Abstract
The ability to form associations between previously unrelated items of information, such as names and faces, is an essential aspect of episodic memory function. The neural substrate that determines success vs. failure in learning these associations remains to be elucidated. Using event-related functional MRI during the encoding of novel face-name associations, we found that successfully remembered face-name pairs showed significantly greater activation in the anterior hippocampal formation bilaterally and left inferior prefrontal cortex, compared to pairs that were forgotten. Functional connectivity analyses revealed significant correlated activity between the right and left hippocampus and neocortical regions during successful, but not attempted, encoding. These findings suggest that anterior regions of the hippocampal formation, in particular, are crucial for successful associative encoding and that the degree of coordination between hippocampal and neocortical activity may predict the likelihood of subsequent memory.
Introduction
The hippocampus and related structures in the medial temporal lobe are thought to be critical for episodic memory, but the precise function of the hippocampus in creating successful memories is not yet fully understood. Converging evidence from animal models of amnesia (Squire and Zola-Morgan, 1991; Bunsey and Eichenbaum, 1995; Nakazawa et al., 2002), animal electrophysiological studies (Wood et al., 1999; Suzuki and Eichenbaum, 2000), and amnestic patients (Squire, 1992; Schacter and Church, 1995; Chalfonte et al., 1996; Ryan et al., 2000) suggests that one primary role of the hippocampal formation in episodic memory encoding is to form new associations between previously unrelated items of information (Squire and Zola-Morgan, 1991; Eichenbaum et al., 1996). Recent functional imaging studies have provided additional support for this role of the hippocampal formation in associative memory processes (Henke et al., 1997; Yonelinas et al., 2001; Zeineh et al., 2003). Many of these studies, as well as a meta-analysis by Schacter and Wagner (1999), have suggested that tasks that require relational or associative processing between stimuli are more likely to activate anterior regions of the hippocampal formation, as opposed to tasks that require processing of single stimuli, which tend to activate posterior hippocampal and parahippocampal regions (Stern et al., 1996; Brewer et al., 1998; Wagner et al., 1998; Kirchhoff et al., 2000). The majority of associative memory studies published to date, however, have used block design paradigms, which did not allow for the separation of signal associated with individual stimuli, and thus could not adequately control for other contributing factors, such as whether the associations were successfully remembered.
Advances in functional neuroimaging paradigm design, specifically event-related fMRI designs (Dale and Buckner, 1997), have allowed the separation of specific stimuli based on whether the stimuli are subsequently remembered or forgotten. The “subsequent memory” fMRI studies published to date have primarily examined nonassociative encoding. These studies have reported that the degree of activation in parahippocampal and some posterior hippocampal regions, as well as prefrontal cortex, predicts the likelihood of subsequent memory for single stimuli, such as words or scenes (Brewer et al., 1998; Wagner et al., 1998; Kirchhoff et al., 2000). It is unknown, however, whether activation in the same regions would also predict successful encoding of associations between unrelated items.
One particularly difficult associative encoding task encountered in daily life is learning the name associated with a new person one meets. Face-name association may be especially challenging because it requires the formation of a novel association between inherently unrelated items of information across the verbal and visual domains. We have previously reported that encoding novel face-name associations activates a set of brain regions, which include the hippocampus, fusiform, and prefrontal cortices (Sperling et al., 2001). We subsequently found that pharmacologically induced associative memory impairment selectively decreases activation in these same regions (Sperling et al., 2002). We undertook the current study, with an event-related face-name associative encoding paradigm with subsequent memory testing, to examine which brain regions are the most critical for successful encoding of novel face-name associations and whether the degree of coordination between regions predicts the likelihood of successful associative memory. We hypothesized that the hippocampal formation, specifically the anterior hippocampus, is critical for successful associative encoding, and that the degree of activation in the anterior hippocampus during encoding would predict success in subsequent associative memory performance.
It is unlikely that the hippocampus, or any other region, functions in isolation to form successful associations. If, in fact, the hippocampal formation is acting to “bind” together distributed representations of the information into a cohesive memory trace (Squire and Zola-Morgan, 1991; Eichenbaum et al., 1996), it is likely that this process requires coordinated activity between the hippocampal formation and neocortical regions. Given the extensive afferent and efferent neocortical connections of the anterior hippocampal formation, this region seems ideally situated to integrate neural activity from widespread neocortical inputs. Thus, we hypothesized that successful encoding would demonstrate a high degree of correlation between activity in the hippocampal formation and neocortical regions involved in the process of forming face-name associations.
Methods
Subjects
Sixteen right-handed, healthy young subjects (ages 20-33 years) participated in this study. Nine were female and seven were male. Participants were screened for neurologic and psychiatric illness, as well as any contraindications to MRI. None of the subjects were taking medications with known central nervous system effects. All subjects provided written informed consent in accordance with the Human Research Committee at Massachusetts General Hospital, Boston, MA.
fMRI activation task
During functional image acquisition, participants viewed face-name stimuli that consisted of a digital color photograph of an unfamiliar face presented against a black background with a fictional first name printed in white underneath the face. For each face-name pair, subjects were asked to indicate with a button press whether each name “fit” the face (pressing a button with their right index finger to indicate that the name did fit the face or with their right middle finger to indicate that the name did not fit the face). Subjects were informed that this was a purely subjective decision designed to augment the associative encoding process. Subjects were also instructed to try to remember the name associated with each face for later testing. These instructions were given verbally prior to beginning the experiment, and in between each functional run. Each face-name pair was presented only once during the experiment for 1.75 s. The face-name stimuli were randomly intermixed with trials of visual fixation of varying lengths (0.25 to 10 s, mean fixation length = 2.84 s). The fixation stimulus was a white fixation cross (+) centered on a black background.
A total of 455 face-name pairs were shown over the course of five runs in a jittered event-related design. The order of the stimuli presentation was determined using Opt-Seq (http://surfer.nmr.mgh.harvard.edu/). Stimuli were presented using MacStim 2.5 on a Macintosh computer and projected via a Sharp XG-2000V color LCD projector (Osaka, Japan) through a collimating lens (Buhl Optical) onto a rear projection screen. Subjects viewed the screen through a mirror attached to the head coil.
Postscan memory test
Immediately after the imaging session, subjects completed a memory test for all 455 face-name pairs that were shown during image acquisition. The postscan stimuli consisted of the same face images on a black background now shown with two names printed in white underneath the face: the correct name that was actually paired with the face during encoding, and an incorrect name that was paired with a different face during encoding. The location of the correct name was balanced for equal numbers on the right and on the left. Subjects were asked to indicate which name was correct and whether they had high or low confidence that they made the correct choice.
Image acquisition
Subjects were scanned using a Siemens Allegra 3.0-T scanner (Siemens Medical Systems, Iselin, NJ) with a 3-axis gradient head coil. Twenty-six slices (5 mm, skip 1 mm) were acquired in an oblique coronal orientation, perpendicular to the anterior commissure-posterior commissure (AC-PC) line, to maximize in-plane resolution (3.125 × 3.125 × 6 mm) and reduce susceptibility artifact within the hippocampus. Functional data were acquired using a gradient echo sequence (TR = 2000, TE = 30, flip angle = 90°). Five functional runs were acquired for each subject with 140 time points per run.
Data analysis
Functional MRI data were preprocessed and analyzed using Statistical Parametric Mapping (SPM99; Wellcome Department of Cognitive Neurology) for Matlab (Math-works, Inc.). The data were realigned and normalized to the standard SPM99 EPI template. The registration of the EPI data to the template was checked for each individual subject. The fMRI data were smoothed with a gaussian kernel of 8 mm. No slice time correction was applied, and the data were modeled with the canonical hemodynamic response function only. No scaling was implemented for global effects. A high pass filter of 70 s was used to filter out low frequency variations (i.e., drift across each run). Comparisons of interest (All Face-Name pairs vs. Fixation; High Confidence Correct vs. Incorrect) were implemented as linear contrasts, first at the individual subject level, averaging together the five runs for each subject. The contrast images for each subject were then used in a random effects analysis to determine which regions were the most consistently activated across subjects using a one-sample t test. Statistical maps were thresholded at a significance level of P < 0.001 with a minimum extent threshold of 20 contiguous voxels (resampled SPM voxel size +3 × 3 × 3 mm). Activations were considered significant at a level of P < 0.05 after correction for multiple comparisons at the cluster level. All P values are reported at the corrected cluster level. Percent signal change for regions of interest (ROIs) functionally defined from the statistical activation maps were determined using the SPMROI Toolbox (http://spm-toolbox.sourceforge.net).
Conjunction analyses were performed using the method described by Cabeza et al. (2002). Masks containing the significantly active voxels for the contrasts of interest (HC-Correct vs. LC-Correct and HC-Incorrect vs. LC-Incorrect) were multiplied together using the ImCalc feature in SPM99. This resulted in a mask that contains only the voxels that are significantly active in both contrasts to examine the relationship between confidence and accuracy.
Functional connectivity analyses were performed on the random effects level group data using the signal extracted from seed regions (4 mm radius) from the contrast maps (All Face-Name pairs vs. Fixation; High Confidence Correct vs. Incorrect), and were compared with all other voxels across subjects using simple correlation analyses (Friston et al., 1996; Poldrack et al., 2001). Connectivity maps were thresholded at a significance level of P < 0.001 with an extent threshold of 20 contiguous voxels. Regions were considered to show significant connectivity if the regions exhibited positive or negative correlations at a level of P < 0.05 after correction for multiple comparisons at the cluster level. Correlation coefficients between the functionally defined ROI (seed regions with 4-mm radius) were then generated using Pearson correlation.
Behavioral results for the encoding task and postscan memory test were analyzed using Student's t test and ANOVA.
Results
Behavioral results
Subjects correctly identified the name associated with the face in 66.2 ± 4.2% of the trials overall, and 77.5 ± 7.8% of the trials in which they indicated a high confidence level (HC-Correct) (see Table 1). Subjects were more likely to indicate that they thought the name fit the face than not during encoding (P < 0.0001); however, there was no difference in the percentage of HC-Correct responses among those face-name pairs that were classified as “fits” vs. “not-fits.” There was no difference in the reaction time during encoding for subsequently correctly compared to incorrectly remembered face-name associations. There was no effect of stimulus order within or across the runs on the percentage of correctly or incorrectly remembered face-name pairs.
Table 1. Performance on postscan memory testing (n = 16).
| Correct | Incorrect | |
|---|---|---|
| Overall | 66.2 ± 4.2%b | 33.8 ± 4.2% |
| High confidence | 77.5 ± 7.8%b | 22.5 ± 7.8% |
| Low confidence | 57.6 ± 3.9%a | 42.4 ± 3.9% |
P < 0.00001;
P < 0.000000001.
Activation patterns in “Attempted” and “Successful” associative encoding
We first examined the pattern of activation in Attempted associative encoding vs. baseline (i.e., All Face-Name pairs vs. Fixation), regardless of subsequent memory performance. This contrast yielded activation in regions consistent with previous studies of novel associative encoding (Sperling et al., 2001, 2002; Zeineh et al., 2003) including: bilateral hippocampus, bilateral fusiform regions, thalamus, and lateral inferior and orbitofrontal regions (Table 2). The activation within the hippocampal formation for All Attempted Encoding was primarily located in mid- to posterior hippocampus (peakMNI coordinates x, y, z: left: −24, −27, −6; right: 21, −30, −3).
Table 2. Significantly activated regions during Attempted and Successful Encodinga.
| Contrast | Region | x | y | z | Z score | P (corrected)b |
|---|---|---|---|---|---|---|
| Attempted encoding (All face-name pairs vs. fixation) | Left fusiform gyrus | −42 | −48 | −24 | 5.58 | <0.000001 |
| Right fusiform gyrus | 42 | −39 | −27 | 5.66 | <0.000001 | |
| Left hippocampus | −24 | −27 | −6 | 4.26 | <0.000001 | |
| Right hippocampus | 21 | −30 | −3 | 4.38 | 0.024 | |
| Left thalamus | − 12 | − 12 | −9 | 4.02 | <0.000001 | |
| Right thalamus | 9 | −9 | 0 | 4.16 | 0.0076 | |
| Left precentral gyrus | −36 | −3 | 57 | 4.16 | <0.000001 | |
| Left inferior frontal gyrus | −60 | 9 | 18 | 4.97 | <0.000001 | |
| Right inferior frontal gyrus | 42 | 12 | 24 | 6.14 | <0.000001 | |
| Left cingulate gyrus | −9 | 15 | 42 | 4.85 | <0.000001 | |
| Right cingulate gyrus | 6 | 18 | 45 | 4.91 | <0.000001 | |
| Left inferior frontal/orbitofrontal gyrus | −33 | 24 | 0 | 4.44 | <0.000001 | |
| Right inferior frontal/orbitofrontal gyrus | 27 | 24 | −6 | 5.09 | <0.000001 | |
| Successful encoding (HC-Correct vs. Incorrect) | Left hippocampus | −30 | −21 | − 18 | 4.57 | 0.000063 |
| Right hippocampus entorhinal cortex | 33 | − 15 | −33 | 4.33 | 0.000097 | |
| Left putamen | −36 | 0 | 12 | 3.94 | 0.024 | |
| Left insula | −45 | 0 | 27 | 3.89 | 0.043 | |
| Left inferior frontal gyrus | − 51 | 21 | 6 | 3.95 | 0.00031 |
Coordinates for the peak voxel are listed as MNI coordinates.
Significance values are corrected for multiple comparisons at the cluster level.
We then examined the pattern of activation in Successful vs. Failed Encoding. To minimize the contribution of correct responses that occurred by chance, activation maps were generated for those face-name pairs that were subsequently remembered with high confidence compared to face-name pairs that were subsequently forgotten (i.e., HC-Correct vs. Incorrect). Random effects group analyses showed significant activation bilaterally in the hippocampal formation (left: −30, −21, −18, P < 0.0001 corrected for multiple comparisons at the cluster level; right: 33, −15, − 33, P < 0.0001) and left inferior prefrontal cortex (−51, 21, 6, P < 0.001). The activation within the hippocampal formation for successful associative encoding extended from the mid-hippocampus anteriorly to the hippocampal-amygdaloid border bilaterally, and included the entorhinal cortex on the right (see Fig. 1).
Fig. 1.

Successful vs. Failed Encoding. Statistical activation maps demonstrating greater response during encoding of Face-Name pairs that were subsequently successfully remembered (High Confidence Correct) compared to Face-Name Pairs that were forgotten (Incorrect). Activation within the hippocampal formation is shown in three image planes: coronal (top), sagittal (middle), and transaxial (bottom), and was located in the anterior hippocampus bilaterally, and the right entorhinal cortex. Analyses performed with SPM99, and statistical activation maps (color bar represents T scores) of the event-related data averaged across subjects are displayed on an anatomic T1 image template.
To examine activity in both the posterior and anterior hippocampal formation in greater detail, we used a region of interest (ROI) approach (Poldrack et al., 2001). This involved extracting the MR signal time course within functionally defined ROIs for three stimulus types: HC-Correct, LC-Correct, and Incorrect responses (see Fig. 2). In the posterior hippocampus, we found a significant increase in MR signal for all stimulus types compared to fixation; that is, there were no significant differences between HC-Correct, LC-Correct, and Incorrect conditions. In the anterior hippocampus, however, the MR signal response was significantly greater (P < 0.0001) for HC-Correct than LC-Correct and Incorrect face-name pairs.
Fig. 2.
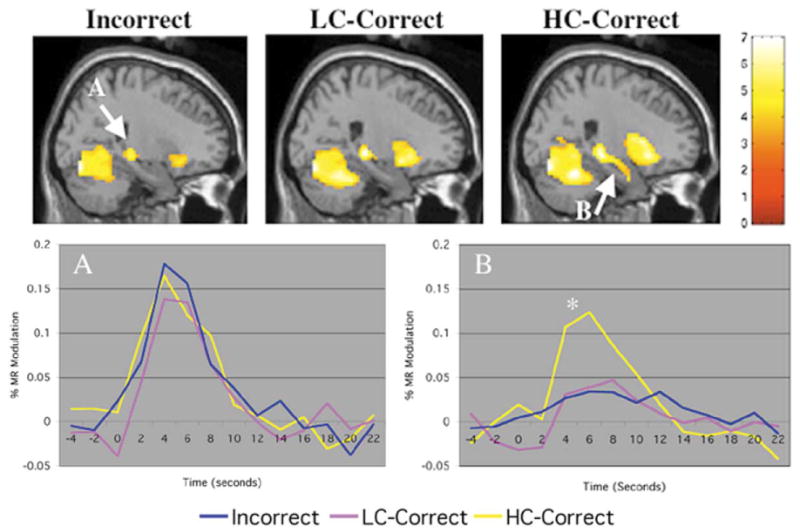
Attempted Encoding vs. Fixation. Activation maps for each of the attempted encoding conditions compared to visual fixation (color bar represents T scores): Face-Name pairs that were subsequently forgotten (Incorrect), Remembered with Low Confidence (LC-Correct), and Remembered with High Confidence (HC-Correct). Within the hippocampal formation, Incorrect (top left) and LC-Correct (top middle) demonstrated significant activation in mid-posterior hippocampus only, while HC-Correct (top right) demonstrated activation along the entire longitudinal axis of the hippocampus, including the anterior hippocampus. MR signal time courses were extracted for a three-dimensional region of interest (4-mm radius) for the posterior hippocampus (bottom left, A: centered at 21, −30, −3) and the anterior hippocampus (bottom right, B: centered at 24, −12, −15). The percent MR signal modulation for each condition is indicated on the y-axis and time in seconds is indicated on the x-axis. All encoding conditions (HC-Correct, LC-Correct, and Incorrect) showed similar response in mid- to posterior hippocampus, while HC-Correct responses were significantly higher (P < 0.00001) than LC-Correct and Incorrect in the anterior hippocampus.
We then explored the possibility that the hippocampal activation was related to the subjective confidence level by using a conjunction analysis (e.g., Cabeza et al., 2002) to examine the relationship between accuracy and confidence. We partitioned the data into HC-Correct, LC-Correct, HC-Incorrect, and LC-Incorrect, and generated two contrasts: HC-Correct vs. LC-Correct and HC-Incorrect vs. LC-Incorrect. These two contrasts showed brain regions that demonstrated significantly greater activation for high confidence responses compared to low confidence responses for correct and incorrect responses, respectively. There were no regions that showed a significant overlap between confidence and accuracy at the significance level of P < 0.001, which was used for all of the other analyses. At a less stringent threshold of P < 0.01, however, there was a small region in the left inferior prefrontal cortex (−42, 24, 18), which showed activation for both HC-Correct vs. LC-Correct and HC-Incorrect vs. LC-Incorrect contrasts (see Fig. 3). There was no activation within the hippocampal formation for the HC-Incorrect vs. LC-Incorrect contrast, even at the lower significance threshold.
Fig. 3.

Analysis of confidence and accuracy. Activation maps for HC-Correct vs. LC-Correct (yellow) and HC-Incorrect vs. LC-Incorrect (red) are displayed on coronal slices: prefrontal regions shown on left at y = 27, and hippocampal regions shown on the right at y = −12). No significant overlap was found using a conjunction analysis at the threshold of P < 0.001, but at the lower threshold atP < 0.01 (shown in figure), the only region showing overlap between confidence and accuracy was found in the left inferior prefrontal cortex. There was bilateral activation in the anterior hippocampal formation for the HC-correct vs. LC-correct contrast, but no activation within the hippocampal formation for the HC-Incorrect vs. LC-Incorrect even at the lower threshold of P < 0.01.
Functional connectivity analyses
To determine which regions exhibited correlated activity with the anterior hippocampus during successful associative encoding, random effects functional connectivity analyses were performed (Friston et al., 1996; see Table 3). The MR signal estimates from the peak activation (maximal task-dependent activation for HC-Correct vs. Incorrect responses) for left and right hippocampal formation were extracted and entered into a voxel-by-voxel correlation analysis across all subjects (Poldrack et al., 2001). These analyses for the left anterior hippocampal formation showed significant positive correlations with the right anterior hippocampus, bilateral fusiform cortices, and bilateral inferior prefrontal regions. We then extracted the MR signal time course within functionally defined ROIs to further examine the strength of the relationship (correlation coefficient and significance value) between activity in specific regions (Poldrack et al., 2001). The strongest correlation was found between the left and right anterior hippocampus (r = 0.89, P < 0.00001). The correlation between left anterior hippocampus MR signal change and left inferior prefrontal cortex MR signal change was also highly significant (r = 0.84, P < 0.0001). Functional connectivity analysis for the right anterior hippocampal formation showed significant correlations with the right anterior fusiform gyrus and the left inferior prefrontal cortex. Using the ROI approach yielded a similarly strong correlation between the right hippocampal formation and left inferior prefrontal cortex (r = 0.83, P < 0.0001).
Table 3. Functional connectivity HC-correct vs. incorrecta.
| Seed Region | Connected Region | x | y | z | r | P |
|---|---|---|---|---|---|---|
| Left hippocampal formation | Left fusiform gyrus | −27 | −69 | −21 | 0.74 | 0.0011 |
| Right fusiform gyrus | 54 | −60 | − 18 | 0.76 | 0.00068 | |
| Right medial temporal gyrus | 42 | −60 | 18 | 0.83 | 0.000083 | |
| Right hippocampus | 27 | − 15 | − 15 | 0.89 | 0.000005 | |
| Left thalamus | −9 | − 12 | 15 | 0.87 | 0.000012 | |
| Left superior temporal gyrus | −30 | 9 | −30 | 0.88 | 0.000008 | |
| Left inferior frontal gyrus | −33 | 36 | 6 | 0.84 | 0.000045 | |
| Right inferior frontal gyrus | 42 | 27 | 21 | 0.86 | 0.000018 | |
| Right hippocampal formation | Right anterior fusiform | 39 | −12 | −36 | 0.89 | 0.000004 |
| Right middle frontal gyrus | 30 | −24 | 36 | 0.89 | 0.000003 | |
| Left inferior frontal gyrus | −48 | 24 | 30 | 0.83 | 0.000076 |
Regions showing significant connectivity at a P < 0.05 corrected for multiple comparisons at the cluster level using SPM functional connectivity analyses. Coordinates for the peak voxel are listed as MNI coordinates. Pearson's correlation coefficients (r) listed with correlation significance values (P).
Random effects functional connectivity analyses were also performed for attempted encoding (All Face-Name pairs vs. Fixation contrast). Despite the increased statistical power afforded by the greater number of stimulus events included in this contrast, there were no regions that showed significant correlation at the corrected cluster level with either the right or left hippocampus, or with any other peaks from the All Face-Name vs. Fixation contrast.
Discussion
This study provides evidence that the successful encoding of novel cross-modal associations is subserved by a specific set of brain regions, in particular, anterior portions of the hippocampal formation bilaterally and the left inferior prefrontal cortex. Furthermore, our data suggest that the degree of coordination between activity in the hippocampal formation and prefrontal cortex may contribute to the likelihood of successful subsequent associative memory.
Our findings are consistent with previous event-related fMRI studies reporting that activation in the medial temporal lobe and prefrontal cortex during encoding is predictive of subsequent memory performance (Brewer et al., 1998; Wagner et al., 1998; Kirchhoff et al., 2000; Otten et al., 2001). More specifically, our results suggest that the location of activation within the medial temporal lobe may be dependent on the specific demands of the memory task (i.e., whether the formation of a novel association is required). Several previous “block design” neuroimaging studies (Henke et al., 1999; Schacter and Wagner, 1999; Mitchell et al., 2000; Yonelinas et al., 2001, Zeineh et al. 2003), including our own (Sperling et al., 2001), have suggested that associative memory tasks preferentially activate anterior regions of the hippocampal formation. Zeineh et al. (2003) recently published a block design fMRI experiment during the encoding and retrieval of face-name pairs. Their study used high resolution MR acquisition and “flat mapping techniques” to examine activation within specific subregions of the hippocampal formation. They demonstrated activation in the anterior CA fields 2 and 3, and the dentate gyrus during blocks of encoding novel face-name pairs compared to fixation. Consistent with our findings, Zeineh et al. (2003) reported that the activation was maximal in these regions during the first of the four blocks of encoding, when the group of subjects learned the greatest number of face-name pairs. Our results using an event-related design, which allows the separation of the MR signal during encoding of face-name pairs that were subsequently remembered from those that were subsequently forgotten for each individual subject, furthermore suggests that activation of these anterior hippocampal regions is directly related to the likelihood of successfully forming these cross-modal associations.
In this study, we did not directly compare associative encoding (i.e., binding together previously unrelated items of information) to nonassociative encoding (single items). However, the location of the activation within the hippocampus for successful encoding of associative stimuli in our study is more anterior than what has now been reported in multiple studies for successful encoding of single item stimuli (Brewer et al., 1998; Wagner et al., 1998; Kirchhoff et al., 2000). For example, Wagner et al. (1998) reported that increased left posterior parahippocampal activation predicted successful memory for single words, and Brewer et al. (1998) reported that increased right posterior parahippocampal activation predicted subsequent memory for single pictures. Kirchoff et al. (2000) found that activation in the posterior hippocampus (28, −30, −6) and parahippocampus (−28, −37, −9) predicted subsequent memory for single pictures and single words, respectively. In our study, the analysis comparing each of the conditions (HC-Correct, LC-Correct, and Incorrect) vs. Fixation as shown in Fig. 2 suggests that, while attempted encoding activates more posterior regions of the hippocampal formation, it is only the successful encoding of novel associations that activated the anterior portions of the hippocampal formation.
A recent event-related fMRI study by Davachi and Wagner (2002) compared relational and item based learning of word triplets. They found greater hippocampal activation for relational processing of items, and greater parahippocampal activation for item based processing. Consistent with our results, Davachi and Wagner (2002) also found that hippocampal activation predicted subsequent memory for items encoded with relational processing, but not with item-based processing. The relational aspect in the Davachi and Wagner (2002) study differs somewhat from the associative encoding task in our study. They used an “elaborative” encoding condition, which required the rating of relative ordering of words according to desirability, compared to a “rote” learning condition. An additional difference is that the subsequent memory testing in the Davachi and Wagner study was performed for each individual word rather than for the triplet associations, whereas in our study, the subsequent memory test required subjects to indicate which of two names, both seen during the experiment, was associated with the face during encoding. Another study from Davachi et al. (2003) demonstrated greater activation in the hippocampus bilaterally for words that were encoded through associative mental imagery compared to words that were read. Furthermore, greater hippocampal activation was seen during encoding when the subject subsequently remembered whether they had encoded the word during imagery or reading, suggesting that the hippocampus is involved in encoding “source” associations. Also consistent with our results is the finding of Otten et al. (2001), who reported greater activation in the anterior hippocampus for “semantically” encoded words that were subsequently remembered, which the authors speculated might represent the binding together of item and contextual information.
A block design fMRI study by Small et al. (2001) examined associative vs. single item encoding for faces and names. This study reported that the encoding of faces alone activated posterior hippocampal regions, while hearing names alone activated more anterior hippocampal regions, consistent with previous studies using auditory encoding paradigms. The encoding of face-name pairs activated a unique spatial distribution, which included mid-hippocampal regions. Because the Small et al. (2001) study was performed with a block design paradigm, it did not address the issue of whether activation was dependent on successful encoding of associations between the stimuli. Our data examining attempted encoding, using a visual presentation of face-name pairs, is consistent with the results of Small et al. (2001), but our data comparing successful vs. failed encoding suggests that it is the anterior hippocampal formation, which is important for creating enduring associative memories. Further study directly comparing the activation pattern during the successful and failed encoding of novel cross-modal associations to successful and failed encoding of single items should clarify whether the anterior hippocampal formation is selectively activated only during successful associative memory processes.
It is also possible, however, that the anterior-posterior differential in activation is an artifact of functional mapping with limited spatial resolution. The difference in results seen with single-item encoding versus associative encoding could represent a distinction between activation in the hippocampus proper versus activation in the posterior parahippocampus. Several of the fMRI studies using single-item encoding parahippocampal and posterior hippocampal activation have used complex scenes (Stern et al., 1996; Brewer et al., 1998; Kirchhoff et al., 2000). The concept of a specific “parahippocampal place area” has thus been suggested (Epstein et al., 1999). This explanation is unlikely to fully explain the anterior-posterior distinction in all of these studies, because posterior parahippocampal activation has also been reported with single-item word encoding (Wagner et al., 1998; Fernandez et al., 1998). It is also possible that, in our study, amygdala activation is contributing to the activation at the border of the anterior hippocampus. It is difficult to accurately distinguish the anterior hippocampus, entorhinal cortex, and amygdala using relative thick slice acquisition parameters (5 mm, skip 1 mm) and additional “smoothing” (8 mm) in the fMRI analyses. However, in a previous study using a similar face-name association task with block design, we anatomically defined the hippocampus, parahippocampus, and amygdala using each individual subject's anatomy, and the activation was clearly centered in the anterior hippocampus (Sperling et al., 2002). In the current study, the activation peaks were also centered in the anterior hippocampus, although we cannot rule out some contribution from amygdalar activation, which is also heavily connected with the entorhinal cortex (Van Hoesen, 1982).
An alternative theory for the role of the hippocampus that has been suggested by several neuroimaging studies (Tulving et al., 1994; Strange et al., 1999) involves the detection of novel stimuli. For example, a recent subsequent memory paper by Strange et al. (2002) reported anterior hippocampal activation, in addition to perirhinal and para-hippocampal activation, for subsequent memory of word lists. In their study, the anterior hippocampal activation was found to predict subsequent memory only for the initial words on each list, which the authors postulated might represent a “primacy” or novelty effect. Our findings in mid- and posterior hippocampal regions are somewhat supportive of the novelty detection theory, since all of the face-name pairs were novel to the subjects and showed a similar increase in the MR signal in these more posterior hippocampal regions compared to baseline. However, novelty detection is unlikely to fully explain our results in the anterior hippocampal formation, as it was only the face-name pairs that were subsequently remembered correctly with high confidence that showed significantly higher MR signal response in these anterior regions. Furthermore, each run included a very large number of face-name pairs, and the distribution of HC-Correct, LC-Correct, and Incorrect responses was independent of the presentation order of the stimuli within each run. Additionally, each subject remembered a unique subset of the face-name pairs; thus, it is unlikely that any other features of specific stimuli explain our results.
There is some neuroanatomical and clinical support for a differential role of the anterior and posterior regions of the hippocampal formation in associative memory function. The most distinguishing features of the anterior hippocampal formation lie in its connections to neocortex (for review, see Moser and Moser, 1998). The primary input from multimodal association neocortical areas into the hippocampus occurs anteriorly, via the entorhinal cortex, and is known as the perforant pathway (Van Hoesen, 1982). The perforant pathway is thought to be of critical importance in memory function, and disruption of this pathway, as is known to occur early in the pathophysiologic progression of Alzheimer's disease (AD), produces profound memory impairment (Hyman et al., 1984). Of particular relevance to this study is the finding that AD patients exhibit significant deficits in associative memory function very early in the clinical course of their disease (Morris et al., 1991; Fowler et al., 2002), and difficulty remembering proper names continues to be the most common complaint of older individuals visiting memory clinics (Zelinski and Gilewski, 1988; Leirer et al., 1990).
Our findings are also consistent with evidence from both functional imaging and animal studies suggesting that the longitudinal axis of the hippocampus may function as a series of interconnected “memory circuits,” a feature that may be particularly important for associative encoding (Small, 2002; Nakazawa et al., 2002). In this context, activation of the anterior hippocampus may be particularly important when it occurs simultaneously with activation of other hippocampal regions, thus effectively activating the entire longitudinal axis of the hippocampus. Our data comparing each of the conditions (HC-Correct, LC-Correct, and Incorrect vs. Fixation as shown in Fig. 2) suggest that while activation in mid- and posterior hippocampus occurs during “attempted” encoding, it may be the addition of the anterior hippocampal activation, which completes the “circuit” along the longitudinal axis of the hippocampus, that might be important for creating successful associations.
In addition to activating anterior regions of the hippocampal formation, successful associative encoding also produced significant activation in the left inferior prefrontal cortex. This finding is consistent with multiple previous subsequent memory event-related designs examining non-associative encoding that have reported that activation in left inferior prefrontal cortex predicts the likelihood of subsequent memory (Brewer et al., 1998; Wagner et al., 1998; Kirchhoff et al., 2000; Otten et al., 2001; Buckner et al., 2001). It remains unclear what the precise role the prefrontal cortex plays during encoding, but this region is likely important for modulating complex attentional networks (Rees and Lavie, 2001; Cabeza et al., 2003). We did not observe any differences in the reaction time during the encoding task between face-name pairs that were subsequently remembered vs. forgotten, but it is possible that the increased left inferior prefrontal cortex activation reflects increased vigilance during the encoding of stimuli that will be later remembered. We also found that a small region in the left inferior prefrontal cortex was activated in highly confident responses for both correct and incorrect answers. Previous studies have suggested that specific regions in the left pre-frontal cortex may be involved in the subjective experience accompanying memory formation and retrieval (Henson et al., 1999; Dobbins et al., 2002). Interestingly, although there was significant activation within the hippocampal formation for HC-Correct vs. LC-Correct contrast, there was no activation within the hippocampal formation for the HC-Incorrect vs. LC-Incorrect contrast. This finding suggests that the primary role of the anterior hippocampus in successful associative memory formation may be in accurately “binding” together items of information rather than in imparting the subjective feeling of confidence for subsequent memory.
As one of the unique attributes of the anterior hippocampal formation is its strong neuroanatomical connectivity with neocortex, we hypothesized that successful associative encoding may depend not only on activation of the anterior hippocampal formation, but also on the synchronization of neural activity with neocortical regions. We used random effects functional connectivity analyses to examine which other brain regions showed evidence of correlated MR activity with the hippocampal formation. These analyses demonstrated that activity in the hippocampal formation was highly correlated with activity in several neocortical regions during successful encoding but not in attempted encoding. Interestingly, only a subset of the regions from the main successful vs. failed encoding contrast showed evidence of significant functional connectivity, namely the left and right hippocampal peaks. In addition, the functional connectivity analyses revealed evidence of correlated activity between the hippocampal formation and additional neocortical regions that were not significantly activated in the main contrast. In successful encoding, the regions showing significant positive correlations with the hippocampus included the fusiform cortices bilaterally, a region which is known to be important for the processing of human faces in the right hemisphere (Kanwisher et al., 1997) and in the successful encoding of faces (Kuskowski and Pardo, 1999). A similar fusiform region in the left hemisphere has also been shown to activate with processing of famous names (Tempini et al., 1998), as well as in other naming paradigms (Martin et al., 1996). A recent PET study of learning face-name associations also demonstrated that fusiform and neighboring inferior temporal cortices were activated during successful encoding (Herholz et al., 2001). Our previous block design studies have also indicated that the anterior regions of the fusiform in particular are involved in the encoding of novel face-name associations (Sperling et al., 2002, 2003). The functional connectivity analyses in this study furthermore suggest that correlated activity between the hippocampus and fusiform cortices may be particularly important for successful encoding of face-name associations.
The hippocampal formation bilaterally also showed evidence of significantly correlated activity with the prefrontal cortex during successful encoding. The prefrontal cortex is known to have both afferent and efferent projections from the hippocampal formation (Goldman-Rakic et al., 1984; Insausti et al., 1987), including specific prefrontal connections projections to the anterior hippocampal formation (Barbas and Blatt, 1995). Interestingly, although only the left inferior prefrontal cortex was significantly activated in the main successful vs. failed encoding contrast, both left and right prefrontal cortices demonstrated significant correlated activity with the hippocampal formation. Our connectivity analyses suggest that, in addition to activation of these regions, the degree of coordination in the activity between the hippocampal formation and neocortical regions may predict successful memory formation. New multivariate methods for image analyses that are being adapted for functional MRI, such as singular value decomposition, may prove particularly useful in investigating the pattern of co-variance between these regions.
Although the prefrontal activation was lateralized to the left during the main successful vs. failed encoding contrast, we observed activation that was quite symmetric in the left and right anterior hippocampal formation in the main contrast. This finding may be related to the fact that our face-name association task requires integration across both verbal and visual domains, since previous studies have suggested that there may be lateralization of hippocampal activation in verbal vs. nonverbal memory tasks (Kelley et al., 1998). In addition, the functional connectivity analyses suggested that activity in the left and right anterior hippocampal formations was strongly intercorrelated during successful encoding, and both left and right anterior hippocampal formations showed correlated activity with inferior prefrontal cortices bilaterally during successful encoding. It is unknown whether the coordination of right and left hippocampal activity is mediated through some other neocortical region, but one might speculate that it is the prefrontal cortex that serves in this capacity.
Acknowledgments
This work was supported by NINDS: K23-NS02189 (R.S.); NIMH: MH60941 (D.S.); and NIA: P01-AG-04953 (M.A.). We gratefully acknowledge Mary Foley, Larry White, and Jennifer Holmes for assistance with scan acquisition.
References
- Barbas H, Blatt GJ. Topographically specific hippocampal projections target functionally distinct prefrontal areas in the rhesus monkey. Hippocampus. 1995;5:511–533. doi: 10.1002/hipo.450050604. [DOI] [PubMed] [Google Scholar]
- Brewer JB, Zhao Z, Desmond JE, Glover GH, Gabrieli JD. Making memories: brain activity that predicts how well visual experience will be remembered. Science. 1998;281:1185–1187. doi: 10.1126/science.281.5380.1185. [DOI] [PubMed] [Google Scholar]
- Buckner RL, Wheeler ME, Sheridan MA. Encoding processes during retrieval tasks. J Cogn Neurosci. 2001;13:406–415. doi: 10.1162/08989290151137430. [DOI] [PubMed] [Google Scholar]
- Bunsey M, Eichenbaum H. Selective damage to the hippocampal region blocks long-term retention of a natural and nonspatial stimulus-stimulus association. Hippocampus. 1995;5:546–556. doi: 10.1002/hipo.450050606. [DOI] [PubMed] [Google Scholar]
- Cabeza R, Dolcos F, Graham R, Nyberg L. Similarities and differences in the neural correlates of episodic memory retrieval and working memory. NeuroImage. 2002;16:317–330. doi: 10.1006/nimg.2002.1063. [DOI] [PubMed] [Google Scholar]
- Cabeza R, Dolcos F, Prince SE, Rice HJ, Weissman DH, Nyberg L. Attention-related activity during episodic memory retrieval: a cross-function fMRI study. Neuropsychologia. 2003;41:390–399. doi: 10.1016/s0028-3932(02)00170-7. [DOI] [PubMed] [Google Scholar]
- Chalfonte BL, Verfaellie M, Johnson MK, Reiss L. Spatial location memory in amnesia: binding item and location information under incidental and intentional encoding conditions. Memory. 1996;4:591–614. doi: 10.1080/741940998. [DOI] [PubMed] [Google Scholar]
- Dale AM, Buckner RL. Selective averaging of rapidly presented individual trials using fMRI. Hum Brain Mapp. 1997;5:329–340. doi: 10.1002/(SICI)1097-0193(1997)5:5<329::AID-HBM1>3.0.CO;2-5. [DOI] [PubMed] [Google Scholar]
- Davachi L, Mitchell JP, Wagner AD. Multiple routes to memory: distinct medial temporal lobe processes build item and source memories. Proc Natl Acad Sci USA. 2003;100:2157–2162. doi: 10.1073/pnas.0337195100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davachi L, Wagner AD. Hippocampal contributions to episodic encoding: insights from relational and item-based learning. J Neuro-physiol. 2002;88:982–990. doi: 10.1152/jn.2002.88.2.982. [DOI] [PubMed] [Google Scholar]
- Dobbins I, Foley H, Schacter D, Wagner A. Executive control during episodic retrieval. Multiple prefrontal processes subserve source memory. Neuron. 2002;35:989. doi: 10.1016/s0896-6273(02)00858-9. [DOI] [PubMed] [Google Scholar]
- Eichenbaum H, Schoenbaum G, Young B, Bunsey M. Functional organization of the hippocampal memory system. Proc Natl Acad Sci USA. 1996;93:13500–13507. doi: 10.1073/pnas.93.24.13500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Epstein R, Harris A, Stanley D, Kanwisher N. The parahippocampal place area: recognition, navigation, or encoding? Neuron. 1999;23:115–125. doi: 10.1016/s0896-6273(00)80758-8. [DOI] [PubMed] [Google Scholar]
- Fernandez G, Weyerts H, Schrader-Bolsche M, et al. Successful verbal encoding into episodic memory engages the posterior hippocampus: a parametrically analyzed functional magnetic resonance imaging study. J Neurosci. 1998;18:1841–1847. doi: 10.1523/JNEUROSCI.18-05-01841.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fowler KS, Saling MM, Conway EL, Semple JM, Louis WJ. Paired associate performance in the early detection of DAT. J the Int Neuropsychol Soc. 2002;8:58–71. [PubMed] [Google Scholar]
- Friston KJ, Frith CD, Fletcher P, Liddle PF, Frackowiak RS. Functional topography: multidimensional scaling and functional connectivity in the brain. Cereb Cortex. 1996;6:156–164. doi: 10.1093/cercor/6.2.156. [DOI] [PubMed] [Google Scholar]
- Goldman-Rakic PS, Selemon LD, Schwartz ML. Dual pathways connecting the dorsolateral prefrontal cortex with the hippocampal formation and parahippocampal cortex in the rhesus monkey. Neuroscience. 1984;12:719–743. doi: 10.1016/0306-4522(84)90166-0. [DOI] [PubMed] [Google Scholar]
- Henke K, Buck A, Weber B, Wieser HG. Human hippocampus establishes associations in memory. Hippocampus. 1997;7:249–256. doi: 10.1002/(SICI)1098-1063(1997)7:3<249::AID-HIPO1>3.0.CO;2-G. [DOI] [PubMed] [Google Scholar]
- Henke K, Weber B, Kneifel S, Wieser HG, Buck A. Human hippocampus associates information in memory. Proc Natl Acad Sci USA. 1999;96:5884–5889. doi: 10.1073/pnas.96.10.5884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henson RN, Rugg MD, Shallice T, Josephs O, Dolan RJ. Recollection and familiarity in recognition memory: an event-related functional magnetic resonance imaging study. J Neurosci. 1999;19:3962–3972. doi: 10.1523/JNEUROSCI.19-10-03962.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herholz K, Ehlen P, Kessler J, Strotmann T, Kalbe E, Markowitsch HJ. Learning face-name associations and the effect of age and performance: a PET activation study. Neuropsychologia. 2001;39:643–650. doi: 10.1016/s0028-3932(00)00144-5. [DOI] [PubMed] [Google Scholar]
- Hyman BT, Van Hoesen GW, Damasio AR, Barnes CL. Alzheimer's disease: cell-specific pathology isolates the hippocampal formation. Science. 1984;225:1168–1170. doi: 10.1126/science.6474172. [DOI] [PubMed] [Google Scholar]
- Insausti R, Amaral DG, Cowan WM. The entorhinal cortex of the monkey: II. Cortical afferents. J Comp Neurol. 1987;264:356–395. doi: 10.1002/cne.902640306. [DOI] [PubMed] [Google Scholar]
- Kanwisher N, McDermott J, Chun MM. The fusiform face area: a module in human extrastriate cortex specialized for face perception. J Neurosci. 1997;17:4302–4311. doi: 10.1523/JNEUROSCI.17-11-04302.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelley WM, Miezin FM, McDermott KB, et al. Hemispheric specialization in human dorsal frontal cortex and medial temporal lobe for verbal and nonverbal memory encoding. Neuron. 1998;20:927–936. doi: 10.1016/s0896-6273(00)80474-2. [DOI] [PubMed] [Google Scholar]
- Kirchhoff BA, Wagner AD, Maril A, Stern CE. Prefrontal-temporal circuitry for episodic encoding and subsequent memory. J Neurosci. 2000;20:6173–6180. doi: 10.1523/JNEUROSCI.20-16-06173.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuskowski MA, Pardo JV. The role of the fusiform gyrus in successful encoding of face stimuli. NeuroImage. 1999;9:599–610. doi: 10.1006/nimg.1999.0442. [DOI] [PubMed] [Google Scholar]
- Leirer VO, Morrow DG, Sheikh JI, Pariante GM. Memory skills elders want to improve. Exp Aging Res. 1990;16:155–158. doi: 10.1080/07340669008251544. [DOI] [PubMed] [Google Scholar]
- Martin A, Wiggs CL, Ungerleider LG, Haxby JV. Neural correlates of category-specific knowledge. Nature. 1996;379:649–652. doi: 10.1038/379649a0. [DOI] [PubMed] [Google Scholar]
- Mitchell KJ, Johnson MK, Raye CL, D'Esposito M. fMRI evidence of age-related hippocampal dysfunction in feature binding in working memory. Brain Res Cogn Brain Res. 2000;10:197–206. doi: 10.1016/s0926-6410(00)00029-x. [DOI] [PubMed] [Google Scholar]
- Morris JC, McKeel DW, Jr, Storandt M, Rubin EH, Price JL, Grant EA, Ball MJ, Berg L. Very mild Alzheimer's disease: informant-based clinical, psychometric, and pathologic distinction from normal aging [see comments] Neurology. 1991;41:469–478. doi: 10.1212/wnl.41.4.469. [DOI] [PubMed] [Google Scholar]
- Moser MB, Moser EI. Functional differentiation in the hippocampus. Hippocampus. 1998;8:608–619. doi: 10.1002/(SICI)1098-1063(1998)8:6<608::AID-HIPO3>3.0.CO;2-7. [DOI] [PubMed] [Google Scholar]
- Nakazawa K, Quirk MC, Chitwood RA, et al. Requirement for hippocampal CA3 NMDA receptors in associative memory recall. Science. 2002;30:30. doi: 10.1126/science.1071795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Otten LJ, Henson RN, Rugg MD. Depth of processing effects on neural correlates of memory encoding: relationship between findings from across- and within-task comparisons. Brain. 2001;124(Pt 2):399–412. doi: 10.1093/brain/124.2.399. [DOI] [PubMed] [Google Scholar]
- Poldrack RA, Clark J, Pare-Blagoev EJ, Shohamy D, Creso Moyano J, Myers C, Gluck MA. Interactive memory systems in the human brain. Nature. 2001;414:546–550. doi: 10.1038/35107080. [DOI] [PubMed] [Google Scholar]
- Rees G, Lavie N. What can functional imaging reveal about the role of attention in visual awareness? Neuropsychologia. 2001;39:1343–1353. doi: 10.1016/s0028-3932(01)00122-1. [DOI] [PubMed] [Google Scholar]
- Ryan JD, Althoff RR, Whitlow S, Cohen NJ. Amnesia is a deficit in relational memory. Psychol Sci. 2000;11:454–461. doi: 10.1111/1467-9280.00288. [DOI] [PubMed] [Google Scholar]
- Schacter DL, Church B. Implicit memory in amnesic patients: when is auditory priming spared? J Int Neuropsychol Soc. 1995;1:434–442. doi: 10.1017/s1355617700000539. [DOI] [PubMed] [Google Scholar]
- Schacter DL, Wagner AD. Medial temporal lobe activations in fMRI and PET studies of episodic encoding and retrieval. Hippocampus. 1999;9:7–24. doi: 10.1002/(SICI)1098-1063(1999)9:1<7::AID-HIPO2>3.0.CO;2-K. [DOI] [PubMed] [Google Scholar]
- Small SA. The longitudinal axis of the hippocampal formation: its anatomy, circuitry, and role in cognitive function. Rev Neurosci. 2002;13:183–194. doi: 10.1515/revneuro.2002.13.2.183. [DOI] [PubMed] [Google Scholar]
- Small SA, Nava AS, Perera GM, DeLaPaz R, Mayeux R, Stern Y. Circuit mechanisms underlying memory encoding and retrieval in the long axis of the hippocampal formation. Nat Neurosci. 2001;4:442–449. doi: 10.1038/86115. [DOI] [PubMed] [Google Scholar]
- Sperling R, Bates J, Chua E, Cocchiarella A, Schacter DL, Rosen B, Albert M. fMRI studies of associative encoding in young and elderly controls and mild AD patients. J Neurol Neurosurg Psychiatry. 2003;74:44–50. doi: 10.1136/jnnp.74.1.44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sperling R, Bates J, Cocchiarella A, Schacter D, Rosen B, Albert M. Encoding novel face-name associations: a functional MRI study. Hum Brain Mapp. 2001;14:129–139. doi: 10.1002/hbm.1047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sperling RA, Greve D, Dale A, et al. fMRI detection of pharmacologically induced memory impairment. Proc Natl Acad Sci USA. 2002;99:455–460. doi: 10.1073/pnas.012467899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Squire LR. Memory and the hippocampus: a synthesis from findings with rats, monkeys, and humans [published erratum appears in Psychol Rev 1992 Jul;99(3):582] Psychol Rev. 1992;99:195–231. doi: 10.1037/0033-295x.99.2.195. [DOI] [PubMed] [Google Scholar]
- Squire LR, Zola-Morgan S. The medial temporal lobe memory system. Science. 1991;253:1380–1386. doi: 10.1126/science.1896849. [DOI] [PubMed] [Google Scholar]
- Stern CE, Corkin S, Gonzalez RG, et al. The hippocampal formation participates in novel picture encoding: evidence from functional magnetic resonance imaging. Proc Natl Acad Sci USA. 1996;93:8660–8665. doi: 10.1073/pnas.93.16.8660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strange BA, Fletcher PC, Henson RN, Friston KJ, Dolan RJ. Segregating the functions of human hippocampus. Proc Natl Acad Sci USA. 1999;96:4034–4039. doi: 10.1073/pnas.96.7.4034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strange BA, Otten LJ, Josephs O, Rugg MD, Dolan RJ. Dissociable human perirhinal, hippocampal, and parahippocampal roles during verbal encoding. J Neurosci. 2002;22:523–528. doi: 10.1523/JNEUROSCI.22-02-00523.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suzuki WA, Eichenbaum H. The neurophysiology of memory. Ann NY Acad Sci. 2000;911:175–191. doi: 10.1111/j.1749-6632.2000.tb06726.x. [DOI] [PubMed] [Google Scholar]
- Tempini ML, Price CJ, Josephs O, Vandenberghe R, Cappa SF, Kapur N, Frackowiak RS. The neural systems sustaining face and proper-name processing. Brain. 1998;121(Pt 11):2103–2118. doi: 10.1093/brain/121.11.2103. [DOI] [PubMed] [Google Scholar]
- Tulving E, Markowitsch HJ, Kapur S, Habib R, Houle S. Novelty encoding networks in the human brain: positron emission tomography data. NeuroReport. 1994;5:2525–2528. doi: 10.1097/00001756-199412000-00030. [DOI] [PubMed] [Google Scholar]
- Van Hoesen GW. The parahippocampal gyrus. Trends Neurosci. 1982;5:345–350. [Google Scholar]
- Wagner AD, Schacter DL, Rotte M, Koutstaal W, Maril A, Dale AM, Rosen BR, Buckner RL. Building memories: remembering and forgetting of verbal experiences as predicted by brain activity. Science. 1998;281:1188–1191. doi: 10.1126/science.281.5380.1188. [DOI] [PubMed] [Google Scholar]
- Wood ER, Dudchenko PA, Eichenbaum H. The global record of memory in hippocampal neuronal activity. Nature. 1999;397:613–616. doi: 10.1038/17605. [DOI] [PubMed] [Google Scholar]
- Yonelinas AP, Hopfinger JB, Buonocore MH, Kroll NE, Baynes K. Hippocampal, parahippocampal and occipital-temporal contributions to associative and item recognition memory: an fMRI study. NeuroReport. 2001;12:359–363. doi: 10.1097/00001756-200102120-00035. [DOI] [PubMed] [Google Scholar]
- Zeineh MM, Engel SA, Thompson PM, Bookheimer SY. Dynamics of the hippocampus during encoding and retrieval of face-name pairs. Science. 2003;299:577–580. doi: 10.1126/science.1077775. [DOI] [PubMed] [Google Scholar]
- Zelinski EM, Gilewski MJ. Assessment of memory complaints by rating scales and questionnaires. Psychopharmacol Bull. 1988;24:523–529. [PubMed] [Google Scholar]


