Abstract
Each photoexcited rhodopsin (R*) molecule catalyzes binding of GTP to many copies of the guanine nucleotide-binding protein transducin, which, in its GTP-binding form, then activates cGMP phosphodiesterase (PDEase). Subsequent deactivation of this light-activated enzyme cascade involves hydrolysis of the GTP bound to transducin, as well as decay of the activating capacity of R*. We report here that deactivation of PDEase in rod outer segment suspensions is highly enhanced by addition of ATP and purified 48-kDa protein, which is an intrinsic rod outer segment protein that is soluble in the dark but binds to photolyzed rhodopsin that has been phosphorylated by rhodopsin kinase and ATP [Kühn, H., Hall, S.W. & Wilden, U. (1984) FEBS Lett. 176, 473-478]. To analyze the mechanism by which ATP and 48-kDa protein deactivate PDEase, we used an ATP-free system consisting of thoroughly washed disk membranes, whose rhodopsin had been previously phosphorylated and chromophore-regenerated, and to which purified PDEase and transducin were reassociated. Such phosphorylated membranes exhibited a significantly lower (by a factor less than or equal to 5) light-induced PDEase-activating capacity than unphosphorylated controls. Addition of purified 48-kDa protein to phosphorylated membranes further suppressed their PDEase-activating capacity; suppression could be as high as 98% (as compared to unphosphorylated membranes), depending on the amount of 48-kDa protein and the flash intensity. Addition of ATP had little further effect. In contrast, PDEase activation or deactivation with unphosphorylated control membranes was not influenced by 48-kDa protein, even in the presence of ATP, provided rhodopsin kinase was absent. Our data suggest that 48-kDa protein binds to phosphorylated R* and thereby quenches its capacity to activate transducin and PDEase.
Full text
PDF
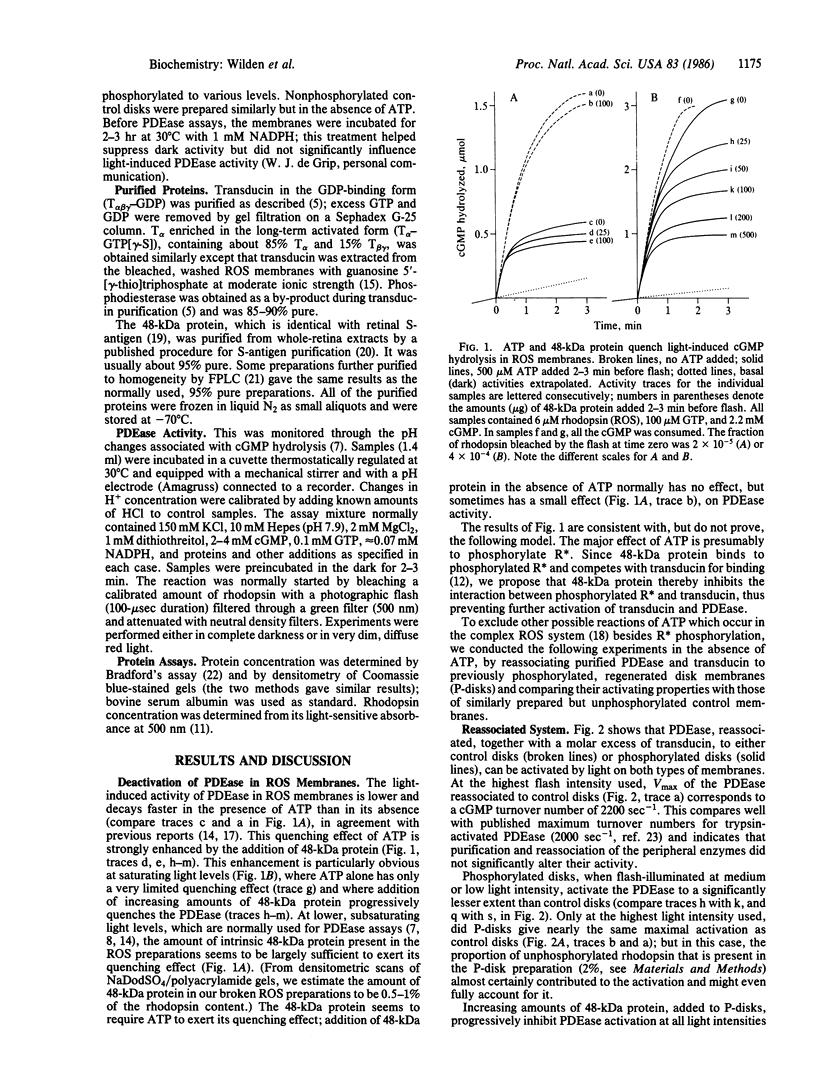
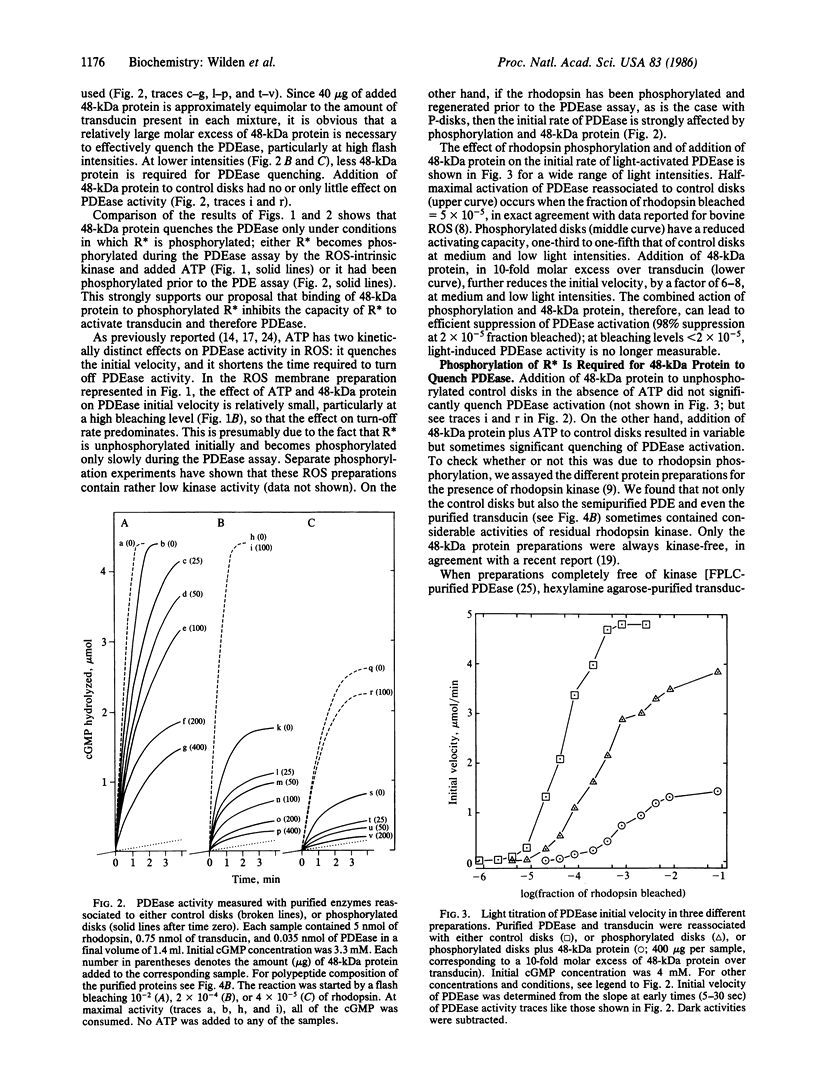
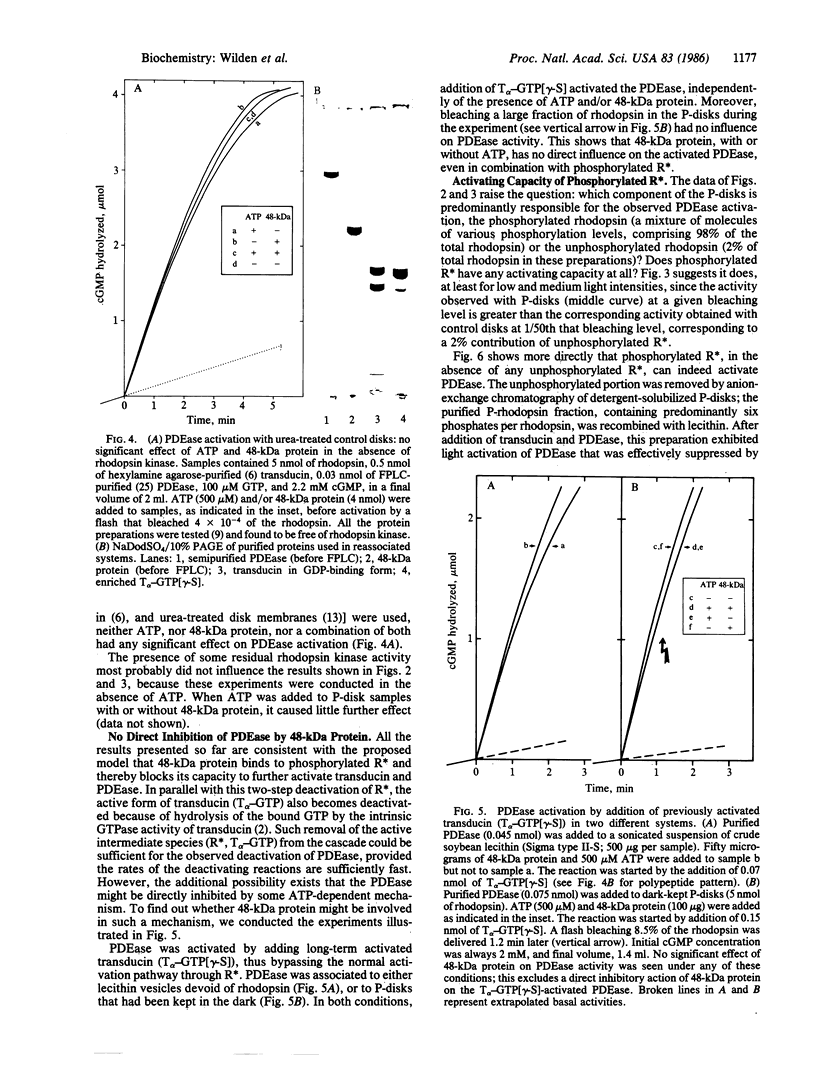
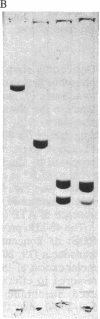
Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Baehr W., Devlin M. J., Applebury M. L. Isolation and characterization of cGMP phosphodiesterase from bovine rod outer segments. J Biol Chem. 1979 Nov 25;254(22):11669–11677. [PubMed] [Google Scholar]
- Bennett N., Michel-Villaz M., Kühn H. Light-induced interaction between rhodopsin and the GTP-binding protein. Metarhodopsin II is the major photoproduct involved. Eur J Biochem. 1982 Sep;127(1):97–103. doi: 10.1111/j.1432-1033.1982.tb06842.x. [DOI] [PubMed] [Google Scholar]
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1006/abio.1976.9999. [DOI] [PubMed] [Google Scholar]
- Dorey C., Cozette J., Faure J. P. A simple and rapid method for isolation of retinal S antigen. Ophthalmic Res. 1982;14(4):249–255. doi: 10.1159/000265199. [DOI] [PubMed] [Google Scholar]
- Emeis D., Kühn H., Reichert J., Hofmann K. P. Complex formation between metarhodopsin II and GTP-binding protein in bovine photoreceptor membranes leads to a shift of the photoproduct equilibrium. FEBS Lett. 1982 Jun 21;143(1):29–34. doi: 10.1016/0014-5793(82)80266-4. [DOI] [PubMed] [Google Scholar]
- Fung B. K., Hurley J. B., Stryer L. Flow of information in the light-triggered cyclic nucleotide cascade of vision. Proc Natl Acad Sci U S A. 1981 Jan;78(1):152–156. doi: 10.1073/pnas.78.1.152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hermolin J., Karell M. A., Hamm H. E., Bownds M. D. Calcium and cyclic GMP regulation of light-sensitive protein phosphorylation in frog photoreceptor membranes. J Gen Physiol. 1982 Apr;79(4):633–655. doi: 10.1085/jgp.79.4.633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hurwitz R. L., Bunt-Milam A. H., Chang M. L., Beavo J. A. cGMP phosphodiesterase in rod and cone outer segments of the retina. J Biol Chem. 1985 Jan 10;260(1):568–573. [PubMed] [Google Scholar]
- Kwok-Keung Fung B., Stryer L. Photolyzed rhodopsin catalyzes the exchange of GTP for bound GDP in retinal rod outer segments. Proc Natl Acad Sci U S A. 1980 May;77(5):2500–2504. doi: 10.1073/pnas.77.5.2500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kühn H., Cook J. H., Dreyer W. J. Phosphorylation of rhodopsin in bovine photoreceptor membranes. A dark reaction after illumination. Biochemistry. 1973 Jun 19;12(13):2495–2502. doi: 10.1021/bi00737a020. [DOI] [PubMed] [Google Scholar]
- Kühn H., Hall S. W., Wilden U. Light-induced binding of 48-kDa protein to photoreceptor membranes is highly enhanced by phosphorylation of rhodopsin. FEBS Lett. 1984 Oct 29;176(2):473–478. doi: 10.1016/0014-5793(84)81221-1. [DOI] [PubMed] [Google Scholar]
- Kühn H. Light- and GTP-regulated interaction of GTPase and other proteins with bovine photoreceptor membranes. Nature. 1980 Feb 7;283(5747):587–589. doi: 10.1038/283587a0. [DOI] [PubMed] [Google Scholar]
- Kühn H. Light-regulated binding of rhodopsin kinase and other proteins to cattle photoreceptor membranes. Biochemistry. 1978 Oct 17;17(21):4389–4395. doi: 10.1021/bi00614a006. [DOI] [PubMed] [Google Scholar]
- Liebman P. A., Pugh E. N., Jr ATP mediates rapid reversal of cyclic GMP phosphodiesterase activation in visual receptor membranes. Nature. 1980 Oct 23;287(5784):734–736. doi: 10.1038/287734a0. [DOI] [PubMed] [Google Scholar]
- Liebman P. A., Pugh E. N., Jr The control of phosphodiesterase in rod disk membranes: kinetics, possible mechanisms and significance for vision. Vision Res. 1979;19(4):375–380. doi: 10.1016/0042-6989(79)90097-x. [DOI] [PubMed] [Google Scholar]
- Miller J. L., Dratz E. A. Phosphorylation at sites near rhodopsin's carboxyl-terminus regulates light initiated cGMP hydrolysis. Vision Res. 1984;24(11):1509–1521. doi: 10.1016/0042-6989(84)90313-4. [DOI] [PubMed] [Google Scholar]
- Pfister C., Chabre M., Plouet J., Tuyen V. V., De Kozak Y., Faure J. P., Kühn H. Retinal S antigen identified as the 48K protein regulating light-dependent phosphodiesterase in rods. Science. 1985 May 17;228(4701):891–893. doi: 10.1126/science.2988124. [DOI] [PubMed] [Google Scholar]
- Sitaramayya A., Liebman P. A. Mechanism of ATP quench of phosphodiesterase activation in rod disc membranes. J Biol Chem. 1983 Jan 25;258(2):1205–1209. [PubMed] [Google Scholar]
- Wheeler G. L., Bitensky M. W. A light-activated GTPase in vertebrate photoreceptors: regulation of light-activated cyclic GMP phosphodiesterase. Proc Natl Acad Sci U S A. 1977 Oct;74(10):4238–4242. doi: 10.1073/pnas.74.10.4238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilden U., Kühn H. Light-dependent phosphorylation of rhodopsin: number of phosphorylation sites. Biochemistry. 1982 Jun 8;21(12):3014–3022. doi: 10.1021/bi00541a032. [DOI] [PubMed] [Google Scholar]
- Yee R., Liebman P. A. Light-activated phosphodiesterase of the rod outer segment. Kinetics and parameters of activation and deactivation. J Biol Chem. 1978 Dec 25;253(24):8902–8909. [PubMed] [Google Scholar]
- Zigler J. S., Jr, Mochizuki M., Kuwabara T., Gery I. Purification of retinal S-antigen to homogeneity by the criterion of gel electrophoresis silver staining. Invest Ophthalmol Vis Sci. 1984 Aug;25(8):977–980. [PubMed] [Google Scholar]