Summary
Phenobarbital and phenytoin, two drugs commonly used for the treatment of neonatal seizures, have been well-documented to induce neuronal apoptosis throughout many regions of the developing rat brain. However, several limbic regions have not been included in previous analyses. Because drug-induced damage to limbic brain regions in infancy could contribute to emotional and psychiatric sequelae, it is critical to determine the extent to which these regions are vulnerable to developmental neurotoxicity. To evaluate the impact of AED exposure on limbic nuclei, we treated postnatal day 7 rat pups with phenobarbital, phenytoin, carbamazepine, or vehicle, and examined nucleus accumbens, septum, amygdala, piriform cortex and frontal cortex for cell death. Histological sections were processed using the TUNEL assay to label apoptotic cells. Nucleus accumbens displayed the highest level of baseline cell death (vehicle group), as well as the greatest net increase in cell death following phenobarbital or phenytoin. Phenobarbital exposure resulted in a significant increase in cell death in all brain regions, while phenytoin exposure increased cell death only in the nucleus accumbens. Carbamazepine was without effect on cell death in any brain region analyzed, suggesting that the neurotoxicity observed is not an inherent feature of AED action. Our findings demonstrate pronounced cell death in several important regions of the rat limbic system following neonatal administration of phenobarbital, the first-line treatment for neonatal seizures in humans. These findings raise the possibility that AED exposure in infancy may contribute to adverse neuropsychiatric outcomes later in life.
Keywords: apoptosis, development, phenobarbital, phenytoin, carbamazepine
Introduction
Early postnatal brain development is marked by a combination of neuronal pruning via programmed cell death, and extensive synaptogenesis. This balance between neuronal elimination and survival is altered by exposure to anesthetic agents and antiepileptic drugs (AEDs) (Bittigau et al., 2002; Kim et al., 2007a, 2007b). Certain AEDs, including phenobarbital, phenytoin, and valproic acid induce neuronal apoptosis in the neonatal rodent brain when given during the first postnatal week (Bittigau et al., 2002; Kim et al., 2007a, 2007b). This effect occurs at doses in the therapeutic range and is observed in a variety of brain regions including cortex, thalamic nuclei, hippocampus, and striatum (Bittigau et al., 2002).
Certain limbic system regions have not been characterized with respect to vulnerability to AED-induced cell death. These regions, which mediate reward and mood, are of particular interest for understanding psychiatric sequelae of early-life seizures. Because early-life seizures are typically treated with AEDs, it is important to establish the extent to which AED exposure damages these regions.
In the present study, we examined neuronal apoptosis in the nucleus accumbens, amygdala, septum, piriform cortex, and frontal cortex of the rat following neonatal exposure to phenobarbital or phenytoin. We also examined the effect of carbamazepine, an AED that we have previously reported to be devoid of a proapoptotic effect on multiple non-limbic regions of the neonatal rat brain (Kim et al., 2007).
Methods
Animals
Postnatal day (P)7, male and female Sprague-Dawley rat pups (Harlan, Indianapolis, IN) were used. Treatment was counterbalanced within and across litter and sex. Pups were born to timed-pregnant dams; P0 = date of parturition. Animals were maintained in a temperature-controlled (21°C) room with a 12-h light cycle. All experiments were approved by the Georgetown University Animal Care and Use Committee.
Drug Treatments
The drug doses used fell within the anticonvulsant range in neonatal rats (Kubova and Mares, 1991; Stankova et al., 1992; Kubová and Mares, 1993). For phenobarbital, the dose selected (75mg/kg) was just below the dose (80mg/kg) that was found to provided complete protection against pentylenetetrazole (PTZ)- induced seizures (both minimal and maximal) in P7 rat pups. (Kubova and Mares, 1991). This dose of phenobarbital was in the middle of the effective dose range previously reported for induction of neuronal apoptosis (Bittigau et al., 2002). For phenytoin, the dose selected (50mg/kg) was within the range (30–60 mg/kg) that reduced the frequency of PTZ seizures in P7 rats (Stankova et al., 1992) and corresponded to the upper end of the dose range previously reported to induce neuronal apoptosis at P7 (Bittigau et al., 2002). The dose of carbamazepine used (100mg/kg) was equivalent to twice the highest dose previously shown to protect against maximal PTZ seizures in P7 rats (Kubova and Mares, 1993). Despite the fact that this is a high dose of carbamazepine, this dose previously was found not to cause significant neuronal apoptosis in P7 rat pups (Kim et al., 2007). Pups were injected (i.p.) with sodium phenobarbital in saline (75mg/kg, n=8, Sigma), phenytoin (sodium diphenylhydantoin) in alkalinized saline (pH 10, 50mg/kg, n=10, Sigma), or a suspension of carbamazepine (100mg/kg, n=6, Sigma) in saline containing 1.0% Tween 80 (Sigma). Control groups received equivalent volumes of vehicle (0.01ml/g body weight, n=11). Treatments occurred on P7, 24h before sacrifice as in prior studies (Bittigau et al., 2002; Kim et al., 2007a, 2007b).
Tissue Preparation
24h after drug treatment, pups were decapitated with sharp scissors, brains were removed, quickly frozen in isopentane, and stored at −80°C until cryosectioning (20µm coronal sections).
Histology and Quantification of Cell Death
Tissues were stained for TUNEL (terminal deoxynucleotidyl transferase-mediated dUTP nick-end labeling) using the Apoptag peroxidase in situ apoptosis detection kit (Chemicon International, Temecula, CA) as described previously (Kim et al., 2007a, 2007b). Photomicrographs (4x) of three sequential sections at 200-µm intervals were taken from each brain area. TUNEL-positive cells (nuclei that were stained darkly with DAB) were counted and summed for each brain area across three sections. Counting was performed on high-resolution photomicrographs in ImageJ, with cells counted manually by a treatment-blind observer (P.A.F.). Cells were counted only within the anatomical boundaries of the brain regions of interest, as defined in the developmental rat atlas of Sherwood and Timeras (1970).
Statistics
Statistical comparisons were performed by ANOVA followed by Bonferroni-Holm’s post-hoc test with p < 0.05 indicating significant difference.
Results
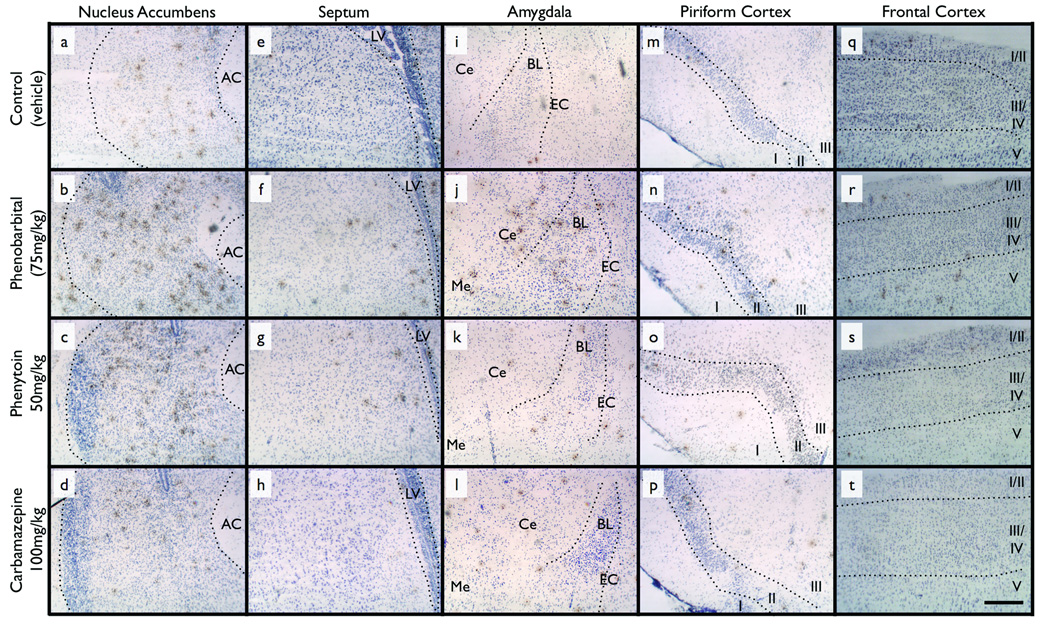
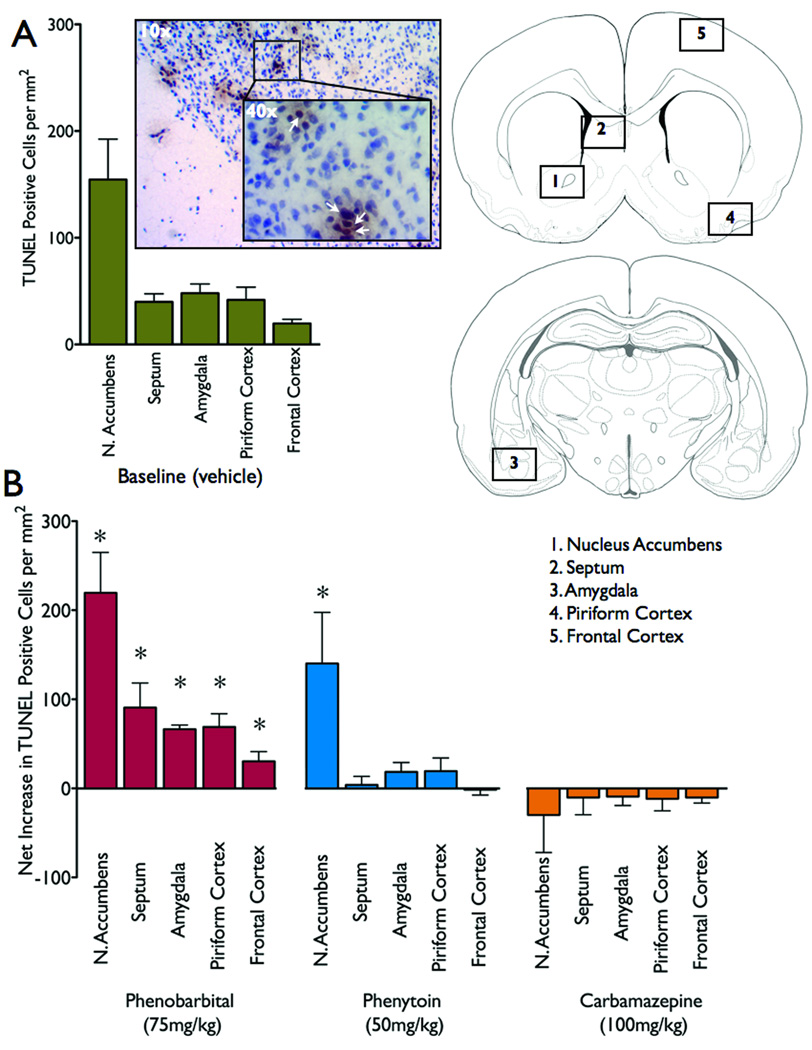
Representative photomicrographs from each brain area and drug treatment are shown in Figure 1. Nucleus accumbens exhibited the greatest number of TUNEL-positive cells under control conditions (Figure 1a and Figure 2a), significantly higher than frontal cortex, piriform cortex and amygdala (Repeated measures ANOVA, F4,28=7.640, p<0.05, post-hoc P<0.05).
Figure 1.
Representative photomicrographs of TUNEL-stained sections for each brain region from animals treated with: vehicle (control) (a, e, i, m, q), phenobarbital (b, f, j, n, r), phenytoin (c, g, k, o, s), and carbamazepine (d, h, l, p, t). Dotted lines illustrate anatomical boundaries. TUNEL positive cells are stained brown. Scale bar = 250µm. AC = anterior commissure, LV = lateral ventricle, Ce = Central amygdala, BL = basal and lateral amygdala, EC = external capsule, Roman numerals I to IV = cortical layers one to five. See Figure 2 for schematic representations of brain regions.
Figure 2.
Quantification of cell death. (a) Baseline levels of cell death following vehicle (control). (b) Net increase (over baseline cell death, as presented in Figure 2a) for each brain area and drug treatment. * = significantly different than baseline levels of cell death, P<0.05. Schematics are modified from the atlas of Sherwood and Timiras (1970). Photomicrograph (10x) of nucleus accumbens with the inset showing TUNEL-positive cells (40x, indicated by white arrowhead).
Following exposure to phenobarbital (Figure 1b) or phenytoin (Figure 1c), the number of TUNEL-positive cells in nucleus accumbens was significantly increased (Figure 2). Phenobarbital, but not phenytoin, significantly increased the number of TUNEL-positive cells in septum (Figure 1e, Figure 2), amygdala (Figure 1j, Figure 2), piriform cortex (Figure 1p, Figure 2) and frontal cortex (Figure 1r, Figure 2). Carbamazepine was without effect in all regions.
In nucleus accumbens, phenobarbital and phenytoin significantly increased the number of TUNEL-positive cells (F3,25=7.503, P<0.001, post-hoc P<0.05). Phenobarbital, but not phenytoin or carbamazepine increased cell death significantly over baseline levels in the septum (F3,30=8.316, P<0.0004, post-hoc, P<0.001), amygdale (F3,29=15.576, P<0.00001, post-hoc, P<0.001), piriform cortex (F3,30=7.288, P<0.001, post-hoc, P<0.005), and frontal cortex (F3,27=6.124, P<0.005, post-hoc, P<0.005).
Discussion
Here we have shown that exposure of neonatal rat pups to Phenobarbital resulted in an increase in cell death in all limbic brain regions analyzed. In contrast, phenytoin-induced cell death was limited to the nucleus accumbens. As previously observed for other brain regions (Kim et al., 2007), carbamazepine exposure was not associated with cell death in any regions. These findings demonstrate that limbic regions are vulnerable to cell death induction by certain AEDs. The timing of drug exposure in our studies was limited to a single administration on P7. This age in the rat fits within a period corresponding to the late third trimester of gestation through early infancy in humans (Dobbing and Sands, 1979).
Increased cell death following phenobarbital was observed throughout the mediolateral and dorsoventral extent of the nucleus accumbens, throughout the subnuclei of the amygdala (basolateral, central, and medial), and in the lateral aspect of the septum to a greater extent than the medial. Increased cell death was also observed in layers II and III of piriform cortex, with little seen in layer I, while in frontal cortex it was present throughout layers I-IV (layer VI was not examined).
As compared to the other brain regions examined, the nucleus accumbens exhibited especially high cell death under control conditions, consistent with a previous report (Maciejewska et al., 1998). Moreover, nucleus accumbens exhibited especially high increases in cell death after phenobarbital or phenytoin, an effect that was at least as pronounced as that previously observed in the dorsomedial striatum (caudate-putamen) (Katz et al., 2007), a region composed of cell types comparable to those found in the nucleus accumbens (ventral striatum). When the brains from the animals used in the present study previously were examined for cell death in several other brain regions (Kim et al., 2007), the most pronounced cell death induced by phenobarbital and phenytoin was found in dorsomedial striatum (50–100 cells/mm2) and in ventromedial thalamus (>100 cells/mm2) (Kim et al., 2007). These observations may explain why humans exposed in utero to AEDs showed a decrease in striatal volume in adulthood (Ikonomidou et al., 2007). The pronounced vulnerability of the dorsal striatum to drug-induced cell death during development has also been demonstrated following exposure to ethanol and anesthetic agents in mice and non-human primates during equivalent developmental windows (Olney et al., 2002; Brambrink et al., 2010; Farber et al., 2010).
In the present study, we evaluated only a single dose of each drug, doses that represent the upper end of the range of clinically relevant concentrations. Thus it should be noted that our findings probably define outcomes associated with the worst-case scenario for an acute treatment. However, since AEDs are typically administered on a chronic basis, it is likely that repeated drug exposure over several days to weeks may exacerbate the neurotoxicity.
Our data suggest that neurotoxicity associated with exposure to Phenobarbital and phenytoin during late gestation or infancy can adversely affect regions of the brain that contribute to emotional and psychiatric conditions. In animal models, chronic early life exposure to phenobarbital has been shown to result in abnormalities in adult behaviors that are dependent on the limbic system. In particular, decreased anxiety-like behavior in the elevated plus maze, impaired prepulse inhibition, and deficient fear conditioning are long-term adverse sequelae of phenobarbital exposure during the period of P7 to P14 (Forcelli et al., 2010). It is conceivable that the cell death we describe here may contribute to these outcomes. If cell death is a critical factor for the behavioral toxicity, we would expect that carbamazepine exposure should be less likely to cause long-term behavioral changes.
The contrast between phenytoin and carbamazepine with respect to developmental neurotoxicity suggests that this toxicity is not related to their common mechanism of therapeutic action (i.e., blockade of voltage-gated sodium channels). A dissociation between these drugs has also been reported with respect to neurobehavioral outcomes following in utero exposure, with children exposed to phenytoin, but not carbamazepine, exhibiting reduced IQ as compared to controls (Scolnik et al., 1994).
In the clinical context, AED exposure typically occurs against the background of recurrent seizure episodes, whereas our studies only examined the impact of AED exposure in otherwise normal animals. Only one preclinical study has examined the interaction between repeated seizures and AED exposure in neonatal rats, evaluating neuronal cell death as an endpoint (Kim et al, 2007b). This study found that repeated electroshock seizures did not change the severity of valproate-induced cell death at P7. Moreover, seizure exposure did not change the extent of either phenobarbital or phenytoin-induced cell death (Unpublished results described in Kim, 2007). In contrast, neuronal cell death induced by the NMDA-receptor antagonist MK-801 was significantly attenuated by the same seizure regimen (Kim et al., 2007b), suggesting that the stimulation caused by the electroshock seizures exerted a neuroprotective action against MK-801 neurotoxicity, an action that did not extend to the neurotoxicity associated with AEDs. Further preclinical studies of the interaction between AEDs exposure and seizures are warranted, especially in conditions in which the seizures and AED treatment recur over a prolonged period, to more closely model chronic treatment conditions in the clinical setting.
Clinically, early life seizures have been identified as risk factors for neuropsychiatric disorders (Vestergaard et al., 2005). While this has been interpreted as evidence for early life seizures contributing to psychiatric disease pathogenesis it is also possible that neurotoxicity associated with therapeutic treatment with commonly used AEDs (phenobarbital, phenytoin) may contribute to neuropsychiatric disorders by disrupting limbic system maturation. An epidemiological analysis of psychiatric outcomes as a function of exposure to drugs that cause cell death in the limbic system, versus those that do not, would be an important first step to address this question.
Acknowledgments
This research was supported by: NIH F31NS066822 and a predoctoral Epilepsy Foundation Fellowships to PAF and JSK, NIH training grants T32DA007291 and T32NS041231, as well as a research grant from the Partnership for Pediatric Epilepsy Research, administered through the Epilepsy foundation to KG, and National Institutes of Health Grants NS20576, NS041231, MH02040.
Footnotes
Disclosure of Conflicts of Interest:
None of the authors has any conflict of interest to disclose. “We confirm that we have read the Journal’s position on issues involved in ethical publication and affirm that this report is consistent with those guidelines.”
References
- Bittigau P, Sifringer M, Genz K, Reith E, Pospischil D, Govindarajalu S, Dzietko M, Pesditschek S, Mai I, Dikranian K, Olney JW, Ikonomidou C. Antiepileptic drugs and apoptotic neurodegeneration in the developing brain. Proc Natl Acad Sci U S A. 2002;99:15089–15094. doi: 10.1073/pnas.222550499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brambrink AM, Evers AS, Avidan MS, Farber NB, Smith DJ, Zhang X, Dissen GA, Creeley CE, Olney JW. Isoflurane-induced neuroapoptosis in the neonatal rhesus macaque brain. Anesthesiology. 2010;112:834–841. doi: 10.1097/ALN.0b013e3181d049cd. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dobbing J, Sands J. Comparative aspects of the brain growth spurt. Early Hum Dev. 1979;3:79–83. doi: 10.1016/0378-3782(79)90022-7. [DOI] [PubMed] [Google Scholar]
- Farber NB, Creeley CE, Olney JW. Alcohol-induced neuroapoptosis in the fetal macaque brain. Neurobiol. Dis. 2010;40:200–206. doi: 10.1016/j.nbd.2010.05.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forcelli PA, Janssen MJ, Stamps LA, Sweeney C, Vicini S, Gale K. Therapeutic strategies to avoid long-term adverse outcomes of neonatal antiepileptic drug exposure. Epilepsia. 2010;51 Suppl 3:18–23. doi: 10.1111/j.1528-1167.2010.02603.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ikonomidou C, Scheer I, Wilhelm T, Juengling FD, Titze K, Stöver B, Lehmkuhl U, Koch S, Kassubek J. Brain morphology alterations in the basal ganglia and the hypothalamus following prenatal exposure to antiepileptic drugs. Eur. J. Paediatr. Neurol. 2007;11:297–301. doi: 10.1016/j.ejpn.2007.02.006. [DOI] [PubMed] [Google Scholar]
- Kim J, Kondratyev A, Gale K. (a) Antiepileptic Drug-Induced Neuronal Cell Death in the Immature Brain: Effects of Carbamazepine, Topiramate, and Levetiracetam as Monotherapy versus Polytherapy. J Pharmacol Exp Ther. 2007a;323:165–173. doi: 10.1124/jpet.107.126250. [DOI] [PubMed] [Google Scholar]
- Kim JS, Kondratyev A, Tomita Y, Gale K. (b) Neurodevelopmental impact of antiepileptic drugs and seizures in the immature brain. Epilepsia. 2007b;48 Suppl 5:19–26. doi: 10.1111/j.1528-1167.2007.01285.x. [DOI] [PubMed] [Google Scholar]
- Kim J. Doctoral dissertation. Georgetown University; 2007. Effects of repeated brief seizures and antiepileptic drugs in the developing rat brain. 2007. [Google Scholar]
- Maciejewska B, Lipowska M, Kowiański P, Domaradzka-Pytel B, Moryś J. Postnatal development of the rat striatum--a study using in situ DNA end labeling technique. Acta Neurobiol Exp (Wars) 1998;58:23–28. doi: 10.55782/ane-1998-1255. [DOI] [PubMed] [Google Scholar]
- Olney JW, Tenkova T, Dikranian K, Qin Y-Q, Labruyere J, Ikonomidou C. Ethanol-induced apoptotic neurodegeneration in the developing C57BL/6 mouse brain. Brain Res. Dev. Brain Res. 2002;133:115–126. doi: 10.1016/s0165-3806(02)00279-1. [DOI] [PubMed] [Google Scholar]
- Scolnik D, Nulman I, Rovet J, Gladstone D, Czuchta D, Gardner HA, Gladstone R, Ashby P, Weksberg R, Einarson T. Neurodevelopment of children exposed in utero to phenytoin and carbamazepine monotherapy. JAMA. 1994;271:767–770. [PubMed] [Google Scholar]
- Sherwood N, Timiras P. A Stereotaxic Atlas of the Developing Rat Brain. Berkeley, CA: University of California Press; 1970. [Google Scholar]
- Vestergaard M, Pedersen C, Christensen J, Madsen K, Olsen J, Mortensen P. Febrile seizures and risk of schizophrenia. Schizophrenia Research. 2005;73:343–349. doi: 10.1016/j.schres.2004.07.004. [DOI] [PubMed] [Google Scholar]