Abstract
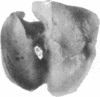
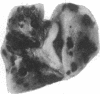
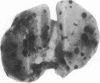
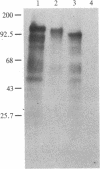
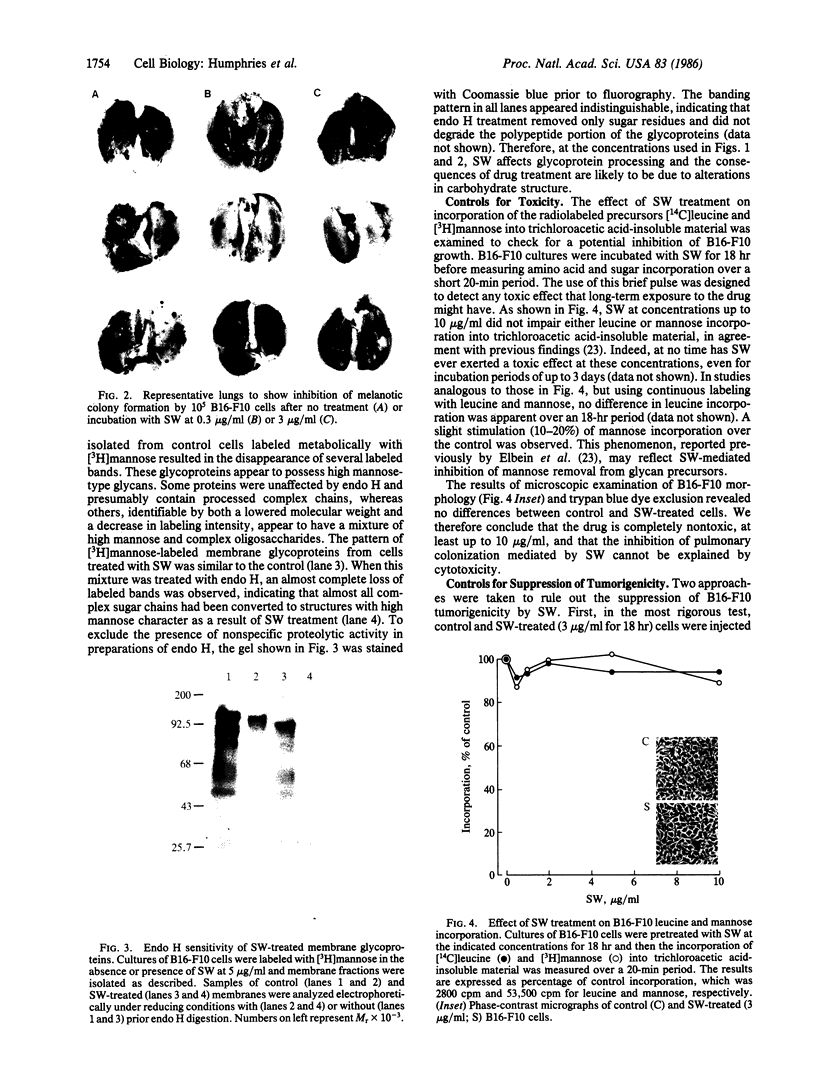
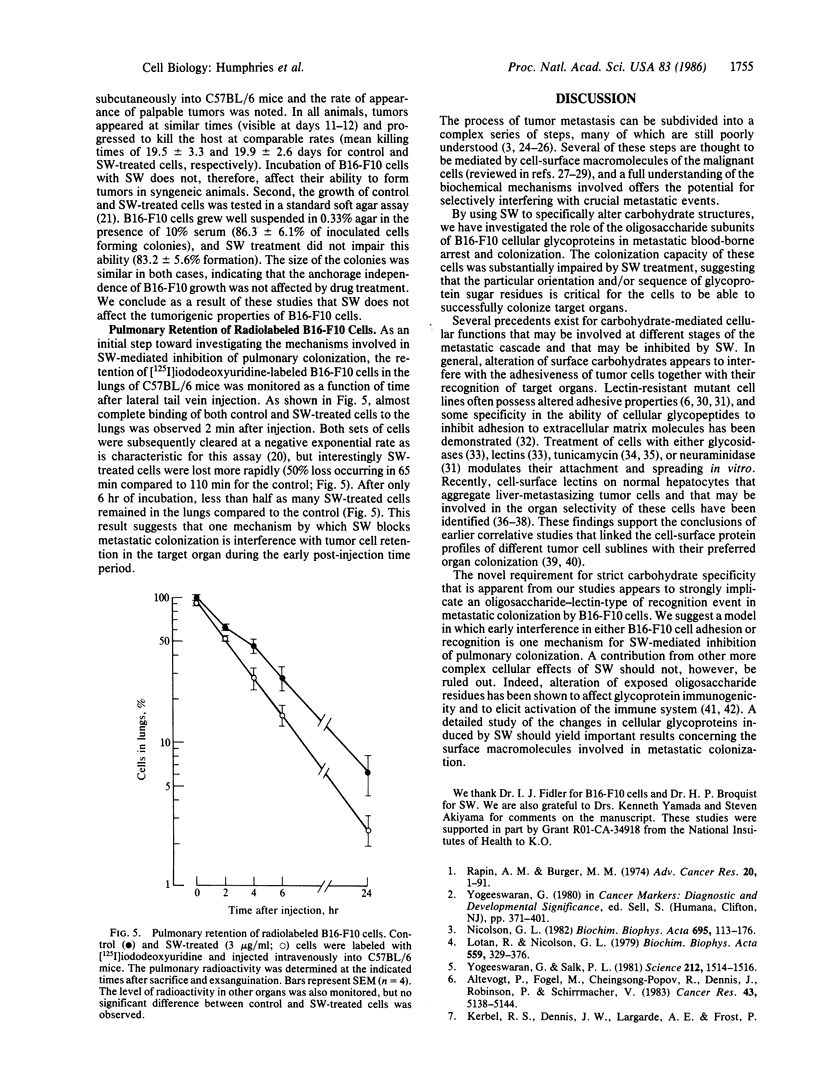
Oligosaccharide moieties of cell-surface glycoconjugates are thought to be involved in recognition events associated with tumor metastasis and invasion. Using swainsonine (SW), an inhibitor of Golgi alpha-mannosidase II that results in the formation of hybrid-type oligosaccharides on N-linked glycoproteins, we have tested the hypothesis that specific glycan structures are required for pulmonary colonization by tumor cells. B16-F10 murine melanoma cells were treated with SW in growth medium and then injected intravenously into syngeneic C57BL/6 mice. This treatment resulted in dramatic inhibition of colonization, but it had no effect on B16-F10 viability or on cellular tumorigenicity after subcutaneous implantation. SW-treated radiolabeled B16-F10 cells were cleared from the lungs at a greater rate than control cells, suggesting that one effect of treatment is to alter tumor cell retention in the target organ. Our results implicate specific glycan structures in pulmonary colonization and offer a potential approach for identification of specific macromolecules involved in tumor cell-organ recognition during metastasis.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Altevogt P., Fogel M., Cheingsong-Popov R., Dennis J., Robinson P., Schirrmacher V. Different patterns of lectin binding and cell surface sialylation detected on related high- and low-metastatic tumor lines. Cancer Res. 1983 Nov;43(11):5138–5144. [PubMed] [Google Scholar]
- Brunson K. W., Beattie G., Nicolsin G. L. Selection and altered properties of brain-colonising metastatic melanoma. Nature. 1978 Apr 6;272(5653):543–545. doi: 10.1038/272543a0. [DOI] [PubMed] [Google Scholar]
- Brunson K. W., Nicolson G. L. Selection of malignant melanoma variant cell lines for ovary colonization. J Supramol Struct. 1979;11(4):517–528. doi: 10.1002/jss.400110410. [DOI] [PubMed] [Google Scholar]
- Butters T. D., Devalia V., Aplin J. D., Hughes R. C. Inhibition of fibronectin-mediated adhesion of hamster fibroblasts to substratum: effects of tunicamycin and some cell surface modifying reagents. J Cell Sci. 1980 Aug;44:33–58. doi: 10.1242/jcs.44.1.33. [DOI] [PubMed] [Google Scholar]
- Cheingsong-Popov R., Robinson P., Altevogt P., Schirrmacher V. A mouse hepatocyte carbohydrate-specific receptor and its interaction with liver-metastasizing tumor cells. Int J Cancer. 1983 Sep 15;32(3):359–366. doi: 10.1002/ijc.2910320316. [DOI] [PubMed] [Google Scholar]
- Dennis J. W., Donaghue T. P., Carlow D. A., Kerbel R. S. Demonstration of a correlation between tumor cell H-2 antigen content, immunogenicity, and tumorigenicity using lectin-resistant tumor variants. Cancer Res. 1981 Oct;41(10):4010–4019. [PubMed] [Google Scholar]
- Dennis J. W., Waller C. A., Schirrmacher V. Identification of asparagine-linked oligosaccharides involved in tumor cell adhesion to laminin and type IV collagen. J Cell Biol. 1984 Oct;99(4 Pt 1):1416–1423. doi: 10.1083/jcb.99.4.1416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dennis J., Waller C., Timpl R., Schirrmacher V. Surface sialic acid reduces attachment of metastatic tumour cells to collagen type IV and fibronectin. Nature. 1982 Nov 18;300(5889):274–276. doi: 10.1038/300274a0. [DOI] [PubMed] [Google Scholar]
- Duksin D., Bornstein P. Changes in surface properties of normal and transformed cells caused by tunicamycin, an inhibitor of protein glycosylation. Proc Natl Acad Sci U S A. 1977 Aug;74(8):3433–3437. doi: 10.1073/pnas.74.8.3433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elbein A. D. Inhibitors of the biosynthesis and processing of N-linked oligosaccharides. CRC Crit Rev Biochem. 1984;16(1):21–49. doi: 10.3109/10409238409102805. [DOI] [PubMed] [Google Scholar]
- Elbein A. D., Pan Y. T., Solf R., Vosbeck K. Effect of swainsonine, an inhibitor of glycoprotein processing, on cultured mammalian cells. J Cell Physiol. 1983 Jun;115(3):265–275. doi: 10.1002/jcp.1041150309. [DOI] [PubMed] [Google Scholar]
- Elbein A. D., Solf R., Dorling P. R., Vosbeck K. Swainsonine: an inhibitor of glycoprotein processing. Proc Natl Acad Sci U S A. 1981 Dec;78(12):7393–7397. doi: 10.1073/pnas.78.12.7393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fidler I. J., Gersten D. M., Hart I. R. The biology of cancer invasion and metastasis. Adv Cancer Res. 1978;28:149–250. doi: 10.1016/s0065-230x(08)60648-x. [DOI] [PubMed] [Google Scholar]
- Fidler I. J. Metastasis: quantitative analysis of distribution and fate of tumor emboli labeled with 125 I-5-iodo-2'-deoxyuridine. J Natl Cancer Inst. 1970 Oct;45(4):773–782. [PubMed] [Google Scholar]
- Fidler I. J. Selection of successive tumour lines for metastasis. Nat New Biol. 1973 Apr 4;242(118):148–149. doi: 10.1038/newbio242148a0. [DOI] [PubMed] [Google Scholar]
- Grimstad I. A., Varani J., McCoy J. P., Jr Contribution of alpha-D-galactopyranosyl end groups to attachment of highly and low metastatic murine fibrosarcoma cells to various substrates. Exp Cell Res. 1984 Dec;155(2):345–358. doi: 10.1016/0014-4827(84)90195-2. [DOI] [PubMed] [Google Scholar]
- Irimura T., Gonzalez R., Nicolson G. L. Effects of tunicamycin on B16 metastatic melanoma cell surface glycoproteins and blood-borne arrest and survival properties. Cancer Res. 1981 Sep;41(9 Pt 1):3411–3418. [PubMed] [Google Scholar]
- Irimura T., Nicolson G. L. Carbohydrate chain analysis by lectin binding to electrophoretically separated glycoproteins from murine B16 melanoma sublines of various metastatic properties. Cancer Res. 1984 Feb;44(2):791–798. [PubMed] [Google Scholar]
- Irimura T., Nicolson G. L. The role of glycoconjugates in metastatic melanoma blood-borne arrest and cell surface properties. J Supramol Struct Cell Biochem. 1981;17(4):325–336. doi: 10.1002/jsscb.380170404. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Liotta L. A., Rao C. N., Barsky S. H. Tumor invasion and the extracellular matrix. Lab Invest. 1983 Dec;49(6):636–649. [PubMed] [Google Scholar]
- Lotan R., Nicolson G. L. Purification of cell membrane glycoproteins by lectin affinity chromatography. Biochim Biophys Acta. 1979 Dec 20;559(4):329–376. doi: 10.1016/0304-4157(79)90010-8. [DOI] [PubMed] [Google Scholar]
- Morin M. J., Bernacki R. J. Biochemical effects and therapeutic potential of tunicamycin in murine L1210 leukemia. Cancer Res. 1983 Apr;43(4):1669–1674. [PubMed] [Google Scholar]
- Nagata K., Ichikawa Y. Requirements for RNA and protein synthesis in the induction of several differentiation-markers in a myeloid leukemia cell line. J Cell Physiol. 1979 Jan;98(1):167–175. doi: 10.1002/jcp.1040980118. [DOI] [PubMed] [Google Scholar]
- Nicolson G. L. Cancer metastasis. Organ colonization and the cell-surface properties of malignant cells. Biochim Biophys Acta. 1982 Dec 21;695(2):113–176. doi: 10.1016/0304-419x(82)90020-8. [DOI] [PubMed] [Google Scholar]
- Nicolson G. L., Irimura T., Gonzalez R., Ruoslahti E. The role of fibronectin in adhesion of metastatic melanoma cells to endothelial cells and their basal lamina. Exp Cell Res. 1981 Oct;135(2):461–465. doi: 10.1016/0014-4827(81)90191-9. [DOI] [PubMed] [Google Scholar]
- Nicolson G. L. Trans-membrane control of the receptors on normal and tumor cells. II. Surface changes associated with transformation and malignancy. Biochim Biophys Acta. 1976 Apr 30;458(1):1–72. doi: 10.1016/0304-419x(76)90014-7. [DOI] [PubMed] [Google Scholar]
- Olden K., Pratt R. M., Yamada K. M. Selective cytotoxicity of tunicamycin for transformed cells. Int J Cancer. 1979 Jul 15;24(1):60–66. doi: 10.1002/ijc.2910240111. [DOI] [PubMed] [Google Scholar]
- Pena S. D., Hughes R. C. Fibronectin-plasma membrane interactions in the adhesion and spreading of hamster fibroblasts. Nature. 1978 Nov 2;276(5683):80–83. doi: 10.1038/276080a0. [DOI] [PubMed] [Google Scholar]
- Poste G., Fidler I. J. The pathogenesis of cancer metastasis. Nature. 1980 Jan 10;283(5743):139–146. doi: 10.1038/283139a0. [DOI] [PubMed] [Google Scholar]
- Poste G., Nicolson G. L. Arrest and metastasis of blood-borne tumor cells are modified by fusion of plasma membrane vesicles from highly metastatic cells. Proc Natl Acad Sci U S A. 1980 Jan;77(1):399–403. doi: 10.1073/pnas.77.1.399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rapin A. M., Burger M. M. Tumor cell surfaces: general alterations detected by agglutinins. Adv Cancer Res. 1974;20:1–91. doi: 10.1016/s0065-230x(08)60108-6. [DOI] [PubMed] [Google Scholar]
- Robin R., Chou I. N., Black P. H. Proteolytic enzymes, cell surface changes, and viral transformation. Adv Cancer Res. 1975;22:203–260. doi: 10.1016/s0065-230x(08)60178-5. [DOI] [PubMed] [Google Scholar]
- Schirrmacher V., Cheingsong-Popov R., Arnheiter H. Hepatocyte-tumor cell interaction in vitro. I. Conditions for rosette formation and inhibition by anti-H-2 antibody. J Exp Med. 1980 Apr 1;151(4):984–989. doi: 10.1084/jem.151.4.984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwarz R. T., Datema R. The lipid pathway of protein glycosylation and its inhibitors: the biological significance of protein-bound carbohydrates. Adv Carbohydr Chem Biochem. 1982;40:287–379. doi: 10.1016/s0065-2318(08)60111-0. [DOI] [PubMed] [Google Scholar]
- Springer G. F., Cheingsong-Popov R., Schirrmacher V., Desai P. R., Tegtmeyer H. Proposed molecular basis of murine tumor cell-hepatocyte interaction. J Biol Chem. 1983 May 10;258(9):5702–5706. [PubMed] [Google Scholar]
- Tulsiani D. R., Harris T. M., Touster O. Swainsonine inhibits the biosynthesis of complex glycoproteins by inhibition of Golgi mannosidase II. J Biol Chem. 1982 Jul 25;257(14):7936–7939. [PubMed] [Google Scholar]
- Yogeeswaran G., Salk P. L. Metastatic potential is positively correlated with cell surface sialylation of cultured murine tumor cell lines. Science. 1981 Jun 26;212(4502):1514–1516. doi: 10.1126/science.7233237. [DOI] [PubMed] [Google Scholar]