Abstract
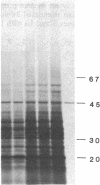
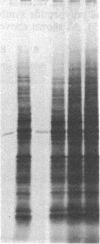
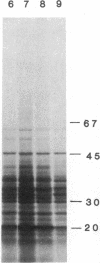
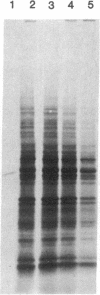
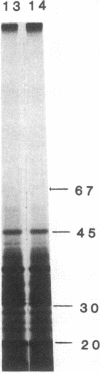
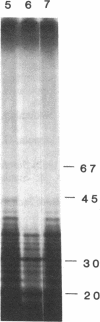
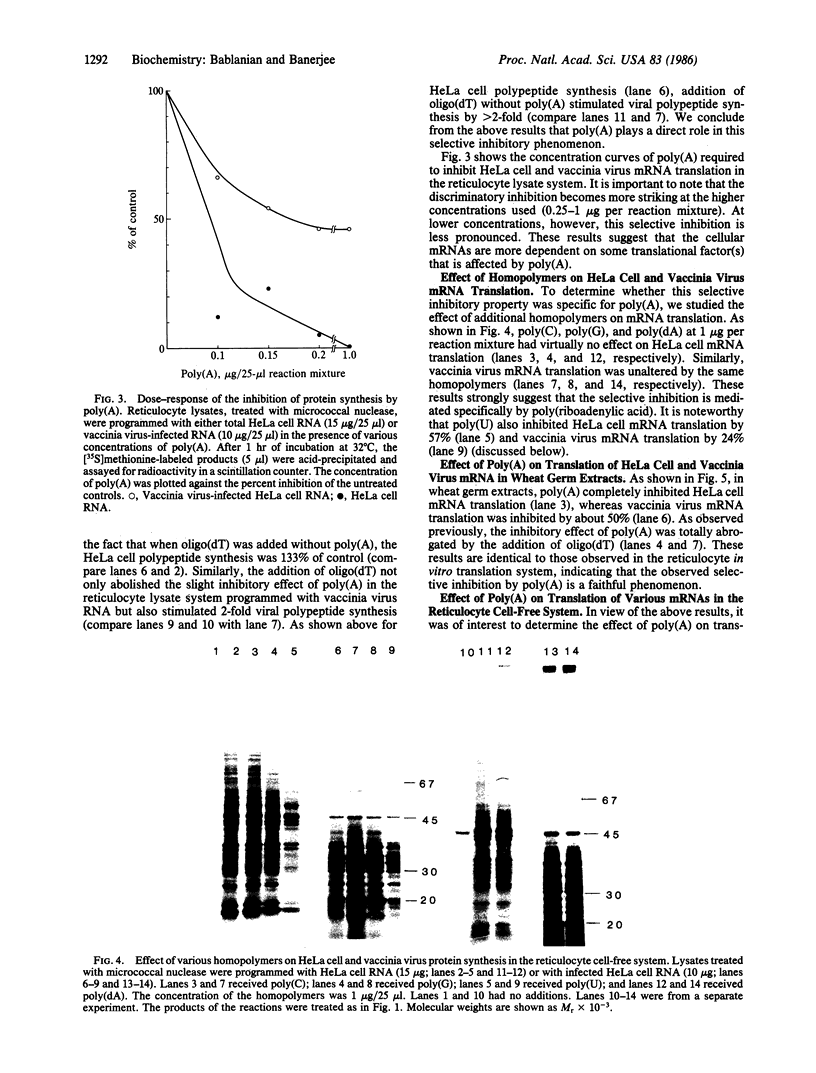
Vaccinia virus-induced inhibition of host protein synthesis seems to be mediated by viral transcripts based on their differential inhibition of cellular mRNA translation in a rabbit reticulocyte lysate system. In this study, we demonstrated that the removal of poly(riboadenylic acid) [poly(A)] from the in vitro viral transcripts abolished this inhibition in the same cell-free system. This observation led us to the finding that less than 1 microM poly(A) completely inhibited HeLa cell mRNA translation in the reticulocyte lysate, whereas only 50% inhibition of vaccinia virus mRNA translation was observed at the same concentration. Similar results were also obtained in a wheat germ protein-synthesizing system. This inhibitory effect of poly(A) was totally abrogated by the addition of polydeoxythymidylate. This selective inhibition was highly specific for poly(A) since other homopolymers, including poly(G), poly(C), and poly(dA), were not capable of causing such an inhibition. Poly(U), however, had a moderate selective inhibitory effect. Among the several mRNAs tested, the translation of L-cell, encephalomyocarditis virus, and reovirus RNAs was also sensitive to poly(A). However, vesicular stomatitis virus mRNA translation was strikingly more resistant. These results suggest that poly(A), which is also synthesized by the virion-associated poly(A) polymerase may be involved in vaccinia virus-mediated host cell shutoff.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bablanian R., Coppola G., Scribani S., Esteban M. Inhibition of protein synthesis by vaccinia virus. IV. The role of low-molecular-weight viral RNA in the inhibition of protein synthesis. Virology. 1981 Jul 15;112(1):13–24. doi: 10.1016/0042-6822(81)90607-3. [DOI] [PubMed] [Google Scholar]
- Bablanian R., Esteban M., Baxt B., Sonnabend J. A. Studies on the mechanisms of vaccina virus cytopathic effects. I. Inhibition of protein synthesis in infected cells is associated with virus-induced RNA synthesis. J Gen Virol. 1978 Jun;39(3):391–402. doi: 10.1099/0022-1317-39-3-391. [DOI] [PubMed] [Google Scholar]
- Balanian R. Structural and functional alterations in cultured cells infected with cytocidal viruses. Prog Med Virol. 1975;19:40–83. [PubMed] [Google Scholar]
- Both G. W., Lavi S., Shatkin A. J. Synthesis of all the gene products of the reovirus genome in vivo and in vitro. Cell. 1975 Feb;4(2):173–180. doi: 10.1016/0092-8674(75)90124-5. [DOI] [PubMed] [Google Scholar]
- Coppola G., Bablanian R. Discriminatory inhibition of protein synthesis in cell-free systems by vaccinia virus transcripts. Proc Natl Acad Sci U S A. 1983 Jan;80(1):75–79. doi: 10.1073/pnas.80.1.75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De B. P., Banerjee A. K. Requirements and functions of vesicular stomatitis virus L and NS proteins in the transcription process in vitro. Biochem Biophys Res Commun. 1985 Jan 16;126(1):40–49. doi: 10.1016/0006-291x(85)90568-6. [DOI] [PubMed] [Google Scholar]
- Dratewka-Kos E., Kiss I., Lucas-Lenard J., Mehta H. B., Woodley C. L., Wahba A. J. Catalytic utilization of eIF-2 and mRNA binding proteins are limiting in lysates from vesicular stomatitis virus infected L cells. Biochemistry. 1984 Dec 4;23(25):6184–6190. doi: 10.1021/bi00320a045. [DOI] [PubMed] [Google Scholar]
- Dunigan D. D., Lucas-Lenard J. M. Two transcription products of the vesicular stomatitis virus genome may control L-cell protein synthesis. J Virol. 1983 Feb;45(2):618–626. doi: 10.1128/jvi.45.2.618-626.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farrell P. J., Balkow K., Hunt T., Jackson R. J., Trachsel H. Phosphorylation of initiation factor elF-2 and the control of reticulocyte protein synthesis. Cell. 1977 May;11(1):187–200. doi: 10.1016/0092-8674(77)90330-0. [DOI] [PubMed] [Google Scholar]
- Gershowitz A., Moss B. Abortive transcription products of vaccinia virus are guanylylated, methylated, and polyadenylylated. J Virol. 1979 Sep;31(3):849–853. doi: 10.1128/jvi.31.3.849-853.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grill L. K., Sun J. D., Kandel J. Effect of double stranded RNA on protein synthesis in an in vitro wheat germ embryo system. Biochem Biophys Res Commun. 1976 Nov 8;73(1):149–156. doi: 10.1016/0006-291x(76)90509-x. [DOI] [PubMed] [Google Scholar]
- Hunter T., Hunt T., Jackson R. J., Robertson H. D. The characteristics of inhibition of protein synthesis by double-stranded ribonucleic acid in reticulocyte lysates. J Biol Chem. 1975 Jan 25;250(2):409–417. [PubMed] [Google Scholar]
- JOKLIK W. K. The preparation and characteristics of highly purified radioactively labelled poxvirus. Biochim Biophys Acta. 1962 Aug 20;61:290–301. doi: 10.1016/0926-6550(62)90091-9. [DOI] [PubMed] [Google Scholar]
- Jacobson A., Favreau M. Possible involvement of poly(A) in protein synthesis. Nucleic Acids Res. 1983 Sep 24;11(18):6353–6368. doi: 10.1093/nar/11.18.6353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kates J., Beeson J. Ribonucleic acid synthesis in vaccinia virus. II. Synthesis of polyriboadenylic acid. J Mol Biol. 1970 May 28;50(1):19–33. doi: 10.1016/0022-2836(70)90101-4. [DOI] [PubMed] [Google Scholar]
- Katze M. G., Chen Y. T., Krug R. M. Nuclear-cytoplasmic transport and VAI RNA-independent translation of influenza viral messenger RNAs in late adenovirus-infected cells. Cell. 1984 Jun;37(2):483–490. doi: 10.1016/0092-8674(84)90378-7. [DOI] [PubMed] [Google Scholar]
- Kerr I. M., Martin E. M. Simple method for the isolation of encephalomyocarditis virus ribonucleic acid. J Virol. 1972 Mar;9(3):559–561. doi: 10.1128/jvi.9.3.559-561.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krystosek A., Cawthon M. L., Kabat D. Improved methods for purification and assay of eukaryotic messenger ribonucleic acids and ribosomes. Quantitative analysis of their interaction in a fractionated reticulocyte cell-free system. J Biol Chem. 1975 Aug 10;250(15):6077–6084. [PubMed] [Google Scholar]
- Lodish H. F., Nathan D. G. Regulation of hemoglobin synthesis. Preferential inhibition of and globin synthesis. J Biol Chem. 1972 Dec 10;247(23):7822–7829. [PubMed] [Google Scholar]
- Paoletti E., Lipinskas B. R., Panicali D. Capped and polyadenylated low-molecular-weight RNA synthesized by vaccinia virus in vitro. J Virol. 1980 Jan;33(1):208–219. doi: 10.1128/jvi.33.1.208-219.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pelham H. R., Jackson R. J. An efficient mRNA-dependent translation system from reticulocyte lysates. Eur J Biochem. 1976 Aug 1;67(1):247–256. doi: 10.1111/j.1432-1033.1976.tb10656.x. [DOI] [PubMed] [Google Scholar]
- Reijnders L., Aalberg A. M., Kammen A. K., Berns A. J. The effect of double-stranded cowpea mosaic viral RNA on protein synthesis. Biochim Biophys Acta. 1975 Apr 16;390(1):69–77. doi: 10.1016/0005-2787(75)90009-x. [DOI] [PubMed] [Google Scholar]
- Rosemond-Hornbeak H., Moss B. Inhibition of host protein synthesis by vaccinia virus: fate of cell mRNA and synthesis of small poly (A)-rich polyribonucleotides in the presence of actinomycin D. J Virol. 1975 Jul;16(1):34–42. doi: 10.1128/jvi.16.1.34-42.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schneider R. J., Weinberger C., Shenk T. Adenovirus VAI RNA facilitates the initiation of translation in virus-infected cells. Cell. 1984 May;37(1):291–298. doi: 10.1016/0092-8674(84)90325-8. [DOI] [PubMed] [Google Scholar]
- Schreier M. H., Staehelin T. Initiation of mammalian protein synthesis: the importance of ribosome and initiation factor quality for the efficiency of in vitro systems. J Mol Biol. 1973 Feb 19;73(3):329–349. doi: 10.1016/0022-2836(73)90346-x. [DOI] [PubMed] [Google Scholar]
- Schrom M., Bablanian R. Inhibition of protein synthesis by vaccinia virus. I. Characterization of an inhibited cell-free protein-synthesizing system from infected cells. Virology. 1979 Dec;99(2):319–328. doi: 10.1016/0042-6822(79)90011-4. [DOI] [PubMed] [Google Scholar]
- Schrom M., Bablanian R. Inhibition of protein synthesis by vaccinia virus. II. Studies on the role of virus-induced RNA synthesis. J Gen Virol. 1979 Sep;44(3):625–638. doi: 10.1099/0022-1317-44-3-625. [DOI] [PubMed] [Google Scholar]
- Testa D., Chanda P. K., Banerjee A. K. Unique mode of transcription in vitro by Vesicular stomatitis virus. Cell. 1980 Aug;21(1):267–275. doi: 10.1016/0092-8674(80)90134-8. [DOI] [PubMed] [Google Scholar]
- Thimmappaya B., Weinberger C., Schneider R. J., Shenk T. Adenovirus VAI RNA is required for efficient translation of viral mRNAs at late times after infection. Cell. 1982 Dec;31(3 Pt 2):543–551. doi: 10.1016/0092-8674(82)90310-5. [DOI] [PubMed] [Google Scholar]