Abstract
In earlier work, we synthesized a cyclic 9-amino acid peptide (AFPep, cyclo[EKTOVNOGN]) and showed it to be useful for prevention and therapy of breast cancer. In an effort to explore the structure-function relationships of AFPep, we have designed analogs that bear a short ‘tail’ (one or two amino acids) attached to the cyclic peptide distal to its pharmacophore. Analogs that bore a tail of either one or two amino acids, either of which had a hydrophilic moiety in the side chain (example: cyclo[EKTOVNOGN]FS) exhibited greatly diminished biological activity (inhibition of estrogen-stimulated uterine growth) relative to AFPep. Analogs that bore a tail of either one or two amino acids which had hydrophobic (aliphatic or aromatic) side chains (example: cyclo[EKTOVNOGN]FI) retained (or had enhanced) growth inhibition activity. Combining in the same biological assay a hydrophilic-tailed analog with either AFPep or a hydrophobic-tailed analog resulted in decreased activity relative to that for AFPep or for the hydrophobic-tailed analog alone, suggesting that hydrophilic-tailed analogs are binding to a biologically active receptor. An analog with a disrupted pharmacophore (cyclo[EKTOVGOGN]) exhibited little or no growth inhibition activity. An analog with a hydrophilic tail and a disrupted pharmacophore (cyclo[EKTOVGOGN]FS) exhibited no growth inhibition activity of its own and did not affect the activity of a hydrophobic-tailed analog, but enhanced the growth inhibition activity of AFPep. These results are discussed in the context of a two-receptor model for binding of AFPep and ring-and-tail analogs. We suggest that tails on cyclic peptides may comprise a useful method to enhance diversity of peptide design and specificity of ligand-receptor interactions.
Keywords: Cyclic peptide, ring-and-tail synthetic peptide, rational design of synthetic peptide, two-receptor model, antagonist, homobiotic peptide
1. Introduction
Peptides are useful pharmaceutical agents that can interact with cellular receptors to block or enhance signal transfers. According to a recent review [44], more than 60 synthetic peptides are in use to treat an array of pathologies including cardiovascular diseases, arthritis, diabetes, growth problems, immunity diseases, and many others. In comparison to antibodies and proteins, peptides are smaller in size, are less immunogenic, have lower manufacturing costs, are high in activity per unit mass, have greater stability at room temperature, and in some cases can provide full biological activity after oral administration. It is important to develop, and have at hand, as many strategies as possible for designing synthetic peptides.
1.1 Design of Synthetic Peptides
Many approaches are useful for the design of synthetic peptides intended for use as pharmaceutical agents [38]. When little is known about the target (e.g., a receptor) or the ligand (the intended synthetic peptide), combinatorial syntheses can be used to identify lead compounds [7, 8, 20]. Relying on powerful screening methods to identify biological function, vast libraries of peptides can be scanned rapidly. Rational design approaches can be useful when there is substantial information available about the ligand or the binding site with which it interacts [29]. Focused, intentional modifications of existing molecules can obviate the need for large numbers of syntheses and screenings. While these illustrative approaches may seem to be at opposite ends of the design spectrum they are by no means mutually exclusive. Lead compounds identified by combinatorial synthesis can be optimized through rational approaches to identify the best analog. Similarly, peptides designed through rational approaches may be optimized by iterative replacement of each amino acid [36]. Molecular modeling contributes importantly to all approaches [9, 28, 31]. Development of peptides as innovative pharmaceutical agents should utilize a variety of design approaches [35].
Important reasons that peptides are attractive as potential pharmaceutical agents include the diversity of constituent building blocks that comprise a peptide, and the minimal toxicity associated with peptides. The 20 naturally occurring amino acids found in proteins offer a tremendous number of permutations for a peptide sequence, even though most of those sequences would have none of the desired biological activity. To enhance the potency of a lead peptide, additional diversity [18] is available through incorporation of amino acids that are not typically found in proteins, (e.g., D-amino acids, ornithine, hydroxyproline, etc.) [14, 15, 19] or through structural modifications of peptides. Incorporation of moieties that are not amino acids, e.g., to generate peptidomimetics, can offer enhanced diversity [35], specificity and functionality but may come with an attendant risk of toxicity beyond that associated with simple amino acids. Minimal host toxicity (conceptualized in terms of a continuum from simple side effects to frank cytotoxicity) is desirable and achievable when using peptides as pharmaceutical agents because peptides: (a) can offer exquisite specificity to limit side effects, (b) eventually get metabolized only to byproducts that are non-toxic (i.e., amino acids), and (c) deliver sustained efficacy because peptides mimic the epitope-based action of naturally occurring proteins in regulating biological response. Efficacy without toxicity is the ultimate goal of drug development, and peptides offer one of the few avenues by which to approach that goal.
Structural modifications of synthetic peptides (for example, cyclization to induce conformational constraint) offer another level of diversity in pharmaceutical design. Cyclic peptides [18] may provide enhanced stability (shelf life, serum stability, etc.) [29], retain biological activity, provide protection against exopeptidases and have increased conformational stability [10]. Cyclization of peptides can be achieved through various techniques such as formation of a disulfide bond or a peptide bond [6, 10, 27, 40]. Disulfide cyclizations are easy to generate but require that two cysteines be included in the sequence which would increase the size of a potential drug (if the linear sequence had no Cys of its own). Additionally, the disulfide bond may be subject to reduction and re-oxidation in unintended configurations. C-terminal to N-terminal amide bond formation results in stable cyclic peptides that need not incorporate any additional amino acids not inherently a part of the sequence. In the development of AFPep [29], we used the cyclization method of Kates et al. [26] and incorporated one additional amino acid, namely Asn, to facilitate both synthesis and cyclization, using the C-terminal to N-terminal amide bond formation thus allowing for minimal size of the peptide.
Further innovative modifications to peptide structure, and likely to their function, could be achieved by the addition of an amino acid ‘tail’ to cyclic structures [12, 13]. Noted biological peptides that have a ring-and-tail structure include arginine vasopressin and analogs. These are 9-amino acid molecules consisting of a 6-amino acid ring and a 3-amino acid tail, and are cyclic by means of a disulfide bond (i.e., a side chain bond). In vasopressin, the 3-mer tail includes a lysine residue. If that residue is exchanged for arginine, the molecule changes its cellular target, binds to a different receptor and provokes a completely different response. Delforge et al. [12, 13] used a C-terminus to N-terminus peptide bond (i.e., main chain atoms) to generate synthetic cyclic peptides, then added a tail of amino acids for desired functionality (including serving as a linker to an affinity resin). Additional amino acids, outside of the cyclic portion of the peptide (i.e., attached to a side chain), may be expected to alter physical properties of the peptide in terms of hydrophobicity or hydrophilicity (perhaps to enhance solubility) and could potentially do so without disrupting the pharmacophore portion of a cyclized peptide. A tail could also conceivably have an effect on orienting a peptide ligand to a receptor. In the very common situation in which two related receptors accept the same ligand but which lead to differences in biological activity, amino acid tails might be useful to direct a cyclic peptide to one or the other of the receptors, thus mimicking the vasopressin observations. Like cyclizations, tails may be useful to increase molecular design possibilities and outcomes.
1.2 AFPep is a cyclic anti-cancer peptide
Earlier we elaborated an inexpensive, synthetic, 9-amino acid cyclic peptide that is useful for the treatment or prevention of breast cancer in animal models, and which retains activity after oral administration [2, 11, 25, 29, 30]. We call this peptide AFPep because it is a peptide mimic of the anti-oncogenic active site of α-fetoprotein (AFP, a protein to which the human fetus is exposed in high concentration). AFPep is cyclo[EKTOVNOGN] (in which O is hydroxyproline). Because background knowledge of AFP provided substantial structural information, rational design approaches were useful to conceptualize development of the active site peptide [11, 25, 29]. AFPep is composed exclusively of naturally occurring amino acids including hydroxyproline (which replaced Pro from the AFP sequence so as to enhance solubility of the peptide) [11, 29]. There are no peptidomimetic moieties, and AFPep has shown no toxicity in any animal model [1, 2, 34, 42]. In animal models, AFPep prevents carcinogen-induced breast cancer [1, 34], stops the growth of human tumor xenografts [2], is very well tolerated [1, 2, 34, 42], and is active when administered p.o., i.p., or s.c. [2]. Although AFPep is anti-estrotrophic, it does not disrupt the estrous cycle, fertility, or fecundity in rats [42], and has a unique mechanism of action that involves inhibition of phosphorylation of the estrogen receptor [34, 39, 41]. AFPep can be used as a stand-alone agent or in combination with other breast cancer agents such as tamoxifen [1]. While AFPep appears to have substantial potential as an anti-breast cancer drug, it may nevertheless be possible to enhance further the potency and specificity of this cyclic peptide by employing additional design concepts.
Earlier we explored the structure-function relationships of AFPep [11] and mapped the pharmacophore to the amino acids TOVNO [25, 29]. Other amino acids in the AFPep sequence are available for replacement or modification [11]. In this contribution, we chose to examine the effect of adding a tail to AFPep, and restricted the tail to being composed exclusively of amino acids so as to retain the very low toxicity profile of the peptide. We chose to put the tail on the non-pharmacophoric Asn side chain [i.e., the underlined Asn in cyclo(EKTOVNOGN)], thus generating analogs of AFPep which have a tail of 1 or 2 amino acids covalently attached (amide bond) through the side chain of Asn. In addition, we explored the utility of disrupting the TOVNO pharmacophore so as to generate an antagonist.
The purposes of this study were to demonstrate the utility, diversity and exquisite specificity of ring-and-tail analogs of AFPep as peptide drugs and as agents for receptor characterization. We show that a simple modification in an extra-annular portion (i.e., a tail) of a cyclic peptide provides surprisingly useful specificity. Further, by modifying the pharmacophore region, we capitalize on that specificity to develop analogs that can antagonize one receptor but not a closely related second receptor.
2. Materials and Methods
2.1 Materials
9-Fluorenylmethoxycarbonyl (FMOC)-protected amino acids were obtained from Calbiochem-Novabiochem (San Diego, CA, USA). Reagents for peptide synthesis, including 1,1,3,3-tetramethyluronium hexafluorophosphate (HATU), FMOC-PAL-PEG-PS resin, 20 % piperidine in dimethylformamide (DMF), and diisopropylethylamine (DIPEA) were obtained from Applied Biosystems, Inc (Framingham, MA USA). Tetrakis(triphenylphosphine)palladium (Pd(PPh3)4) catalyst, isopropanol, N-4-methylmorpholine, diethyldithiocarbamate trihydrate (DEDC), anisole, dichloromethane and chloroform were obtained from Sigma (St. Louis, MO, USA). Trifluroacetic acid (TFA), acetic acid, ethyl ether, and ethyl acetate were purchased from Fisher (Morris Plains, NJ, USA). 1,2-Ethanedithiol (EDT) was purchased from Fluka (Milwaukee, WI, USA).
2.2 Methods
2.2.1 Peptide synthesis
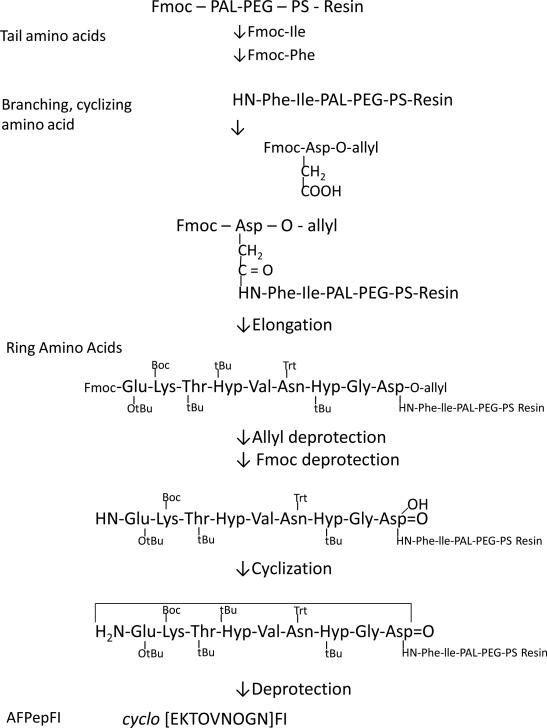
Peptides were prepared using FMOC solid phase synthesis as previously described [25, 29]. Automated or manual approaches were used to assemble the growing peptide chain on FMOC-PAL-PEG-PS resin beginning with the C-terminus using Nα-protected amino acids. Activation of the C-terminus of incoming amino acids was accomplished by treatment with HATU and DIPEA. Peptides were also obtained from commercial suppliers; for example, AmbioPharm (North Augusta, SC, USA) consistently produced active AFPep and ring-and-tail analogs of AFPep.
2.2.1.1 Cyclic peptides
After linear peptide synthesis, cyclization was accomplished using methods described by Kates et a.l [6, 26, 27] as described [25, 29]. Briefly, Nα-FMOC-L-aspartic acid-α-allyl ester (as the C-terminus of the synthetic peptide) was coupled to the resin via the γ carboxylic acid. Removal of the Nα-FMOC allowed the remaining amino acids to be incorporated sequentially into the growing peptide. To generate a free α-carboxyl group by removal of the allyl group from the C-terminal Asp [17, 26, 29], the peptidyl-resin was dried and flushed with nitrogen delivered through a septum. A catalyst solution was prepared separately by mixing 3 equivalents of Pd(PPh3)4 in CHCl3/acetic acid/N-4-methylmorpholine (37:2:1 v/v) (15 mL/g of resin) and dissolved by bubbling nitrogen through the solution. The catalyst was transferred to the tube containing peptidyl-resin using a gas-tight syringe, and mixed for 2 h. Peptidyl-resin was washed consecutively with 0.5% DIPEA in DMF and 0.5% w/w DEDC in DMF to remove the catalyst. Subsequently, the FMOC moiety was removed from the N-terminus which was then coupled to the free α-carboxyl group (while on the resin) in order to generate the cyclic peptide. The cyclized peptide was then removed from the resin in such a way as to yield the γ-carboxamido derivative (i.e., Asn). For AFPep: purity 99.4 %; MW expected: 967.0 Da; MW observed (mass spec): 968.7 Da; elemental analysis: 49.68 % C, 6.46 % H, 17.38 % N. For cyclo[EKTOVGOGN]: purity 99.7 %; MW expected 911.4 Da; MW observed (mass spec): 911.6 Da.
2.2.1.2 Ring-and-tail peptides
Peptides were assembled on PAL-PEG-PS resin beginning with the amino acids that formed the ‘tail.’ The C-terminus of the tail was attached to the resin and the peptide was assembled in routine fashion. FMOC-Asp-O-allyl was coupled through its γ-carboxylic acid to the N-terminus of the tail, leaving the α-carboxylic group protected (with the allyl group) for later cyclization. The remainder of the peptide was assembled and cyclization was achieved as described above, after the allyl group and the FMOC protection moieties were removed. The cyclic peptide was cleaved from the resin which yielded the ‘tail’-forming amino acid(s) outside the peptide ring, and as the C-terminal carboxamide, attached in amide link through the N-terminal amino acid to the γ-carboxamido moiety of the branching (non-pharmacophoric) Asn. This methodology is shown in Scheme 1. For cyclo[EKTOVNOGN]FS, purity 97.1 %; MW expected: 1202.6 Da; observed (mass spec): 1203.3 Da.
Scheme 1.
Synthetic process for generating ring-and-tail cyclic peptides.
2.2.2 Mouse uterine growth inhibition assay
As a rapid, inexpensive, sensitive surrogate for anti-cancer activity, the anti-estrotrophic activity of the peptides was determined using the immature mouse uterine growth inhibition assay as described by Festin et al. [16]. In this screening assay, inhibition of estrogen-stimulated uterine growth is closely correlated with inhibition of estrogen-stimulated tumor growth in xenograft assays. Administration of 0.5 μg of 17β-estradiol (E2) intraperitoneally (i.p.) to immature mice has been demonstrated [32] to double the uterine weight in 24 h (with a corresponding increase in mitotic figures), and this response (similar to estrogen-dependent cancer growth) is inhibited by AFPep [3]. Swiss Webster female mice (13-15 day old, 6-8 g body weight; Taconic Farms, Germantown, NY, USA) were weighed and distributed into treatment groups typically consisting of five mice each such that groups contained mice of comparable weight ranges. Each group received two sequential i.p. injections (0.2 mL) spaced 1 h apart. The first injection contained test substance or vehicle control. The second injection contained 0.5 μg E2 or vehicle. Twenty-two hours after the second injection, mice were sacrificed and weighed. Uteri were dissected and immediately weighed. Uterine weights were normalized to body weight (mg uterine weight per g of body weight) to compensate for differences between body weights of littermates. There were a minimum of five animals per group. Inhibition of estrogen-stimulated uterine growth was calculated from the average normalized uterine weights in each group using the following equation:
Statistical significance of the inhibition of estrogen-stimulated growth was assessed using the Wilcoxon sum of ranks test. A difference between groups of 15-20 % or greater was usually significant at the p < 0.05 level.
3. Results
AFPep analogs were generated, each of which bore a tail of either one or two amino acids, and with hydrophobic or hydrophilic side chains, or both. Analogs were screened at a single dose (1 μg/mouse) for their anti-estrotrophic activity, specifically the ability to inhibit estrogen-stimulated growth of the immature mouse uterus, and the results are listed in Table 1. At this dose, AFPep inhibits estrogen-stimulation of uterine growth by 38 ± 3 %. Analogs that bore a tail of one amino acid with a hydrophilic moiety in the side chain exhibited substantially diminished activity, ranging from 17 to 21 % growth inhibition. This is statistically different from AFPep. Analogs that bore a tail of two amino acids, in which either of the residues included a hydrophilic moiety in the side chain (i.e., Ser or Tyr) exhibited even less activity (less than 10 %). On the other hand, analogs that bore a tail of one amino acid with an exclusively hydrophobic side chain (i.e., Phe or Ile) had as much activity as did AFPep; an analog with two hydrophobic amino acids in the tail (both F and I) had slightly better activity than did AFPep in this screening assay. Analogs with a disrupted pharmacophore had very low activity.
Table 1.
Antiestrotrophic activity of AFPep and ring-and-tail analogs.
| Analog | Tail Amino Acid(s) | Nature of the Side Chain | Inhibition of Uterine Growtha % | Inhibition of T47D Cell Proliferationb % |
|---|---|---|---|---|
| cyclo[EKTOVNOGN] | None | (AFPep) | 38 ± 3* | 51 ± 4* |
| cyclo[EKTOVNOGN] S | S | Hydrophilic | 18 ± 2 | 22 ± 6 |
| cyclo[EKTOVNOGN] Y | Y | Hydrophilic | 21 ± 2 | 22 ± 3 |
| cyclo[EKTOVNOGN] K | K | Hydrophilic | 17 ± 3 | 18 ± 6 |
| cyclo[EKTOVNOGN] E | E | Hydrophilic | 18 ± 3 | 20 ± 2 |
| cyclo[EKTOVNOGN] FS | FS | Hydrophilic | 7 ± 3 | NA |
| cyclo[EKTOVNOGN] YI | YI | Hydrophilic | 9 ± 5 | 24 ± 3 |
| cyclo[EKTOVNOGN] F | F | Hydrophobic | 37 ± 2* | 46 ± 2* |
| cyclo[EKTOVNOGN] I | I | Hydrophobic | 39 ± 2* | 55 ± 6* |
| cyclo[EKTOVNOGN] FI | FI | Hydrophobic | 46 ± 4* | 66 ± 2* |
| Analogs with Disrupted Pharmacophores | ||||
| cyclo[EKTOVGOGN] | None | None | 14 ± 5 | NA |
| cyclo[EKTOVGOGN] FS | FS | Hydrophilic | 3 ± 7 | NA |
All peptides were administered at a dose of 1 μg/mouse intraperitoneally. Values are presented as mean ± S.E. (n = 5).
T47D human cancer cells were treated with the indicated peptides at a concentration of 1 μM. Values are mean ± S.E. (n = 6).
Peptides with inhibition of more than 25 % significantly inhibited estrogen-stimulated growth.
Statistically significantly different from saline control; p < 0.05 Wilcoxon sum of ranks test.
Also shown in Table 1 are the results of an in vitro assay that measures inhibition of proliferation of human breast cancer cells [25]. Inhibition of T47D human breast cancer cell proliferation parallels that of inhibition of growth of mouse uterus, suggesting that any species differences, or differences between in vivo and in vitro assays, are minimal.
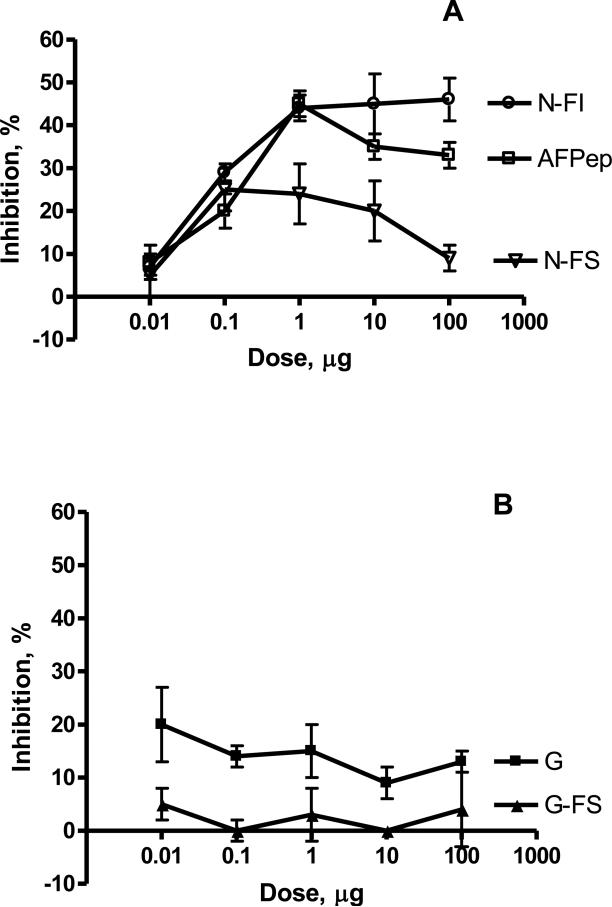
Dose-response curves are shown for selected analogs in Figure 1A. AFPep exhibited a biphasic dose-response curve, with partial loss of growth inhibitory activity at higher concentrations. These results are similar to those seen by Joseph et al. [25], in which the observation was ascribed to a two-receptor model. Putatively, a high affinity receptor binds AFPep at low dose and leads to a desired response, namely inhibition of uterine (or tumor) growth; a lower affinity receptor binds AFPep at higher doses and leads to an undesirable response, namely the suppression of growth inhibition. Analogs with hydrophobic tails (in this example cyclo[EKTOVNOGN]FI) exhibited a sigmoidal dose-response curve without apparent loss of activity at higher analog doses. This may be because hydrophobic-tailed analogs bind preferentially to the putative high affinity receptor. Analogs with a hydrophilic tail (in this example cyclo[EKTOVNOGN]FS) exhibited little activity at any dose, perhaps because they do not bind well to any receptor or because they bind preferentially to the putative low affinity receptor.
Figure 1. Inhibition of estrogen-stimulated uterine growth by analogs of AFPep.
Panel A: AFPep (open squares) exhibits slight loss of inhibition at higher doses; cyclo[EKTOVNOGN]FI (open circles, indicated as N-FI) exhibits sigmoidal dose-response curve without loss of activity at higher doses. cyclo[EKTOVNOGN]FS (open triangles, indicated as N-FS) exhibits low activity at low dose, even less activity at higher doses. Panel B: Analogs with a disrupted pharmacophore exhibit very low activity. cyclo[EKTOVGOGN] (an analog of AFPep with a single point mutation) (closed squares, indicated as G) exhibited modest activity at low dose, no activity at higher doses, whereas cyclo[EKTOVGOGN]FS (closed triangles, indicated as G-FS) had no activity at any dose.
Two additional analogs proved to be quite interesting, shown in Table 1 and Figure 1B. Replacing the pharmacophoric Asn (in the TOVNO region) with Gly resulted in an analog of AFPep (namely cyclo[EKTOVGOGN]) that had very low and statistically non-significant biological activity. As was shown by DeFreest [11], the pharmacophoric Asn appears to be essential for activity. An analog with a similarly disrupted pharmacophore and a hydrophilic tail cyclo[EKTOVGOGN]FS exhibited virtually no activity at all when assayed alone.
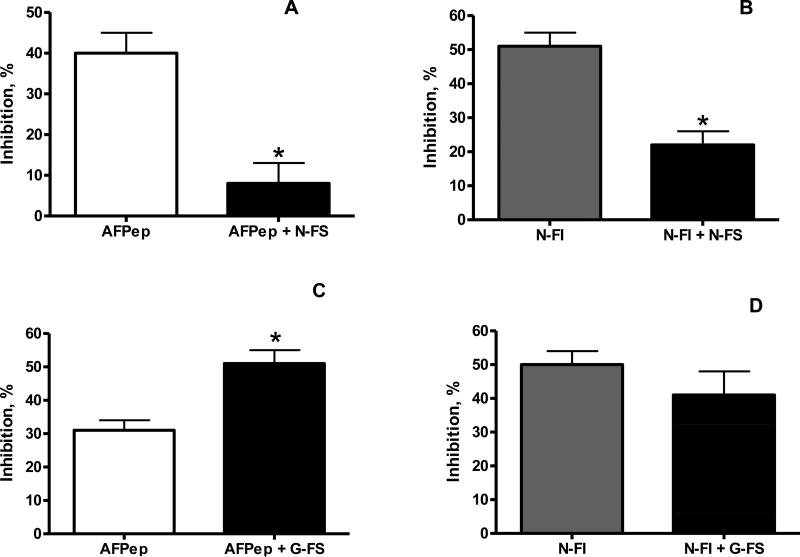
These analogs were then used in various combinations to yield the results shown in Figure 2. The analog with an intact pharmacophore and a hydrophilic tail (namely cyclo[EKTOVNOGN]FS) decreased the activity of AFPep (Fig. 2A) and decreased the activity of cyclo[EKTOVNOGN]FI (Fig. 2B), perhaps because this analog binds preferentially to the low affinity receptor, activates that receptor, and leads to suppression of the activity stimulated by either AFPep or cyclo[EKTOVNOGN]FI. At the very least, this observation suggests that the analog with a hydrophilic tail does bind to a receptor, i.e., that its low level of activity seen in Fig. 1A and Table 1 is not due to lack of binding to a receptor. On the other hand, an analog with a disrupted pharmacophore and a hydrophilic tail (namely cyclo[EKTOVGOGN]FS) enhanced the activity of AFPep (Fig. 2C) and had no effect on the activity of cyclo[EKTOVNOGN]FI (Fig 2D). These results would be expected if that analog bound preferentially to the low affinity receptor but did not activate it. Here, too, this analog must be binding to a receptor.
Figure 2. Combination of analogs affect biological responses.
The activity of AFPep (Panel A) or of cyclo[EKTOVNOGN]FI (Panel B, indicated as N-FI) is decreased in the presence of an analog with an intact pharmacophore and a hydrophilic tail (cyclo[EKTOVNOGN]FS, indicated as N-FS) which may bind preferentially to, and activate, a putative low affinity receptor. The activity of AFPep is enhanced (Panel C), while the activity of cyclo[EKTOVNOGN]FI (indicated as N-FI) is unaffected (Panel D) in the presence of an analog with a disrupted pharmacophore and a hydrophilic tail, (namely cyclo[EKTOVGOGN]FS, indicated as G-FS) which may bind preferentially to, but not activate, the low affinity receptor.
4. Discussion
The purpose of this paper is to highlight the potential of ring-and-tail modifications in the design of synthetic peptides to generate desired properties, including biological activity with exquisite specificity. Mimicking the structure and behavior of biologically derived ring-and-tail peptides such as vasopressin, synthetic peptides with tails may be useful for pharmaceutical purposes. It should be possible to generate peptide drugs that are efficacious, exquisitely specific and which therefore exhibit minimal side effects, and are generally non-toxic owing to the nature of their metabolites (i.e., simple amino acids). It is not the purpose of this contribution to characterize thoroughly the two-receptor system that we invoke to discuss our observations, and it is clear that alternate mechanisms (e.g., receptor desensitization or enzymatic degradation of peptides) could be considered. Some alternates may be formally analogous to the existence of a putative second binding site. We note nevertheless that it is possible to begin probing structure-function relationships of drug candidates without detailed knowledge of their binding partners.
For this study, we utilized analogs of AFPep, an anti-cancer synthetic peptide developed earlier. We restricted our design concepts so that only amino acids would be utilized, as opposed to generating peptidomimetics. Obviously the addition of moieties other than amino acids would broaden further the design opportunities for ring and tail molecules. We use the term ‘homobiotic’ (as opposed to xenobiotic) to describe these analogs because they are derived from a natural human protein and lack non-peptidic moieties. Homobiotic molecules might be expected to (a) exhibit the biological activity of the protein from which they are derived, (b) be low in toxicity (because their metabolites are exclusively amino acids) and side effects (because they have only one epitope/active site), and (c) be less susceptible to drug inactivation mechanisms such as cellular export (because they presumably function through natural pathways to mimic a natural biological response). Efficacy without toxicity may be achievable through use of homobiotic peptides.
AFPep was derived from the naturally occurring protein α-fetoprotein (AFP) [16, 29, 30] which is produced during fetal development [4, 5, 21] and which exhibits anti-oncogenic activity [23]. AFP is reported to be the primary agent responsible for a decreased incidence of breast cancer in populations of women who have experienced a full-term pregnancy compared to the nulliparous population [22, 24, 37]. In earlier work, a series of parsings [16, 30] of the AFP sequence was utilized to find the shortest fragment that retained the anti-estrotrophic activity of the parent protein. We showed that EMTPVNPG was the shortest biologically active peptide, but it exhibited a very short shelf life, probably attributable to aggregation, and a biphasic dose-response curve [25, 30]. To decrease aggregation, hydroxyproline was substituted for proline, creating EMTOVNOG, and to increase synthetic yield and purity, Met was replaced by Lys [11]. REMD simulations [25, 28], studies of smaller analogs, and the observation that a 7-mer had less biological activity than an 8-mer led to the idea that 8-mers (but not 7-mers) assumed pseudo-cyclic structures. A logical conclusion was to cyclize the linear peptide for added conformational constraint. Cyclization of EKTOVNOGN utilizing the method of Kates et al. [6, 25-27, 29] yielded an active cyclic 9-mer peptide (i.e., AFPep) with impressive stability including significantly longer shelf life and a less-pronounced biphasic dose-response curve [11, 29]. Continuing the rational design approach, DeFreest et al. [11] defined precisely the pharmacophore of the cyclic peptide and demonstrated that TOVNO contained the necessary constituents responsible for the activity of AFPep; it was especially noted that disruption of the Asn in this region would obviate activity. On the other hand, the Asn between the Gly and the Glu (i.e., the non-pharmacophoric Asn) can be altered or replaced without loss of activity [11].
Interestingly, AFPep yielded a biphasic dose-response curve [25], at least in the anti-estrogenic screening assay (as opposed to anti-cancer assays), suggesting an opportunity for further optimization of ligand through peptide design. One common explanation for a biphasic dose-response in binding of ligand is that there may be two receptors: a putative high affinity binding site leads to a specific response (in this case, a desired, growth inhibitory response), and a putative low affinity binding site may diminish that response as it becomes loaded at higher ligand concentrations. Indeed, a high affinity and low affinity receptor have been reported for AFPep's parent protein, AFP [33, 43]. In the study reported herein and elsewhere [25], higher AFPep doses led to an undesirable diminishment of the inhibition of estrogen-stimulated uterine growth. These data and the autoregulatory two-receptor systems widely present in biological systems provided direction for the design of next generation analogs of AFPep. If there were a peptide that would bind preferentially to (and activate) a putative high affinity receptor, it should yield a sigmoidal dose-response curve, mitigating the loss of activity at higher drug doses. Similarly, if there were an analog that would bind preferentially to the putative low affinity binding site, it should not exhibit the growth inhibitory activity, whether or not that analog was able to activate that receptor. If an analog were to bind preferentially to the putative low affinity receptor, but not activate the receptor, it may be useful as an antagonist of the low affinity receptor. Such a molecule might be useful in combination with AFPep to obviate the biphasic nature of AFPep's activity profile.
Three types of AFPep analogs are proffered in this communication. (a) An analog with a hydrophobic tail and intact pharmacophore: cyclo[EKTOVNOGN]FI appears to bind preferentially to the putative high affinity receptor since it yields a sigmoidal dose response curve with no loss of activity at high doses (Fig 1A). (b) An analog with a hydrophilic tail and an intact pharmacophore: cyclo[EKTOVNOGN]FS appears to bind preferentially to the putative low affinity receptor since it has little activity of its own (Fig 1A), but it can decrease the activity of AFPep or of cyclo[EKTOVNOGN]FI (Fig. 2A and B). Were it to bind to the high affinity binding site, it should lead to some biological activity since it has the correct pharmacophore. In fact, at low dose there is a non-statistically significant amount of biological activity associated with cyclo[EKTOVNOGN]FS which may suggest that there is some binding to the high affinity receptor (Fig 1A). cyclo[EKTOVNOGN]FS likely is an agonist of the low affinity binding site, leading to diminished activity of cyclo[EKTOVNOGN]FI. (c) An analog with a hydrophilic tail and a disrupted pharmacophore: cyclo[EKTOVGOGN]FS appears to be an antagonist of the low affinity binding site in that it may bind to that receptor but not activate it. Combination of this ligand with AFPep enhanced the activity of AFPep (Fig. 2C), presumably by occupying but not activating the low affinity receptor, thus preventing AFPep from binding there. cyclo[EKTOVGOGN]FS in combination with cyclo[EKTOVNOGN]FI had no effect on the activity of cyclo[EKTOVNOGN]FI (Fig 2D) perhaps because the latter analog does not bind preferentially to the low affinity receptor.
In view of the identity of the ring portion of cyclo[EKTOVNOGN]FS and cyclo[EKTOVNOGN]FI, the ability of these analogs to bind preferentially to related targets must reside in the tail of the peptide, which may interact with a corresponding epitope on the putative receptors. We predict that the putative high affinity receptor has a region near the active site that is complementary to the hydrophobic tail of cyclo[EKTOVNOGN]FI; polar or charged side chains in the tail of these ligands seems to disfavor interaction with this receptor. A low affinity receptor may lack that epitope or have a mutation in that epitope such that it interacts preferentially with peptides that have hydrophilic moieties in their tail. We note that tails containing positively charged, negatively charged, or polar side chains yielded similarly low levels of activity (Table 1). These observations suggest that extra-annular portions of synthetic cyclic peptides may provide another approach to exquisite specificity of ligand-receptor interactions, mimicking that seen in biological peptides such as vasopressin. The observation that substitution of a single amino acid (N to G) in the pharmacophore region of AFPep analogs results in loss of activity suggests that the side chain of this Asn is involved directly in signaling, i.e., activating the receptor, in keeping with the concepts explored by DeFreest et al. [11].
As acknowledged earlier, alternate models could be proffered to consider the results presented here, including differential metabolism of the ligands. Proteolysis of AFPep at high concentrations should not lead to a biphasic dose-response curve (Fig 1A), but rather to a sigmoidal curve, even if at a lower level than that for cyclo[EKTOVNOGN]FI. cyclo[EKTOVNOGN]FI and cyclo[EKTOVNOGN]FS yield very different results, but differ by only one amino acid in the tail. It would seem unlikely that a differential proteolytic site exists within the ring portion of these molecules because those sequences are identical. If a proteolytic site were in the tail of these analogs, proteolysis would convert either analog to AFPep (if proteolysis occurred on the N-terminal side of the F), or to the same analog cyclo[EKTOVNOGN]F if proteolysis occurred on the C-terminal side of F. Those events would not generate the results seen in Figure 1 A. Similarly, it might be postulated that the ligand with a disrupted pharmacophore, cyclo[EKTOVGOGN]FS, might differentially affect proteolysis of AFPep compared to cyclo[EKTOVNOGN]FI, but in the absence of two postulated binding sites, neither enhanced nor inhibited degradation of AFPep would be expected to lead to the enhanced activity seen in Fig 2C. Thus, differential proteolysis is considered unlikely, in keeping with the concepts published earlier [25] . The simplest explanation for the results herein invokes a two-receptor model.
It is noted that opportunities to design a tail portion for cyclic peptides may be non-obvious in the absence of detailed information about a receptor. For this work, we simply used hydrophobicity/hydrophilicity variations and changed the length of the tails (1 or 2 amino acids), and obtained notable alterations of biological response. There seemed to be no need to go to 3 amino acids in the tails, since it is desirable to minimize the size of potential therapeutic agents. Obviously, molecular modeling approaches, rational design considerations, or combinatorial approaches may be employed in the design of effector regions (tails) of peptides, as warranted by the system under study.
Finally, it should be noted that AFPep has substantial potential as an anti-cancer drug, and that the biphasic nature of its binding as demonstrated in this uterine growth inhibition screening assay may or may not obtain when the molecules are used in anti-cancer assays or for therapeutic purposes. cyclo[EKTOVNOGN]FI generates a sigmoidal dose-response curve in the screening assay but its results in the more expensive anti-cancer assays (which utilize many adult animals) have not yet been demonstrated. It may serve as a better agent than would AFPep, though it is slightly more expensive to synthesize. Alternatively it may be useful to add an antagonist of the putative low affinity receptor to a preparation of AFPep to obviate potential loss of activity at higher drug doses. These considerations must await investigations that utilize the more sophisticated cancer chemotherapy and cancer chemoprevention biological assays.
Acknowledgments
Funding
Support from the NIH: 5 R01 CA102540; 5 R25 GM 062460; T34 GM 008718.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Reference List
- 1.Andersen TT, Georgekutty J, DeFreest LA, Amaratunga G, Narendran A, Lemanski N, et al. An alpha-fetoprotein-derived peptide reduces the uterine hyperplasia and increases the antitumour effect of tamoxifen. Br J Cancer. 2007 Jul 31;97(3):327–33. doi: 10.1038/sj.bjc.6603882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bennett JA, Defreest L, Anaka I, Saadati H, Balulad S, Jacobson HI, et al. AFPep: an anti-breast cancer peptide that is orally active. Breast Cancer Res Treat. 2006 Mar 15;98(2):133–41. doi: 10.1007/s10549-005-9140-5. [DOI] [PubMed] [Google Scholar]
- 3.Bennett JA, Mesfin FB, Andersen TT, Gierthy JF, Jacobson HI. A peptide derived from alpha-fetoprotein prevents the growth of estrogen-dependent human breast cancers sensitive and resistant to tamoxifen. Proc Natl Acad Sci U S A. 2002 Feb 19;99(4):2211–5. doi: 10.1073/pnas.251667098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bennett JA, Semeniuk DJ, Jacobson HI, Murgita RA. Similarity between natural and recombinant human alpha-fetoprotein as inhibitors of estrogen-dependent breast cancer growth. Breast Cancer Res Treat. 1997 Sep;45(2):169–79. doi: 10.1023/a:1005841032371. [DOI] [PubMed] [Google Scholar]
- 5.Bennett JA, Zhu S, Pagano-Mirarchi A, Kellom TA, Jacobson HI. Alpha-fetoprotein derived from a human hepatoma prevents growth of estrogen-dependent human breast cancer xenografts. Clin Cancer Res. 1998 Nov;4(11):2877–84. [PubMed] [Google Scholar]
- 6.Blackburn C, Kates SA. Solid-phase synthesis of cyclic homodetic peptides. Methods Enzymol. 1997;289:175–98. doi: 10.1016/s0076-6879(97)89048-9. [DOI] [PubMed] [Google Scholar]
- 7.Blondelle SE, Lohner K. Combinatorial libraries: a tool to design antimicrobial and antifungal peptide analogues having lytic specificities for structure-activity relationship studies. Biopolymers. 2000;55(1):74–87. doi: 10.1002/1097-0282(2000)55:1<74::AID-BIP70>3.0.CO;2-S. [DOI] [PubMed] [Google Scholar]
- 8.Blondelle SE, Pinilla C, Boggiano C. Synthetic combinatorial libraries as an alternative strategy for the development of novel treatments for infectious diseases. Methods Enzymol. 2003;369:322–44. doi: 10.1016/S0076-6879(03)69018-X. [DOI] [PubMed] [Google Scholar]
- 9.Chanda SK, Caldwell JS. Fulfilling the promise: drug discovery in the post-genomic era. Drug Discov Today. 2003 Feb 15;8(4):168–74. doi: 10.1016/s1359-6446(02)02595-3. [DOI] [PubMed] [Google Scholar]
- 10.Cudic M, Wade JD, Otvos L. Convenient synthesis of a head-to-tail cyclic peptide containing an expanded ring. Tetrahedron Letters. 2000 Jun 12;41(23):4527–31. [Google Scholar]
- 11.DeFreest LA, Mesfin FB, Joseph L, McLeod DJ, Stallmer A, Reddy S, et al. Synthetic peptide derived from alpha-fetoprotein inhibits growth of human breast cancer: investigation of the pharmacophore and synthesis optimization. J Pept Res. 2004 May;63(5):409–19. doi: 10.1111/j.1399-3011.2004.00139.x. [DOI] [PubMed] [Google Scholar]
- 12.Delforge D, Art M, Gillon B, Dieu M, Delaive E, Raes M, et al. Automated solid-phase synthesis of cyclic peptides bearing a side-chain tail designed for subsequent chemical grafting. Anal Biochem. 1996 Nov 15;242(2):180–6. doi: 10.1006/abio.1996.0451. [DOI] [PubMed] [Google Scholar]
- 13.Delforge D, Dieu M, Delaive E, Art M, Gillon B, Devreese B, et al. Solid-phase synthesis of tailed cyclic peptides: The use of alpha-allyl-protected aspartic acid leads to aspartimide and tetramethylguanidinium formation. Letters in Peptide Science. 1996 May;3(2):89–97. [Google Scholar]
- 14.Fernandez-Tejada A, Corzana F, Busto JH, Avenoza A, Peregrina JM. Conformational effects of the non-natural alpha-methylserine on small peptides and glycopeptides. J Org Chem. 2009 Dec 18;74(24):9305–13. doi: 10.1021/jo901988w. [DOI] [PubMed] [Google Scholar]
- 15.Fernandez-Tejada A, Corzana F, Busto JH, Jimenez-Oses G, Peregrina JM, Avenoza A. Non-natural amino acids as modulating agents of the conformational space of model glycopeptides. Chemistry. 2008;14(23):7042–58. doi: 10.1002/chem.200800460. [DOI] [PubMed] [Google Scholar]
- 16.Festin SM, Bennett JA, Fletcher PW, Jacobson HI, Shaye DD, Andersen TT. The recombinant third domain of human alpha-fetoprotein retains the antiestrotrophic activity found in the full-length molecule. Biochim Biophys Acta. 1999 Apr 19;1427(2):307–14. doi: 10.1016/s0304-4165(99)00030-6. [DOI] [PubMed] [Google Scholar]
- 17.Flouzat C, Marguerite F, Croizet F, Percebois M, Monteil A, Combourieu M. Solid-phase synthesis of “head-to-side chain” cyclic tripeptides using allyl deprotection. Tetrahedron Letters. 1997 Feb 17;38(7):1191–4. [Google Scholar]
- 18.Gentilucci L, Cardillo G, Tolomelli A, Squassabia F, De MR, Chiriano G. Cyclopeptide analogs for generating new molecular and 3D diversity. Comb Chem High Throughput Screen. 2009 Dec;12(10):929–39. doi: 10.2174/138620709789824754. [DOI] [PubMed] [Google Scholar]
- 19.Gentilucci L, De MR, Cerisoli L. Chemical modifications designed to improve peptide stability: incorporation of non-natural amino acids, pseudo-peptide bonds, and cyclization. Curr Pharm Des. 2010;16(28):3185–203. doi: 10.2174/138161210793292555. [DOI] [PubMed] [Google Scholar]
- 20.Houghten RA. Combinatorial libraries. Finding the needle in the haystack. Curr Biol. 1994 Jun 1;4(6):564–7. doi: 10.1016/s0960-9822(00)00127-5. [DOI] [PubMed] [Google Scholar]
- 21.Jacobson HI, Bennett JA, Mizejewski GJ. Inhibition of estrogen-dependent breast cancer growth by a reaction product of alpha-fetoprotein and estradiol. Cancer Res. 1990 Jan 15;50(2):415–20. [PubMed] [Google Scholar]
- 22.Jacobson HI, Lemanski N, Agarwal A, Narendran A, Turner KE, Bennett JA, et al. A Proposed Unified Mechanism for the Reduction of Human Breast Cancer Risk by the Hormones of Pregnancy. Cancer Prev Res (Phila Pa) 2009 Nov 24; doi: 10.1158/1940-6207.CAPR-09-0050. [DOI] [PubMed] [Google Scholar]
- 23.Jacobson HI, Lemanski N, Agarwal A, Narendran A, Turner KE, Bennett JA, et al. A proposed unified mechanism for the reduction of human breast cancer risk by the hormones of pregnancy. Cancer Prev Res (Phila Pa) 2010 Feb;3(2):212–20. doi: 10.1158/1940-6207.CAPR-09-0050. [DOI] [PubMed] [Google Scholar]
- 24.Jacobson HI, Lemanski N, Narendran A, Agarwal A, Bennett JA, Andersen TT. Hormones of Pregnancy, AFP, and Reduction of Breast Cancer Risk. In: Li JJ, editor. Hormonal Carcinogenesis V. Springer; Berlin: 2000. [Google Scholar]
- 25.Joseph LC, Bennett JA, Kirschner KN, Shields GC, Hughes J, Lostritto N, et al. Antiestrogenic and anticancer activities of peptides derived from the active site of alpha-fetoprotein. J Pept Sci. 2009 Apr;15(4):319–25. doi: 10.1002/psc.1119. [DOI] [PubMed] [Google Scholar]
- 26.Kates SA, Daniels SB, Albericio F. Automated allyl cleavage for continuous-flow synthesis of cyclic and branched peptides. Anal Biochem. 1993 Aug 1;212(2):303–10. doi: 10.1006/abio.1993.1334. [DOI] [PubMed] [Google Scholar]
- 27.Kates SA, Sole NA, Johnson CR, Hudson D, Barany G, Albericio F. A Novel, Convenient, 3-Dimensional Orthogonal Strategy for Solid-Phase Synthesis of Cyclic-Peptides. Tetrahedron Letters. 1993 Mar 5;34(10):1549–52. [Google Scholar]
- 28.Kirschner KN, Lexa KW, Salisburg AM, Alser KA, Joseph L, Andersen TT, et al. Computational design and experimental discovery of an antiestrogenic peptide derived from alpha-fetoprotein. J Am Chem Soc. 2007 May 16;129(19):6263–8. doi: 10.1021/ja070202w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Mesfin FB, Andersen TT, Jacobson HI, Zhu S, Bennett JA. Development of a synthetic cyclized peptide derived from alpha-fetoprotein that prevents the growth of human breast cancer. J Pept Res. 2001 Sep;58(3):246–56. doi: 10.1034/j.1399-3011.2001.00922.x. [DOI] [PubMed] [Google Scholar]
- 30.Mesfin FB, Bennett JA, Jacobson HI, Zhu S, Andersen TT. Alpha-fetoprotein-derived antiestrotrophic octapeptide. Biochim Biophys Acta. 2000 Apr 15;1501(1):33–43. doi: 10.1016/s0925-4439(00)00008-9. [DOI] [PubMed] [Google Scholar]
- 31.Mitsutake A, Sugita Y, Okamoto Y. Generalized-ensemble algorithms for molecular simulations of biopolymers. Biopolymers. 2001;60(2):96–123. doi: 10.1002/1097-0282(2001)60:2<96::AID-BIP1007>3.0.CO;2-F. [DOI] [PubMed] [Google Scholar]
- 32.Mizejewski GJ, Vonnegut M, Jacobson HI. Estradiol-activated alpha-fetoprotein suppresses the uterotropic response to estrogens. Proc Natl Acad Sci U S A. 1983 May;80(9):2733–7. doi: 10.1073/pnas.80.9.2733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Naval J, Villacampa MJ, Goguel AF, Uriel J. Cell-type-specific receptors for alpha-fetoprotein in a mouse T-lymphoma cell line. Proc Natl Acad Sci U S A. 1985 May;82(10):3301–5. doi: 10.1073/pnas.82.10.3301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Parikh RR, Gildener-Leapman N, Narendran A, Lin HY, Lemanski N, Bennett JA, et al. Prevention of N-methyl-N-nitrosourea-induced breast cancer by alpha-fetoprotein (AFP)-derived peptide, a peptide derived from the active site of AFP. Clin Cancer Res. 2005 Dec 1;11(23):8512–20. doi: 10.1158/1078-0432.CCR-05-1651. [DOI] [PubMed] [Google Scholar]
- 35.Qin C, Bu X, Wu X, Guo Z. A chemical approach to generate molecular diversity based on the scaffold of cyclic decapeptide antibiotic tyrocidine A. J Comb Chem. 2003 Jul;5(4):353–5. doi: 10.1021/cc0300255. [DOI] [PubMed] [Google Scholar]
- 36.Qin C, Bu X, Zhong X, Ng NL, Guo Z. Optimization of antibacterial cyclic decapeptides. J Comb Chem. 2004 May;6(3):398–406. doi: 10.1021/cc030117u. [DOI] [PubMed] [Google Scholar]
- 37.Richardson BE, Hulka BS, Peck JL, Hughes CL, van den Berg BJ, Christianson RE, et al. Levels of maternal serum alpha-fetoprotein (AFP) in pregnant women and subsequent breast cancer risk. Am J Epidemiol. 1998 Oct 15;148(8):719–27. doi: 10.1093/oxfordjournals.aje.a009691. [DOI] [PubMed] [Google Scholar]
- 38.Sams-Dodd F. Drug discovery: selecting the optimal approach. Drug Discovery Today. 2006 May;11(9-10):465–72. doi: 10.1016/j.drudis.2006.03.015. [DOI] [PubMed] [Google Scholar]
- 39.Sierralta WD, Epunan MJ, Reyes JM, Valladares LE, Andersen TT, Bennett JA, et al. A peptide derived from alpha-fetoprotein inhibits the proliferation induced by estradiol in mammary tumor cells in culture. Oncol Rep. 2008 Jan;19(1):229–35. [PubMed] [Google Scholar]
- 40.Spinella MJ, Malik AB, Everitt J, Andersen TT. Design and synthesis of a specific endothelin 1 antagonist: effects on pulmonary vasoconstriction. Proc Natl Acad Sci U S A. 1991 Aug 15;88(16):7443–6. doi: 10.1073/pnas.88.16.7443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Torres CG, Pino AM, Sierralta WD. A cyclized peptide derived from alpha fetoprotein inhibits the proliferation of ER-positive canine mammary cancer cells. Oncol Rep. 2009 Jun;21(6):1397–404. doi: 10.3892/or_00000367. [DOI] [PubMed] [Google Scholar]
- 42.Tower AM, Trinward A, Lee K, Joseph L, Jacobson HI, Bennett JA, et al. AFPep, a novel drug for the prevention and treatment of breast cancer, does not disrupt the estrous cycle or fertility in rats. Oncol Rep. 2009 Jul;22(1):49–56. doi: 10.3892/or_00000405. [DOI] [PubMed] [Google Scholar]
- 43.Villacampa MJ, Moro R, Naval J, Failly-Crepin C, Lampreave F, Uriel J. Alpha-fetoprotein receptors in a human breast cancer cell line. Biochem Biophys Res Commun. 1984 Aug 16;122(3):1322–7. doi: 10.1016/0006-291x(84)91236-1. [DOI] [PubMed] [Google Scholar]
- 44.Vlieghe P, Lisowski V, Martinez J, Khrestchatisky M. Synthetic therapeutic peptides: science and market. Drug Discov Today. 2010 Jan;15(1-2):40–56. doi: 10.1016/j.drudis.2009.10.009. [DOI] [PubMed] [Google Scholar]