Abstract
In the past, segments were defined by landmarks such as muscle attachments, notably by Snodgrass, the king of insect anatomists. Here, we show how an objective definition of a segment, based on developmental compartments, can help explain the dorsal abdomen of adult Drosophila. The anterior (A) compartment of each segment is subdivided into two domains of cells, each responding differently to Hedgehog. The anterior of these domains is non-neurogenic and clones lacking Notch develop normally; this domain can express stripe and form muscle attachments. The posterior domain is neurogenic and clones lacking Notch do not form cuticle; this domain is unable to express stripe or form muscle attachments. The posterior (P) compartment does not form muscle attachments. Our in vivo films indicate that early in the pupa the anterior domain of the A compartment expresses stripe in a narrowing zone that attracts the extending myotubes and resolves into the attachment sites for the dorsal abdominal muscles. We map the tendon cells precisely and show that all are confined to the anterior domain of A. It follows that the dorsal abdominal muscles are intersegmental, spanning from one anterior domain to the next. This view is tested and supported by clones that change cell identity or express stripe ectopically. It seems that growing myotubes originate in posterior A and extend forwards and backwards until they encounter and attach to anterior A cells. The dorsal adult muscles are polarised in the anteroposterior axis: we disprove the hypothesis that muscle orientation depends on genes that define planar cell polarity in the epidermis.
Keywords: Segmentation, Muscle pattern, Notch, Patched, Hedgehog, Compartments, Drosophila
INTRODUCTION
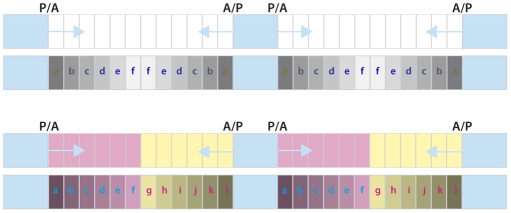
Most multicellular animals, such as arthropods, are largely built as a chain of homologous segments. In the past, those studying development and morphology of insects tried to delineate the anatomical limits of segments precisely (Snodgrass, 1935). Then, their definition of a segment depended on landmarks (muscle attachments, folds in the embryo or adult, pigmentation, etc.) but arguments based on such evidence were subjective and engendered many disputes (Rempel, 1975; Zrzavy and Stys, 1995). Later, it became clear that segments can be defined more objectively and accurately by looking at their cell lineage during development. Cell-marking experiments in insects showed that each ectodermal segment comprises two separate and immiscible sets of cells: anterior (A) and posterior (P) compartments (García-Bellido et al., 1973; Lawrence, 1973) (Fig. 1). A ‘parasegment’ was also recognised and consisted of a pair of compartments that are out of register with the segment (a segment consists of A + P, whereas a parasegment consists of P + A) (Martínez-Arias and Lawrence, 1985) (Fig. 1). Surprisingly, the basic unit of development and of genetic control is the parasegment (Struhl, 1984) – the main evidence is that, in the Drosophila embryo, the parasegments (not the segments) appear first in development and are co-extensive with the expression and requirement for some pair-rule and many homeotic genes (reviewed in Akam, 1987). Further study of the wing disc of Drosophila showed that morphogen gradients (for example, Hedgehog, Decapentaplegic) that drive pattern in the disc depend on cell interaction across the compartment boundaries, interfaces where A and P cells meet (reviewed in Lawrence and Struhl, 1996).
Fig. 1.
Compartments and morphogens in pattern formation. The insect body plan consists of a chain of metameres divided into anterior (A) and posterior (P) compartments. Hedgehog (Hh) is produced by all the P cells and spreads forwards and backwards into the adjacent A compartments, forming concentration gradients (blue arrows) that pattern the A cells. One scenario is shown at the top: all the A cells might respond to Hh alike giving a reflexed pattern of cell types (a-f-a). However, this pattern is not observed and instead there is a single sequence of cuticle types (a-l); our explanation depends on there being two types of A cells (shown in magenta and yellow) each responding differently to Hh (Struhl et al., 1997b).
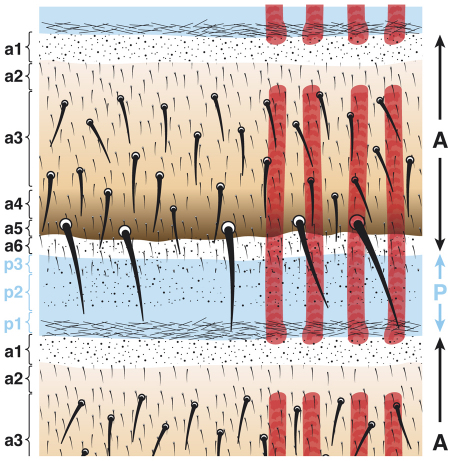
The Drosophila abdomen is ideal material to investigate because, unlike an imaginal disc, it comprises the entire metameric pattern including both types of compartment borders (A/P and P/A). Here, the A compartments are patterned by Hedgehog, a morphogen secreted by each P compartment that spreads both forwards and backwards to form gradients in the flanking A compartments (Struhl et al., 1997b). Based on this model one might expect all cells of the A compartment, front and back, to respond to incoming Hedgehog in the same way and this should lead to a reflexed sequence of cuticle types ‘abcdeffedcba’ (Fig. 1). However, this is not the case; in each segment of the Drosophila abdomen there is a single sequence of cuticle types spanning the whole A compartment ‘abcdefghijkl’ (Struhl et al., 1997b), therefore presenting us with a conundrum. Already in 1982 and based on theoretical arguments, Meinhardt had raised the same puzzle (Meinhardt, 1982). Our solution is that, pre-existing and independent of Hh, there appear to be two types of cells in the A compartment: cells of the anterior domain and cells of the posterior domain. These two types of cells each respond differently to Hh (Struhl et al., 1997a). We also refine earlier findings that these two cell types have differing requirements for the Notch (N) gene (Lawrence et al., 1999; Lawrence et al., 2002). Here, we map the two domains: the anterior domain is N-independent and non-neurogenic and includes all of a1 and a2 whereas the posterior domain is N-dependent and neurogenic and appears to comprise a3-a6 (Fig. 2). The P compartment is also N-independent and non-neurogenic.
Fig. 2.
The cuticle of an adult abdominal segment. The epidermis of the Drosophila adult abdomen derives from nests of histoblasts that are set aside in the embryo and remain quiescent throughout the larval stages. In each adult segment, nine different cuticle types can be distinguished by means of surface structure, pigmentation and bristles. The A compartment is subdivided into a smaller anterior domain composed of a1 and a2 cuticle and a larger posterior domain formed by a3-a6 cuticle. These two regions are distinguished because their cells respond to Hh signalling differently (see Fig. 1) (Struhl et al., 1997b) and only the posterior domain requires N (see Fig. 3). Note, it is difficult to place the border precisely between a2 and a3; a2 was distinguished from a3 only by the absence of bristles (Struhl et al., 1997b). The bristles move during development (García-Bellido and Merriam, 1971) and therefore are not a reliable marker of the provenance of the epidermal cells around them. The dorsal abdominal muscles of one side are shown in red. Anterior is at the top and the posterior is at the bottom.
There are implications not only for design of the peripheral nervous system but also for the muscle pattern. In the thorax of Drosophila, there is some mutual exclusion between two kinds of territories. The first expresses achaete-scute (ac-sc), requires N and can make bristles and other sensilla. The second kind of territory cannot make sensilla, but expresses and requires stripe (sr) (Usui et al., 2004), a transcription factor whose expression specifies tendon cells that are essential for muscle attachment (reviewed in Schweitzer et al., 2010; Frommer et al., 1996). In the abdomen it might follow that muscles could attach only to that part of the A compartment able to express sr. To investigate, we filmed the development of the dorsal abdominal muscles in vivo and found a zone of early sr expression that appears to be co-extensive with the anterior domain of the A compartment. Mapping the muscle attachments of the dorsal abdomen in terms of their cellular provenance shows that all the dorsal abdominal muscles indeed attach only to cells belonging to this same non-neurogenic, sr-competent domain; these adult muscles span from the rear part of the anterior domain in segment n to the front part of the anterior domain in segment n+1. As a further test for the special function of the anterior domain we switched the identity of patches of epidermal cells using genetic mosaics and altered muscle attachments accordingly.
We also investigate what orients the muscles; these are mostly precisely arranged in the anteroposterior axis. In embryos, a signal from sr-expressing cells attracts the myotubes (Frommer et al., 1996). Our films of the pupa show that the muscles extend in the anteroposterior axis as they grow forwards towards the sr-expressing (anterior) domain in front and backwards towards the homologous sr-expressing domain in the next segment behind. We postulate that the muscle cells are oriented by and towards these sources of Sr Alternatively, there is evidence and an earlier view that positional information in the epidermis determines the orientation of the muscles (Sahota and Beckel, 1967; Williams and Caveney, 1980) and as we now know the bristles and hairs in the epidermis are oriented by the mechanisms of planar cell polarity (PCP) (reviewed in Klein and Mlodzik, 2005), we tested whether the PCP genes help orient the underlying muscles. We present evidence that rejects this hypothesis: mutations in the PCP genes wreck the polarity of the epidermis but there are no effects on muscle polarity.
MATERIALS AND METHODS
Mutations and transgenes
FlyBase (Tweedie et al., 2009) entries of the mutations and transgenes referred to in the text are as follows.
CD2y+: CD2hs.PJ
ds–: dsUAO71
en.Gal4: Scer\GAL4en–e16E
FRT39: P{FRT(whs)}39
FRT42: P{ry[+t7.2]=neoFRT}42D
H2A::GFP: His2AvT:Avic\GFP–S65T
H2A:: RFP: His2AvT:Disc\RFP–mRFP
hs.FLP: FLP1hs.PS, Saccharomyces cerevisiae FLIP recombinase under the control of the hsp70 promoter
ILK::GFP: IlkT:Avic\GFP–m6
mef2.Gal: Scer\GAL4Mef2.PR
smo–: smo3, an amorphic allele of the smo gene
N–: NXK11, an amorphic allele of the N gene
Dp(1;2)w-ec, a duplication of the N gene
sr.Gal4: srmd710
stan–: stan3/stanE59 allelic combination
tub.Gal4: Scer\GAL4alphaTub84B.PL
UAS.srA: srA.Scer\UAS
UAS.srB: srB.Scer\UAS
wt: Canton-S
tub>y+>hh: hhScer\FRT.Rnor\CD2.aTub84B
UAS.GFP-nls: Avic\GFPScer\UAS.T:Hsap\MYC,T:SV40\nls2
UAS.GFP::act: Act5CScer\UAS.T:Avic\GFP
UAS.mCD8::GFP: Mmus\Cd8aScer\UAS.T:Avic\GFP
UAS.RFP: Disc\RFPDsRedT4.Scer\UAS.T:nls5
Experimental genotypes
Flies of the following genotypes were used.
ds– stan–: y w hs.FLP; ds– y+ FRT42 pwn stan59 sha/ds-FRT42 stan3
en.Gal4 UAS.GFP::act: w; en.Gal4 UAS.GFP::act/ CyO
en.Gal4 UAS.mCD8::GFP: w; en.Gal4; UAS.mCD8::GFP
H2A:: RFP mef2.Gal4 UAS.mCD8::GFP: w; H2A::RFP; mef2.Gal4/ UAS.mCD8GFP
H2A::GFP mef2.Gal4 UAS.RFP: w, H2A::GFP; mef2.Gal4/ UAS.RFP
H2A::GFP sr.Gal4 UAS.RFP: w; H2A::GFP; sr.Gal4/ UAS.RFP
Ilk::GFP: w; Ilk::GFP
All clones were generated by Flipase-mediated mitotic recombination (Golic, 1991) and induced by heat shocking third instar larvae of the following genotypes.
srA-expressing clones: y w hs.FLP, tub.Gal4 UAS.GFP-nls; UAS.srA; tub FRT42D Gal80 y+ FRT42 Gal4 (1 hour, 36°C)
srB-expressing clones: y w hs.FLP, tub.Gal4, UAS.GFP-nls;UAS.srB; tub FRT42D Gal80 y+ FRT42 Gal4 (1 hour, 36°C)
hh-expressing clones: y w hs.FLP; tub> y+> hh (15 minutes, 35°C)
N– clones: y N– hs.FLP; CD2y+ stc FRT40/ Dp(1;2)w-ec FRT40 (1 hour, 35°C)
Clones were induced during or soon after the blastoderm stage prior to the allocation of cells to the dorsal histoblast nests in the embryos of the following genotype.
smo– clones: y w hs.FLP/ y w; FRT39 Dp(1;2)sc19 w+30c /FRT39 smo3 stc (1 hour, 37°C).
Fixation, immunostaining and imaging
All dissections were made in PBS (pH 7.4). Ventral and lateral parts of pupal and adult abdomens were cut away and the internal organs gently removed. The dorsal parts were pinned out flat on a Sylgard-coated dish using steel insect pins (Ø 0.10 mm). The samples were fixed in 4% formaldehyde (Polysciences, Eppelheim, Germany) in PBT (PBS containing 0.1% Triton X-100) for 20 minutes. After several washes in PBT, they were incubated with primary antibodies (guinea pig anti-SrB, a gift from T. Volk, Rehovot, Israel; or 22c10 from the Developmental Studies Hybridoma Bank, used at 1:100) overnight at 4°C, washed again and incubated with secondary antibodies (donkey anti-guinea pig FITC- or Cy5-conjugated used at 1:500 from Jackson ImmunoResearch, Stratech Scientific, Newmarket, UK) for 4 hours at room temperature. After antibody incubation, samples were stained with phalloidin (Texas Red- or Alexa 488-phalloidin from Invitrogen, Paisley, UK) for ∼30 minutes at room temperature, washed several times and mounted in Vectashield (Vector Laboratories, Peterborough, UK) and examined using a Leica SP5 confocal microscope or an Axiophot microscope connected to a Nikon D-300 camera. Nikon Camera Control Pro was used to control the camera. Images were processed with Helicon Focus Pro and Adobe Photoshop CS4.
4D-imaging of pupae
A window in the pupal case was made and the pupae were filmed as described previously (Escudero et al., 2007; Bischoff and Cseresnyes, 2009). Note that all the studied pupae developed into pharate adults following imaging. z-stacks of ∼100 μm with a step size of 2.5 μm were recorded every ∼4 minutes for ∼13 hours using a Leica SP5 confocal microscope at 23-25°C.
RESULTS
Mapping dependence on the N gene
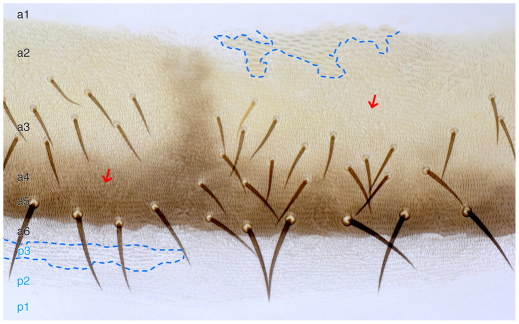
One can identify and map epidermal territories that depend on N: if N is removed from groups of cells within neurogenic territories (Lehmann et al., 1983; Heitzler and Simpson, 1991), the N– cells all form sensory precursors; they sort out from the epidermis and none of the N– cells forms cuticle. If N is removed from non-neurogenic territories there is no effect and the N– cells make normal cuticle. In our experiments with the adult abdomen, N– cells only survived to make cuticle in an anterior domain that is a1 and a2 of the A compartment (Struhl et al., 1997b) (Figs 2, 3) and in the P compartment. Our earlier brief reports were partly in error (Lawrence et al., 1999; Lawrence et al., 2002); using a better marker we now report that N– clones do not appear to make cuticle in a6 (Fig. 3, legend). Thus, we postulate that the entire posterior domain of the A compartment (a3-a6) is neurogenic and N-dependent whereas the anterior domain of the A compartment (a1 and a2) and the P compartment are non-neurogenic and N-independent (Fig. 2). The N– clones allow a better estimate of a2 and suggest it reaches back to the zone (a3) that forms bristles (Fig. 3). The N– clones are not associated with any changes in muscle attachments (data not shown).
Fig. 3.
Differential requirement for N in the A compartment. Marked N– clones in the dorsal cuticle (outlined with blue dashed lines). Clones arising in the posterior domain of A (a3-a6) do not make cuticle. We now correct our earlier report (Lawrence et al., 2002) when a6 was not included in that posterior zone: of 24 N– clones found close to and on either side of the a6/P border in 11 abdomens only two were found in a6. These two exceptions were near the midline where N– clones sometimes can be seen, even in a3 territory. Apart from these occasional exceptions, a3 epidermis is all unmarked (N+ cells) but has areas that lack bristles (indicated by arrows). These bald patches could be due to N– clones that made neurons there and inhibited nearby bristles. We stained for neurons and found clusters of neurons of different sizes underlying the bald patches of cuticle. Of four bald patches, varying in size from one-third to most of a hemisegment, we saw underneath the cuticle five, eight, eight and ten clusters of neurons. No such clusters were seen in the normal territory flanking the bald patches. Perhaps, as in the notum of Drosophila, each N– cell forms neurons that emit a strong Delta signal, inhibiting the formation of N+ bristles nearby (Heitzler and Simpson, 1991). However, clones form normally in the anterior domain of the A compartment and also in the P compartment and this image shows both.
Mapping the muscle attachment sites
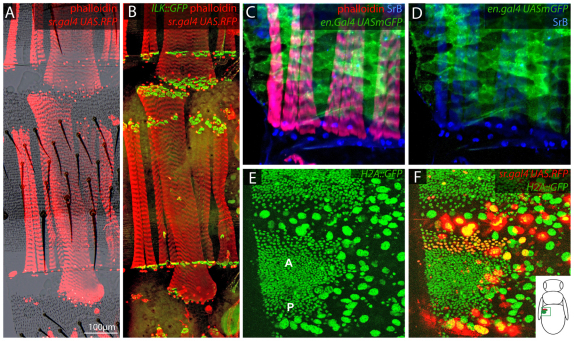
Each hemisegment bears 17-22 dorsal abdominal muscles that are aligned in the anteroposterior axis (D. A. Currie, PhD thesis, University of Cambridge, 1991). In male but not female flies, the fifth abdominal segment has a pair of muscles, the Muscles of Lawrence (MOL, supplementary material Fig. S1) (Lawrence and Johnston, 1984; D. A. Currie, PhD thesis, University of Cambridge, 1991). Also, several larval muscles persist in the adult until about 48 hours after eclosion (Kimura and Truman, 1990). We have mapped the attachment sites of these three types of muscles in a typical segment, relative to the cuticle, and find that all attachment sites, front and back, are restricted to the A compartment within a1 and a2 (Fig. 4A-D; supplementary material Fig. S1). In the adult, the anterior muscle attachments of the dorsal abdominal muscles and their associated tendon cells are localised close to or in front of the a2 and a3 junction, whereas the posterior tendon cells are found confined to a1 of the next segment back (Fig. 4A-F) (D. A. Currie, PhD thesis, University of Cambridge, 1991). Conclusive evidence that these posterior attachment sites form within a1 epidermis and not within the adjacent P [engrailed (en)-expressing] cells is shown when abdomens are stained for En and Sr (Fig. 4C,D); the cells express either en or sr but not both. In the larva, the muscles that will persist into the adult also attach to the most anterior cells of the A compartment, just behind the cells expressing en. During metamorphosis, the larval persistent muscles move to attach a few cell rows back from the P compartment and well into a2 cuticle (data not shown). Thus, in the adult, both the larval persistent muscles and the MOL have anterior and posterior attachments within a2 of contiguous segments. All the adult tendon cells are, therefore, restricted to a narrow zone within a1 and a2 (Fig. 2, Fig. 4A,D).
Fig. 4.
Muscle attachments in the dorsal abdominal epidermis. (A,B) Both anterior and posterior attachment sites of the Drosophila larval persistent muscles (larger muscles) are located in a2 cuticle, whereas the anterior attachment sites for adult muscles (the smaller muscles) are located near to the boundary between a2 and a3 (see Fig. 2). The nuclei of tendon cells are labelled red with sr.Gal4 UAS.RFP, the muscular attachments are marked in green with Ilk::GFP and the muscles are marked in red with phalloidin. (C,D) Posterior attachment sites of adult muscles (tendon nuclei, marked blue with anti-SrB antibody) are located in the first rows of the A compartment, just behind the cells of the en-expressing P compartment (patchy green). C and D show that the tendon nuclei and the P cells are adjacent but do not overlap. (E,F) Dorsolateral view of a segment ∼21 hours after puparium formation. (E) Two histoblast nests: the bigger anterior (A) and the smaller posterior (P) fuse to form the adult epidermis of one segment. All nuclei, larval (large) and adult (small), express H2A::GFP. (F) A merge of red and green channels shows that the sr-expressing adult cells (red) are located at the front of the anterior nest. The diagram shows the position of the analysed histoblast nests in the pupa.
Filming the development of the muscles in vivo
Films were made during muscle development in the pupa. Nascent myotubes are clearly seen only after about 24 hours following puparium formation. These first become visible in the middle of the anterior histoblast nest and are already remarkably well oriented in the anteroposterior axis (supplementary material Movies 1, 2). They extend both anteriorly and posteriorly by means of cytoplasmic extensions (supplementary material Movie 3), as do the embryonic muscles (Bate, 1990). The dorsal abdominal muscles first reach their posterior attachment sites at the back, that is just behind the anterior limit of the next segment posteriorly. However, they continue to put out extensions at the front and elongate to reach their anterior attachment sites later (Bate et al., 1991; Dutta et al., 2004). The MOL develop on the same schedule but grow past the anterior attachment sites of the dorsal abdominal muscles to attach further forward (data not shown).
Hh is secreted from the P compartment of the pupa and spreads forwards and backwards to enter the adjacent A histoblast nests, where it signals its arrival by upregulating patched (ptc). Hh arrives first at the back and later at the front of the A compartment, about 40 hours after puparium formation (Kopp et al., 1997) (M. Bischoff, personal communication). Long before Hh arrives at the front, Sr is already present there (Fig. 4E,F). Later, the Sr band fades, narrows and resolves into a series of tendon cells in the anterior region of the A compartment (Fig. 4B). These tendon cells constitute the posterior attachments of the dorsal abdominal muscles from the previous segment as well as the anterior attachments of the equivalent muscles of the same segment; they continue to express sr strongly in the adult (Fig. 4B).
The muscle attachments are limited to sr-expressing non-neurogenic territory
smo– clones suggest muscles attach only to a1 and a2, not a3
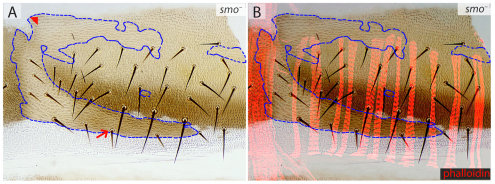
There is some ambiguity in the description above because we cannot define precisely where a2 and a3 cuticle meet (Fig. 2, legend) and cannot therefore determine unequivocally whether the dorsal abdominal muscles are attaching to a2 or to a3. To investigate this, we removed the smoothened (smo) gene, which is necessary for Hh reception (Chen and Struhl, 1996; Ingham et al., 2000). When smo– cells are induced in the back of the A compartment they develop as ectopic a3 (Struhl et al., 1997a). However, even these large patches of ectopic a3 cuticle are not associated with additional attachment sites, suggesting that muscles do not or cannot attach to a3. Figure 5 shows a large smo– clone (or several fused clones) spanning much of the segment, yet there is no alteration in the musculature, arguing again that a3 is not competent to form tendon cells. If found in the anterior region of the A compartment, smo– clones in a1 autonomously form a2 cuticle (Struhl et al., 1997a), and muscle attachments are normal. Thus, transformation of a1 into a2 does not translocate or damage the muscle attachments, perhaps because both a1 and a2 are equally competent to form tendons. These results suggest that, in the wild type, it is the part of a2 near to a3 that sponsors the anterior attachment sites for the dorsal abdominal muscles. The films and the wild-type pattern (Fig. 4) argue that these muscles form these attachments at the first competent epidermis (a2) that they meet as they extend anteriorly through a3 territory.
Fig. 5.
smo– clones in the cuticle. (A,B) smo– clones (marked with y and stc, encircled by blue dashed lines). The identity of the smo– cells in the posterior A compartment is changed into a3 (small bristles, arrow) and in the anterior A compartment a1 is transformed into a2 (arrowhead) We believe that the muscles (red, B) and their attachment sites are not affected by these transformations because a3 and P are incompetent to form attachments, and a1 and a2 are competent and equivalent.
UAS.hh clones also suggest muscles attach only to a1 or a2
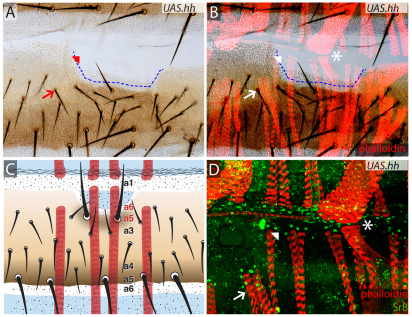
To test whether the identity of the epidermis determines the position of the muscle attachment sites, we transformed cell identity by manipulating Hh signalling. The Flp-out technique was used to generate clones of Hh-secreting cells (Struhl and Basler, 1993). A typical clone anterior within the A compartment forms an ectopic P compartment that itself secretes Hh and, as a consequence, patterns the A cells nearby (Fig. 6A-C). The main effect of this is to transform the region where the muscles normally attach (a2, as we have just argued), so that this non-neurogenic territory is replaced by neurogenic territory (a3-a6) as well as P. This leaves no candidate attachment sites within or behind the clone. The result is that both larval persistent and adult muscles underlying the clone become longer than normal; they pass over the clone and attach to wild-type a1 and a2 cuticle located anterior to the clone (Fig. 6B). Staining with an anti-SrB antibody confirmed that the ectopic attachment sites express sr and are formed normally (Fig. 6D). It would seem that the myoblasts, while extending anteriorwards, continue their journey until they first encounter a zone competent to form tendon cells, which in this case will be a1. A scheme of the identity transformations and their effects on muscle attachments is shown in Fig. 6C.
Fig. 6.
UAS.hh clones change the identities of the epidermal cells as well as the positions of muscle attachments. (A-C) UAS.hh-expressing clone (boundary estimated with a blue dashed line) located in a2/a3 area, transforms its cells towards P identity and as Hh spreads from the clone into surrounding A territory it changes the identity and orientation of neighbouring cells, replacing a2/a3 and a3 with a6-a4 (Struhl et al., 1997b) as shown schematically in C. The muscles underlying the transformed cuticle do not contact the newly formed a4-a6 cuticle and instead attach to a1 cuticle that is still present (asterisk). Note that muscles from the preceding segment attach correctly even in the neighbourhood of a UAS.hh clone, presumably because a1 is not changed by the clone. (D) Detail showing the muscle attachments and apparently normal tendon cells (green, marked with anti-SrB). Asterisks, arrows and arrowheads indicate the corresponding points in the three figures.
Overexpression of sr attracts the muscles
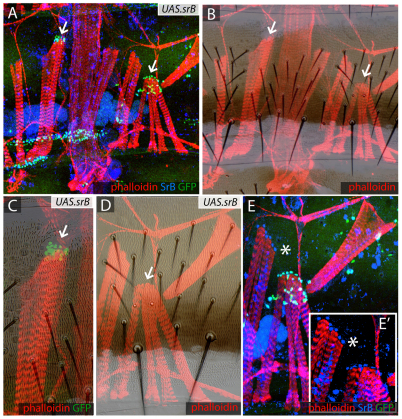
Is ectopic sr expression sufficient to induce muscle attachment in cells that normally do not form attachment sites? We induced marked clones in abdomens expressing one of the two spliced forms of Sr (Frommer et al., 1996). In both UAS.srA and UAS.srB clones, cells expressing sr attracted muscles to the ectopic attachment sites within the anterior and the posterior domains of the A compartments (Fig. 7). They even formed ectopic attachment sites within the P compartments (data not shown). Those marked clones located in the tendon-competent epidermis of the anterior domain sometimes attracted muscles, causing them to deviate from their usual paths (Fig. 7).
Fig. 7.
UAS.sr clones attract muscles and divert them from their usual points of attachment. (A,B) UAS.srB clones (marked with GFP, green) attract the muscles (stained with phalloidin, red). All tendon nuclei are marked with anti-SrB antibody (blue). Some muscles attach to ectopic sites where sr is expressed (arrows). (C-E′) Details of clones shown in A and B. Note the altered cuticle in one sr-expressing clone (arrow, C) while another clone can make maimed bristles (below arrow, D). (E) The same region stained for anti-SrB antibody (blue) and GFP for UAS.srB (green); (E′) A detail of E lacking the green channel showing that both the endogenous and ectopic tendons express sr at similar levels. It appears that myotubes ignore their usual attachment sites and prefer to form ectopic contacts with the clones, either because the ectopic sr expression starts earlier or is more persistent; if the latter, this could be due to autoregulation of sr (Vorbruggen and Jackle, 1997). Asterisk in E,E′ indicates the same location.
PCP and muscle orientation
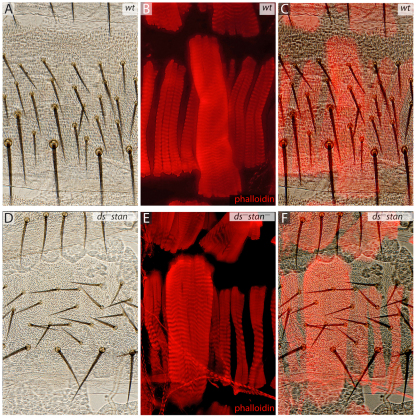
Movies of the developing muscles showed that the nascent myotubes are oriented precisely in the anteroposterior axis from the beginning and that they extend both anteriorly and posteriorly along that axis (supplementary material Movies 1-3). This polarity could reflect an intrinsic polarity of the founder cell and/or an external signal. Either way the genes of the PCP systems could be responsible (reviewed in Klein and Mlodzik, 2005; Lawrence et al., 2007; Zallen, 2007). To test for any role of the two PCP systems in muscle orientation we removed both essential elements dachsous (ds) and starry night (stan): ds– stan– double mutant embryos develop to pharate adults with a strong PCP phenotype and show randomisation of hair and bristle orientation (Casal et al., 2006). Nevertheless, phalloidin stains of these pharate adults show no orientation defects in the abdominal muscles (Fig. 8). This result gives us two crucial pieces of information. First, the ds and stan systems are not required in the myoblasts for proper orientation of the muscles. Second, our experiments show that normal polarity of the epidermal cells is dispensable for tendon formation and muscle orientation. It is important to stress that, in ds– stan– double mutants, apart from the polarity defects, the epidermal cells maintain their normal identities as signalled by cuticle pigmentation and the positioning of hairs and bristles; it follows that it is not the polarities of the epidermal cells but their identities that help to define the muscle attachments.
Fig. 8.
In ds– stan– mutant flies, muscle orientation is not affected. The bristles and hairs are oriented by the Stan and Ds systems, the twin mechanisms of planar cell polarity (PCP) (Casal et al., 2006; Lawrence et al., 2007). (A-C) Wild-type dorsal epidermis and the underlying muscles. (D-F) ds– stan– flies have disoriented hairs and bristles but the identity of the epidermal cells is not changed. Muscle orientation is not affected by lack of organised polarity in the cuticle. Muscles are stained with phalloidin.
DISCUSSION
We show that all the dorsal muscles of a typical segment of the abdomen, including the larval persistent muscles and the MOL, attach only to a small subregion: part of the anterior (A) compartment of each adult segment. In this discussion, we first consider the mechanisms responsible for the muscle pattern and then the general significance of what we have found.
The two domains of the anterior compartment
It appears that the pupal A compartment contains two distinct types of cells even before Hh arrives to pattern it. In an anterior domain of the A compartment, the cells develop normally without N and do not form bristles and, above a certain level, Hh specifies a1 cuticle. By contrast, when presumptive a3 cells of the posterior domain of the A compartment receive Hh, they now make a4-a6 cuticle as well as the appropriate types of bristles (Struhl et al., 1997b; Lawrence et al., 2002). Here, we confirm that epidermal cells of this posterior domain absolutely depend on N activity as N– clones do not form cuticle in that territory and instead make clusters of neurons (Fig. 3, legend). We add more evidence for the two domains: the anterior domain appears to be competent to express sr and to form muscle attachments whereas the posterior domain does neither. Also, in the thorax of Drosophila, there is earlier evidence that sensory bristles and tendon cells cannot be both formed by the same progenitors. If sr is overexpressed in the thorax, bristle formation is inhibited. Reciprocally, expression of achaete-scute in the sr domain leads to impairment of the flight muscles (Usui et al., 2004). This antagonism between achaete-scute and sr is conserved in many dipteran groups (Usui et al., 2004). These findings provide a genetic correlate with the two domains of the A compartment.
Specification of muscle attachment sites
In general, the pattern of attachment of muscles is related to the patterned expression of sr in the ectoderm (Schnorrer and Dickson, 2004). In the embryo, as soon as the myotubes arrive nearby, they become attracted to the sr-expressing cells and contact them (Becker et al., 1997). This is crucial; only the epidermal cells that establish contact with a myotube can maintain a high level of sr expression and can mature properly (Becker et al., 1997; Volohonsky et al., 2007). In the pupal abdomen, as only the anterior domain of the A compartment can express sr, one would expect muscle attachments to be limited to those cells, as we have found. The P compartment is a special case; N– clones form normally showing the cells to be non-neurogenic, consistent with an absence of bristles and sensilla in P compartments. However, muscle attachments do not form there either, perhaps because the action of en on cell identity abrogates the expression of sr.
Consistently, we find that ectopic expression of either srA or srB bypasses the original positional identity (Fig. 7), even P compartment cells will attach muscles if they express ectopic sr. In these circumstances, muscles may lose their normal anteroposterior orientation and bend to reach the clones expressing sr (Fig. 7). This is more evidence that the orientation of the dorsal abdominal muscles is a response to an Sr-dependent signal and not a response to polarity information in the epidermis (Fig. 8).
The pattern of muscle attachments therefore depends on information specified in the ectoderm, raising the question of how far the mesodermal founder cells are also programmed. In pupal myogenesis, the myoblasts and histoblasts develop in close association (D. A. Currie, PhD thesis, University of Cambridge, 1991) and our movies illustrate this in vivo (supplementary material Movies 1, 2) suggesting that they interact. Our most concrete evidence for some interaction between the ectoderm and the mesoderm comes from the MOL. The MOL are longer than other adult muscles, extending further both at the front and at the back, even though both ends attach within the tendon-competent zones (supplementary material Fig. S1). The peculiar attachment sites and size of the MOL depend entirely on the intervention of a neuron that is active only in the fifth abdominal segment of males; without this intervention, these myotubes form the same attachment sites as the normal dorsal abdominal muscles (Lawrence and Johnston, 1984; Lawrence and Johnston, 1986).
We have presented some additional evidence that signals are sent from the epidermis to the developing myotubes. Clones in the epidermis with changed cell identities were induced in third instar larvae, before the formation of myotubes. These clones affected the growing myotubes: if the tendon-competent cells of the anterior domain of the A compartment were replaced by tendon-incompetent cells of the posterior domain, the muscles passed over the transformed cells to attach to anterior domain cells further away, suggesting that a signal emanating from these tendon-competent (sr-expressing) cells had attracted the extending myotubes.
Our films show that precise anteroposterior orientation of the developing myotubes is maintained from the beginning. How is this achieved? One possibility is that polarity information originating in the epidermis might orient the muscle migration (Sahota and Beckel, 1967; Williams and Caveney, 1980) but we show this is not correct, at least with regard to the PCP genes. The films also show that, just as the myotubes are forming, a band of cells in the anterior region of each A compartment expresses sr. Probably, as in the embryo (Becker et al., 1997), these sr-expressing cells of the pupa attract and orient the growing myotubes. Later still, sr expression is increased at the actual sites of attachment, presumably as a result of short-range interaction between the specified myoblasts and the epidermal cells; again, as in the embryo (Becker et al., 1997).
Muscle attachments and anatomy of the segment
“Organisms...contain an internal description of their structure, function, development and history encoded in the DNA sequences of their genes” (Brenner, 1999).
For many decades, the definition of a segment was contentious – scientists argued using anatomical criteria about the homology of parts of one species with similar parts of another. Most had been content with Snodgrass’ perspective that the integumentary plates of harder cuticle (tergites in the dorsal abdomen of Drosophila) form the essential segments and these are separated by more flexible cuticle he called the intersegmental membranes (Snodgrass, 1935; Gullan and Cranston, 2010). Muscle attachments were traditionally used as landmarks for the borders of segments, particularly in juvenile and primitive soft-bodied insects. Using these criteria, it was concluded that most of the longitudinal muscles were ‘intrasegmental’. But, in adults or in more advanced arthropods, Snodgrass noted that most muscles spanned from one integumentary plate to the next, and he therefore described those muscles as ‘intersegmental’. He concluded that the muscle attachments could shift during development and evolution. Following Brenner, one could call this rather vague picture an ‘external description’ of a segment. However, a different way of defining a metamere came from studies of cell lineage during development and from mapping domains of gene expression, and these methods redefined a segment as the sum of two precisely defined group of cells: the A and the P compartments (García-Bellido et al., 1973; Lawrence, 1973; Blair, 1995). This definition amounted to an ‘internal description’ (Brenner, 1999).
We show here that the dorsal abdominal muscles of the abdomen can attach to a limited anterior domain of the A compartment at the front of one segment and, as they extend, they cross over the posterior domain of the A compartment as well as the P compartment to reach the anterior domain of the next segment. It follows that all the three types of muscles of the dorsal abdomen that we have studied are intersegmental in extent. It is likely that these same fundamental subdivisions of the segments are conserved in other insects and, if so, would confine most muscle attachment sites to particular locations, thereby constraining the range of muscle patterns that can be built by evolution (Maynard-Smith et al., 1985).
Supplementary Material
Acknowledgments
We thank Michael Bate and Pat Simpson for constructive and illuminating criticism; Javier Ortega Hernández and Gary Struhl for useful suggestions; Marcus Bischoff for teaching us how to make pupal movies; Talila Volk and the Developmental Studies Hybridoma Bank for the antibodies; Marcus Bischoff, Nick Brown, Manuel Calleja, Michèle Crozatier, Pedro Saavedra, Mar Ruíz Gómez and the Bloomington Stock Center for flies; Tulay Atamert for taking care of the fly stocks; and Zoltan Cseresnyes and Matt Wayland for assistance with confocal microscopy.
Footnotes
Funding
This work was supported by the Wellcome Trust [WD086986MA]. J.K. was supported by a long-term fellowship from the European Molecular Biology Organization (EMBO). Deposited in PMC for release after 6 months.
Competing interests statement
The authors declare no competing financial interests.
Supplementary material
Supplementary material available online at http://dev.biologists.org/lookup/suppl/doi:10.1242/dev.073692/-/DC1
References
- Akam M. (1987). The molecular basis for metameric pattern in the Drosophila embryo. Development 101, 1–22 [PubMed] [Google Scholar]
- Bate M. (1990). The embryonic development of larval muscles in Drosophila. Development 110, 791–804 [DOI] [PubMed] [Google Scholar]
- Bate M., Rushton E., Currie D. A. (1991). Cells with persistent twist expression are the embryonic precursors of adult muscles in Drosophila. Development 113, 79–89 [DOI] [PubMed] [Google Scholar]
- Becker S., Pasca G., Strumpf D., Min L., Volk T. (1997). Reciprocal signaling between Drosophila epidermal muscle attachment cells and their corresponding muscles. Development 124, 2615–2622 [DOI] [PubMed] [Google Scholar]
- Bischoff M., Cseresnyes Z. (2009). Cell rearrangements, cell divisions and cell death in a migrating epithelial sheet in the abdomen of Drosophila. Development 136, 2403–2411 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blair S. S. (1995). Compartments and appendage development in Drosophila. BioEssays 17, 299–309 [DOI] [PubMed] [Google Scholar]
- Brenner S. (1999). Theoretical biology in the third millennium. Philos. Trans. R. Soc. Lond. B Biol. Sci. 354, 1963–1965 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casal J., Lawrence P. A., Struhl G. (2006). Two separate molecular systems, Dachsous/Fat and Starry night/Frizzled, act independently to confer planar cell polarity. Development 133, 4561–4572 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Y., Struhl G. (1996). Dual roles for patched in sequestering and transducing Hedgehog. Cell 87, 553–563 [DOI] [PubMed] [Google Scholar]
- Dutta D., Anant S., Ruiz-Gomez M., Bate M., VijayRaghavan K. (2004). Founder myoblasts and fibre number during adult myogenesis in Drosophila. Development 131, 3761–3772 [DOI] [PubMed] [Google Scholar]
- Escudero L. M., Bischoff M., Freeman M. (2007). Myosin II regulates complex cellular arrangement and epithelial architecture in Drosophila. Dev. Cell 13, 717–729 [DOI] [PubMed] [Google Scholar]
- Frommer G., Vorbruggen G., Pasca G., Jackle H., Volk T. (1996). Epidermal egr-like zinc finger protein of Drosophila participates in myotube guidance. EMBO J. 15, 1642–1649 [PMC free article] [PubMed] [Google Scholar]
- García-Bellido A., Merriam J. R. (1971). Clonal parameters of tergite development in Drosophila. Dev. Biol. 26, 264–276 [DOI] [PubMed] [Google Scholar]
- García-Bellido A., Ripoll P., Morata G. (1973). Developmental compartmentalisation of the wing disk of Drosophila. Nat. New Biol. 245, 251–253 [DOI] [PubMed] [Google Scholar]
- Golic K. G. (1991). Site-specific recombination between homologous chromosomes in Drosophila. Science 252, 958–961 [DOI] [PubMed] [Google Scholar]
- Gullan P. J., Cranston P. S. (2010). The Insects: an Outline of Entomology. Chichester: Wiley-Blackwell; [Google Scholar]
- Heitzler P., Simpson P. (1991). The choice of cell fate in the epidermis of Drosophila. Cell 64, 1083–1092 [DOI] [PubMed] [Google Scholar]
- Ingham P. W., Nystedt S., Nakano Y., Brown W., Stark D., van den Heuvel M., Taylor A. M. (2000). Patched represses the Hedgehog signalling pathway by promoting modification of the Smoothened protein. Curr. Biol. 10, 1315–1318 [DOI] [PubMed] [Google Scholar]
- Kimura K. I., Truman J. W. (1990). Postmetamorphic cell death in the nervous and muscular systems of Drosophila melanogaster. J. Neurosci. 10, 403–411 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klein T. J., Mlodzik M. (2005). Planar cell polarization: an emerging model points in the right direction. Annu. Rev. Cell Dev. Biol. 21, 155–176 [DOI] [PubMed] [Google Scholar]
- Kopp A., Muskavitch M. A., Duncan I. (1997). The roles of hedgehog and engrailed in patterning adult abdominal segments of Drosophila. Development 124, 3703–3714 [DOI] [PubMed] [Google Scholar]
- Lawrence P. A. (1973). A clonal analysis of segment development in Oncopeltus (Hemiptera). J. Embryol. Exp. Morphol. 30, 681–699 [PubMed] [Google Scholar]
- Lawrence P. A., Johnston P. (1984). The genetic specification of pattern in a Drosophila muscle. Cell 36, 775–782 [DOI] [PubMed] [Google Scholar]
- Lawrence P. A., Johnston P. (1986). The muscle pattern of a segment of Drosophila may be determined by neurons and not by contributing myoblasts. Cell 45, 505–513 [DOI] [PubMed] [Google Scholar]
- Lawrence P. A., Struhl G. (1996). Morphogens, compartments, and pattern: lessons from Drosophila? Cell 85, 951–961 [DOI] [PubMed] [Google Scholar]
- Lawrence P. A., Casal J., Struhl G. (1999). hedgehog and engrailed: pattern formation and polarity in the Drosophila abdomen. Development 126, 2431–2439 [DOI] [PubMed] [Google Scholar]
- Lawrence P. A., Casal J., Struhl G. (2002). Towards a model of the organisation of planar polarity and pattern in the Drosophila abdomen. Development 129, 2749–2760 [DOI] [PubMed] [Google Scholar]
- Lawrence P. A., Struhl G., Casal J. (2007). Planar cell polarity: one or two pathways? Nat. Rev. Genet. 8, 555–563 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lehmann R., Jiménez F., Dietrich U., Campos-Ortega J. A. (1983). On the phenotype and development of mutants of early neurogenesis in Drosophila melanogaster. Wilhelm Roux’s Arch. Dev. Biol. 192, 62–74 [DOI] [PubMed] [Google Scholar]
- Martínez-Arias A., Lawrence P. A. (1985). Parasegments and compartments in the Drosophila embryo. Nature 313, 639–642 [DOI] [PubMed] [Google Scholar]
- Maynard-Smith J., Burian R., Kauffman S., Alberch P., Campbell J., Goodwin B., Lande R., Raup D., Wolpert L. (1985). Developmental constraints and evolution: a perspective from the Mountain Lake conference on development and evolution. Q. Rev. Biol. 60, 265–287 [Google Scholar]
- Meinhardt H. (1982). Models of Biological Pattern Formation. New York: Academic Press; [Google Scholar]
- Rempel J. G. (1975). The evolution of the insect head: the endless dispute. Quaest. Entomol. 11, 7–25 [Google Scholar]
- Sahota T. S., Beckel W. (1967). The influence of epidermis on the developing flight muscles in Galleria mellonella. Can. J. Zool. 45, 421–434 [Google Scholar]
- Schnorrer F., Dickson B. J. (2004). Muscle building; mechanisms of myotube guidance and attachment site selection. Dev. Cell 7, 9–20 [DOI] [PubMed] [Google Scholar]
- Schweitzer R., Zelzer E., Volk T. (2010). Connecting muscles to tendons: tendons and musculoskeletal development in flies and vertebrates. Development 137, 2807–2817 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Snodgrass R. E. (1935). Principles of Insect Morphology. New York: McGraw-Hill; [Google Scholar]
- Struhl G. (1984). Splitting the bithorax complex of Drosophila. Nature 308, 454–457 [Google Scholar]
- Struhl G., Basler K. (1993). Organizing activity of wingless protein in Drosophila. Cell 72, 527–540 [DOI] [PubMed] [Google Scholar]
- Struhl G., Barbash D. A., Lawrence P. A. (1997a). Hedgehog acts by distinct gradient and signal relay mechanisms to organise cell type and cell polarity in the Drosophila abdomen. Development 124, 2155–2165 [DOI] [PubMed] [Google Scholar]
- Struhl G., Barbash D. A., Lawrence P. A. (1997b). Hedgehog organises the pattern and polarity of epidermal cells in the Drosophila abdomen. Development 124, 2143–2154 [DOI] [PubMed] [Google Scholar]
- Tweedie S., Ashburner M., Falls K., Leyland P., McQuilton P., Marygold S., Millburn G., Osumi-Sutherland D., Schroeder A., Seal R., et al. (2009). FlyBase: enhancing Drosophila gene ontology annotations. Nucleic Acids Res. 37, D555–D559 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Usui K., Pistillo D., Simpson P. (2004). Mutual exclusion of sensory bristles and tendons on the notum of dipteran flies. Curr. Biol. 14, 1047–1055 [DOI] [PubMed] [Google Scholar]
- Volohonsky G., Edenfeld G., Klambt C., Volk T. (2007). Muscle-dependent maturation of tendon cells is induced by post-transcriptional regulation of stripeA. Development 134, 347–356 [DOI] [PubMed] [Google Scholar]
- Vorbruggen G., Jackle H. (1997). Epidermal muscle attachment site-specific target gene expression and interference with myotube guidance in response to ectopic stripe expression in the developing Drosophila epidermis. Proc. Natl. Acad. Sci. USA 94, 8606–8611 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams G. J., Caveney S. (1980). A gradient of morphogenetic information involved in muscle patterning. J. Embryol. Exp. Morphol. 58, 35–61 [PubMed] [Google Scholar]
- Zallen J. A. (2007). Planar polarity and tissue morphogenesis. Cell 129, 1051–1063 [DOI] [PubMed] [Google Scholar]
- Zrzavy J., Stys P. (1995). Evolution of metamerism in arthropoda: developmental and morphological perspectives. Q. Rev. Biol. 70, 279–295 [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.