tightly regulated by the level of pHi [192]. They showed that, bath perfusion of quinine, an agent known to cause intracellular alkalization, dramatically increases the amplitude of the ASIC current. In contrast, manipulations that cause intracellular acidification, for example, addition and withdrawal of NH4Cl [189,193] or perfusion of propionate [194,195], produce an opposite effect on the ASIC current. The effects of intracellular alkalizing/acidifying agents are also mimicked by using intracellular solutions with the pH directly buffered at high/low values. In addition to affecting the current amplitude, increasing pHi induces a shift in H+ dose-response curve toward less acidic pH but a shift in the steady-state inactivation curve toward more acidic pH. Furthermore, alkalizing pHi induces an increase in the recovery rate of ASICs from desensitization. Therefore, the overall effect of decreasing pHi is an inhibition of ASIC activity, whereas the effect of increasing pHi is an enhancement of ASIC activity. Consistent with the changes in ASIC current, acid-induced increases of intracellular Ca2+ concentration, membrane depolarization, and acidosis-mediated neuronal injury are dramatically affected by the level of pHi.
Considering the variable, non-uniform changes of pHo and pHi in ischemic brain [3], this study suggests that the activities of ASICs in ischemic brain and ASIC-mediated neuronal injury are non-uniform in different brain regions. In regions where the drop of pHo is accompanied by a normal or alkaline pHi, the activities of ASICs and ASIC-mediated cell injury would be greater than the regions where both pHo and pHi decrease.
5. CONCLUSION
ASICs represent new biological components in peripheral sensory and CNS neurons. Increasing evidence indicates the involvement of these channels in both physiological and pathological processes such as nociception, mechanosensation, taste transduction, synaptic plasticity, learning/memory, and acidosis-mediated neu-rodegeneration. By combining biochemical, molecular biological, structural, and functional studies, much progress has been made in recent years in the comprehension of the mechanisms by which extracellular and intracellular signaling molecules can affect the ASIC activity [196]. Although in most electrophysiological recordings ASIC responses appear to be transient in nature, various biochemical changes, largely occur in pathological conditions such as brain ischemia, dramatically enhance the amplitude, reduce the desensitization, or increase the recovery of ASICs from desensitization. Delineating the mechanisms underlying the modulation of ASICs by signaling molecules is, with no doubt, critical for understanding the physiological/pathological roles of these novel channels and for establishing future therapeutic interventions.
Acknowledgments
XPC is supported by American Heart Association Scientist Development Grant 0735092N, University of Missouri Research Board and University of Missouri-Kansas City School of Medicine Start-up Funding. The work in ZGX's lab is supported in part by NIH R01NS047506, R01NS066027, UL1 RR025008, U54 RR026137, AHA 0840132N, and ALZ IIRG-10-173350.
References
- 1.Chesler M. The regulation and modulation of pH in the nervous system. Prog Neurobiol. 1990;34(5):401–427. doi: 10.1016/0301-0082(90)90034-e. [DOI] [PubMed] [Google Scholar]
- 2.Nedergaard M, Kraig RP, Tanabe J, Pulsinelli WA. Dynamics of interstitial and intracellular pH in evolving brain infarct. Am J Physiol. 1991;260(3 Pt 2):R581–R588. doi: 10.1152/ajpregu.1991.260.3.R581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Back T, Hoehn M, Mies G, Busch E, Schmitz B, Kohno K, Hossmann KA. Penumbral tissue alkalosis in focal cerebral ischemia: relationship to energy metabolism, blood flow, and steady potential. Ann Neurol. 2000;47(4):485–492. [PubMed] [Google Scholar]
- 4.CROWELL JW, KAUFMANN BN. Changes in tissue pH after circulatory arrest. Am J Physiol. 1961;200:743–745. doi: 10.1152/ajplegacy.1961.200.4.743. [DOI] [PubMed] [Google Scholar]
- 5.Ljunggren B, Norberg K, Siesjo BK. Influence of tissue acidosis upon restitution of brain energy metabolism following total ischemia. Brain Res. 1974;77(2):173–186. doi: 10.1016/0006-8993(74)90782-3. [DOI] [PubMed] [Google Scholar]
- 6.Rehncrona S. Brain acidosis. Ann Emerg Med. 1985;14(8):770–776. doi: 10.1016/s0196-0644(85)80055-x. [DOI] [PubMed] [Google Scholar]
- 7.Siesjo BK. Acidosis and ischemic brain damage. Neurochem Pathol. 1988;9:31–88. doi: 10.1007/BF03160355. [DOI] [PubMed] [Google Scholar]
- 8.Siesjo BK, Katsura K, Kristian T. Acidosis-related damage. Adv Neurol. 1996;71:209–233. [PubMed] [Google Scholar]
- 9.Tombaugh GC, Sapolsky RM. Evolving concepts about the role of acidosis in ischemic neuropathology. J Neurochem. 1993;61(3):793–803. doi: 10.1111/j.1471-4159.1993.tb03589.x. [DOI] [PubMed] [Google Scholar]
- 10.Revici E, Stoopen E, Frenk E, Ravich RA. The painful focus. II. The relation of pain to local physico-chemical changes. Bull Inst Appl Biol. 1949;1:21. [Google Scholar]
- 11.Sutherland SP, Cook SP, McCleskey EW. Chemical mediators of pain due to tissue damage and ischemia. Prog Brain Res. 2000;129:21–38. doi: 10.1016/S0079-6123(00)29003-1. [DOI] [PubMed] [Google Scholar]
- 12.Tang CM, Dichter M, Morad M. Modulation of the N-methyl-D-aspartate channel by extracellular H+ Proc Natl Acad Sci U S A. 1990;87(16):6445–6449. doi: 10.1073/pnas.87.16.6445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Traynelis SF, Cull-Candy SG. Proton inhibition of N-methyl-D-aspartate receptors in cerebellar neurons. Nature. 1990;345(6273):347–350. doi: 10.1038/345347a0. [DOI] [PubMed] [Google Scholar]
- 14.Li YF, Wu LJ, Li Y, Xu L, Xu TL. Mechanisms of H+ modulation of glycinergic response in rat sacral dorsal commissural neurons. J Physiol. 2003;552(Pt 1):73–87. doi: 10.1113/jphysiol.2003.047324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Waldmann R, Lazdunski M. H(+)-gated cation channels: neuronal acid sensors in the ENaC/DEG family of ion channels. Curr Opin Neurobiol. 1998;8(3):418–424. doi: 10.1016/s0959-4388(98)80070-6. [DOI] [PubMed] [Google Scholar]
- 16.Waldmann R, Champigny G, Bassilana F, Heurteaux C, Lazdunski M. A proton-gated cation channel involved in acid-sensing. Nature. 1997;386(6621):173–177. doi: 10.1038/386173a0. [DOI] [PubMed] [Google Scholar]
- 17.Baron A, Waldmann R, Lazdunski M. ASIC-like, proton-activated currents in rat hippo-campal neurons. J Physiol. 2002;539(Pt 2):485–494. doi: 10.1113/jphysiol.2001.014837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Price MP, Snyder PM, Welsh MJ. Cloning and expression of a novel human brain Na+ channel. J Biol Chem. 1996;271(14):7879–7882. doi: 10.1074/jbc.271.14.7879. [DOI] [PubMed] [Google Scholar]
- 19.Wemmie JA, Chen J, Askwith CC, Hruska-Hageman AM, Price MP, Nolan BC, Yoder PG, Lamani E, Hoshi T, Freeman JH, Welsh MJ. The acid-activated ion channel ASIC contributes to synaptic plasticity, learning, and memory. Neuron. 2002;34(3):463–477. doi: 10.1016/s0896-6273(02)00661-x. [DOI] [PubMed] [Google Scholar]
- 20.De La Rosa DA, Krueger SR, Kolar A, Shao D, Fitzsimonds RM, Canessa CM. Distribution, subcellular localization and ontogeny of ASIC1 in the mammalian central nervous system. J Physiol. 2003;546(Pt 1):77–87. doi: 10.1113/jphysiol.2002.030692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Krishtal OA, Pidoplichko VI. A receptor for protons in the nerve cell membrane. Neurosci-ence. 1980;5(12):2325–2327. doi: 10.1016/0306-4522(80)90149-9. [DOI] [PubMed] [Google Scholar]
- 22.Kovalchuk Y, Krishtal OA, Nowycky MC. The proton-activated inward current of rat sensory neurons includes a calcium component. Neurosci Lett. 1990;115(2-3):237–242. doi: 10.1016/0304-3940(90)90461-h. [DOI] [PubMed] [Google Scholar]
- 23.Grantyn R, Perouansky M, Rodriguez-Tebar A, Lux HD. Expression of depolarizing voltage- and transmitter-activated currents in neuronal precursor cells from the rat brain is preceded by a proton- activated sodium current. Brain Res Dev Brain Res. 1989;49(1):150–155. doi: 10.1016/0165-3806(89)90070-9. [DOI] [PubMed] [Google Scholar]
- 24.Ueno S, Nakaye T, Akaike N. Proton-induced sodium current in freshly dissociated hypothalamic neurones of the rat. J Physiol (Lond) 1992;447:309–327. doi: 10.1113/jphysiol.1992.sp019004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Varming T. Proton-gated ion channels in cultured mouse cortical neurons. Neuropharmacology. 1999;38(12):1875–1881. doi: 10.1016/s0028-3908(99)00079-9. [DOI] [PubMed] [Google Scholar]
- 26.Alvarez dlR, Krueger SR, Kolar A, Shao D, Fitzsimonds RM, Canessa CM. Distribution, subcellular localization and ontogeny of ASIC1 in the mammalian central nervous system. J Physiol. 2003;546(Pt 1):77–87. doi: 10.1113/jphysiol.2002.030692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Voilley N. Acid-sensing ion channels (ASICs): new targets for the analgesic effects of non-steroid anti-Inflammatory drugs (NSAIDs) Curr Drug Targets Inflamm Allergy. 2004;3(1):71–79. doi: 10.2174/1568010043483980. [DOI] [PubMed] [Google Scholar]
- 28.Xiong ZG, Chu XP, Simon RP. Acid sensing ion channels–novel therapeutic targets for ischemic brain injury. Front Biosci. 2007;12:1376–1386. doi: 10.2741/2154. [DOI] [PubMed] [Google Scholar]
- 29.Wemmie JA, Price MP, Welsh MJ. Acidsensing ion channels: advances, questions and therapeutic opportunities. Trends Neurosci. 2006;29(10):578–586. doi: 10.1016/j.tins.2006.06.014. [DOI] [PubMed] [Google Scholar]
- 30.Xiong ZG, Zhu XM, Chu XP, Minami M, Hey J, Wei WL, MacDonald JF, Wemmie JA, Price MP, Welsh MJ, Simon RP. Neuroprotection in ischemia: blocking calcium-permeable Acidsensing ion channels. Cell. 2004;118(6):687–698. doi: 10.1016/j.cell.2004.08.026. [DOI] [PubMed] [Google Scholar]
- 31.Benveniste M, Dingledine R. Limiting stroke-induced damage by targeting an acid channel. N Engl J Med. 2005;352(1):85–86. doi: 10.1056/NEJMcibr045010. [DOI] [PubMed] [Google Scholar]
- 32.Huang Y, McNamara JO. Ischemic stroke: “acidotoxicity” is a perpetrator. Cell. 2004;118(6):665–666. doi: 10.1016/j.cell.2004.09.004. [DOI] [PubMed] [Google Scholar]
- 33.Yermolaieva O, Leonard AS, Schnizler MK, Abboud FM, Welsh MJ. Extracellular acidosis increases neuronal cell calcium by activating acid-sensing ion channel 1a. Proc Natl Acad Sci U S A. 2004;101(17):6752–6757. doi: 10.1073/pnas.0308636100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Yu Y, Chen Z, Li WG, Cao H, Feng EG, Yu F, Liu H, Jiang H, Xu TL. A nonproton ligand sensor in the acid-sensing ion channel. Neuron. 2010;68(1):61–72. doi: 10.1016/j.neuron.2010.09.001. [DOI] [PubMed] [Google Scholar]
- 35.Alvarez dlR, Canessa CM, Fyfe GK, Zhang P. Structure and regulation of amiloridesensitive sodium channels. Annu Rev Physiol. 2000;62:573–594. doi: 10.1146/annurev.physiol.62.1.573. [DOI] [PubMed] [Google Scholar]
- 36.Krishtal O. The ASICs: signaling molecules? Modulators? Trends Neurosci. 2003;26(9):477–483. doi: 10.1016/S0166-2236(03)00210-8. [DOI] [PubMed] [Google Scholar]
- 37.Grifoni SC, Jernigan NL, Hamilton G, Drummond HA. ASIC proteins regulate smooth muscle cell migration. Microvasc Res. 2008;75(2):202–210. doi: 10.1016/j.mvr.2007.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Jahr H, van Driel M, van Osch GJ, Weinans H, van Leeuwen JP. Identification of acidsensing ion channels in bone. Biochem Biophys Res Commun. 2005;337(1):349–354. doi: 10.1016/j.bbrc.2005.09.054. [DOI] [PubMed] [Google Scholar]
- 39.Grunder S, Chen X. Structure, function, and pharmacology of acid-sensing ion channels (ASICs): focus on ASIC1a. Int J Physiol Pathophysiol Pharmacol. 2010;2(1):73–94. [PMC free article] [PubMed] [Google Scholar]
- 40.Bassler EL, Ngo-Anh TJ, Geisler HS, Ruppersberg JP, Grunder S. Molecular and functional characterization of acid-sensing ion channel (ASIC) 1b. J Biol Chem. 2001;276(36):33782–33787. doi: 10.1074/jbc.M104030200. [DOI] [PubMed] [Google Scholar]
- 41.Jasti J, Furukawa H, Gonzales EB, Gouaux E. Structure of acid-sensing ion channel 1 at 1.9 A resolution and low pH. Nature. 2007;449(7160):316–323. doi: 10.1038/nature06163. [DOI] [PubMed] [Google Scholar]
- 42.Bevan S, Yeats J. Protons activate a cation conductance in a sub-population of rat dorsal root ganglion neurones. J Physiol (Lond) 1991;433:145–161. doi: 10.1113/jphysiol.1991.sp018419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Krishtal OA, Pidoplichko VI. A receptor for protons in the membrane of sensory neurons may participate in nociception. Neuroscience. 1981;6(12):2599–2601. doi: 10.1016/0306-4522(81)90105-6. [DOI] [PubMed] [Google Scholar]
- 44.Ugawa S, Ueda T, Ishida Y, Nishigaki M, Shibata Y, Shimada S. Amiloride-blockable acid-sensing ion channels are leading acid sensors expressed in human nociceptors. J Clin Invest. 2002;110(8):1185–1190. doi: 10.1172/JCI15709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Sluka KA, Price MP, Breese NM, Stucky CL, Wemmie JA, Welsh MJ. Chronic hyperalgesia induced by repeated acid injections in muscle is abolished by the loss of ASIC3, but not ASIC1. Pain. 2003;106(3):229–239. doi: 10.1016/S0304-3959(03)00269-0. [DOI] [PubMed] [Google Scholar]
- 46.Chen CC, Zimmer A, Sun WH, Hall J, Brownstein MJ, Zimmer A. A role for ASIC3 in the modulation of high-intensity pain stimuli. Proc Natl Acad Sci U S A. 2002;99(13):8992–8997. doi: 10.1073/pnas.122245999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Wu LJ, Duan B, Mei YD, Gao J, Chen JG, Zhuo M, Xu L, Wu M, Xu TL. Characterization of acid-sensing ion channels in dorsal horn neurons of rat spinal cord. J Biol Chem. 2004;279(42):43716–43724. doi: 10.1074/jbc.M403557200. [DOI] [PubMed] [Google Scholar]
- 48.Benson CJ, Eckert SP, McCleskey EW. Acid-evoked currents in cardiac sensory neurons: A possible mediator of myocardial ischemic sensation. Circ Res. 1999;84(8):921–928. doi: 10.1161/01.res.84.8.921. [DOI] [PubMed] [Google Scholar]
- 49.Price MP, Lewin GR, McIlwrath SL, Cheng C, Xie J, Heppenstall PA, Stucky CL, Mannsfeldt AG, Brennan TJ, Drummond HA, Qiao J, Benson CJ, Tarr DE, Hrstka RF, Yang B, Williamson RA, Welsh MJ. The mammalian sodium channel BNC1 is required for normal touch sensation. Nature. 2000;407(6807):1007–1011. doi: 10.1038/35039512. [DOI] [PubMed] [Google Scholar]
- 50.Price MP, McIlwrath SL, Xie J, Cheng C, Qiao J, Tarr DE, Sluka KA, Brennan TJ, Lewin GR, Welsh MJ. The DRASIC cation channel contributes to the detection of cutaneous touch and acid stimuli in mice. Neuron. 2001;32(6):1071–1083. doi: 10.1016/s0896-6273(01)00547-5. [DOI] [PubMed] [Google Scholar]
- 51.Page AJ, Brierley SM, Martin CM, Price MP, Symonds E, Butler R, Wemmie JA, Blackshaw LA. Different contributions of ASIC channels 1a, 2, and 3 in gastrointestinal mechanosensory function. Gut. 2005;54(10):1408–1415. doi: 10.1136/gut.2005.071084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Ugawa S, Yamamoto T, Ueda T, Ishida Y, Inagaki A, Nishigaki M, Shimada S. Amilorideinsensitive currents of the acid-sensing ion channel-2a (ASIC2a)/ASIC2b heteromeric sour-taste receptor channel. J Neurosci. 2003;23(9):3616–3622. doi: 10.1523/JNEUROSCI.23-09-03616.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Ugawa S. Identification of sour-taste receptor genes. Anat Sci Int. 2003;78(4):205–210. doi: 10.1046/j.0022-7722.2003.00062.x. [DOI] [PubMed] [Google Scholar]
- 54.Lin W, Ogura T, Kinnamon SC. Acidactivated cation currents in rat vallate taste receptor cells. J Neurophysiol. 2002;88(1):133–141. doi: 10.1152/jn.2002.88.1.133. [DOI] [PubMed] [Google Scholar]
- 55.Wemmie JA, Askwith CC, Lamani E, Cassell MD, Freeman JH, Jr, Welsh MJ. Acidsensing ion channel 1 is localized in brain regions with high synaptic density and contributes to fear conditioning. J Neurosci. 2003;23(13):5496–5502. doi: 10.1523/JNEUROSCI.23-13-05496.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Ettaiche M, Deval E, Cougnon M, Lazdunski M, Voilley N. Silencing acid-sensing ion channel 1a alters cone-mediated retinal function. J Neurosci. 2006;26(21):5800–5809. doi: 10.1523/JNEUROSCI.0344-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Render JA, Howe KR, Wunsch AM, Guionaud S, Cox PJ, Wemmie JA. Histologic examination of the eye of acid-sensing ion channel 1a knockout mice. Int J Physiol Pathophysiol Pharmacol. 2010;2(1):69–72. [PMC free article] [PubMed] [Google Scholar]
- 58.Ettaiche M, Guy N, Hofman P, Lazdunski M, Waldmann R. Acid-sensing ion channel 2 is important for retinal function and protects against light-induced retinal degeneration. J Neurosci. 2004;24(5):1005–1012. doi: 10.1523/JNEUROSCI.4698-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Lu Y, Ma X, Sabharwal R, Snitsarev V, Morgan D, Rahmouni K, Drummond HA, Whiteis CA, Costa V, Price M, Benson C, Welsh MJ, Chapleau MW, Abboud FM. The ion channel ASIC2 is required for baroreceptor and autonomic control of the circulation. Neuron. 2009;64(6):885–897. doi: 10.1016/j.neuron.2009.11.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Johnson MB, Jin K, Minami M, Chen D, Simon RP. Global ischemia induces expression of acid-sensing ion channel 2a in rat brain. J Cereb Blood Flow Metab. 2001;21(6):734–740. doi: 10.1097/00004647-200106000-00011. [DOI] [PubMed] [Google Scholar]
- 61.Gao J, Duan B, Wang DG, Deng XH, Zhang GY, Xu L, Xu TL. Coupling between NMDA receptor and acid-sensing ion channel contributes to ischemic neuronal death. Neuron. 2005;48(4):635–646. doi: 10.1016/j.neuron.2005.10.011. [DOI] [PubMed] [Google Scholar]
- 62.Pignataro G, Cuomo O, Esposito E, Sirabella R, Di Renzo G, Annunziato L. ASIC1a contributes to neuroprotection elicited by ischemic preconditioning and postconditioning. Int J Physiol Pathophysiol Pharmacol. 2011;3(1):1–8. [PMC free article] [PubMed] [Google Scholar]
- 63.Friese MA, Craner MJ, Etzensperger R, Vergo S, Wemmie JA, Welsh MJ, Vincent A, Fugger L. Acid-sensing ion channel-1 contributes to axonal degeneration in autoimmune inflammation of the central nervous system. Nat Med. 2007;13(12):1483–1489. doi: 10.1038/nm1668. [DOI] [PubMed] [Google Scholar]
- 64.Li MH, Inoue K, Si HF, Xiong ZG. Calciumpermeable ion channels involved in glutamate receptor-independent ischemic brain injury. Acta Pharmacol Sin. 2011;32(6):734–740. doi: 10.1038/aps.2011.47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Berdiev BK, Xia J, McLean LA, Markert JM, Gillespie GY, Mapstone TB, Naren AP, Jovov B, Bubien JK, Ji HL, Fuller CM, Kirk KL, Benos DJ. Acid-sensing ion channels in malignant gliomas. J Biol Chem. 2003;278(17):15023–15034. doi: 10.1074/jbc.M300991200. [DOI] [PubMed] [Google Scholar]
- 66.Li M, Xiong ZG. Ion channels as targets for cancer therapy. Int J Physiol Pathophysiol Pharmacol. 2011;3(2):156–166. [PMC free article] [PubMed] [Google Scholar]
- 67.Mamet J, Baron A, Lazdunski M, Voilley N. Proinflammatory mediators, stimulators of sensory neuron excitability via the expression of acid-sensing ion channels. J Neurosci. 2002;22(24):10662–10670. doi: 10.1523/JNEUROSCI.22-24-10662.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Voilley N, de Weille J, Mamet J, Lazdunski M. Nonsteroid anti-inflammatory drugs inhibit both the activity and the inflammationinduced expression of acid-sensing ion channels in nociceptors. J Neurosci. 2001;21(20):8026–8033. doi: 10.1523/JNEUROSCI.21-20-08026.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Zhang GC, Mao LM, Wang JQ, Chu XP. Upregulation of acid-sensing ion channel 1 protein expression by chronic administration of cocaine in the mouse striatum in vivo. Neurosci Lett. 2009;459(3):119–122. doi: 10.1016/j.neulet.2009.05.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Suman A, Mehta B, Guo ML, Chu XP, Fibuch EE, Mao LM, Wang JQ. Alterations in subcellular expression of acid-sensing ion channels in the rat forebrain following chronic amphetamine administration. Neurosci Res. 2010;68(1):1–8. doi: 10.1016/j.neures.2010.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Immke DC, McCleskey EW. Lactate enhances the acid-sensing Na+ channel on ischemia-sensing neurons. Nat Neurosci. 2001;4(9):869–870. doi: 10.1038/nn0901-869. [DOI] [PubMed] [Google Scholar]
- 72.Allen NJ, Attwell D. Modulation of ASIC channels in rat cerebellar Purkinje neurons by ischemia-related signals. J Physiol (Lond) 2002;543(2):521–529. doi: 10.1113/jphysiol.2002.020297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Askwith CC, Cheng C, Ikuma M, Benson C, Price MP, Welsh MJ. Neuropeptide FF and FMRFamide potentiate acid-evoked currents from sensory neurons and proton-gated DEG/ENaC channels. Neuron. 2000;26(1):133–141. doi: 10.1016/s0896-6273(00)81144-7. [DOI] [PubMed] [Google Scholar]
- 74.Ostrovskaya O, Moroz L, Krishtal O. Modulatory action of RFamide-related peptides on acid-sensing ionic channels is pH dependent: the role of arginine. J Neurochem. 2004;91(1):252–255. doi: 10.1111/j.1471-4159.2004.02688.x. [DOI] [PubMed] [Google Scholar]
- 75.Sherwood TW, Askwith CC. Dynorphin opioid peptides enhance acid-sensing ion channel 1a activity and acidosis-induced neuronal death. J Neurosci. 2009;29(45):14371–14380. doi: 10.1523/JNEUROSCI.2186-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Duan B, Wang YZ, Yang T, Chu XP, Yu Y, Huang Y, Cao H, Hansen J, Simon RP, Zhu MX, Xiong ZG, Xu TL. Extracellular spermine exacerbates ischemic neuronal injury through sensitization of ASIC1a channels to extracellular acidosis. J Neurosci. 2011;31(6):2101–2112. doi: 10.1523/JNEUROSCI.4351-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Vukicevic M, Kellenberger S. Modulatory effects of acid-sensing ion channels on action potential generation in hippocampal neurons. Am J Physiol Cell Physiol. 2004;287(3):C682–C690. doi: 10.1152/ajpcell.00127.2004. [DOI] [PubMed] [Google Scholar]
- 78.Chen CC, England S, Akopian AN, Wood JN. A sensory neuron-specific, proton-gated ion channel. Proc Natl Acad Sci U S A. 1998;95(17):10240–10245. doi: 10.1073/pnas.95.17.10240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Bassilana F, Champigny G, Waldmann R, De Weille JR, Heurteaux C, Lazdunski M. The acid-sensitive ionic channel subunit ASIC and the mammalian degenerin MDEG form a heteromultimeric H+-gated Na+ channel with novel properties. J Biol Chem. 1997;272(46):28819–28822. doi: 10.1074/jbc.272.46.28819. [DOI] [PubMed] [Google Scholar]
- 80.Lingueglia E, De Weille JR, Bassilana F, Heurteaux C, Sakai H, Waldmann R, Lazdunski M. A modulatory subunit of acid sensing ion channels in brain and dorsal root ganglion cells. J Biol Chem. 1997;272(47):29778–29783. doi: 10.1074/jbc.272.47.29778. [DOI] [PubMed] [Google Scholar]
- 81.Champigny G, Voilley N, Waldmann R, Lazdunski M. Mutations causing neurodegeneration in Caenorhabditis elegans drastically alter the pH sensitivity and inactivation of the mammalian H+-gated Na+ channel MDEG1. J Biol Chem. 1998;273(25):15418–15422. doi: 10.1074/jbc.273.25.15418. [DOI] [PubMed] [Google Scholar]
- 82.Dube GR, Lehto SG, Breese NM, Baker SJ, Wang X, Matulenko MA, Honore P, Stewart AO, Moreland RB, Brioni JD. Electrophysiological and in vivo characterization of A-317567, a novel blocker of acid sensing ion channels. Pain. 2005;117(1-2):88–96. doi: 10.1016/j.pain.2005.05.021. [DOI] [PubMed] [Google Scholar]
- 83.Escoubas P, De Weille JR, Lecoq A, Diochot S, Waldmann R, Champigny G, Moinier D, Menez A, Lazdunski M. Isolation of a tarantula toxin specific for a class of proton-gated Na+ channels. J Biol Chem. 2000;275(33):25116–25121. doi: 10.1074/jbc.M003643200. [DOI] [PubMed] [Google Scholar]
- 84.Baron A, Deval E, Salinas M, Lingueglia E, Voilley N, Lazdunski M. Protein kinase C stimulates the acid-sensing ion channel ASIC2a via the PDZ domain-containing protein PICK1. J Biol Chem. 2002;277(52):50463–50468. doi: 10.1074/jbc.M208848200. [DOI] [PubMed] [Google Scholar]
- 85.Chen X, Kalbacher H, Grunder S. The tarantula toxin psalmotoxin 1 inhibits acidsensing ion channel (ASIC) 1a by increasing its apparent H+ affinity. J Gen Physiol. 2005;126(1):71–79. doi: 10.1085/jgp.200509303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Chen X, Kalbacher H, Grunder S. Interaction of acid-sensing ion channel (ASIC) 1 with the tarantula toxin psalmotoxin 1 is state dependent. J Gen Physiol. 2006;127(3):267–276. doi: 10.1085/jgp.200509409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Salinas M, Rash LD, Baron A, Lambeau G, Escoubas P, Lazdunski M. The receptor site of the spider toxin PcTx1 on the protongated cation channel ASIC1a. J Physiol. 2006;570(Pt 2):339–354. doi: 10.1113/jphysiol.2005.095810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Diochot S, Baron A, Rash LD, Deval E, Escoubas P, Scarzello S, Salinas M, Lazdunski M. A new sea anemone peptide, APETx2, inhibits ASIC3, a major acid-sensitive channel in sensory neurons. EMBO J. 2004;23(7):1516–1525. doi: 10.1038/sj.emboj.7600177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Chagot B, Escoubas P, Diochot S, Bernard C, Lazdunski M, Darbon H. Solution structure of APETx2, a specific peptide inhibitor of ASIC3 proton-gated channels. Protein Sci. 2005;14(8):2003–2010. doi: 10.1110/ps.051378905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Pidoplichko VI, Dani JA. Acid-sensitive ionic channels in midbrain dopamine neurons are sensitive to ammonium, which may contribute to hyperammonemia damage. Proc Natl Acad Sci U S A. 2006;103(30):11376–11380. doi: 10.1073/pnas.0600768103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Baron A, Schaefer L, Lingueglia E, Champigny G, Lazdunski M. Zn2+ and H+ are coactivators of acid-sensing ion channels. J Biol Chem. 2001;276(38):35361–35367. doi: 10.1074/jbc.M105208200. [DOI] [PubMed] [Google Scholar]
- 92.Chu XP, Wemmie JA, Wang WZ, Zhu XM, Saugstad JA, Price MP, Simon RP, Xiong ZG. Subunit-dependent high-affinity zinc inhibition of acid-sensing ion channels. J Neurosci. 2004;24(40):8678–8689. doi: 10.1523/JNEUROSCI.2844-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Gao J, Wu LJ, Xu L, Xu TL. Properties of the proton-evoked currents and their modulation by Ca2+ and Zn2+ in the acutely dissociated hippocampus CA1 neurons. Brain Res. 2004;1017(1-2):197–207. doi: 10.1016/j.brainres.2004.05.046. [DOI] [PubMed] [Google Scholar]
- 94.Babini E, Paukert M, Geisler HS, Grunder S. Alternative splicing and interaction with diand polyvalent cations control the dynamic range of acid-sensing ion channel 1 (ASIC1) J Biol Chem. 2002;277(44):41597–41603. doi: 10.1074/jbc.M205877200. [DOI] [PubMed] [Google Scholar]
- 95.Babinski K, Catarsi S, Biagini G, Seguela P. Mammalian ASIC2a and ASIC3 subunits coassemble into heteromeric proton- gated channels sensitive to Gd3+ J Biol Chem. 2000;275(37):28519–28525. doi: 10.1074/jbc.M004114200. [DOI] [PubMed] [Google Scholar]
- 96.Immke DC, McCleskey EW. Protons open acid-sensing ion channels by catalyzing relief of Ca2+ blockade. Neuron. 2003;37(1):75–84. doi: 10.1016/s0896-6273(02)01130-3. [DOI] [PubMed] [Google Scholar]
- 97.Zhang P, Sigworth FJ, Canessa CM. Gating of acid-sensitive ion channel-1: release of Ca2+ block vs. allosteric mechanism. J Gen Physiol. 2006;127(2):109–117. doi: 10.1085/jgp.200509396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Andrey F, Tsintsadze T, Volkova T, Lozovaya N, Krishtal O. Acid sensing ionic channels: modulation by redox reagents. Biochim Biophys Acta. 2005;1745(1):1–6. doi: 10.1016/j.bbamcr.2005.01.008. [DOI] [PubMed] [Google Scholar]
- 99.Chu XP, Close N, Saugstad JA, Xiong ZG. ASIC1a-specific modulation of acid-sensing ion channels in mouse cortical neurons by redox reagents. J Neurosci. 2006;26(20):5329–5339. doi: 10.1523/JNEUROSCI.0938-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Xie J, Price MP, Wemmie JA, Askwith CC, Welsh MJ. ASIC3 and ASIC1 mediate FMRFamide-related peptide enhancement of H+-gated currents in cultured dorsal root ganglion neurons. J Neurophysiol. 2003;89(5):2459–2465. doi: 10.1152/jn.00707.2002. [DOI] [PubMed] [Google Scholar]
- 101.Catarsi S, Babinski K, Seguela P. Selective modulation of heteromeric ASIC protongated channels by neuropeptide FF. Neuropharmacology. 2001;41(5):592–600. doi: 10.1016/s0028-3908(01)00107-1. [DOI] [PubMed] [Google Scholar]
- 102.Poirot O, Vukicevic M, Boesch A, Kellenberger S. Selective regulation of acid-sensing ion channel 1 by serine proteases. J Biol Chem. 2004;279(37):38448–38457. doi: 10.1074/jbc.M407381200. [DOI] [PubMed] [Google Scholar]
- 103.Vukicevic M, Weder G, Boillat A, Boesch A, Kellenberger S. Trypsin cleaves acid-sensing ion channel 1a in a domain that is critical for channel gating. J Biol Chem. 2006;281(2):714–722. doi: 10.1074/jbc.M510472200. [DOI] [PubMed] [Google Scholar]
- 104.Donier E, Rugiero F, Okuse K, Wood JN. Annexin II light chain p11 promotes functional expression of acid-sensing ion channel ASIC1a. J Biol Chem. 2005;280(46):38666–38672. doi: 10.1074/jbc.M505981200. [DOI] [PubMed] [Google Scholar]
- 105.Duggan A, Garcia-Anoveros J, Corey DP. The PDZ domain protein PICK1 and the sodium channel BNaC1 interact and localize at mechanosensory terminals of dorsal root ganglion neurons and dendrites of central neurons. J Biol Chem. 2002;277(7):5203–5208. doi: 10.1074/jbc.M104748200. [DOI] [PubMed] [Google Scholar]
- 106.Hruska-Hageman AM, Wemmie JA, Price MP, Welsh MJ. Interaction of the synaptic protein PICK1 (protein interacting with C kinase 1) with the non-voltage gated sodium channels BNC1 (brain Na+ channel 1) and ASIC (acid-sensing ion channel) Biochem J. 2002;361(Pt 3):443–450. doi: 10.1042/0264-6021:3610443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Hruska-Hageman AM, Benson CJ, Leonard AS, Price MP, Welsh MJ. PSD-95 and Lin-7b interact with acid-sensing ion channel-3 and have opposite effects on H+- gated current. J Biol Chem. 2004;279(45):46962–46968. doi: 10.1074/jbc.M405874200. [DOI] [PubMed] [Google Scholar]
- 108.Leonard AS, Yermolaieva O, Hruska-Hageman A, Askwith CC, Price MP, Wemmie JA, Welsh MJ. cAMP-dependent protein kinase phosphorylation of the acid-sensing ion channel-1 regulates its binding to the protein interacting with C-kinase-1. Proc Natl Acad Sci U S A. 2003;100(4):2029–2034. doi: 10.1073/pnas.252782799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Deval E, Salinas M, Baron A, Lingueglia E, Lazdunski M. ASIC2b-dependent regulation of ASIC3, an essential acid-sensing ion channel subunit in sensory neurons via the partner protein PICK-1. J Biol Chem. 2004;279(19):19531–19539. doi: 10.1074/jbc.M313078200. [DOI] [PubMed] [Google Scholar]
- 110.Kusama N, Harding AM, Benson CJ. Extracellular chloride modulates the desensitization kinetics of acid-sensing ion channel 1a (ASIC1a) J Biol Chem. 2010;285(23):17425–17431. doi: 10.1074/jbc.M109.091561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Paukert M, Babini E, Pusch M, Grunder S. Identification of the Ca2+ blocking site of acidsensing ion channel (ASIC) 1: implications for channel gating. J Gen Physiol. 2004;124(4):383–394. doi: 10.1085/jgp.200308973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.de Weille J, Bassilana F. Dependence of the acid-sensitive ion channel, ASIC1a, on extracellular Ca(2+) ions. Brain Res. 2001;900(2):277–281. doi: 10.1016/s0006-8993(01)02345-9. [DOI] [PubMed] [Google Scholar]
- 113.Wang W, Duan B, Xu H, Xu L, Xu TL. Calcium-permeable acid-sensing ion channel is a molecular target of the neurotoxic metal ion lead. J Biol Chem. 2006;281(5):2497–2505. doi: 10.1074/jbc.M507123200. [DOI] [PubMed] [Google Scholar]
- 114.Zhang Y, McBride DWJ, Hamill OP. The ion selectivity of a membrane conductance inactivated by extracellular calcium in Xenopus oocytes. J Physiol (Lond) 1998;508(Pt 3):763–776. doi: 10.1111/j.1469-7793.1998.763bp.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Xiong Z, Lu W, MacDonald JF. Extracellular calcium sensed by a novel cation channel in hippocampal neurons. Proc Natl Acad Sci U S A. 1997;94(13):7012–7017. doi: 10.1073/pnas.94.13.7012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Assaf SY, Chung SH. Release of endogenous Zn2+ from brain tissue during activity. Nature. 1984;308(5961):734–736. doi: 10.1038/308734a0. [DOI] [PubMed] [Google Scholar]
- 117.Howell GA, Welch MG, Frederickson CJ. Stimulation-induced uptake and release of zinc in hippocampal slices. Nature. 1984;308(5961):736–738. doi: 10.1038/308736a0. [DOI] [PubMed] [Google Scholar]
- 118.Poirot O, Berta T, Decosterd I, Kellenberger S. Distinct ASIC currents are expressed in rat putative nociceptors and are modulated by nerve injury. J Physiol. 2006;576(Pt 1):215–234. doi: 10.1113/jphysiol.2006.113035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Jiang Q, Papasian CJ, Wang JQ, Xiong ZG, Chu XP. Inhibitory regulation of acid-sensing ion channel 3 by zinc. Neuroscience. 2010;169(2):574–583. doi: 10.1016/j.neuroscience.2010.05.043. [DOI] [PubMed] [Google Scholar]
- 120.Jiang Q, Inoue K, Wu X, Papasian CJ, Wang JQ, Xiong ZG, Chu XP. Cysteine 149 in the extracellular finger domain of acid-sensing ion channel 1b subunit is critical for zincmediated inhibition. Neuroscience. 2011;193:89–99. doi: 10.1016/j.neuroscience.2011.07.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Wang W, Yu Y, Xu TL. Modulation of acidsensing ion channels by Cu2+ in cultured hypothalamic neurons of the rat. Neuroscienc. 2007;16:631–641. doi: 10.1016/j.neuroscience.2006.12.009. [DOI] [PubMed] [Google Scholar]
- 122.Staruschenko A, Dorofeeva NA, Bolshakov KV, Stockand JD. Subunit-dependent cadmium and nickel inhibition of acid-sensing ion channels. J Neurobiol. 2006;67:97–107. doi: 10.1002/dneu.20338. [DOI] [PubMed] [Google Scholar]
- 123.Hamill OP, McBride DW., Jr The pharmacology of mechanogated membrane ion channels. Pharmacol Rev. 1996;48(2):231–252. [PubMed] [Google Scholar]
- 124.Aizenman E, Lipton SA, Loring RH. Selective modulation of NMDA responses by reduction and oxidation. Neuron. 1989;2(3):1257–1263. doi: 10.1016/0896-6273(89)90310-3. [DOI] [PubMed] [Google Scholar]
- 125.Kohr G, Eckardt S, Luddens H, Monyer H, Seeburg PH. NMDA receptor channels: subunit- specific potentiation by reducing agents. Neuron. 1994;12(5):1031–1040. doi: 10.1016/0896-6273(94)90311-5. [DOI] [PubMed] [Google Scholar]
- 126.DiChiara TJ, Reinhart PH. Redox modulation of hslo Ca2+-activated K+ channels. J Neurosci. 1997;17(13):4942–4955. doi: 10.1523/JNEUROSCI.17-13-04942.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Amato A, Connolly CN, Moss SJ, Smart TG. Modulation of neuronal and recombinant GABAA receptors by redox reagents. J Physiol. 1999;517(Pt 1):35–50. doi: 10.1111/j.1469-7793.1999.0035z.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Todorovic SM, Jevtovic-Todorovic V, Meyenburg A, Mennerick S, Perez-Reyes E, Romano C, Olney JW, Zorumski CF. Redox modulation of T-type calcium channels in rat peripheral nociceptors. Neuron. 2001;31(1):75–85. doi: 10.1016/s0896-6273(01)00338-5. [DOI] [PubMed] [Google Scholar]
- 129.Schulz JB, Lindenau J, Seyfried J, Dichgans J. Glutathione, oxidative stress and neurodegeneration. Eur J Biochem. 2000;267(16):4904–4911. doi: 10.1046/j.1432-1327.2000.01595.x. [DOI] [PubMed] [Google Scholar]
- 130.Nakamura H, Nakamura K, Yodoi J. Redox regulation of cellular activation. Annu Rev Immunol. 1997;15:351–369. doi: 10.1146/annurev.immunol.15.1.351. [DOI] [PubMed] [Google Scholar]
- 131.Ueda S, Masutani H, Nakamura H, Tanaka T, Ueno M, Yodoi J. Redox control of cell death. Antioxid Redox Signal. 2002;4(3):405–414. doi: 10.1089/15230860260196209. [DOI] [PubMed] [Google Scholar]
- 132.Cho JH, Askwith CC. Potentiation of acidsensing ion channels by sulfhydryl compounds. Am J Physiol Cell Physiol. 2007;292(6):C2161–C2174. doi: 10.1152/ajpcell.00598.2006. [DOI] [PubMed] [Google Scholar]
- 133.Zha XM, Wang R, Collier DM, Snyder PM, Wemmie JA, Welsh MJ. Oxidant regulated inter-subunit disulfide bond formation between ASIC1a subunits. Proc Natl Acad Sci U S A. 2009;106(9):3573–3578. doi: 10.1073/pnas.0813402106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Jaffrey SR, Erdjument-Bromage H, Ferris CD, Tempst P, Snyder SH. Protein Snitrosylation: a physiological signal for neuronal nitric oxide. Nat Cell Biol. 2001;3(2):193–197. doi: 10.1038/35055104. [DOI] [PubMed] [Google Scholar]
- 135.Cadiou H, Studer M, Jones NG, Smith ES, Ballard A, McMahon SB, McNaughton PA. Modulation of acid-sensing ion channel activity by nitric oxide. J Neurosci. 2007;27(48):13251–13260. doi: 10.1523/JNEUROSCI.2135-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Lingueglia E, Deval E, Lazdunski M. FMRFamide-gated sodium channel and ASIC channels: a new class of ionotropic receptors for FMRFamide and related peptides. Peptides. 2006;27(5):1138–1152. doi: 10.1016/j.peptides.2005.06.037. [DOI] [PubMed] [Google Scholar]
- 137.Xie J, Price MP, Berger AL, Welsh MJ. DRASIC contributes to pH-gated currents in large dorsal root ganglion sensory neurons by forming heteromultimeric channels. J Neurophysiol. 2002;87(6):2835–2843. doi: 10.1152/jn.2002.87.6.2835. [DOI] [PubMed] [Google Scholar]
- 138.Askwith CC, Wemmie JA, Price MP, Rokhlina T, Welsh MJ. ASIC2 modulates ASIC1 H+- activated currents in hippocampal neurons. J Biol Chem. 2004;279(18):18296–18305. doi: 10.1074/jbc.M312145200. [DOI] [PubMed] [Google Scholar]
- 139.Deval E, Baron A, Lingueglia E, Mazarguil H, Zajac JM, Lazdunski M. Effects of neuropeptide SF and related peptides on acid sensing ion channel 3 and sensory neuron excitability. Neuropharmacology. 2003;44(5):662–671. doi: 10.1016/s0028-3908(03)00047-9. [DOI] [PubMed] [Google Scholar]
- 140.Chen X, Paukert M, Kadurin I, Pusch M, Grunder S. Strong modulation by RFamide neuropeptides of the ASIC1b/3 heteromer in competition with extracellular calcium. Neuropharmacology. 2006;50(8):964–974. doi: 10.1016/j.neuropharm.2006.01.007. [DOI] [PubMed] [Google Scholar]
- 141.Steen KH, Reeh PW. Sustained graded pain and hyperalgesia from harmless experimental tissue acidosis in human skin. Neurosci Lett. 1993;154(1-2):113–116. doi: 10.1016/0304-3940(93)90184-m. [DOI] [PubMed] [Google Scholar]
- 142.Steen KH, Issberner U, Reeh PW. Pain due to experimental acidosis in human skin: evidence for non-adapting nociceptor excitation. Neurosci Lett. 1995;199(1):29–32. doi: 10.1016/0304-3940(95)12002-l. [DOI] [PubMed] [Google Scholar]
- 143.Issberner U, Reeh PW, Steen KH. Pain due to tissue acidosis: a mechanism for inflammatory and ischemic myalgia? Neurosci Lett. 1996;208(3):191–194. doi: 10.1016/0304-3940(96)12576-3. [DOI] [PubMed] [Google Scholar]
- 144.Jones NG, Slater R, Cadiou H, McNaughton P, McMahon SB. Acid-induced pain and its modulation in humans. J Neurosci. 2004;24(48):10974–10979. doi: 10.1523/JNEUROSCI.2619-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Ballou LR, Botting RM, Goorha S, Zhang J, Vane JR. Nociception in cyclooxygenase isozyme-deficient mice. Proc Natl Acad Sci U S A. 2000;97(18):10272–10276. doi: 10.1073/pnas.180319297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Brune K, Beck WS, Geisslinger G, Menzel-Soglowek S, Peskar BM, Peskar BA. Aspirin-like drugs may block pain independently of prostaglandin synthesis inhibition. Experientia. 1991;47(3):257–261. doi: 10.1007/BF01958153. [DOI] [PubMed] [Google Scholar]
- 147.Tonussi CR, Ferreira SH. Mechanism of diclofenac analgesia: direct blockade of inflammatory sensitization. Eur J Pharmacol. 1994;251(2-3):173–179. doi: 10.1016/0014-2999(94)90398-0. [DOI] [PubMed] [Google Scholar]
- 148.Steen KH, Reeh PW, Kreysel HW. Dosedependent competitive block by topical acetylsalicylic and salicylic acid of low pH-induced cutaneous pain. Pain. 1996;64(1):71–82. doi: 10.1016/0304-3959(95)00087-9. [DOI] [PubMed] [Google Scholar]
- 149.Dray A. Inflammatory mediators of pain. Br J Anaesth. 1995;75(2):125–131. doi: 10.1093/bja/75.2.125. [DOI] [PubMed] [Google Scholar]
- 150.Kress M, Reeh PW, Vyklicky L. An interaction of inflammatory mediators and protons in small diameter dorsal root ganglion neurons of the rat. Neurosci Lett. 1997;224(1):37–40. doi: 10.1016/s0304-3940(97)13450-4. [DOI] [PubMed] [Google Scholar]
- 151.Steen KH, Steen AE, Kreysel HW, Reeh PW. Inflammatory mediators potentiate pain induced by experimental tissue acidosis. Pain. 1996;66(2-3):163–170. doi: 10.1016/0304-3959(96)03034-5. [DOI] [PubMed] [Google Scholar]
- 152.Bevan S, Winter J. Nerve growth factor (NGF) differentially regulates the chemosensitivity of adult rat cultured sensory neurons. J Neurosci. 1995;15(7 Pt 1):4918–4926. doi: 10.1523/JNEUROSCI.15-07-04918.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Garza A, Lopez-Ramirez O, Vega R, Soto E. The aminoglycosides modulate the acidsensing ionic channel currents in dorsal root ganglion neurons from the rat. J Pharmacol Exp Ther. 2010;332(2):489–499. doi: 10.1124/jpet.109.152884. [DOI] [PubMed] [Google Scholar]
- 154.Chen X, Qiu L, Li M, Durrnagel S, Orser BA, Xiong ZG, MacDonald JF. Diarylamidines: high potency inhibitors of acid-sensing ion channels. Neuropharmacology. 2010;58(7):1045–1053. doi: 10.1016/j.neuropharm.2010.01.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Nicole O, Docagne F, Ali C, Margaill I, Carmeliet P, MacKenzie ET, Vivien D, Buisson A. The proteolytic activity of tissue-plasminogen activator enhances NMDA receptor-mediated signaling. Nat Med. 2001;7(1):59–64. doi: 10.1038/83358. [DOI] [PubMed] [Google Scholar]
- 156.Casado M, Ascher P. Opposite modulation of NMDA receptors by lysophospholipids and arachidonic acid: common features with mechanosensitivity [In Process Citation] J Physiol (Lond) 1998;513(Pt 2):317–330. doi: 10.1111/j.1469-7793.1998.317bb.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Wang LY, Orser BA, Brautigan DL, Mac-Donald JF. Regulation of NMDA receptors in cultured hippocampal neurons by protein phosphatases 1 and 2A. Nature. 1994;369(6477):230–232. doi: 10.1038/369230a0. [DOI] [PubMed] [Google Scholar]
- 158.Xiong ZG, Raouf R, Lu WY, Wang LY, Orser BA, Dudek EM, Browning MD, MacDonald JF. Regulation of N-methyl-D-aspartate receptor function by constitutively active protein kinase C. Mol Pharmacol. 1998;54(6):1055–1063. [PubMed] [Google Scholar]
- 159.Gingrich MB, Traynelis SF. Serine proteases and brain damage - is there a link? Trends Neurosci. 2000;23(9):399–407. doi: 10.1016/s0166-2236(00)01617-9. [DOI] [PubMed] [Google Scholar]
- 160.Vivien D, Buisson A. Serine protease inhibitors: novel therapeutic targets for stroke? J Cereb Blood Flow Metab. 2000;20(5):755–764. doi: 10.1097/00004647-200005000-00001. [DOI] [PubMed] [Google Scholar]
- 161.Chraibi A, Vallet V, Firsov D, Hess SK, Horisberger JD. Protease modulation of the activity of the epithelial sodium channel expressed in Xenopus oocytes. J Gen Physiol. 1998;111(1):127–138. doi: 10.1085/jgp.111.1.127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Holt JC, Lioudyno M, Athas G, Garcia MM, Perin P, Guth PS. The effect of proteolytic enzymes on the alpha9-nicotinic receptormediated response in isolated frog vestibular hair cells. Hear Res. 2001;152(1-2):25–42. doi: 10.1016/s0378-5955(00)00225-2. [DOI] [PubMed] [Google Scholar]
- 163.Clark EB, Jovov B, Rooj AK, Fuller CM, Benos DJ. Proteolytic cleavage of human acidsensing ion channel 1 by the serine protease matriptase. J Biol Chem. 2010;285(35):27130–27143. doi: 10.1074/jbc.M110.153213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Sang N, Chen C. Lipid signaling and synaptic plasticity. Neuroscientist. 2006;12(5):425–434. doi: 10.1177/1073858406290794. [DOI] [PubMed] [Google Scholar]
- 165.Farooqui AA, Horrocks LA. Phospholipase A2-generated lipid mediators in the brain" the good, the bad, and the ugly. Neuroscientist. 2006;12(3):245–260. doi: 10.1177/1073858405285923. [DOI] [PubMed] [Google Scholar]
- 166.Muralikrishna AR, Hatcher JF. Phospholipase A2, reactive oxygen species, and lipid peroxidation in cerebral ischemia. Free Radic Biol Med. 2006;40(3):376–387. doi: 10.1016/j.freeradbiomed.2005.08.044. [DOI] [PubMed] [Google Scholar]
- 167.Farooqui AA, Ong WY, Horrocks LA. Inhibitors of brain phospholipase A2 activity: their neuropharmacological effects and therapeutic importance for the treatment of neurologic disorders. Pharmacol Rev. 2006;58(3):591–620. doi: 10.1124/pr.58.3.7. [DOI] [PubMed] [Google Scholar]
- 168.Rehncrona S, Westerberg E, Akesson B, Siesjo BK. Brain cortical fatty acids and phospholipids during and following complete and severe incomplete ischemia. J Neurochem. 1982;38(1):84–93. doi: 10.1111/j.1471-4159.1982.tb10857.x. [DOI] [PubMed] [Google Scholar]
- 169.Miller B, Sarantis M, Traynelis SF, Attwell D. Potentiation of NMDA receptor currents by arachidonic acid. Nature. 1992;355(6362):722–725. doi: 10.1038/355722a0. [DOI] [PubMed] [Google Scholar]
- 170.Nagano N, Imaizumi Y, Watanabe M. Modulation of calcium channel currents by arachidonic acid in single smooth muscle cells from vas deferens of the guinea-pig. Br J Pharmacol. 1995;116(2):1887–1893. doi: 10.1111/j.1476-5381.1995.tb16678.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Mignen O, Thompson JL, Shuttleworth TJ. Arachidonate-regulated Ca2+-selective (ARC) channel activity is modulated by phosphorylation and involves an A-kinase anchoring protein. J Physiol. 2005;567(Pt 3):787–798. doi: 10.1113/jphysiol.2005.090209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Angelova P, Muller W. Oxidative modulation of the transient potassium current IA by intracellular arachidonic acid in rat CA1 pyramidal neurons. Eur J Neurosci. 2006;23(9):2375–2384. doi: 10.1111/j.1460-9568.2006.04767.x. [DOI] [PubMed] [Google Scholar]
- 173.Hu HZ, Xiao R, Wang C, Gao N, Colton CK, Wood JD, Zhu MX. Potentiation of TRPV3 channel function by unsaturated fatty acids. J Cell Physiol. 2006;208(1):201–212. doi: 10.1002/jcp.20648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Keros S, McBain CJ. Arachidonic acid inhibits transient potassium currents and broadens action potentials during electrographic seizures in hippocampal pyramidal and inhibitory interneurons. J Neurosci. 1997;17(10):3476–3487. doi: 10.1523/JNEUROSCI.17-10-03476.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Paoletti P, Ascher P. Mechanosensitivity of NMDA receptors in cultured mouse central neurons. Neuron. 1994;13:645–655. doi: 10.1016/0896-6273(94)90032-9. [DOI] [PubMed] [Google Scholar]
- 176.Smith ES, Cadiou H, McNaughton PA. Arachidonic acid potentiates acid-sensing ion channels in rat sensory neurons by a direct action. Neuroscience. 2007;145(2):686–698. doi: 10.1016/j.neuroscience.2006.12.024. [DOI] [PubMed] [Google Scholar]
- 177.Schurr A, Rigor BM. Brain anaerobic lactate production: a suicide note or a survival kit? Dev Neurosci. 1998;20(4-5):348–357. doi: 10.1159/000017330. [DOI] [PubMed] [Google Scholar]
- 178.Schurr A. Lactate, glucose and energy metabolism in the ischemic brain (Review) Int J Mol Med. 2002;10(2):131–136. [PubMed] [Google Scholar]
- 179.Xiong ZG, MacDonald JF. Sensing of extracellular calcium by neurones. Can J Physiol Pharmacol. 1999;77(9):715–721. [PubMed] [Google Scholar]
- 180.Hess P, Lansman JB, Tsien RW. Calcium channel selectivity for divalent and monovalent cations. Voltage and concentration dependence of single channel current in ventricular heart cells. J Gen Physiol. 1986;88(3):293–319. doi: 10.1085/jgp.88.3.293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Zhou W, Jones SW. Surface charge and calcium channel saturation in bullfrog sympathetic neurons. J Gen Physiol. 1995;105(4):441–462. doi: 10.1085/jgp.105.4.441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Chai S, Li M, Branigan D, Xiong ZG, Simon RP. Activation of acid-sensing ion channel 1a (ASIC1a) by surface trafficking. J Biol Chem. 2010;285(17):13002–13011. doi: 10.1074/jbc.M109.086041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Jiang Q, Li MH, Papasian CJ, Branigan D, Xiong ZG, Wang JQ, Chu XP. Characterization of acid-sensing ion channels in medium spiny neurons of mouse striatum. Neuroscience. 2009;162(1):55–66. doi: 10.1016/j.neuroscience.2009.04.029. [DOI] [PubMed] [Google Scholar]
- 184.Mamet J, Lazdunski M, Voilley N. How nerve growth factor drives physiological and inflammatory expressions of acid-sensing ion channel 3 in sensory neurons. J Biol Chem. 2003;278(49):48907–48913. doi: 10.1074/jbc.M309468200. [DOI] [PubMed] [Google Scholar]
- 185.Zha XM, Wemmie JA, Green SH, Welsh MJ. Acid-sensing ion channel 1a is a postsynaptic proton receptor that affects the density of dendritic spines. Proc Natl Acad Sci U S A. 2006;103(44):16556–16561. doi: 10.1073/pnas.0608018103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Zha XM, Costa V, Harding AM, Reznikov L, Benson CJ, Welsh MJ. ASIC2 subunits target acid-sensing ion channels to the synapse via an association with PSD-95. J Neuro sci. 2009;29(26):8438–8446. doi: 10.1523/JNEUROSCI.1284-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Anzai N, Deval E, Schaefer L, Friend V, Lazdunski M, Lingueglia E. The multivalent PDZ domain-containing protein CIPP is a partner of acid-sensing ion channel 3 in sensory neurons. J Biol Chem. 2002;277(19):16655–16661. doi: 10.1074/jbc.M201087200. [DOI] [PubMed] [Google Scholar]
- 188.Chai S, Li M, Lan J, Xiong ZG, Saugstad JA, Simon RP. A kinase-anchoring protein 150 and calcineurin are involved in regulation of acid-sensing ion channels ASIC1a and ASIC2a. J Biol Chem. 2007;282(31):22668–22677. doi: 10.1074/jbc.M703624200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Chu XP, Zhu XM, Wei WL, Li GH, Simon RP, MacDonald JF, Xiong ZG. Acidosis decreases low Ca(2+)-induced neuronal excitation by inhibiting the activity of calciumsensing cation channels in cultured mouse hippocampal neurons. J Physiol. 2003;550(Pt 2):385–399. doi: 10.1113/jphysiol.2003.043091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Zhu G, Chanchevalap S, Cui N, Jiang C. Effects of intra- and extracellular acidifications on single channel Kir2.3 currents. J Physiol. 1999;516(Pt 3):699–710. doi: 10.1111/j.1469-7793.1999.0699u.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Hougaard C, Jorgensen F, Hoffmann EK. Modulation of the volume-sensitive K+ current in Ehrlich ascites tumour cells by pH. Pflugers Arch. 2001;442(4):622–633. doi: 10.1007/s004240100585. [DOI] [PubMed] [Google Scholar]
- 192.Wang WZ, Chu XP, Li MH, Seeds J, Simon RP, Xiong ZG. Modulation of acid-sensing ion channel currents, acid-induced increase of intracellular Ca2+, and acidosis-mediated neuronal injury by intracellular pH. J Biol Chem. 2006;281(39):29369–29378. doi: 10.1074/jbc.M605122200. [DOI] [PubMed] [Google Scholar]
- 193.Deitmer JW, Ellis D. Interactions between the regulation of the intracellular pH and sodium activity of sheep cardiac Purkinje fibres. J Physiol. 1980;304:471–488. doi: 10.1113/jphysiol.1980.sp013337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Roos A, Boron WF. Intracellular pH. Physiol Rev. 1981;61(2):296–434. doi: 10.1152/physrev.1981.61.2.296. [DOI] [PubMed] [Google Scholar]
- 195.Sharp AP, Thomas RC. The effects of chloride substitution on intracellular pH in crab muscle. J Physiol. 1981;312:71–80. doi: 10.1113/jphysiol.1981.sp013616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Xu TL, Xiong ZG. Dynamic regulation of acid-sensing ion channels by extracellular and intracellular modulators. Curr Med Chem. 2007;14(16):1753–1763. doi: 10.2174/092986707781058977. [DOI] [PubMed] [Google Scholar]