Abstract
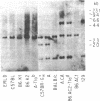
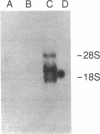
The thymus leukemia (TL) antigens, encoded by class I genes in the Tla subregion of the major histocompatibility complex (MHC), are cell surface molecules expressed on thymocytes of certain strains of mice and on certain T-cell leukemias. In order to study the fine structure and interrelationships of genes of the Tla subregion, a Tla-specific probe was isolated from the TL-encoding T13c gene of BALB/c mice (Tlac haplotype). The probe hybridized with two Tla genes in the Tlac haplotype (T13c and T3c) and with only one in the Tlab haplotype (T3b). Examination of this subset of Tla genes (T3b, T3c, and T13c) by restriction enzyme analysis and oligonucleotide hybridization studies confirmed that T3b is the allele of T3c and that T3c and T13c may have arisen by duplication. The T3b gene, while not transcribed in the tissues of the TL- strain C57BL6, was shown to be transcriptionally active in the TL-expressing leukemic cell line ERLD derived from that strain. The T3b gene was cloned and its complete DNA sequence was determined. These data permit complete comparison of two Tla-region genes, T3b and its homologue T13c, and allow us to conclude that these genes show extraordinarily high sequence conservation, in contrast to alleles of the H-2K- and H-2D-region genes. Comparison of T3b with other class I sequences in the H-2 and Qa subregions suggests that the T3-subset genes are the most divergent from other class I genes.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Auffray C., Rougeon F. Purification of mouse immunoglobulin heavy-chain messenger RNAs from total myeloma tumor RNA. Eur J Biochem. 1980 Jun;107(2):303–314. doi: 10.1111/j.1432-1033.1980.tb06030.x. [DOI] [PubMed] [Google Scholar]
- Breathnach R., Chambon P. Organization and expression of eucaryotic split genes coding for proteins. Annu Rev Biochem. 1981;50:349–383. doi: 10.1146/annurev.bi.50.070181.002025. [DOI] [PubMed] [Google Scholar]
- Cook R. G., Landolfi N. F. Expression of the thymus leukemia antigen by activated peripheral T lymphocytes. J Exp Med. 1983 Sep 1;158(3):1012–1017. doi: 10.1084/jem.158.3.1012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fisher D. A., Hunt S. W., 3rd, Hood L. Structure of a gene encoding a murine thymus leukemia antigen, and organization of Tla genes in the BALB/c mouse. J Exp Med. 1985 Aug 1;162(2):528–545. doi: 10.1084/jem.162.2.528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodenow R. S., McMillan M., Nicolson M., Sher B. T., Eakle K., Davidson N., Hood L. Identification of the class I genes of the mouse major histocompatibility complex by DNA-mediated gene transfer. Nature. 1982 Nov 18;300(5889):231–237. doi: 10.1038/300231a0. [DOI] [PubMed] [Google Scholar]
- Klein J., Figueroa F., David C. S. H-2 haplotypes, genes and antigens: second listing. II. The H-2 complex. Immunogenetics. 1983;17(6):553–596. doi: 10.1007/BF00366126. [DOI] [PubMed] [Google Scholar]
- Kozak M. Compilation and analysis of sequences upstream from the translational start site in eukaryotic mRNAs. Nucleic Acids Res. 1984 Jan 25;12(2):857–872. doi: 10.1093/nar/12.2.857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krayev A. S., Kramerov D. A., Skryabin K. G., Ryskov A. P., Bayev A. A., Georgiev G. P. The nucleotide sequence of the ubiquitous repetitive DNA sequence B1 complementary to the most abundant class of mouse fold-back RNA. Nucleic Acids Res. 1980 Mar 25;8(6):1201–1215. doi: 10.1093/nar/8.6.1201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krayev A. S., Markusheva T. V., Kramerov D. A., Ryskov A. P., Skryabin K. G., Bayev A. A., Georgiev G. P. Ubiquitous transposon-like repeats B1 and B2 of the mouse genome: B2 sequencing. Nucleic Acids Res. 1982 Dec 11;10(23):7461–7475. doi: 10.1093/nar/10.23.7461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kress M., Barra Y., Seidman J. G., Khoury G., Jay G. Functional insertion of an Alu type 2 (B2 SINE) repetitive sequence in murine class I genes. Science. 1984 Nov 23;226(4677):974–977. doi: 10.1126/science.6095445. [DOI] [PubMed] [Google Scholar]
- Kvist S., Roberts L., Dobberstein B. Mouse histocompatibility genes: structure and organisation of a Kd gene. EMBO J. 1983;2(2):245–254. doi: 10.1002/j.1460-2075.1983.tb01413.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lalanne J. L., Transy C., Guerin S., Darche S., Meulien P., Kourilsky P. Expression of class I genes in the major histocompatibility complex: identification of eight distinct mRNAs in DBA/2 mouse liver. Cell. 1985 Jun;41(2):469–478. doi: 10.1016/s0092-8674(85)80020-9. [DOI] [PubMed] [Google Scholar]
- Mellor A. L., Weiss E. H., Kress M., Jay G., Flavell R. A. A nonpolymorphic class I gene in the murine major histocompatibility complex. Cell. 1984 Jan;36(1):139–144. doi: 10.1016/0092-8674(84)90082-5. [DOI] [PubMed] [Google Scholar]
- Michaelson J., Boyse E. A., Chorney M., Flaherty L., Fleissner E., Hämmerling U., Reinisch C., Rosenson R., Shen F. W. The biochemical genetics of the Qa-Tla region. Transplant Proc. 1983 Dec;15(4):2033–2038. [PubMed] [Google Scholar]
- OLD L. J., BOYSE E. A. ANTIGENIC PROPERTIES OF EXPERIMENTAL LEUKEMIAS. I. SEROLOGICAL STUDIES IN VITRO WITH SPONTANEOUS AND RADIATION-INDUCED LEUKEMIAS. J Natl Cancer Inst. 1963 Oct;31:977–995. [PubMed] [Google Scholar]
- Obata Y., Chen Y. T., Stockert E., Old L. J. Structural analysis of TL genes of the mouse. Proc Natl Acad Sci U S A. 1985 Aug;82(16):5475–5479. doi: 10.1073/pnas.82.16.5475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pease L. R., Nathenson S. G., Leinwand L. A. Mapping class I gene sequences in the major histocompatibility complex. Nature. 1982 Jul 22;298(5872):382–385. doi: 10.1038/298382a0. [DOI] [PubMed] [Google Scholar]
- Rogers J. H. Family organization of mouse H-2 class I genes. Immunogenetics. 1985;21(4):343–353. doi: 10.1007/BF00430800. [DOI] [PubMed] [Google Scholar]
- Sanger F., Nicklen S., Coulson A. R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Southern E. M. Detection of specific sequences among DNA fragments separated by gel electrophoresis. J Mol Biol. 1975 Nov 5;98(3):503–517. doi: 10.1016/s0022-2836(75)80083-0. [DOI] [PubMed] [Google Scholar]
- Steinmetz M., Moore K. W., Frelinger J. G., Sher B. T., Shen F. W., Boyse E. A., Hood L. A pseudogene homologous to mouse transplantation antigens: transplantation antigens are encoded by eight exons that correlate with protein domains. Cell. 1981 Sep;25(3):683–692. doi: 10.1016/0092-8674(81)90175-6. [DOI] [PubMed] [Google Scholar]
- Steinmetz M., Winoto A., Minard K., Hood L. Clusters of genes encoding mouse transplantation antigens. Cell. 1982 Mar;28(3):489–498. doi: 10.1016/0092-8674(82)90203-3. [DOI] [PubMed] [Google Scholar]
- Weiss E. H., Golden L., Fahrner K., Mellor A. L., Devlin J. J., Bullman H., Tiddens H., Bud H., Flavell R. A. Organization and evolution of the class I gene family in the major histocompatibility complex of the C57BL/10 mouse. Nature. 1984 Aug 23;310(5979):650–655. doi: 10.1038/310650a0. [DOI] [PubMed] [Google Scholar]
- Weiss E., Golden L., Zakut R., Mellor A., Fahrner K., Kvist S., Flavell R. A. The DNA sequence of the H-2kb gene: evidence for gene conversion as a mechanism for the generation of polymorphism in histocompatibilty antigens. EMBO J. 1983;2(3):453–462. doi: 10.1002/j.1460-2075.1983.tb01444.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yokoyama K., Stockert E., Old L. J., Nathenson S. G. Structural comparisons of TL antigens derived from normal and leukemia cells of Tl+ and TL- strains and relationship to genetically linked H-2 major histocompatibility complex products. Proc Natl Acad Sci U S A. 1981 Nov;78(11):7078–7082. doi: 10.1073/pnas.78.11.7078. [DOI] [PMC free article] [PubMed] [Google Scholar]