Abstract
Overexpression of steroid receptor coactivator (SRC)-1 and SRC-3 is associated with cancer initiation, metastasis, advanced disease, and resistance to chemotherapy. In most of these cases, SRC-1 and SRC-3 have been shown to promote tumor cell growth by activating nuclear receptor and multiple growth factor signaling cascades that lead to uncontrolled tumor cell growth. Up until now, most targeted chemotherapeutic drugs have been designed largely to block a single pathway at a time, but cancers frequently acquire resistance by switching to alternative growth factor pathways. We reason that the development of chemotherapeutic agents against SRC coactivators that sit at the nexus of multiple cell growth signaling networks and transcriptional factors should be particularly effective therapeutics. To substantiate this hypothesis, we report the discovery of 2,2′-bis-(Formyl-1,6,7-trihydroxy-5-isopropyl-3-methylnaphthalene (gossypol) as a small molecule inhibitor of coactivator SRC-1 and SRC-3. Our data indicate that gossypol binds directly to SRC-3 in its receptor interacting domain. In MCF-7 breast cancer cells, gossypol selectively reduces the cellular protein concentrations of SRC-1 and SRC-3 without generally altering overall protein expression patterns, SRC-2, or other coactivators, such as p300 and coactivator-associated arginine methyltransferase 1. Gossypol reduces the concentration of SRC-3 in prostate, lung, and liver cancer cell lines. Gossypol inhibits cell viability in the same cancer cell lines where it promotes SRC-3 down-regulation. Additionally, gossypol sensitizes lung and breast cancer cell lines to the inhibitory effects of other chemotherapeutic agents. Importantly, gossypol is selectively cytotoxic to cancer cells, whereas normal cell viability is not affected. This data establish the proof-of-principle that, as a class, SRC-1 and SRC-3 coactivators are accessible chemotherapeutic targets. Given their function as integrators of multiple cell growth signaling systems, SRC-1/SRC-3 small molecule inhibitors comprise a new class of drugs that have potential as novel chemotherapeutics able to defeat aspects of acquired cancer cell resistance mechanisms.
Nuclear receptors (NR) comprise a large superfamily of ligand-regulated (and orphan) transcription factors that transduce steroid, retinoid, thyroid hormone, and lipophilic endocrine signaling into distinct physiological responses. Agonist ligand binding to NR leads to the recruitment of coactivator proteins that are required for their transcriptional activity. The first identified NR coactivator, steroid receptor coactivator (SRC)-1 was found to interact with NR in a ligand-dependent manner and to robustly enhance their transcriptional activity (1). Soon after this, two other proteins, transcriptional intermediary factor-2/SRC-2 (2, 3) and amplified in breast cancer-1/SRC-3 (SRC-3) (4–7) were identified as NR coactivators that comprise the SRC coactivator family. The SRC family functions as coactivators not only for NR but also for multiple other transcriptional factors (TF), such as nuclear factor κB, E2F1, and growth factor-dependent kinases and IGF-I-dependent TFs (8–10). All members of the SRC family can modulate diverse growth gene expression programs both by NR and other TFs and have been found to drive physiological and pathophysicological processes.
In human breast cancers, both SRC-1 and SRC-3 are frequently overexpressed. In approximately 20% of primary breast cancers, higher levels of SRC-1 protein have been detected, and this increase is positively associated with avian erythroblastosis oncogene B 2 (ERBB2) expression, disease recurrence, and poor disease survival (11, 12). Knockouts of SRC-1 in an mouse mammary tumor virus-polyoma middle T antigen mammary tumor-prone mouse cancer model system markedly inhibit tumor cell metastasis to the lung (13). For SRC-3, gene amplification has been found in 9.5% of breast cancers, and its mRNA was found to be overexpressed as high as 64% of the time (4). Overexpression of SRC-3 in mammary epithelial cells has been shown to be sufficient to promote mammary tumor formation, directly implicating it in breast cancer initiation (14). Consistent with this finding, SRC-3 knockout mice had suppressed oncogene- and carcinogen-induced breast cancer initiation, progression, and metastasis (15–18). In a variety of other cancer types, overexpression of SRC-3 has been frequently observed in ovarian (19), endometrial (20), prostate (21–23), liver (24), pancreatic (25), colorectal (26), and lung cancers (27).
Expression levels of SRC coactivators are known to be associated with specific responses to selective estrogen receptor (ER) modulators in different body tissues. For instance, high levels of SRC-1 in endometrial cells and low levels in mammary cells can determine the agonist or antagonist behavior of 4-hydroxytamoxifen (4HT) in each respective tissue (28). High expression of both ERBB2 and SRC-1 is associated with 4HT therapy resistance in breast cancer (11). High expression of both SRC-3 and ERBB2 also was shown to significantly increase the agonist activities of 4HT, resulting in resistance to 4HT treatment (29). In ERBB2-overexpressing breast cancer cells, overexpression of SRC-3 contributes to resistance against ERBB2 targeting treatment with trastuzumab (Herceptin) through activation of IGF signaling pathways (30).
Other studies have explored additional molecular mechanisms underlying the role of SRC coactivators in driving cancer cell growth. For instance, SRC-1 overexpression has been shown to increase ERBB2, colony-stimulating factor-1, and Twist gene expression (13, 18). SRC-3 overexpression has been shown to stimulate IGF and E2F1-mediated pathways, also pointing to its broad ability to activate multiple cancer growth pathways (10, 14–17).
Among others, the current arsenal of targeted chemotherapeutic agents to treat breast cancers includes selective ER modulators, such as 4HT, the pure antiestrogen Imperial Chemcial Industries (ICI) 182,780, and aromatase inhibitors that block estrogen synthesis, along with Herceptin to target ERBB2/human epidermal growth factor receptor 2. As discussed above, a limitation of these therapies is the acquired resistance, possibly due to the overexpression of SRC-1 and SRC-3 and the attendant activation of alternative cell growth pathways. Therefore, newly designed drugs to perturb “networks” rather than discrete pathways are needed. Considering the ability of SRC coactivators to integrate and activate multiple pathways in cancers, the SRC coactivator family represents such a prototype of drug targets. Accumulating evidence has shown that experimental targeting of SRC-3 can limit cancer cells growth in multiple cancer types. For example, small interfering RNA-mediated disruption of SRC-3 expression impairs epidermal growth factor (EGF) activity in a variety of cell lines (31) in addition to its role in IGF and ERBB2-mediated signaling discussed above.
Taken together, these studies point to a strong potential for coactivators such as SRC-1 and SRC-3 as important drug targets and provide a strong impetus to identify small molecule inhibitors (SMI) to inhibit these oncogenes. This goal is hampered by the fact that SRC are large disordered proteins that lack high affinity ligand binding sites or other features that exist in most “druggable” targets. Nevertheless, attempts to discover SMI against other regulatory proteins were successful (32).
A component of the National Institute of Health's Molecular Libraries Roadmap Initiative has been to develop the PubChem bioassay bioinformatic resource that contains data on high throughput screening of small molecule libraries in distinct biological assays from a multitude of depositors (http://pubchem.ncbi.nlm.nih.gov) (33). This resource contains data from a variety of different binary, cell-free fluorescence resonance energy transfer (FRET)-based assays designed to identify small molecules capable of disrupting NR-SRC protein-protein interactions. Because these assays are unable to determine whether a particular SMI specifically targets the receptor or the coactivator, using our laboratory's expertise, we have examined a subset of these “hits” to identify those which might target the coactivator. Using both coactivator activity- and stability-based assays, we reexamined these hits to identify SRC-targeted compounds, a cadre of compounds that target SRC-3 (and SRC-1) for degradation. We selected 2,2′-bis-(Formyl-1,6,7-trihydroxy-5-isopropyl-3-methylnaphthalene (gossypol) as a SMI selective for SRC-1 and SRC-3 to provide “proof-of-principle” data to demonstrate that coactivators indeed are a new class of druggable targets.
Results
Identification of gossypol as a selective SRC coactivator inhibitor
To effectively screen test compounds, we have employed two high throughput compatible screening assays. To explore the effect of test compounds on SRC protein stability, we employed SRC-1-A and SRC-3 luciferase fusion protein vectors (SRC-1A-LUC and SRC-3-LUC, respectively) that we developed previously (34). To evaluate the effects of test compounds on the intrinsic transcriptional activities of SRC family coactivators, we measured the output of a GAL4-responsive luciferase reporter in the presence of GAL4 DNA-binding domain (DBD) SRC coactivator fusion proteins (35). Gossypol was identified from a screen of selected compound hits indicated to interfere with binding between the SRC-1 NR-binding domain [receptor interacting domain (RID)] and ERα ligand-binding domain based on the PubChem databases (AID 629; AID 713).
Gossypol is a natural polyphenol found in cotton seeds (Fig. 1A), once considered as a promising male antifertility agent that was ultimately deemed unsuitable due to an unacceptable risk of permanent infertility and some instances of hypokalemia (36, 37). More recently, gossypol has been repositioned for its potential anticancer properties in prostate (38–41). In HeLa cells transiently transfected with a GAL4-responsive luciferase reporter gene and expression vectors for GAL4 DBD-SRC-1A or GAL4 DBD-SRC-3, gossypol reduced luciferase reporter activities in a dose-dependent manner, indicating that gossypol inhibits the intrinsic transcriptional activities of both SRC-1A and SRC-3 (Fig. 1B). Furthermore, in HeLa cells transiently transfected with SRC-1A-LUC or SRC-3-LUC, gossypol was found to reduce luciferase activities, indicating that gossypol was able to reduce the steady state levels of either coactivator fusion protein in the cell (Fig. 1C). These data indicated that the loss in SRC-1A and SRC-3 activity is due to the ability of gossypol inhibitor to reduce the cellular concentration of either coactivator in the cell.
Fig. 1.
Gossypol reduces the intrinsic transcriptional activities and stabilities of exogenously expressed SRC-1 and SRC-3 in HeLa cells. A, Chemical structure of gossypol. B, Gossypol reduces the intrinsic transcriptional activities of SRC-1A and SRC-3. Luciferase assays were performed in HeLa cells transiently cotransfected with expression vectors for the yeast transcription activator protein (GAL4)-DBD-SRC-1A and GAL4-DBD-SRC-3 fusion proteins along with the GAL4-responsive luciferase reporter expression vectors before incubation with gossypol of different concentrations (0, 1, 2.5, 5, and 10 μm) for 24 h. Empty GAL4 DBD vector was used as a negative control. C, Gossypol reduces steady state levels of SRC-1A-LUC and SRC-3-LUC proteins. Luciferase assays were performed in HeLa cells transiently transfected with SRC-1A-LUC or SRC-3-LUC expression vectors before incubation with gossypol of different concentrations (0, 1, 2.5, 5, and 10 μm) for 24 h.
Gossypol reduces cellular SRC-1 and SRC-3 protein concentration selectively and in an ERα ligand-independent manner
To test the effects of gossypol on endogenous SRC family member protein expression levels, we examined SRC-1, SRC-2, and SRC-3 protein content in MCF-7 cells after 24 h of incubation with 10 μm gossypol. SRC-1 and SRC-3 protein levels were markedly reduced by gossypol (Fig. 2A, lower panel). Next, to assess whether there is a global effect of gossypol on the proteome, we resolved these samples by SDS-PAGE followed by silver staining. Gossypol incubation did not induce any gross changes in cellular protein, whereas SRC-1 and SRC-3 protein levels were noticeably reduced (Fig. 2A, upper panel).
Fig. 2.
Gossypol selectively reduces endogenous SRC-1 and SRC-3 protein levels in an ERα ligand-independent manner. A–C, Gossypol reduces endogenous SRC-1 and SRC-3 protein levels without inducing any gross changes in the cellular protein expression. MCF-7 cells were serum starved overnight before incubation with DMSO as the control or with 10 μm gossypol for 24 h. A, Cells were harvested for silver staining. The same cell extracts were subjected to Western blotting using antibodies against SRC-1, SRC-3, and actin. B, Cell extracts were subjected to Western blotting using antibodies against p300, CARM1, SRC-3, and actin. C, Cell extracts were subjected to Western blotting using antibodies against SRC-2, and actin or SRC-3 and actin. Numbers in the blot indicate the relative intensities normalized by actin. D, Gossypol-mediated down-regulation of SRC-3 was not affected by the presence of E2, tamoxifen (4HT), and ICI 182,780 (ICI). Serum-starved MCF-7 cells as above were treated with DMSO or with 10 μm gossypol together with vehicle ethanol (ETOH), E2 (10−9 m), 4HT (10−7 m), or ICI (10−7 m) for 24 h. Western blotting was performed using antibodies against SRC-3 and actin.
The effect of gossypol on the steady state levels of other coactivators was investigated. The protein level of other coactivators, such as p300 and coactivator-associated arginine methyltransferase 1 (CARM1), which have been shown to be recruited to SRC-3 to form multiprotein coactivator complexes, were examined next (5, 42, 43). Importantly, gossypol did not alter either p300 or CARM1 protein levels (Fig. 2B), revealing that gossypol selectively reduces endogenous SRC-1 and SRC-3 protein levels without generally altering cellular protein expression and associated coactivator proteins. In contrast to the decrease of SRC-3, the protein level of the homologous family members, SRC-2, was not affected by gossypol administration (Fig. 2C).
Previous reports demonstrated that SRC-1 and SRC-3 protein levels are modulated by different ERα ligands (34). We asked whether the inhibitory effect of gossypol on SRC-3 protein is modulated when different ERα ligands are present. MCF-7 cells were treated with estradiol (E2), 4HT, and ICI 182,780 in the absence or presence of gossypol (Fig. 2D). Gossypol-mediated down-regulation of SRC-3 was not affected by the presence of different ERα ligands. Consistent with this, gossypol was able to down-regulate SRC-3 protein levels in a variety of other cancer cell lines that are ERα negative, indicating that gossypol-mediated down-regulation of SRC-3 is independent of ERα (see below).
Gossypol-mediated protein down-regulation occurs through a proteasome-independent mechanism
To understand the mechanism underlying the decreased level of SRC-3 protein in more detail, we examined the endogenous mRNA for SRC-3 and found that it was increased by gossypol treatment for 24 h (Fig. 3A). In agreement with previous reports (44), B-cell lymphoma 2 (Bcl-2) mRNA was strongly reduced by gossypol treatment (Fig. 3A), although this may be a result of gossypol targeting of SRC-3 (see below). Our finding indicates that gossypol is able to reduce SRC-3 protein posttranscriptionally.
Fig. 3.
Gossypol-mediated SRC-3 protein down-regulation occurs through a proteasome-independent mechanism. A, Gossypol increases SRC-3 but decreases Bcl-2 mRNA levels. Serum-starved MCF-7 cells were incubated with DMSO or with 10 μm gossypol for 24 h. Total RNA was extracted and subjected to quantitative PCR (Q-PCR). Collected Q-PCR data were presented using the comparative Ct method, in which the actin mRNA was used as the normalizer. Differences between the control and the treated samples were analyzed for the significance of difference using a Student's t test; ***, P < 0.005. B, Proteasome inhibitor MG132 does not prevent the SRC-3 protein reduction induced by gossypol. Serum-starved MCF-7 cells were incubated with DMSO or 5 μm gossypol in the absence or presence of MG132 for 24 h. Cell extracts were collected and subjected to Western blotting using antibodies against SRC-3 and actin. C, Gossypol down-regulates SRC-3 protein in a time-dependent manner. Serum-starved MCF-7 cells were incubated with 10 μm gossypol for 0, 1, 2, 3, 4, 5, 6, or 7 h before cell extracts were subjected to Western blotting using antibodies against SRC-3 and actin. Ct, Cycle threshold.
Previously, we have shown that endogenous SRC-1 and SRC-3 (as well as SRC-2) are targets of the proteasome. This conclusion was supported by the fact that treating with the proteasome inhibitor MG132 results in an increase in the level of SRC proteins (35). We asked whether the SRC-3 degradation induced by gossypol is similarly mediated by proteasomal degradation. To test this, we examined the effect of gossypol on SRC-3 protein after treating with MG132. Consistent with previous reports, the endogenous level of SRC-3 is strongly increased after MG132 treatment alone. In contrast, the SRC-3 reduction induced by gossypol was not prevented by MG132 (Fig. 3B). Our results suggest that gossypol leads to SRC-3 protein degradation by a nonproteasome proteolytic mechanism.
To further characterize the effects of gossypol on SRC-3 protein down-regulation, we performed a time-course analysis of SRC-3 protein levels upon administration of gossypol. A steady decrease in SRC-3 protein levels was observed after 4–5 h (Fig. 3C). This decrease occurs fairly rapidly, because the normal half-life of SRC-3 protein is 3–4 h (34).
Gossypol directly binds to SRC-3
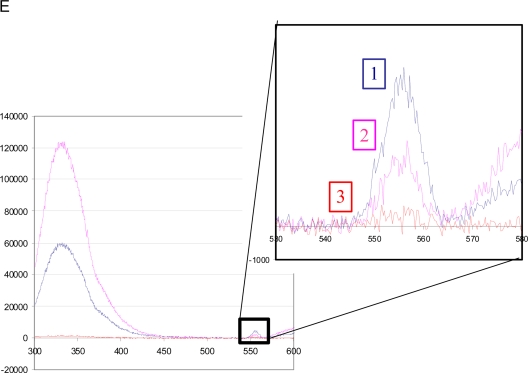
Because gossypol was identified in a cell-free FRET-based compound screen based on its ability to interfere with binding between the SRC-1 RID domain and ERα ligand-binding domain, we presumed that its ability to down-regulate SRC-3 protein proceeds through its direct physical interaction with SRC-3. To confirm this, we examined the ability of gossypol to quench the intrinsic fluorescence of different portions of the SRC-3 protein (45, 46). As shown in Fig. 4A, the fluorescence emission maximum of glutathione S-transferase (GST) SRC-3 RID at 330 nm was quenched progressively by increasing concentrations of gossypol. To determine the binding parameters, the fluorescence intensities at 338 nm were fitted vs. gossypol concentration, which indicates that the half quenching concentration (QC50) of gossypol for the RID domain is 2.5 μm (Fig. 4B). The ability of gossypol to quench the fluorescence of the GST SRC-3 CBP ineraction domain (CID) and basic helix-loop-helix (bHLH) constructs were similarly evaluated. Compared with the quenching with the SRC-3 RID, much higher concentrations of gossypol were required to quench the GST SRC-3 CID and bHLH proteins, with QC50 of 15 and 12 μm, respectively (Fig. 4C). These data indicate that gossypol binds directly and preferentially to the RID domain of SRC-3. To confirm this result, sizing chromatography also was used to analyze the interaction. Elution fractions containing the GST SRC-3 RID proteins were collected (Fig. 4D) and measured for the intrinsic fluorescence spectrum of gossypol at 550 nm. Fluorescence spectra indicated that pools containing GST SRC-3 RID with the emission maximum at 330 nm also exhibited a fluorescence peak signal at approximately 555 nm, which is the signature peak of gossypol (Fig. 4E). The result that GST SRC-3 RID and gossypol coeluted on a sizing column is consistent with a binding interaction between these two components. Taken together, these fluorescence based assays indicate that gossypol interacts directly with the RID domain of SRC-3. Furthermore, isothermal titration calorimetry (47) also revealed that gossypol can interact with the SRC-3 RID, although the high heat of dilution of gossypol made it difficult to accurately determine the precise affinity of gossypol for the SRC-3 RID via this approach (data not shown).
Fig. 4.
Gossypol directly binds to the RID of GST SRC-3. A, Intrinsic tryptophan fluorescence emission spectra of 1.5 μm GST SRC-3 RID (λExcitation = 278 nm) was quenched with increasing concentrations of gossypol. Blue, Without gossypol; pink, 1 μm; yellow, 5 μm; green, 10 μm; purple, 20 μm; brown, 30 μm gossypol. B, The plots of peak intensity at 338 nm vs. gossypol concentration show a dose-response relationship. The fitting of these plots to a binary binding model indicates that gossypol has a QC50 of 2.5 μm. C, Normalized fluorescence signals of different portions of the SRC-3 protein with increasing concentrations of gossypol, with QC50 shown as indicated. D, Sizing chromatography of mixture of GST SRC-3 RID and gossypol. A 1-ml mixture of GST SRC-3 RID and gossypol was fractionated on the sizing column and analyzed for protein content at 254 nm. Four 0.5-ml fractions were pooled as shown and labeled as 1 (blue), 2 (pink), and 3 (red). These three pools were measured for the intrinsic fluorescence spectrum of GST SRC-3 RID. E, Fluorescence spectra of the pools from sizing chromatography. The inset is a magnification of the region corresponding to the intrinsic fluorescence peak of gossypol at approximately 555 nm. The fractions containing GST SRC-3 RID (blue and pink), which were indicated by the emission maximum at 330 nm, also exhibited the signature peak of gossypol at 550 nm.
Gossypol degrades SRC-3 protein in multiple cancer cells
Gossypol was next tested to examine whether it has effects on SRC-3 protein levels in other cancer cell lines. In the ERα negative, ERBB2 positive SKBR3 breast cancer cells (48, 49), gossypol reduced SRC-3 protein levels (Fig. 5A). In HepG2 hepatocarcinoma cells (Fig. 5B), PC3 and LNCaP prostate cancer cells (Fig. 5, C and D), and in A549 and H1299 lung cancer cells (Fig. 5, E and F), gossypol also was effective in down-regulating SRC-3 protein. Taken together, these results indicate that gossypol is broadly able to reduce cellular SRC- 3 protein levels in cell lines derived from various forms of cancer.
Fig. 5.
Gossypol degrades SRC-3 protein in multiple cancer cell lines. SKBR3 cells (A), HepG2 cells (B), LNCaP cells (C), PC3 cells (D), H1299 (E), and A549 cells (F) were serum starved overnight before incubation with DMSO as the control or with gossypol for 24 h. Cell extracts were subjected to Western blotting using antibodies against SRC-3 and actin or glyceraldehyde 3-phosphate dehydrogenase (GAPDH). Numbers in the blot indicate the relative intensities normalized by actin or GAPDH.
Gossypol inhibits cell viabilities in cancerous cells but not in normal cells
We sought to test whether gossypol-mediated down-regulation of SRC-3 corresponds with cell viability in these same cancer cell lines. We treated the cell lines tested above with different concentrations of gossypol for 24 h and then conducted 3-(4,5-dimethylthiazol-2-yl)-5-(3-carboxymethoxyphenyl)-2-(4-sulfophenyl)-2H-tetrazoliuminner salt (MTS) assays to determine its effect on cell viability. In the cell lines tested (MCF-7, SKBR3, LNCaP, PC3, and A549), incubation with gossypol resulted in a significant decrease in the number of viable cells, with IC50 values ranging from 2.07 to 4.22 μm (Fig. 6A). To address whether gossypol had similar effects on normal, nonimmortalized, and transformed cells, we compared the dose-response effects of gossypol between the hepatocellular carcinoma HepG2 and mouse primary hepatocytes. The HepG2 hepatocarcinoma cells suffered a sharp decline in the number of viable cells in the presence of 2 μm gossypol or higher. In contrast, mouse primary hepatocytes survived even when the concentration of gossypol was as high as 12 μm. This finding is consistent with the previous observations in normal human primary fibroblast and normal human bronchial epithelia (50). We conclude that gossypol has cytotoxic effects against hepatic cancer cells but not toward normal liver cells.
Fig. 6.
Gossypol preferentially inhibits cell viabilities in cancerous cells but not in normal cells. A, Gossypol reduces cell viabilities in multiple cancer lines in a dose-dependent manner. Serum-starved cells were incubated with vehicle (DMSO) as the control or with gossypol of different doses (0.25, 0.5, 1, 2, 4, 6, 8, 10, and 12 μm) for 24 h. The number of viable cells with different treatment was determined by MTS assays. Collected data were normalized to the DMSO treatment control, in which the number of viable cells was set to 100%. Each data point represented the average of relative values derived from three wells of cells treated with gossypol at the indicated dose. The concentration of gossypol providing 50% inhibition of viable cells, IC50, was determined by constructing the dose-response curve. To derive the dose-response curve, the Hill-Slope model, y = Min + was applied to fit the dose-response data. B, Gossypol preferentially reduces cell viabilities in cancerous cells but not in normal cells. Serum-starved mouse primary hepatocytes and hepatocarcinoma HepG2 cells were incubated with gossypol of different concentrations for 24 h, and viable cell numbers were determined as above. In HepG2 cells, gossypol caused a sharp decrease in the number of viable cells with doses higher than 2 μm. By contrast, primary hepatocytes survived even when the concentration of gossypol is as high as 12 μm.
Gossypol can sensitize cancer cells to growth factor signaling pathway inhibitors
Numerous studies have revealed that SRC-1 and SRC-3 lie at a nexus that links diverse growth factor signaling cascades, such as IGF, EGF receptor (EGFR), and HER2 signaling pathways, and that coactivator overexpression contributes to acquired chemotherapy resistance (see introductory section). Thus, we reasoned that depletion of SRC-3 by gossypol should sensitize cancers cells to inhibitors by breaking the link that allows for growth factor signaling cross talk.
We and other groups have shown that MAPK kinase (MEK) and ERK1/2 signaling leads to phosphorylation and the subsequent transcriptional activation of SRC-3 (51, 52). On the other hand, activation of ERK1/2 signaling was impaired in mice with reduced SRC-3 levels during Neu-induced tumorigenesis (15). Given the interaction between SRC-3 and MEK signaling, we examined the inhibitory effects on cell viability of a MEK inhibitor in combination of gossypol. MEK inhibitor AZD6244 is a highly selective MEK inhibitor with potential for targeting tumors carrying Ras/Raf mutations (53). The A549 cell line is derived from a lung cancer tumor possessing an activating mutation in the K-ras gene (54, 55). Treating A549 cells with AZD6244 alone at concentrations ranging from 100 nm to 5 μm had no effect on cell viabilities (Fig. 7A). However, when combined with 1 and 2 μm gossypol, AZD6244 noticeably reduced the number of viable cells in a dose-dependent manner. We also examined the inhibitory effects of an EGFR tyrosine kinase inhibitor AG1478 (56) and an IGF-I receptor (IGF-IR) inhibitor AG1024 (57) on breast cancer cell viability in combination with gossypol. Exposure to a low concentration of gossypol significantly sensitized SKBR3 and MCF-7 breast cancer cells to AG1478 and AG1024, respectively (Fig. 7, B and C). Taken together, we conclude that gossypol can effectively sensitize cancer cells to treatment with growth factor signaling pathway inhibitors.
Fig. 7.
Gossypol sensitizes cancer cells to growth factor signaling pathway inhibitors. A, Forty-eight-hour treatments with the MEK inhibitor AZD6244 significantly reduced lung cancer A549 cell viability in a dose-dependent manner (0.1, 2, 5, and 10 μm) when combined with 1 or 2 μm gossypol. B, Seventy-two-hour treatments with the EGFR inhibitor AG1478 (0.25, 0.5, and 1 μm) combined with 0.25 μm gossypol reduced cell viability in SKBR3 breast cancer cells. C, A 48-h treatment with the IGF-IR inhibitor AG1024 (0.25 and 0.5 μm) combined with 1 μm gossypol reduced cell viability in MCF-7 breast cancer cells. Serum-starved cells were incubated with vehicle (DMSO) as a control or with gossypol combined with inhibitors at indicated doses for 48–72 h. Cell viability with the different treatments was determined by MTS assays. Collected data were normalized to the DMSO treatment control, which was set to 100%. Each data point represented the average of values obtained from three wells of cells for each treatment. Differences between the untreated control and the inhibitor-treated samples were analyzed for the significance of difference using a Student's t test, with P values indicated.
Discussion
The SRC coactivator family has been widely implicated in distinct aspects of carcinogenesis. Overexpression of SRC coactivators is linked to tumor initiation, progression, metastasis, advanced disease, and drug resistance. Most rationally designed targeted cancer therapies have been developed to target distinct growth factor pathways, such as 4HT and ICI 182,780 against ERα, Herceptin and Lapatinib against ERBB2 or erlotinib that targets EGFR. Although these drugs are often effective initially, cancers commonly develop acquired resistance to these agents, which is largely contributed by the ability of cancer cells to switch to other growth factor pathways that allow escape. SRC-1 and SRC-3 integrate and activate multiple pathways responsible for promoting cancer cell growth, invasiveness, and motility, and it is less likely that cancer cells will be able to develop bypass mechanisms to continue to survive the loss of function of these oncogenic proteins.
One of the key challenges in the identification of SMI against SRC-1 and SRC-3 is the fact that SRC-3 is a largely unstructured protein with no high affinity ligand binding sites. By combining information from already published NR-SRC interaction inhibitor screens with the GAL4-SRC-1A, GAL4-SRC-3 intrinsic transcriptional assay, and SRC-1A-LUC and SRC-3-LUC protein stability assay screens, we were able to identify gossypol as an effective SRC-1/SRC-3 SMI that can be used in cell culture. Our data demonstrate that gossypol reduces endogenous SRC-1 and SRC-3 protein levels in MCF-7 breast cancer cells, well known for its overexpression of SRC-3 and its dependence on this protein for proliferation (58). Further testing revealed that gossypol can reduce SRC-3 protein concentrations in a variety of other cancer cell lines and that it is preferentially toxic to cancerous but not normal cells.
In the attempt to understand the mechanism underlying the reduced level of SRC-3 protein by gossypol, we provide evidence that it is mediated posttranscriptionally, because mRNA levels for SRC-3 were not down-regulated by gossypol treatment. Previously, it has been shown that all members of the SRC coactivator family are targeted by the proteasome (35, 59), and this degradation is regulated through extensive posttranslational modifications, including phosphorylation, methylation, acetylation, and ubiquitination (60, 61). Interestingly, treatment of cells with the proteasome inhibitor MG132 could not prevent the SRC-3 protein from being degraded in the presence of gossypol, indicating that a nonproteasome-mediated mechanism is responsible for its degradation.
Silver stain analysis of gossypol-treated cells revealed that this compound did not lead to any broad differences in protein expression in the cell. To better address its selectivity, we analyzed the effects of gossypol on other coactivators, including p300 and CARM1. Expression levels for neither of these two proteins were altered, indicating that gossypol can selectively target SRC-1 and SRC-3. Moreover, our data showed that gossypol has little effect on SRC-2 protein levels. Further analyses of differences in the RID domains of SRC-2 may provide more detailed information about how gossypol promotes the selective degradation of SRC-1 and SRC-3. Interestingly, we found that gossypol decreased ERα protein through proteasomal proteolytic mechanisms (data not shown), a characteristic deemed advantageous for breast cancers. Our findings that gossypol promotes SRC-3 down-regulation in a wide variety of ERα negative cancer cell lines indicates that gossypol-mediated degradation of SRC-3 is independent of ERα.
Chemotherapeutic agents should be able to selectively kill tumor cells while not effecting surrounding normal cells. Gossypol possesses this feature, because it has cytotoxic effects on hepatocellular carcinoma HepG2 cells, whereas primary hepatocytes are insensitive. From its characterization as an antifertility drug and in other clinical trials for its use as a Bcl-2 inhibitor in prostate cancer, it is well known that gossypol is tolerated in human subjects. This is consistent with the fact that mouse SRC-1 and SRC-3 knockouts are viable, indicating that normal tissue can survive without either coactivator (62, 63).
The activation of multiple cell growth signaling networks by SRC family coactivators can lead to acquired resistance to chemotherapeutic therapies. Here, we show that we can significantly enhance the inhibitory effects of the MEK inhibitor AZD6244, the EGFR inhibitor AG1478, and the IGF-IR inhibitor AG1024 on cancer cell growth by cotargeting SRC-3 with gossypol. These observations provide the impetus to evaluate the use of gossypol in combination with other chemotherapeutic agents designed to inhibit MEK, EGF, and IGF signaling pathways.
Clinical trials to evaluate gossypol in prostate cancers, lung cancers, and leukemia are currently being conducted (http://www.clinicaltrials.gov). In these trials, gossypol, also referred to as Ascenta Therapeutics-101 (levorotatory enantiomer), is being tested based on a different mechanism of action than described here, as an inhibitor of Bcl-2 (41, 44, 64). Nuclear magnetic resonance spectroscopy, fluorescence polarization assays, and FRET analyses indicated that gossypol can bind to Bcl-2 and Bcl-XL, and antagonize their antiapoptotic functions (41, 64–66). Gossypol also was shown to decrease protein levels of Bcl-2 (44, 64). However, results from both our study and a previous report show that Bcl-2 mRNA were decreased with gossypol treatment (Fig. 3A) (44). The finding that gossypol reduces Bcl-2 mRNA points to an upstream mechanism in addition to a direct effect on Bcl-2 protein. Genome-wide localization analysis of the SRC-3 cistrome in MCF-7 cells revealed several SRC-3 binding sites within both the Bcl-2 gene and Bcl-XL gene (67), suggesting that SRC-3 is a potential upstream protein involved in driving Bcl-2 family gene expression. Consistent with this, depletion of SRC-3 via small interfering RNA in MCF-7 cells was found to noticeably decrease both Bcl-2 mRNA and protein expression (68). Together, these findings suggest that a large part of gossypol's proapoptotic actions is derived from its ability to down-regulate the cellular concentration of SRC-3.
Their role as key integrators of a wide variety of different cell growth pathways and their frequent overexpression in diverse cancer types make SRC-1 and SRC-3 attractive targets for new cancer drugs. Perhaps most importantly, the present study employing gossypol as a proof-of-principle chemical demonstrates that these oncogenic coactivators indeed are accessible to SMI-based chemotherapy and will encourage expanded high throughput screening for additional therapeutic compounds.
Materials and Methods
Materials
Gossypol, MG132, and AG1478 were obtained from Sigma (St. Louis, MO) and dissolved in dimethylsulfoxide (DMSO). AZD6244 and AG1024 were purchased from SelleckChem (Houston, TX) and dissolved in DMSO. E2, 4HT, and ICI 182,780 were purchased from Sigma and dissolved in ethanol. Antibodies to SRC-1 and SRC-3 were purchased from Cell Signaling (Danvers, MA). Antibodies to CARM1 and SRC-2 were obtained from Bethyl (Montgomery, TX). Antibodies to p300 and β-actin were purchased from Santa Cruz Biotechnology, Inc. (Santa Cruz, CA) and Sigma, respectively.
Cell culture
Human cancer cell lines were obtained from the American Type Culture Collection (Manassas, VA). Cell lines were cultured in DMEM (HeLa and MCF-7), RPMI 1640 (LNCaP, A549, and H1299), DMEM/F12 (PC3), McCoy's 5A (SKBR3), or MEM (HepG2) supplemented with 10% fetal calf serum (FCS) and penicillin and streptomycin (100 U/ml). Primary mouse hepatocytes were isolated from 16-wk-old male mice as described previously (69). Cells were plated in Williams E medium supplemented with 10% FCS and penicillin and streptomycin. Before administration of gossypol, all cells were changed to medium supplemented with 0.5% stripped FCS and penicillin and streptomycin (100 U/ml) overnight. All cells were cultured at 37 C under 5% CO2.
Plasmids and transfections
The expression vectors for the SRC-1A and SRC-3 luciferase fusion protein (pCR3.1 SRC-1A-LUC and pCR3.1 SRC-3-LUC) were described (34). The expression vectors for GAL4-responsive luciferase reporter pG5-LUC and Gal4 DBD fusion proteins with empty vector, SRC-1A and SRC-3, were also described (35). Twenty-four hours before transfection, HeLa cells were plated in 24-well dishes. Cells were transfected with the indicated expression vector plasmids using Lipofectamine 2000 (Invitrogen, Carlsbad, CA) according to the manufacturer's protocol. Transfected cells were changed to medium supplemented with 0.5% stripped FCS and incubated overnight before addition of the indicated concentration of gossypol.
Cell extraction and assays
For luciferase assay, at the indicated times after drug treatment, cell pellets were lysed in passive lysis buffer (Promega, Madison WI) and assayed for luciferase activity. Luciferase activities were normalized against total cellular protein by Bradford analysis (Bio-Rad, Hercules, CA). For Western blotting, cells were harvested and lysed in lysis buffer [50 mm Tris (pH 7.5), 150 mm NaCl, and 0.5% Nonidet P-40] and then centrifuged for 15 min at 21,000 × g. Protein lysates were solved by SDS-7.5% PAGE and transferred to polyvinylidene fluoride membranes (Bio-Rad). Membranes were blocked and incubated with indicated antibodies as previously described (35). Cell pellets for silver staining were harvested in the same way as for Western blotting. Silver staining was performed by SilverQuest Silver Staining kit (Invitrogen) according to the manufacturer's protocol. All experiments were repeated at least two times. Intensities of the bands of Western blotting were quantitated by ImageJ (http://rsb.info.nih.gov/ij/).
Quantitative PCR analysis
MCF-7 cell total RNA were isolated from 24-well culture dishes using the RNeasy mini kit (QIAGEN, Valencia, CA). The mRNA for β-actin, SRC-3, and Bcl-2 were quantitated by TaqMan-based RT-PCR using the ABI Prism 7700 sequence detection system (Applied Biosystems, Foster City, CA). For SRC-3, the primer pair 5′-agctgagctgcgaggaaa-3′ and 5′-gagtccaccatccagcaagt-3′ was used with Universal Roche Probe no. 70 (Roche, Indianapolis, IN). For Bcl-2, the primer pair 5′-ttgacagaggatcatgctgtactt-3′ and 5′-atctttatttcatgaggcacgtt-3′ was used along with Universal Roche Probe no. 6. For β-actin, the Human ACTB Endogenous Control (Applied Biosystems) was used. RT-PCR was performed using 1× TaqMan Universal PCR Master Mix (Applied Biosystems). All mRNA quantities were normalized against β-actin RNA. Experiments were repeated two times.
Fluorescence spectrometry
The GST fusion proteins of different portions of SRC-3 were expressed and purified as described previously (45). The fluorescence spectrometric measurements were performed using a SLM 48000S fluorescence spectrometer (SLM-Aminco, Rochester, NY). A total of 1.5 μm GST SRC-3 RID, CID, or bHLH was placed in the fluorescence cuvette. The samples were excited by UV light at a wavelength of 278 nm with a 2-nm bandwidth, and the emission spectra were recorded from 295 nm to above 400 nm with a bandwidth of 4 nm. After addition of a small aliquot of gossypol to a designated concentration, the fluorescence emission spectrum was recorded. The aliquot size of gossypol was maintained below 5% of the total sample volume to minimize the effects of dilution. The peak fluorescence intensity was 338 nm for GST-RID and 306 nm for GST-CID and GST-HLH. The peak fluorescence intensities were fit vs. gossypol concentration using the tight binding inhibitor equation. For sizing chromatography, a 1-ml mixture of GST SRC-3 RID and gossypol was analyzed on a Superdex 75 sizing column (Superdex75 HR 10/30; Amersham Pharmacia Biotech, Uppsala, Sweden).
Cell viability assay
Cells were seeded in 96-well plates in medium supplemented with 10% FCS and changed to medium supplemented with 0.5% stripped FCS overnight before gossypol was administrated at indicated concentrations. After 24 h, relative numbers of viable cells were measured by luminescence with Cell Titer 96 Aqueous One Solution Cell Proliferation Assay (Promega). IC50 value was determined with OriginPro8 (OriginLab Corp., Northampton, MA).
Acknowledgments
This work was supported by National Institutes of Health Grants HD 07857 and HD 08818 (to B.W.O.), Cancer Prevention Research Institute of Texas (CPRIT) Grants RP100348 and RP101251) (to B.W.O. and D.M.L.), The Welch Foundation (B.W.O.), and the Baylor College of Medicine Comprehensive Cancer Training Program CPRIT RP101499 (to Y.W.). This work was also supported by the joint participation of The Diana Helis Henry Medical Research Foundation through its direct engagement in funding of medical research in conjunction with Baylor College of Medicine (D.M.L.).
Disclosure Summary: The authors have nothing to disclose.
Footnotes
- Bcl-2
- B-cell lymphoma 2
- bHLH
- basic helix-loop-helix
- CARM1
- coactivator-associated arginine methyltransferase 1
- CID
- CBP ineraction domain
- DBD
- DNA-binding domain
- DMSO
- dimethylsulfoxide
- E2
- estradiol
- EGF
- epidermal growth factor
- EGFR
- EGF receptor
- ER
- estrogen receptor
- ERBB2
- avian erythroblastosis oncogene B 2
- FCS
- fetal calf serum
- FRET
- fluorescence resonance energy transfer
- gossypol
- 2,2′-bis-(Formyl-1,6,7-trihydroxy-5-isopropyl-3-methylnaphthalene
- GAL4
- the yeast transcription activator protein
- GST
- glutathione S-transferase
- 4HT
- 4-hydroxytamoxifen
- ICI
- Imperial Chemical Industries
- IGF-IR
- IGF-I receptor
- MEK
- MAPK kinase
- MTS
- 3-(4,5-dimethylthiazol-2-yl)-5-(3-carboxymethoxyphenyl)-2-(4-sulfophenyl)-2H-tetrazolium inner salt
- NR
- nuclear receptor
- QC50
- half quenching concentration
- RID
- receptor interacting domain
- SMI
- small molecule inhibitor
- SRC
- steroid receptor coactivator
- TF
- transcriptional factor.
References
- 1. Oñate SA, Tsai SY, Tsai MJ, O'Malley BW. 1995. Sequence and characterization of a coactivator for the steroid hormone receptor superfamily. Science 270:1354–1357 [DOI] [PubMed] [Google Scholar]
- 2. Hong H, Kohli K, Garabedian MJ, Stallcup MR. 1997. GRIP1, a transcriptional coactivator for the AF-2 transactivation domain of steroid, thyroid, retinoid, and vitamin D receptors. Mol Cell Biol 17:2735–2744 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Voegel JJ, Heine MJ, Zechel C, Chambon P, Gronemeyer H. 1996. TIF2, a 160 kDa transcriptional mediator for the ligand-dependent activation function AF-2 of nuclear receptors. EMBO J 15:3667–3675 [PMC free article] [PubMed] [Google Scholar]
- 4. Anzick SL, Kononen J, Walker RL, Azorsa DO, Tanner MM, Guan XY, Sauter G, Kallioniemi OP, Trent JM, Meltzer PS. 1997. AIB1, a steroid receptor coactivator amplified in breast and ovarian cancer. Science 277:965–968 [DOI] [PubMed] [Google Scholar]
- 5. Chen H, Lin RJ, Schiltz RL, Chakravarti D, Nash A, Nagy L, Privalsky ML, Nakatani Y, Evans RM. 1997. Nuclear receptor coactivator ACTR is a novel histone acetyltransferase and forms a multimeric activation complex with P/CAF and CBP/p300. Cell 90:569–580 [DOI] [PubMed] [Google Scholar]
- 6. Li H, Gomes PJ, Chen JD. 1997. RAC3, a steroid/nuclear receptor-associated coactivator that is related to SRC-1 and TIF2. Proc Natl Acad Sci USA 94:8479–8484 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Torchia J, Rose DW, Inostroza J, Kamei Y, Westin S, Glass CK, Rosenfeld MG. 1997. The transcriptional co-activator p/CIP binds CBP and mediates nuclear-receptor function. Nature 387:677–684 [DOI] [PubMed] [Google Scholar]
- 8. Oh A, List HJ, Reiter R, Mani A, Zhang Y, Gehan E, Wellstein A, Riegel AT. 2004. The nuclear receptor coactivator AIB1 mediates insulin-like growth factor I-induced phenotypic changes in human breast cancer cells. Cancer Res 64:8299–8308 [DOI] [PubMed] [Google Scholar]
- 9. Gao Z, Chiao P, Zhang X, Zhang X, Lazar MA, Seto E, Young HA, Ye J. 2005. Coactivators and corepressors of NF-κB in IκBα gene promoter. J Biol Chem 280:21091–21098 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Louie MC, Zou JX, Rabinovich A, Chen HW. 2004. ACTR/AIB1 functions as an E2F1 coactivator to promote breast cancer cell proliferation and antiestrogen resistance. Mol Cell Biol 24:5157–5171 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Fleming FJ, Myers E, Kelly G, Crotty TB, McDermott EW, O'Higgins NJ, Hill AD, Young LS. 2004. Expression of SRC-1, AIB1, and PEA3 in HER2 mediated endocrine resistant breast cancer; a predictive role for SRC-1. J Clin Pathol 57:1069–1074 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Myers E, Hill AD, Kelly G, McDermott EW, O'Higgins NJ, Buggy Y, Young LS. 2005. Associations and interactions between Ets-1 and Ets-2 and coregulatory proteins, SRC-1, AIB1, and NCoR in breast cancer. Clin Cancer Res 11:2111–2122 [DOI] [PubMed] [Google Scholar]
- 13. Wang S, Yuan Y, Liao L, Kuang SQ, Tien JC, O'Malley BW, Xu J. 2009. Disruption of the SRC-1 gene in mice suppresses breast cancer metastasis without affecting primary tumor formation. Proc Natl Acad Sci USA 106:151–156 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Torres-Arzayus MI, Font de Mora J, Yuan J, Vazquez F, Bronson R, Rue M, Sellers WR, Brown M. 2004. High tumor incidence and activation of the PI3K/AKT pathway in transgenic mice define AIB1 as an oncogene. Cancer Cell 6:263–274 [DOI] [PubMed] [Google Scholar]
- 15. Fereshteh MP, Tilli MT, Kim SE, Xu J, O'Malley BW, Wellstein A, Furth PA, Riegel AT. 2008. The nuclear receptor coactivator amplified in breast cancer-1 is required for Neu (ErbB2/HER2) activation, signaling, and mammary tumorigenesis in mice. Cancer Res 68:3697–3706 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Kuang SQ, Liao L, Wang S, Medina D, O'Malley BW, Xu J. 2005. Mice lacking the amplified in breast cancer 1/steroid receptor coactivator-3 are resistant to chemical carcinogen-induced mammary tumorigenesis. Cancer Res 65:7993–8002 [DOI] [PubMed] [Google Scholar]
- 17. Kuang SQ, Liao L, Zhang H, Lee AV, O'Malley BW, Xu J. 2004. AIB1/SRC-3 deficiency affects insulin-like growth factor I signaling pathway and suppresses v-Ha-ras-induced breast cancer initiation and progression in mice. Cancer Res 64:1875–1885 [DOI] [PubMed] [Google Scholar]
- 18. Qin L, Liao L, Redmond A, Young L, Yuan Y, Chen H, O'Malley BW, Xu J. 2008. The AIB1 oncogene promotes breast cancer metastasis by activation of PEA3-mediated matrix metalloproteinase 2 (MMP2) and MMP9 expression. Mol Cell Biol 28:5937–5950 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Bautista S, Vallès H, Walker RL, Anzick S, Zeillinger R, Meltzer P, Theillet C. 1998. In breast cancer, amplification of the steroid receptor coactivator gene AIB1 is correlated with estrogen and progesterone receptor positivity. Clin Cancer Res 4:2925–2929 [PubMed] [Google Scholar]
- 20. Kershah SM, Desouki MM, Koterba KL, Rowan BG. 2004. Expression of estrogen receptor coregulators in normal and malignant human endometrium. Gynecol Oncol 92:304–313 [DOI] [PubMed] [Google Scholar]
- 21. Fujimoto N, Mizokami A, Harada S, Matsumoto T. 2001. Different expression of androgen receptor coactivators in human prostate. Urology 58:289–294 [DOI] [PubMed] [Google Scholar]
- 22. Gnanapragasam VJ, Leung HY, Pulimood AS, Neal DE, Robson CN. 2001. Expression of RAC 3, a steroid hormone receptor co-activator in prostate cancer. Br J Cancer 85:1928–1936 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Mäki HE, Waltering KK, Wallén MJ, Martikainen PM, Tammela TL, van Weerden WM, Vessella RL, Visakorpi T. 2006. Screening of genetic and expression alterations of SRC1 gene in prostate cancer. Prostate 66:1391–1398 [DOI] [PubMed] [Google Scholar]
- 24. Wang Y, Wu MC, Sham JS, Zhang W, Wu WQ, Guan XY. 2002. Prognostic significance of c-myc and AIB1 amplification in hepatocellular carcinoma. A broad survey using high-throughput tissue microarray. Cancer 95:2346–2352 [DOI] [PubMed] [Google Scholar]
- 25. Henke RT, Haddad BR, Kim SE, Rone JD, Mani A, Jessup JM, et al. 2004. Overexpression of the nuclear receptor coactivator AIB1 (SRC-3) during progression of pancreatic adenocarcinoma. Clin Cancer Res 10(Pt 1):6134–6142 [DOI] [PubMed] [Google Scholar]
- 26. Xie D, Sham JS, Zeng WF, Lin HL, Bi J, Che LH, Hu L, Zeng YX, Guan XY. 2005. Correlation of AIB1 overexpression with advanced clinical stage of human colorectal carcinoma. Hum Pathol 36:777–783 [DOI] [PubMed] [Google Scholar]
- 27. Cai D, Shames DS, Raso MG, Xie Y, Kim YH, Pollack JR, Girard L, Sullivan JP, Gao B, Peyton M, Nanjundan M, Byers L, Heymach J, Mills G, Gazdar AF, Wistuba I, Kodadek T, Minna JD. 2010. Steroid receptor coactivator-3 expression in lung cancer and its role in the regulation of cancer cell survival and proliferation. Cancer Res 70:6477–6485 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Shang Y, Brown M. 2002. Molecular determinants for the tissue specificity of SERMs. Science 295:2465–2468 [DOI] [PubMed] [Google Scholar]
- 29. Shou J, Massarweh S, Osborne CK, Wakeling AE, Ali S, Weiss H, Schiff R. 2004. Mechanisms of tamoxifen resistance: increased estrogen receptor-HER2/neu cross-talk in ER/HER2-positive breast cancer. J Natl Cancer Inst 96:926–935 [DOI] [PubMed] [Google Scholar]
- 30. Lahusen T, Henke RT, Kagan BL, Wellstein A, Riegel AT. 2009. The role and regulation of the nuclear receptor co-activator AIB1 in breast cancer. Breast Cancer Res Treat 116:225–237 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Lahusen T, Fereshteh M, Oh A, Wellstein A, Riegel AT. 2007. Epidermal growth factor receptor tyrosine phosphorylation and signaling controlled by a nuclear receptor coactivator, amplified in breast cancer 1. Cancer Res 67:7256–7265 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Wells JA, McClendon CL. 2007. Reaching for high-hanging fruit in drug discovery at protein-protein interfaces. Nature 450:1001–1009 [DOI] [PubMed] [Google Scholar]
- 33. Rosania GR, Crippen G, Woolf P, States D, Shedden K. 2007. A cheminformatic toolkit for mining biomedical knowledge. Pharm Res 24:1791–1802 [DOI] [PubMed] [Google Scholar]
- 34. Lonard DM, Tsai SY, O'Malley BW. 2004. Selective estrogen receptor modulators 4-hydroxytamoxifen and raloxifene impact the stability and function of SRC-1 and SRC-3 coactivator proteins. Mol Cell Biol 24:14–24 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Lonard DM, Nawaz Z, Smith CL, O'Malley BW. 2000. The 26S proteasome is required for estrogen receptor-α and coactivator turnover and for efficient estrogen receptor-α transactivation. Mol Cell 5:939–948 [DOI] [PubMed] [Google Scholar]
- 36. Hadley MA, Burgos MH. 1986. Gossypol injected in the rat epididymal fat pad inhibits epididymal sperm motility. Microsc Electron Biol Celular 10:133–143 [PubMed] [Google Scholar]
- 37. Oko R, Hrudka F. 1984. Comparison of the effects of gossypol, estradiol-17β and testosterone compensation on male rat reproductive organs. Biol Reprod 30:1198–1207 [DOI] [PubMed] [Google Scholar]
- 38. Gilbert NE, O'Reilly JE, Chang CJ, Lin YC, Brueggemeier RW. 1995. Antiproliferative activity of gossypol and gossypolone on human breast cancer cells. Life Sci 57:61–67 [DOI] [PubMed] [Google Scholar]
- 39. Tuszynski GP, Cossu G. 1984. Differential cytotoxic effect of gossypol on human melanoma, colon carcinoma, and other tissue culture cell lines. Cancer Res 44:768–771 [PubMed] [Google Scholar]
- 40. Wolter KG, Wang SJ, Henson BS, Wang S, Griffith KA, Kumar B, Chen J, Carey TE, Bradford CR, D'Silva NJ. 2006. (−)-Gossypol inhibits growth and promotes apoptosis of human head and neck squamous cell carcinoma in vivo. Neoplasia 8:163–172 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Zhang M, Liu H, Guo R, Ling Y, Wu X, Li B, Roller PP, Wang S, Yang D. 2003. Molecular mechanism of gossypol-induced cell growth inhibition and cell death of HT-29 human colon carcinoma cells. Biochem Pharmacol 66:93–103 [DOI] [PubMed] [Google Scholar]
- 42. Feng Q, Yi P, Wong J, O'Malley BW. 2006. Signaling within a coactivator complex: methylation of SRC-3/AIB1 is a molecular switch for complex disassembly. Mol Cell Biol 26:7846–7857 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Liu Z, Wong J, Tsai SY, Tsai MJ, O'Malley BW. 2001. Sequential recruitment of steroid receptor coactivator-1 (SRC-1) and p300 enhances progesterone receptor-dependent initiation and reinitiation of transcription from chromatin. Proc Natl Acad Sci USA 98:12426–12431 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Huang YW, Wang LS, Chang HL, Ye W, Dowd MK, Wan PJ, et al. 2006. Molecular mechanisms of (−)-gossypol-induced apoptosis in human prostate cancer cells. Anticancer Res 26(3A):1925–1933 [PubMed] [Google Scholar]
- 45. Wu RC, Feng Q, Lonard DM, O'Malley BW. 2007. SRC-3 coactivator functional lifetime is regulated by a phospho-dependent ubiquitin time clock. Cell 129:1125–1140 [DOI] [PubMed] [Google Scholar]
- 46. Epps DE, Raub TJ, Caiolfa V, Chiari A, Zamai M. 1999. Determination of the affinity of drugs toward serum albumin by measurement of the quenching of the intrinsic tryptophan fluorescence of the protein. J Pharm Pharmacol 51:41–48 [DOI] [PubMed] [Google Scholar]
- 47. Velazquez-Campoy A, Ohtaka H, Nezami A, Muzammil S, Freire E. 2004. Isothermal titration calorimetry. Curr Protoc Cell Biol Chapter 17:Unit 17 8 [DOI] [PubMed] [Google Scholar]
- 48. Kao J, Salari K, Bocanegra M, Choi YL, Girard L, Gandhi J, Kwei KA, Hernandez-Boussard T, Wang P, Gazdar AF, Minna JD, Pollack JR. 2009. Molecular profiling of breast cancer cell lines defines relevant tumor models and provides a resource for cancer gene discovery. PLoS One 4:e6146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Márquez-Garbán DC, Chen HW, Fishbein MC, Goodglick L, Pietras RJ. 2007. Estrogen receptor signaling pathways in human non-small cell lung cancer. Steroids 72:135–143 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Yeow WS, Baras A, Chua A, Nguyen DM, Sehgal SS, Schrump DS, Nguyen DM. 2006. Gossypol, a phytochemical with BH3-mimetic property, sensitizes cultured thoracic cancer cells to Apo2 ligand/tumor necrosis factor-related apoptosis-inducing ligand. J Thorac Cardiovasc Surg 132:1356–1362 [DOI] [PubMed] [Google Scholar]
- 51. Wu RC, Qin J, Yi P, Wong J, Tsai SY, Tsai MJ, O'Malley BW. 2004. Selective phosphorylations of the SRC-3/AIB1 coactivator integrate genomic reponses to multiple cellular signaling pathways. Mol Cell 15:937–949 [DOI] [PubMed] [Google Scholar]
- 52. Font de Mora J, Brown M. 2000. AIB1 is a conduit for kinase-mediated growth factor signaling to the estrogen receptor. Mol Cell Biol 20:5041–5047 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Wong KK. 2009. Recent developments in anti-cancer agents targeting the Ras/Raf/ MEK/ERK pathway. Recent Pat Anticancer Drug Discov 4:28–35 [DOI] [PubMed] [Google Scholar]
- 54. Lyons JF, Wilhelm S, Hibner B, Bollag G. 2001. Discovery of a novel Raf kinase inhibitor. Endocr Relat Cancer 8:219–225 [DOI] [PubMed] [Google Scholar]
- 55. Suzuki T, Nakagawa T, Endo H, Mitsudomi T, Masuda A, Yatabe Y, Sugiura T, Takahashi T, Hida T. 2003. The sensitivity of lung cancer cell lines to the EGFR-selective tyrosine kinase inhibitor ZD1839 (‘Iressa’) is not related to the expression of EGFR or HER-2 or to K-ras gene status. Lung Cancer 42:35–41 [DOI] [PubMed] [Google Scholar]
- 56. Lenferink AE, Simpson JF, Shawver LK, Coffey RJ, Forbes JT, Arteaga CL. 2000. Blockade of the epidermal growth factor receptor tyrosine kinase suppresses tumorigenesis in MMTV/Neu + MMTV/TGF-α bigenic mice. Proc Natl Acad Sci USA 97:9609–9614 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Wen B, Deutsch E, Marangoni E, Frascona V, Maggiorella L, Abdulkarim B, Chavaudra N, Bourhis J. 2001. Tyrphostin AG 1024 modulates radiosensitivity in human breast cancer cells. Br J Cancer 85:2017–2021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. List HJ, Lauritsen KJ, Reiter R, Powers C, Wellstein A, Riegel AT. 2001. Ribozyme targeting demonstrates that the nuclear receptor coactivator AIB1 is a rate-limiting factor for estrogen-dependent growth of human MCF-7 breast cancer cells. J Biol Chem 276:23763–23768 [DOI] [PubMed] [Google Scholar]
- 59. Yan F, Gao X, Lonard DM, Nawaz Z. 2003. Specific ubiquitin-conjugating enzymes promote degradation of specific nuclear receptor coactivators. Mol Endocrinol 17:1315–1331 [DOI] [PubMed] [Google Scholar]
- 60. Han SJ, Lonard DM, O'Malley BW. 2009. Multi-modulation of nuclear receptor coactivators through posttranslational modifications. Trends Endocrinol Metab 20:8–15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Lonard DM, Kumar R, O'Malley BW. 2010. Minireview: the SRC family of coactivators: an entree to understanding a subset of polygenic diseases? Mol Endocrinol 24:279–285 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Xu J, Liao L, Ning G, Yoshida-Komiya H, Deng C, O'Malley BW. 2000. The steroid receptor coactivator SRC-3 (p/CIP/RAC3/AIB1/ACTR/TRAM-1) is required for normal growth, puberty, female reproductive function, and mammary gland development. Proc Natl Acad Sci USA 97:6379–6384 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Xu J, Qiu Y, DeMayo FJ, Tsai SY, Tsai MJ, O'Malley BW. 1998. Partial hormone resistance in mice with disruption of the steroid receptor coactivator-1 (SRC-1) gene. Science 279:1922–1925 [DOI] [PubMed] [Google Scholar]
- 64. Meng Y, Tang W, Dai Y, Wu X, Liu M, Ji Q, Ji M, Pienta K, Lawrence T, Xu L. 2008. Natural BH3 mimetic (−)-gossypol chemosensitizes human prostate cancer via Bcl-xL inhibition accompanied by increase of Puma and Noxa. Mol Cancer Ther 7:2192–2202 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Kitada S, Leone M, Sareth S, Zhai D, Reed JC, Pellecchia M. 2003. Discovery, characterization, and structure-activity relationships studies of proapoptotic polyphenols targeting B-cell lymphocyte/leukemia-2 proteins. J Med Chem 46:4259–4264 [DOI] [PubMed] [Google Scholar]
- 66. Wang G, Nikolovska-Coleska Z, Yang CY, Wang R, Tang G, Guo J, Shangary S, Qiu S, Gao W, Yang D, Meagher J, Stuckey J, Krajewski K, Jiang S, Roller PP, Abaan HO, Tomita Y, Wang S. 2006. Structure-based design of potent small-molecule inhibitors of anti-apoptotic Bcl-2 proteins. J Med Chem 49:6139–6142 [DOI] [PubMed] [Google Scholar]
- 67. Lanz RB, Bulynko Y, Malovannaya A, Labhart P, Wang L, Li W, Qin J, Harper M, O'Malley BW. 2010. Global characterization of transcriptional impact of the SRC-3 coregulator. Mol Endocrinol 24:859–872 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Karmakar S, Foster EA, Smith CL. 2009. Unique roles of p160 coactivators for regulation of breast cancer cell proliferation and estrogen receptor-α transcriptional activity. Endocrinology 150:1588–1596 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Louet JF, Chopra AR, Sagen JV, An J, York B, Tannour-Louet M, Saha PK, Stevens RD, Wenner BR, Ilkayeva OR, Bain JR, Zhou S, DeMayo F, Xu J, Newgard CB, O'Malley BW. 2010. The coactivator SRC-1 is an essential coordinator of hepatic glucose production. Cell Metab 12:606–618 [DOI] [PMC free article] [PubMed] [Google Scholar]