Abstract
The phosphatidylinositol-3-kinase-dependent kinase, Akt2, plays a central role in mediating insulin effects in glucose-metabolizing tissues. Akt2 knockout mice display insulin resistance with a reactive increase in pancreatic islet mass and hyperinsulinemia. The related phosphatidylinositol-3-kinase-dependent kinase, serum- and glucocorticoid-regulated kinase 3 (SGK3), is essential for normal postnatal hair follicle development but plays no apparent role in glucose homeostasis. We report here an unexpected role of SGK3 in islet β-cell function, which is revealed in Akt2/SGK3 double-knockout (DKO) mice. DKO mice have markedly worse glucose homeostasis than Akt2 single-null animals, including greater baseline glucose, and greater rise in blood glucose after glucose challenge. However, surprisingly, our data strongly support the idea that this exacerbation of the glucose-handling defect is due to impaired β-cell function, rather than increased insulin resistance in peripheral tissues. DKO mice had lower plasma insulin and C-peptide levels, lower β-cell mass, reduced glucose-stimulated insulin secretion, and greater sensitivity to exogenous insulin than Akt2 single nulls. We further demonstrated that SGK3 is strongly expressed in normal mouse islets and, interestingly, that β-catenin expression is dramatically lower in the islets of DKO mice than in those of Akt2−/−/SGK3+/+ or Akt2−/−/SGK3+/− mice. Taken together, these data strongly suggest that SGK3 plays a previously unappreciated role in glucose homeostasis, likely through direct effects within β-cells, to stimulate proliferation and insulin release, at least in part by controlling the expression and activity of β-catenin.
Defects in insulin action in target tissues together with a failure in compensation by pancreatic islet β-cells both contribute to the development of type 2 diabetes (1, 2). In addition to controlling glucose uptake and metabolism by peripheral tissues, insulin has trophic effects in a variety of cell types, including β-cells (3). Both of these classes of action require insulin receptor signaling through the phosphatidylinositol-3-kinase (PI3K) pathway (2, 4–6), which results in changes in activity and expression of a large number of enzymes, transporters, and regulatory proteins. The Akt family of serine-threonine kinases, particularly Akt2, is a key mediator of insulin effects on glucose metabolism in peripheral tissues. It is a PI3K-dependent kinase, which has as its substrates various proteins central to glucose metabolism, as well as cell growth, proliferation, and apoptosis. These include glycogen synthase kinase 3β (GSK-3β), S6-kinase, and forkhead transcription factor (FoxO) (7–9). Targeted deletion of Akt2 in mice leads to moderate-to-severe insulin resistance, hyperinsulinemia accompanied by increased β-cell proliferation and mass. In some backgrounds, a diabetic phenotype of variable severity results (10, 11), which may be influenced by Akt1 (12), but this is less well defined (13). The functional differences among the Akt isoforms may be explained in part by their relative tissue expression: Akt1 is widely expressed in mammalian tissues (13–15), whereas Akt2 is the most highly expressed isoform in skin and insulin-responsive tissues (10, 16–18), and Akt3 is primarily expressed in brain (19, 20).
The serum- and glucocorticoid-regulated kinase (SGK) family of serine-threonine kinases shares significant homology with Akt, and family members are similarly dependent on PI3K for phosphorylation and activation. They are involved in a wide range of cell functions such as ion transport, hormone release, cell proliferation, and apoptosis (21). There are three SGK isoforms (SGK1-3), which share more than 75% identity in their kinase domains. SGK3, in particular, is most abundantly expressed in epithelial cells including kidney, liver, pancreas, and skin (22, 23), and, despite its name, its expression is not regulated by serum or glucocorticoids (22). In vitro, SGK and Akt share the same core substrate sequence, RXRXXS (single letter amino acid code), as well as a number of downstream targets, including Bcl-2-associated death protein, cAMP response element-binding protein, GSK-3β, Nedd4-2, and FKHRL1 (22, 24–27). Thus, the Akt and SGK family kinases have overlapping substrates, and partially overlapping expression patterns; Akt2 and SGK3, in particular, are both expressed in skin as well as in insulin-responsive tissues including liver, fat, and muscle (22, 28, 29), indicating the possibility that, in the absence of Akt2, SGK3 can convey metabolic signals from insulin or other growth factors.
However, mice deleted for SGK3 have a markedly different phenotype from those deleted for Akt2: they display a pronounced defect in hair growth (30) and subtle abnormalities in a number of transport proteins (31) but not impaired glucose tolerance. Importantly, there is redundancy in these signaling pathways (14, 32–35), and the role of a particular component may be revealed only in the absence of other redundant alternatives. Indeed, within the SGK-Akt superfamily, compound knockouts in some cases have had synergistic effects. For example, Akt1/Akt2 double knockout (DKO) mice have a severe neonatal lethal phenotype, which was not found in either Akt1 or Akt2 single knockouts (36). Akt1/Akt3 DKO mice die around embryonic d 12 with severe impairment in growth, cardiovascular development, and organization of the nervous system (37). On the other hand, SGK1/SGK3 DKO mice demonstrate only a superposition of the two single-null phenotypes (38). In a previous study, we demonstrated that Akt2/SGK3 DKO mice have a defect in hair growth that is markedly worse than that of mice lacking only SGK3 (29), strongly suggesting that there may be compensation by Akt2 for SGK3 in hair follicle development. These observations suggested the possibility that a role for SGK3 in insulin action might be unmasked in the context of an Akt2-null animal, and we therefore investigated glucose metabolism, insulin action, and β-cell function in Akt2/SGK3 DKO mice.
Results
DKO mice are hyperglycemic and glucose intolerant
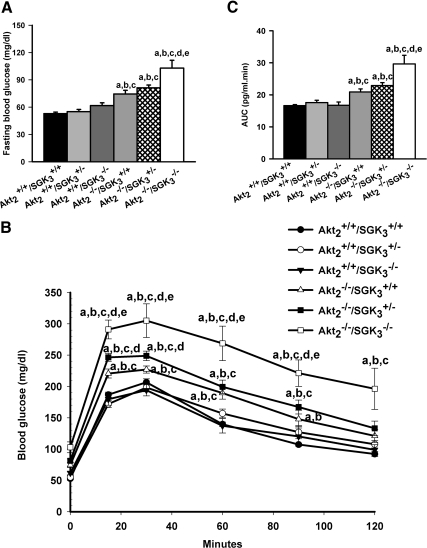
Akt2 single-knockout mice display abnormal glucose homeostasis with mild but statistically significant fasting hyperglycemia, and abnormal glucose tolerance after ip glucose injection (10). These mice also have hyperinsulinemia and a near 4-fold increase in islet mass, reflecting β-cell compensation to peripheral insulin resistance. In contrast, SGK3-null mice have normal glucose tolerance, and histologically normal islets (30). However, SGK3 has been found to affect both Glut-4 and SGLT1-mediated glucose transport in cultured cells (28, 39), and SGK3-null mice have reduced intestinal glucose uptake, and in some genetic backgrounds are mildly hypoglycemic when fasted (40). Hence, it seemed plausible that these kinases could have either similar or opposing effects on glucose homeostasis. As a first step toward understanding their regulatory interactions, we assessed the fasting glucose and response to glucose load of Akt2−/−/SGK3+/+, Akt2−/−/SGK3+/−, Akt2−/−/SGK3−/−, Akt2+/+/SGK3−/−, Akt2+/+/SGK3+/−, and Akt2+/+/SGK3+/+ male mice at 7–9 wk of age. After being fasted overnight, ip glucose tolerance tests were performed on the various genotypes, as described in Materials and Methods. Akt2+/+/SGK3−/− and Akt2+/+/SGK3+/− had normal baseline glucose and glucose tolerance as compared with Akt2+/+/SGK3+/+ (Fig. 1, A and C); this result strongly suggests that the glucose homeostasis of Akt2+/+/SGK3+/− is equivalent to Akt2+/+/SGK3+/+. Consistent with prior reports, the baseline glucose of Akt2−/−/SGK3+/+, Akt2−/−/SGK3+/− were mildly but significantly elevated, and both of these genotypes demonstrated glucose intolerance with significantly higher blood glucose levels at 0, 15, 30, 60, and 90 min, as compared with Akt2+/+/SGK3+/− mice, and increased area under the glucose curve. Interestingly, the glucose handling of Akt2−/−/SGK3−/− (DKO) mice was significantly worse than that of either Akt2−/−/SGK3+/+ or Akt2−/−/SGK3+/− mice (Fig. 1, A and B). Notably, baseline glucose of DKO mice was approximately 30% greater than that of Akt2−/−/SGK3+/+, and was significantly higher at all time points after glucose challenge out to 90 min. Thus, both baseline glucose and glucose tolerance of DKO mice were worse in DKO mice than those of Akt2 single-null mice.
Fig. 1.
Fasting blood glucose and glucose tolerance in DKO mice. A, Blood glucose levels from animals (n ≥7 per genotype) fasted overnight were measured by glucometer. B, Intraperitoneal glucose tolerance test at 7–9 wk of age. Male mice were fasted overnight and subjected to ip injection of 1 g/kg glucose. (n = 7 per genotype). Blood glucose levels were tested at 0, 15, 30, 60, 90, and 120 min. C, Quantification of glycemic excursions as area under the curve. Data are represented as mean ± sem. a, P < 0.05 vs. Akt2+/+/SGK3+/+; b, P < 0.05 vs. Akt2+/+/SGK3+/−; c, P < 0.05 vs. Akt2+/+/SGK3−/−; d, P < 0.05 vs. Akt2−/−/SGK3+/+; e, P < 0.05 vs. Akt2−/−/SGK3+/−.
DKO mice have lower plasma insulin levels than Akt2 single knockout (KO) mice
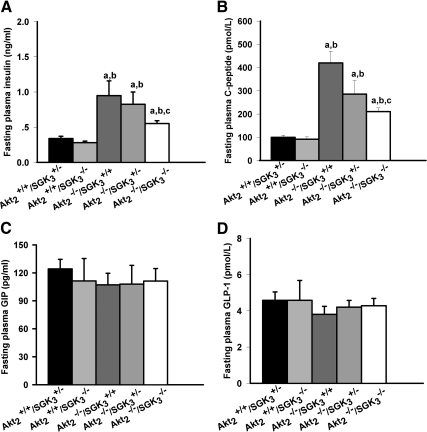
Based on the sequence similarity, shared PI3K dependence, and overlapping substrates and expression patterns of Akt2 and SGK3, we expected the DKO mice to have a further exacerbation of the insulin resistance manifested in Akt2−/−/SGK3+/+ mice, and therefore to have further elevation of plasma insulin. However, to our surprise, both the insulin and C-peptide levels of DKO mice were significantly lower than those of Akt2−/−/SGK3+/+ mice, (Fig. 2, A and B), suggesting that the exacerbated diabetic phenotype is not due to increased insulin resistance. Furthermore, plasma glucagon-like peptide (GLP)-1 and gastric inhibitory peptide (GIP) levels were no different among the five genotypes (Fig. 2, C and D), suggesting that these peptide hormones, which are produced by intestinal epithelia and stimulate β-cell function, are not implicated in the defect.
Fig. 2.
Fasting plasma insulin, C-peptide, GLP-1, and GIP levels in DKO mice. Male mice (n = 5–10) (7–9 wk of age) were fasted overnight and fasting plasma insulin (A), C-peptide (B), GLP-1 (C), and GIP (D) values were measured by commercial ELISA kit. Data are represented as mean ± sem. a, P < 0.05 vs. Akt2+/+/SGK3+/−; b, P < 0.05 vs. Akt2+/+/SGK3−/−; c, P < 0.05 vs. Akt2−/−/SGK3+/+.
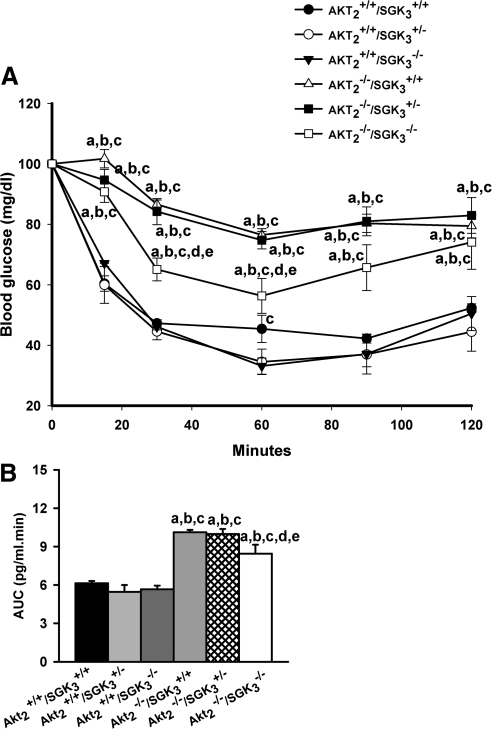
When we compared the sensitivity of the mice to exogenous insulin, we found that all Akt2-null genotypes (Akt2−/−/SGK3+/+, Akt2−/−/SGK3+/−, and Akt2−/−/SGK3−/−) showed significantly impaired insulin sensitivity compared with those that were intact at the Akt2 locus (Akt2+/+/SGK3+/+, Akt2+/+/SGK3+/−, and Akt2+/+/SGK3−/−) (Fig. 3A). However, the area under the curve was modestly, but significantly smaller in DKO (Akt2−/−/SGK3−/−) mice as compared with Akt2−/−/SGK3+/+ and Akt2−/−/SGK3+/− mice (Fig. 3B). These data suggest that the DKO mice have more mild insulin resistance than Akt2−/−/SGK3+/+ and Akt2−/−/SGK3+/− mice. In any case, the insulin resistance of DKO mice is clearly no worse than that of Akt single nulls. It is also notable that the insulin tolerance of Akt2+/+/SGK3+/− and Akt2+/+/SGK3+/+ mice was indistinguishable. This result is also consistent with our earlier studies in which SGK3+/− were indistinguishable from wild type in all respects examined, showing no evidence of haplotype insufficiency (29, 30, 40) Based on these observations, to reduce animal numbers and simplify the breeding strategy, in subsequent experiments, SGK3+/− mice were used as controls, rather than wild type.
Fig. 3.
Insulin tolerance of mice lacking SGK3 and/or Akt2. A, Male mice (7–9 wk of age) were fasted for 4 h, and the blood glucose levels were monitored in response to ip injection of 1 U/kg insulin (n ≥ 7 per genotype) at 0, 15, 30, 60, 90, and 120 min. Genotypes are as shown in the label. B, Quantification of the area under the curve. Data are represented as mean ± sem; a, P < 0.05 vs. Akt2+/+/SGK3+/+; b, P < 0.05 vs. Akt2+/+/SGK3+/−; c, P < 0.05 vs. Akt2+/+/SGK3−/−; d, P < 0.05 vs. Akt2−/−/SGK3+/+; e, P < 0.05 vs. Akt2−/−/SGK3+/−.
Biochemical assessment of insulin action in muscle, liver, and fat of Akt2/SGK3 DKO mice
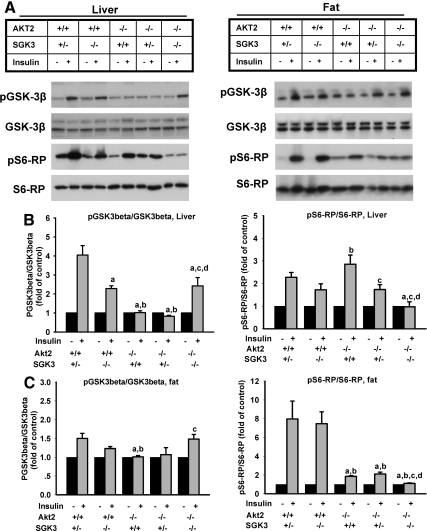
To further examine insulin action in glucose-metabolizing tissues, we measured the effect of insulin on the phosphorylation state of GSK-3β and S6 ribosomal protein (S6-RP), two key insulin targets, in muscle, liver, and fat. Mice were injected ip with insulin, and 20 min later animals were killed and tissues harvested, and prepared for Western blot. As expected, we found that phosphorylation of both GSK-3β and S6-RP (at Ser240/244) were strongly stimulated in liver, muscle, and fat of Akt2+/+/SGK3+/− as in wild-type mice (Fig. 4 and data not shown), consistent with previous reports (41, 42). In the other genotypes, effects of insulin depended on the target, the tissue, and the specific genotype. Notably, in liver of Akt2+/+/SGK3−/− mice, insulin-induced phosphorylation of GSK-3β was moderately blunted, whereas in Akt2−/−/SGK3+/+ and Akt2−/−/SGK3+/− it was completely abrogated. However, interestingly, insulin-stimulated GSK-3β phosphorylation was higher in liver of DKO mice than in liver of Akt2−/− mice with an intact SGK3 allele, and comparable to that of Akt2+/+/SGK3−/− (SGK3 single nulls) (Fig. 4, A and B). The basis of this latter finding is uncertain, but it is consistent with the greater insulin sensitivity of DKO mice relative to Akt2−/−/SGK3+/+ mice and further supports the conclusion that the exacerbated defect in glucose homeostasis of DKO mice is due to lower insulin levels in DKO mice, rather than increased insulin resistance. In fat, although the effect of insulin on GSK-3β phosphorylation in wild type was less pronounced than in liver, the pattern among the various genotypes was qualitatively similar (Fig. 4C, left panel).
Fig. 4.
Insulin signaling in liver and fat of DKO mice. Male mice at 7–9 wk of age were fasted overnight, injected with either saline or insulin at 2 U/kg body weight, and killed 20 min later. A, Levels of phosphorylated GSK-3β (pGSK-3β), total GSK-3β, phosphorylated S6-RP (pS6-RP), and total S6-RP in liver and fat in the absence and presence of insulin were assessed by immunoblot (n = 5–7 per genotype). B, Quantitation of insulin-stimulated phosphorylation of GSK-3β and S6-RP in liver. C, Quantitation of insulin-stimulated phosphorylation of GSK-3β and S6-RP in fat. Phosphoproteins were normalized to the corresponding total proteins. Data are represented as mean ± sem. a, P < 0.05 vs. Akt2+/+/SGK3+/−; b, P < 0.05 vs. Akt2+/+/SGK3−/−; c, P < 0.05 vs. Akt2−/−/SGK3+/+; d, P < 0.05 vs. Akt2−/−/SGK3+/−.
S6-RP phosphorylation showed a markedly different pattern from that of GSK-3β, appearing to be more equally dependent on Akt2 and SGK3. Most notably, although there were minor differences among the various genotypes, only the DKO mice showed complete abrogation of insulin-induced phosphorylation of S6-RP (Fig. 4, panel A, left panel; and panel B, right panel). Although the basis of these tissue- and target-specific findings will require further investigation, it is worth noting in the present context that phosphorylation of S6-RP, which is completely abrogated in DKO mice, is less important for insulin-stimulated glucose disposition (43, 44), than is phosphorylation of GSK-3β, which is less compromised in DKO than in Akt single nulls. Thus, these findings are consistent with the insulin tolerance tests, which suggest that the insulin sensitivity of DKO mice is actually greater than that of Akt2 single nulls.
Abnormal β-cell mass of Akt2/SGK3 DKO mice
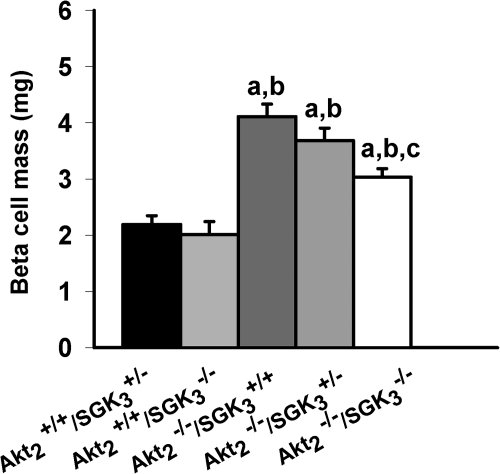
As a first step toward understanding the mechanistic basis of this effect of SGK3 deletion, we examined the β-cell mass of the DKO mice and compared it with Akt2 single nulls and the other compound genotypes. Earlier studies had established that Akt2 deletion results in a marked increase in β-cell mass and islet numbers. We therefore assessed islet morphology in mice of Akt2+/+/SGK3+/−, Akt2+/+/SGK3−/−, Akt2−/−/SGK3+/+, Akt2−/−/SGK3+/−, and Akt2−/−/SGK3−/−, which had been fed a standard mouse chow diet, ad libitum from weaning to 7–9 wk of age. In hematoxylin-stained pancreatic sections, the islets of all mice homozygous null for Akt (Akt2−/−/SGK3+/+, Akt2−/−/SGK3+/−, and Akt2−/−/SGK3−/−) appeared larger than those with wild-type Akt2 (Akt2+/+/SGK3−/− and Akt2+/+/SGK3+/−) (data not shown). Quantitative morphometic analysis (Fig. 5) confirmed that Akt2−/−/SGK3+/+, Akt2−/−/SGK3+/−, and DKO mice have greater β-cell mass than Akt2+/+/SGK3−/− and Akt2+/+/SGK3+/−, and importantly, demonstrated that the β-cell mass of the DKO was significantly less than that of Akt2−/−/SGK3+/+, consistent with the idea that the ability of DKO mice to increase their β-cell mass in response to hyperglycemia is significantly blunted, but not abrogated.
Fig. 5.
β-Cell mass of DKO mice. Nonoverlapping sections from the entire pancreas of 7- to 9-wk-old Akt2+/+/SGK3+/−, Akt2+/+/SGK3−/−, Akt2−/−/SGK3+/+, Akt2−/−/SGK3+/−, and Akt2−/−/SGK3−/− male mice (n = 3 per genotype) were stained with antibody to insulin and counterstained with hematoxylin and eosin to determine fractional β-cell area. β-Cell mass was obtained by multiplying the pancreatic mass by the fractional β-cell area (see Materials and Methods for details). Values represent means ± sem. a, P < 0.05 vs. Akt2+/+/SGK3+/−; b, P < 0.05 vs. Akt2+/+/SGK3−/−; c, P < 0.05 vs. Akt2−/−/SGK3+/+.
DKO mice have normal β-cell apoptosis but abnormal β-cell proliferation
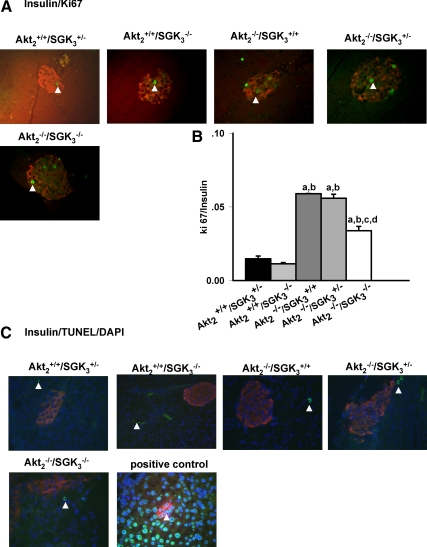
To begin to examine the cause of the reduced capacity of DKO mice to increase their β-cell mass, we next examined β-cell proliferation (using ki-67 staining) and apoptosis [using terminal deoxynucleotide transferase-mediated dUTP nick-end labeling (TUNEL)]. As shown in Fig. 6, A and B, all of Akt2-null (Akt2−/−/SGK3+/+, Akt2−/−/SGK3+/−, and Akt2−/−/SGK3−/−) mice demonstrated increased proliferative rates compared with mice with intact Akt2 (Akt2+/+/SGK3+/− and Akt2+/+/SGK3−/−). However, DKO mice showed a significantly lower proliferation rate than Akt2−/−/SGK3+/+ and Akt2−/−/SGK3+/− mice, suggesting that their capacity to mount a β-cell proliferative response to hyperglycemia was impaired. In contrast, we checked at least 30 islets of randomly selected three sections of each animal, and found TUNEL-positive β-cells were rare in all five genotypes examined, and there was no significant difference among them (Fig. 6C).
Fig. 6.
β-Cell proliferation and apoptosis in DKO mice. A, Pancreas sections from Akt2+/+/SGK3+/−, Akt2+/+/SGK3−/−, Akt2−/−/SGK3+/+, Akt2−/−/SGK3+/−, and Akt2−/−/SGK3−/− mice (7–9 wk of age; n = 3 per genotype) were stained with antibodies to insulin (red), Ki67 (green). Arrowheads indicate proliferating cells. B, The number of cells that were positive for both Ki67 and insulin were quantified as a percentage of total number of insulin-positive cells. At least 30 islets from three nonoverlapping pancreas sections were analyzed for each animal. C, Pancreas sections from above indicated genotypes were stained with TUNEL kit (green), antibodies to insulin (red), and 4′,6-diamidino-2-phenylindole (DAPI) (blue). Data are represented as mean ± sem. a, P < 0.05 vs. Akt2+/+/SGK3+/−; b, P < 0.05 vs. Akt2+/+/SGK3−/−; c, P < 0.05 vs. Akt2−/−/SGK3+/+; d, P < 0.05 vs. Akt2−/−/SGK3+/−.
DKO mice have markedly reduced insulin-secretory capacity in vivo
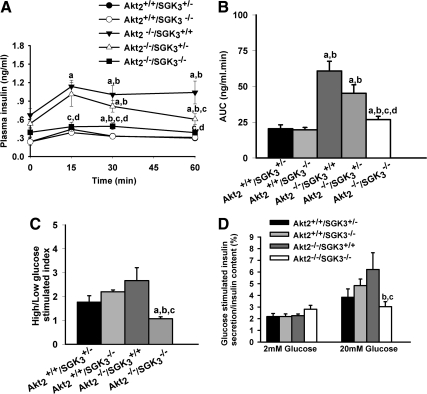
The above data suggested that DKO mice have a defect in β-cell function and proliferative response not manifest in Akt2 single-null animals. To directly examine insulin-secretory capacity, glucose-induced insulin secretion levels were measured during glucose tolerance test, first in vivo, and then in vitro. After an overnight fast, Akt2−/−/SGK3+/+, Akt2−/−/SGK3+/−, and DKO mice had elevated baseline insulin levels. However, the effect of glucose to increase plasma insulin was markedly less in the DKO mice relative to Akt2−/−/SGK3+/+ and Akt2−/−/SGK3+/−, particularly at the earlier time points (15 and 30 min), consistent with blunted insulin secretion as opposed to increased degradation. Furthermore, the area under the insulin curve was decreased by 50–60% in DKO mice as compared with Akt2−/−/SGK3+/+ mice (Fig. 7B).
Fig. 7.
DKO mice have reduced in vivo and in vitro glucose-stimulated insulin secretory capacity. A and B, In vivo glucose-stimulated insulin secretion. Glucose (1 g/kg) was administered ip to overnight fasted male mice. A, Plasma insulin levels were monitored at 0, 15, 30, and 60 min (n = 10 animals per group). B, Quantification of plasma insulin excursions as area under the curve (AUC). C and D, In vitro glucose-stimulated insulin secretory capacity: male mice (7–9 wk of age) were fasted overnight, and islets were isolated and incubated in 2 mm glucose for 10 min and then in 20 mm glucose for another 10 min. C, Insulin secretion index was calculated as ratio of normalized insulin secretion in high glucose to normalized insulin secretion in low glucose. D, Insulin levels under low- and high-glucose stimulation related to total cellular insulin content separately. Insulin secretion into medium and total cellular content were measured. Values shown are mean ± sem. a, P < 0.05 vs. Akt2+/+/SGK3+/−; b, P < 0.05 vs. Akt2+/+/SGK3−/−; c, P < 0.05 vs. Akt2−/−/SGK3+/+; d, P < 0.05 vs. Akt2−/−/SGK3+/−.
We next directly examined glucose-stimulated insulin secretion studies using islets isolated from 7- to 9-wk-old Akt2−/−/SGK3+/+, Akt2−/−/SGK3−/−, Akt2+/+/SGK3−/−, and Akt2+/+/SGK3+/− mice. Islets were incubated in Krebs-Ringer buffer containing 2 mm (low) for 10 min and then incubated in 20 mm (high) glucose for another 10 min, and the insulin concentration of the supernatant was measured by ELISA (see Materials and Methods for details), as previously described (45). When normalized to insulin content, glucose-stimulated insulin secretion was markedly reduced in DKO islets compared with islets isolated from Akt2+/+/SGK3+/−, Akt2+/+/SGK3−/−, and Akt2−/−/SGK3+/+ mice (Fig. 7, C and D). In contrast, normalized insulin secretion from islets of Akt2−/−/SGK3+/+ and Akt2+/+/SGK3−/− mice was indistinguishable from that of Akt2+/+/SGK3+/−. Thus, these data further support the idea that the exacerbated hyperglycemia and glucose intolerance of DKO mice is due to a failure of β-cells to compensate for peripheral insulin resistance. These data also support the idea that most of the increase in insulin secretion in Akt2−/−/SGK3+/+ is due to increased β-cell mass (see Fig. 7, A and B).
Expression of SGK3 and β-catenin in mouse pancreatic islets
To further address the mechanism of SGK3 regulation of β-cell function, we first examined the distribution of SGK3 in mouse pancreas. We found that SGK3 was abundantly expressed throughout the islets but not in exocrine pancreas (Fig. 8A).
Fig. 8.
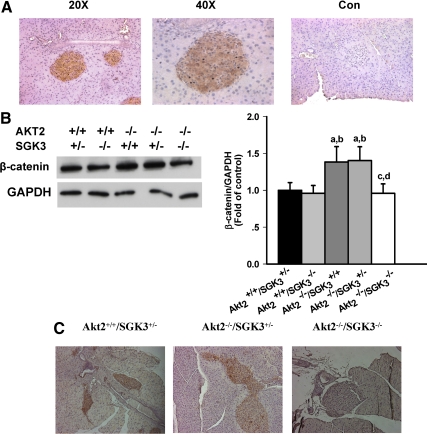
Expression of SGK3 and β-catenin in mouse pancreatic islets. A, Pancreas from Akt2+/+/SGK3+/− mice was sectioned and stained with rabbit anti-SGKL/SGK3 (Imgenex), and visualized with peroxidase-linked secondary (brown signal), and counterstained with hematoxylin as described in Materials and Methods. Magnification, ×20 and ×40. Control lacking primary antibody (×20). B, Levels of β-catenin and glyceraldehyde-3-phosphate dehydrogenase (GAPDH) were assessed by immunoblot (n = 5–7 per genotype) and semiquantitative analysis in islets isolated from Akt2+/+/SGK3+/−, Akt2+/+/SGK3−/−, Akt2−/−/SGK3+/+, Akt2−/−/SGK3+/−, and Akt2−/−/SGK3−/− mice. Proteins were normalized to control (Con). Data are represented as mean ± sem. a, P < 0.05 vs. Akt2+/+/SGK3+/−; b, P < 0.05 vs. Akt2+/+/SGK3−/−; c, P < 0.05 vs. Akt2−/−/SGK3+/+; d, P < 0.05 vs. Akt2−/−/SGK3+/−. C, Pancreas sections from Akt2+/+/SGK3+/−, Akt2−/−/SGK3+/−, and Akt2−/−/SGK3−/− mice were stained with mouse anti-β-catenin antibody and visualized using horseradish peroxidase-linked secondary antibody with 3,3′-diaminobenzidine peroxidase (brown) and counterstained with hematoxylin, as described in Materials and Methods and viewed at ×10 magnification.
Because our previous work demonstrated that β-catenin expression and signaling are abnormal in the hair follicles of SGK3-Akt2 DKO mice (29), and because the WNT/β-catenin signaling pathway has been reported to be involved in β-cell proliferation and insulin secretion (46, 47), we next examined islet β-catenin expression by Western blot and immunohistochemistry (Figs. 8B and 8C). In quantitated Western blots performed on isolated islets, we found that β-catenin expression was significantly greater in the islets of Akt2 single-null mice (Akt2−/−/SGK3+/+ or Akt2−/−/SGK3+/−) than in the other genotypes tested, including Akt2+/+/SGK3+/− or Akt2+/+/SGK3−/−, and DKO mice (Fig. 8B). Importantly, this is despite the fact that the two Akt2+/+ genotypes have normal glucose tolerance whereas that of DKO mice is markedly abnormal. By immunohistochemistry the defect in β-catenin expression in DKO mice was even more apparent: in pancreas sections, islets of both Akt2+/+/SGK3+/− and Akt2 single nulls showed abundant β-catenin staining, whereas DKO islets showed virtually no specific staining (Fig. 8C). Consistent with Fig. 5, islets of Akt2 single nulls were larger than those of either wild-type or DKO mice. Taken together with our earlier work (29, 30), these data suggest a mechanistic role for β-catenin in SGK3-mediated β-cell proliferation and secretion.
Discussion
We have found that mice lacking both Akt2 and SGK3 have abnormalities in glucose homeostasis, which cannot be explained by simple superposition of the two single knockout phenotypes. Consistent with earlier reports, Akt2 single-null (Akt2−/−/SGK3+/+) mice (in a B6; 129 background) had moderate glucose intolerance and enlarged islets when compared with wild-type mice, and SGK3 single nulls (Akt2+/+/SGK3−/−) had normal glucose tolerance, which was indistinguishable from both SGK3 heterozygotes (Akt2+/+/SGK3+/−) and wild-type mice. In sharp contrast, DKO mice had both higher fasting glucose and substantially worse glucose tolerance than Akt2−/−/SGK3+/+ mice (Fig. 1, B and C). Based on the overlapping expression patterns, relatively high degree of homology, and related mechanisms of action of Akt2 and SGK3, we expected the worsened glucose metabolism of the DKO mice to be due to an increase in insulin resistance, as reflected in higher insulin levels, greater islet hyperplasia, and greater glucose-stimulated insulin release compared with Akt2 single nulls. However, surprisingly, DKO mice had lower plasma insulin levels (Figs. 2A and 7B) and smaller islets (Fig. 5) with less β-cell proliferation (Fig. 6), and islets isolated from these mice displayed lower levels of glucose-stimulated insulin secretion, in vitro (Fig. 7). Furthermore, the DKO mice were more responsive to exogenous insulin than Akt single nulls, both with respect to glucose disposition (Fig. 3) and with respect to insulin-induced phosphorylation of GSK-3β in peripheral tissues (Fig. 4, and data not shown). Finally, SGK3 and Akt2 are both expressed in wild-type islets (Fig. 8 and Refs. 48 and 49). Together, these data strongly suggest that SGK3 plays an important role in mediating signals that stimulate β-cell function and proliferation but does not play a stimulatory role in peripheral tissues involved in glucose disposition.
The contribution of insufficient insulin secretion to the development of type 2 diabetes is now widely accepted; however, opinions diverge regarding the relative contribution of a decrease in β-cell mass vs. an intrinsic defect in the secretory machinery of the β-cell (50, 51). In the present study, we found that SGK3 signaling is required for the increase in both β-cell proliferation and insulin secretion seen in the absence of Akt2, which together with high-islet SGK3 expression, points to a direct role of SGK3 in β-cell function. Moreover, the defective β-cell proliferation and insulin secretion of DKO mice also support the idea that adaptive mechanisms of function and mass share common regulatory pathways and can act in concert. With this in mind, it is interesting to note that β-cell-specific insulin receptor knockout mice exhibit impaired glucose tolerance, a selective loss of glucose-stimulated insulin secretion, and decreased β-cell proliferation (2, 52). These parallels are consistent with the idea that SGK3 is downstream of the insulin receptor in β-cell signaling. β-Catenin, which is a downstream mediator of Akt2 and SGK3 function in hair follicles (29, 30), has been shown to play an important role in β-cell proliferation and insulin secretion (46, 47). In this context, it is of considerable interest that β-catenin expression was markedly decreased in DKO islets, which is consistent with the idea that SGK3-mediated β-catenin stabilization is involved in maintaining normal β-cell function and proliferation. However, the detailed mechanism, including mechanistic interconnections between SGK3 and β-catenin, will require further examination.
In light of the present results, it is also interesting to speculate that SGK3 could play a role in the effect of background differences on the severity of glucose tolerance in Akt2 KO mice. Akt2 has been disrupted on two different genetic backgrounds, resulting in glucose homeostasis defects with differing severity. On a mixed B6;129 background (same as the present study), Akt2 knockout mice displayed a mild but statistically significant fasting hyperglycemia, which was more pronounced during fed states (10). Hyperglycemia was accompanied by hyperinsulinemia resulting from an increase in islet mass, consistent with β-cell compensation to insulin resistance. On an inbred DBA/1lacJ background, disruption of the Akt2 gene resulted in a more severe metabolic defect, characterized by progression of insulin resistance, and islet failure in males (11). Importantly, the ability of β-cells to respond to peripheral insulin resistance is an important determinant of these background effects; however, the genes responsible for mediating these differences are unknown. Our present results suggest the interesting possibility that differences in SGK3 expression or activity could play a role in these differences.
Although we favor the view that the role of SGK3 is in the β-cell itself, it is possible that it acts in a different cell type, which influences β-cell function. It was with this possibility in mind that we examined GLP-1 and GIP levels. GLP-1 and GIP, which stimulate β-cell insulin production, are both stimulated by intestinal glucose (53). Because SGK3-deficient mice have reduced intestinal glucose uptake (40), one mechanism for the reduced insulin in DKO mice might have been lowered levels of these mediators. Although further studies will be needed to definitively rule out this possibility, it seems unlikely in view of the finding that GLP-1 and GIP levels were indistinguishable among the different phenotypes, including DKO mice (Fig. 2).
Our present results support the conclusion that SGK3 does not play a positive role in mediating insulin effects in peripheral tissues and might even have an inhibitory effect (Fig. 3). In this context, it is interesting to consider the marked differences in phosphorylation of GSK-3β and S6-RP in liver and fat (Fig. 4). GSK-3β (which was more strongly phosphorylated in DKO than in Akt2 single nulls) is an important mediator of insulin effects on glucose metabolism in peripheral tissue, notably stimulating glycogen synthesis, particularly in liver (44). In contrast, S6K1 (the phosphorylation of which was completely abrogated in DKO mice) is involved in negative feedback regulation of insulin signaling (42, 54–56). These observations raise the interesting possibility that the increased insulin sensitivity of DKO mice may be due, in part, to decreased feedback inhibition, particularly in hepatocytes. However, it is also possible that the enhanced insulin sensitivity of DKO mice may simply be due to their chronically lower insulin levels or lower body weight: at 7–9 wk of age, the body weights of DKO mice were significantly lower than those of Akt2−/−/SGK3+/+ and Akt2−/−/SGK3+/− mice [15.7 g ± 0.30 (sem) vs. 19.606 ± 0.5188 and 18.6 g ± 0.46 (sem), P < 0.001, respectively]. Further studies will be needed to address these issues. In any case, the increased insulin sensitivity of the DKO mice is an ameliorating factor, despite which, they have worse glucose-handling capacity, further underscoring their defect in β-cell function.
In sum, our data strongly support the idea that in mice lacking Akt2, the ability of β-cells to compensate for insulin resistance is, in large part, dependent on SGK3. Unlike Akt2 single nulls, the islets of mice that lack both kinases fail to increase β-catenin expression, demonstrate low β-cell proliferation rate, and have markedly impaired glucose-stimulated insulin secretion. The consequent glucose intolerance is significantly worse than in Akt2 single nulls, despite a mild enhancement in insulin sensitivity. Thus, these data provide in vivo evidence of a physiological role of SGK3 in β-cell function and identify a potentially novel therapeutic target for the prevention and treatment of type 2 diabetes.
Materials and Methods
Generation of Akt2/SGK3 DKO mice
All animal experiments were conducted following institutional Committee on Animal Research Committee approval. Targeted disruption of the Akt2 and SGK3 alleles have been described previously (10, 40). Akt2 KO mice were bred with SGK3 KO mice to generate compound heterozygote (Akt2+/−/SGK3+/−) mice, which were then interbred to generate double-homozygous mice with a mixed B6;129 background.
Genotype analysis
Genomic DNA was prepared from tail biopsies using the GenElute Mammalian Genomic DNA Minprep Kit (Sigma-Adrich, St. Louis, MO), and 250 ng DNA were used for genotyping. PCR analysis of the Ak2 allele has been described previously (10). For analysis of the SGK3 allele, the forward primer 5′-GATTGCGAACTCTTCACTCATTTGC-3′ and the reverse primer 5′-GAACACAGCTGCTCATACAGACACAGG-3′were used. Reactions were performed using LA Taq (TaKaRa, Otsu, Shiga, Japan) with the following conditions: 94 C for 1 min; 94 C for 30 sec, 62 C for 45 sec, and 68 C for 7 min for 14 cycles, then increased extension by 15 sec/cycle with a final extension of 72 C for 15 min. PCR products were resolved on 2% agarose gels.
Measurement of glucose, insulin, C-peptide, GLP-1, and GIP levels
Akt2+/+/SGK3+/−, Akt2+/+/SGK3−/−, Akt2−/−/SGK3+/+, Akt2−/−/SGK3+/−, and Akt2−/−/SGK3−/− male mice (7–9 wk of age) were fasted overnight, and glucose was determined by glucometer (Bayer, Leverkusen, Germany). Plasma insulin concentrations were measured by ELISA (Linco Research, Inc., St. Charles, MO), C-peptide and glucose-dependent insulin-releasing polypeptide (GIP) levels were tested with commercial ELISA kits (Millipore Corp., Bedford, MA), plasma GLP-1 levels were determined by ELISA (ALPCO Diagnostics, Salem, NH) according to the manufacturer's instructions.
Intraperitoneal glucose tolerance test
Akt2+/+/SGK3+/+, Akt2+/+/SGK3+/−, Akt2+/+/SGK3−/−, Akt2−/−/SGK3+/+, Akt2−/−/SGK3+/−, and Akt2−/−/SGK3−/− male mice (7–9 wk of age) were fasted overnight (16 h) and then injected ip with 1 g/kg body weight d-glucose [10% (wt/vol) stock solution in PBS]. Blood samples were collected from the transversely sectioned tip of the tail. Whole-blood glucose was measured at 0 min (just before glucose injection), and at 15-, 30-, 60-, 90-, and 120-min intervals after the glucose load, in the same animals.
Intraperitoneal insulin tolerance test
The insulin tolerance test was performed on 7- to 9-wk-old Akt2+/+/SGK3+/+, Akt2+/+/SGK3+/−, Akt2+/+/SGK3−/−, Akt2−/−/SGK3+/+, Akt2−/−/SGK3+/−, and Akt2−/−/SGK3−/− male mice. Indicated genotypes were fasted for 4 h and then injected ip with 1.0 U/kg body weight insulin (Sigma, St. Louis, MO). Blood samples were collected from the transversely sectioned tip of the tail, and whole-blood glucose was measured by Glucometer, as above, at 0, 15, 30, 60, 90, and 120 min after insulin injection.
Glucose-stimulated insulin secretion
To examine glucose-stimulated insulin secretion, the above indicated genotypes were fasted for 16 h. Blood was collected from submandiular plexus before (0 min) and 15, 30, and 60 min after glucose administration (1 g/kg). Serum samples were immediately obtained by centrifugation at 3000 rpm for 10 min and stored at −20 C. Insulin secretion levels were assessed by insulin ELISA kit.
Glucose-stimulated insulin secretion was further studied on isolated islets from Akt2+/+/SGK3+/−, Akt2+/+/SGK3−/−, Akt2−/−/SGK3+/+, and Akt2−/−/SGK3−/− mice (n = 4 per genotype). Hand-picked islets were isolated after intraductal collagenase P (Roche Molecular Biochemicals, Indianapolis, IN) injection and Ficoll gradient centrifugation as previously described (57). Pools of islets were incubated in 600-1000 μl Krebs-Ringer buffer (119 mm NaCl; 4.7 mm KCl; 2.5 mm CaCl2; 1.2 mm MgSO4; 1.2 mm KH2PO4; 2.5 mm NaHCO3, pH 7.4) containing 2 mm glucose at 37 C for 1 h. After being washed and aliquoted, islets (20/tube) were incubated in 2 mm glucose for 10 min and followed by 10 min incubation in 20 mm glucose. Insulin secretion levels were assessed by ELISA. Insulin output was normalized to the insulin content of the corresponding islets. To measure insulin content, tubes were centrifuged for 10 min at 800 × g, the supernatant was discarded, and acid-ethanol was added [250 μl/sample; 87.5% (vol/vol) ethanol plus 12.5% (vol/vol) 2 n HCl). Samples were kept overnight at 4 C and centrifuged for 10 min at 800 × g, after which the supernatant was harvested and stored at −20 C until measurement.
Western blot analysis
Akt2+/+/SGK3+/−, Akt2+/+/SGK3−/−, Akt2−/−/SGK3+/+, Akt2−/−/SGK3+/−, and Akt2−/−/SGK3−/− male mice (7–9 wk of age) were fasted overnight and killed by cervical dislocation, and protein lysates from muscle, liver and fat, and isolated islets were prepared as previously described (10). Protein extracts were resolved by SDS-PAGE and then transferred onto pore-sized nitrocellulose membranes. The membranes were incubated overnight at 4 C with antibodies against phospho-GSK-3β (Ser21/9) (Cell Signaling Technology, Danvers, MA), GSK-3β (Santa Cruz Biotechnology, Inc., Santa Cruz, CA), phospho-S6 ribosomal protein (S6-RP) (Ser240/244) (Cell Signaling Technology), S6-RP (Cell Signaling Technology). Horseradish peroxidase-conjugated antibodies (GE Healthcare, Piscataway, NJ) were used as secondary antibodies.
Morphometric analysis, immunohistochemistry, and immunofluorescence of the pancreatic islets
Pancreas from 7- to 9-wk-old mice of the various genotypes was isolated, weighed, and fixed. For quantitation of β-cell mass, the entire pancreas was sectioned every 250 μm. All sections were first stained for insulin and then counterstained with hematoxylin and eosin. Images were scanned and analyzed with Metamorph software. The total β-cell mass was then calculated using the following calculation: [(β-cell positive area/total pancreas area) × pancreas weight].
For staining for SGK3 and β-catenin, mouse pancreas that had been sectioned from above and embedded in paraffin was subject to rehydration by soaking in xylene for 5 min for three intervals and then placed into progressively lower concentrations of ETOH (100, 95, 70, and 50%) for 5 min each and finally placed in ddH20 for 5 min. Slides were unmasked by microwaving for 10 min at 80% power in a 1 liter bath of 0.01 m NaCitrate buffer pH 6.0. Subsequently H202 was added to each slide for 30 min to reduce endogenous peroxidase activity. For SGK3 staining, slides were prepared using the Peroxidase Anti-Rabbit IgG Vecta statin ABC Kit (Vector Laboratories, Inc., Burlingame, CA) as per manufacturer's protocol. The primary antibody was diluted 1:1000 of rabbit anti-SGKL/SGK3 (Imgenex Sorrento Valley, CA) in PBS and applied to each sample overnight at 4 C, except the control which had PBS applied overnight. For β-catenin staining, the procedure was identical except the primary antibody, mouse anti-β-catenin (BD Transduction Laboratories, Lexington, KY) was added to the slides at 1:100 dilution for 90 min at room temperature, and biotinylated goat antimouse IgG (Vector Laboratories) was used as secondary antibody. Slides were developed using the 3,3′-Diaminobenzidine Peroxidase Substrate Kit (Vector Laboratories) for 5 min and counterstained with 30 sec of Mayer's Hematoxylin (DAKO Cytomation). Slides were dehydrated and mounted with Mounting Medium (Richard-Allan Scientific, Pasadena, TX), visualized with a MC02 Nikon Eclipse TE300 inverted microscope (Nikon, Inc., Melville, NY) and imaged with a digital Diagnostic Instruments Spot RT color camera.
For preparation and staining for insulin and Ki67, sections were stained as previously described (58). Apoptosis was detected by labeling DNA strand breaks with the TUNEL (TdT-mediated dUTP nick end labeling) kit (Millipore Corp.) according to the manufacturer's protocol, followed by staining for insulin to detect β-cells. Primary antisera were diluted in 1% goat serum/PBS, diluted as follows: guinea pig antiinsulin (Linco Research, St. Charles, MO), 1:4000; rabbit anti-Ki67 (Novocastra Laboratories, Newcastle, UK), 1:500. Secondary antibodies (Jackson ImmunoResearch Laboratories Inc., West Grove, PA) for immunofluorescence were used at the following concentrations: Cy3-conjugated goat antiguinea pig, 1:800, fluorescein isothiocyanate-conjugated goat antirabbit, 1:200. The sections were mounted with Vectashield mounting medium with 4′,6-diamidino-2-phenylindole (Vector Laboratories), visualized with a Zeiss Axioskope II microscope (Carl Zeiss, Thornwood, NY) and imaged with a ORCA100 digital camera (Hamamatsu, Bridgewater, NJ) at a final magnification of ×40. At least 30 islets from three pancreas sections of each animal were mounted and stained for insulin/Ki-67 and insulin/TUNEL. The number of Ki67/insulin and TUNEL/insulin double-positive cells were counted, and the ratio of double-positive to total insulin-positive cells was determined.
Statistical analysis
Data were analyzed using Microsoft excel. Area under the curve was calculated with SigmaPlot software. Statistical analyses were performed using SPSS software. Data were analyzed using unpaired t test or two-way ANOVA and P < 0.05 was taken as statistically significant.
Acknowledgments
We thank Z. Huang (University of California, San Francisco) for technical assistance.
This work was supported by National Institutes of Health (NIH) training grant T32DK007219 (to J.A.M.), NIH grant DK56886 (to M.B.), Deutsche Forschungsgemeinschaft (La 315/4-6) (to F.L.), and NIH grant DK56695 (to D.P.).
Disclosure Summary: All authors have nothing to disclose.
Footnotes
- DKO
- Double knockout
- GIP
- gastric inhibitory peptide
- GLP
- glucagon-like peptide
- GSK
- glycogen synthase kinase
- KO
- knockout
- PI3K
- phosphatidylinositol-3-kinase
- S6-RP
- S6 ribosomal protein
- SGK3
- serum- and glucocorticoid-regulated kinase 3
- TUNEL
- terminal deoxynucleotide transferase-mediated dUTP nick-end labeling.
References
- 1. Kahn CR. 1994. Banting Lecture. Insulin action, diabetogenes, and the cause of type II diabetes. Diabetes 43:1066–1084 [DOI] [PubMed] [Google Scholar]
- 2. Kulkarni RN, Brüning JC, Winnay JN, Postic C, Magnuson MA, Kahn CR. 1999. Tissue-specific knockout of the insulin receptor in pancreatic β cells creates an insulin secretory defect similar to that in type 2 diabetes. Cell 96:329–339 [DOI] [PubMed] [Google Scholar]
- 3. Williams JA, Goldfine ID. 1985. The insulin-pancreatic acinar axis. Diabetes 34:980–986 [DOI] [PubMed] [Google Scholar]
- 4. Bhaskar PT, Hay N. 2007. The two TORCs and Akt. Dev Cell 12:487–502 [DOI] [PubMed] [Google Scholar]
- 5. Parsons R. 2004. Human cancer, PTEN and the PI-3 kinase pathway. Semin Cell Dev Biol 15:171–176 [DOI] [PubMed] [Google Scholar]
- 6. Whiteman EL, Cho H, Birnbaum MJ. 2002. Role of Akt/protein kinase B in metabolism. Trends Endocrinol Metab 13:444–451 [DOI] [PubMed] [Google Scholar]
- 7. Coffer PJ, Jin J, Woodgett JR. 1998. Protein kinase B (c-Akt): a multifunctional mediator of phosphatidylinositol 3-kinase activation. Biochem J 335:1–13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Manning BD, Cantley LC. 2007. AKT/PKB signaling: navigating downstream. Cell 129:1261–1274 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Hay N, Sonenberg N. 2004. Upstream and downstream of mTOR. Genes Dev 18:1926–1945 [DOI] [PubMed] [Google Scholar]
- 10. Cho H, Mu J, Kim JK, Thorvaldsen JL, Chu Q, Crenshaw EB, III, Kaestner KH, Bartolomei MS, Shulman GI, Birnbaum MJ. 2001. Insulin resistance and a diabetes mellitus-like syndrome in mice lacking the protein kinase Akt2 (PKB β). Science 292:1728–1731 [DOI] [PubMed] [Google Scholar]
- 11. Garofalo RS, Orena SJ, Rafidi K, Torchia AJ, Stock JL, Hildebrandt AL, Coskran T, Black SC, Brees DJ, Wicks JR, McNeish JD, Coleman KG. 2003. Severe diabetes, age-dependent loss of adipose tissue, and mild growth deficiency in mice lacking Akt2/PKB β. J Clin Invest 112:197–208 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Chen WS, Peng XD, Wang Y, Xu PZ, Chen ML, Luo Y, Jeon SM, Coleman K, Haschek WM, Bass J, Philipson LH, Hay N. 2009. Leptin deficiency and β-cell dysfunction underlie type 2 diabetes in compound Akt knockout mice. Mol Cell Biol 29:3151–3162 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Cho H, Thorvaldsen JL, Chu Q, Feng F, Birnbaum MJ. 2001. Akt1/PKBα is required for normal growth but dispensable for maintenance of glucose homeostasis in mice. J Biol Chem 276:38349–38352 [DOI] [PubMed] [Google Scholar]
- 14. Yang ZZ, Tschopp O, Hemmings-Mieszczak M, Feng J, Brodbeck D, Perentes E, Hemmings BA. 2003. Protein kinase B α/Akt1 regulates placental development and fetal growth. J Biol Chem 278:32124–32131 [DOI] [PubMed] [Google Scholar]
- 15. Chen WS, Xu PZ, Gottlob K, Chen ML, Sokol K, Shiyanova T, Roninson I, Weng W, Suzuki R, Tobe K, Kadowaki T, Hay N. 2001. Growth retardation and increased apoptosis in mice with homozygous disruption of the Akt1 gene. Genes Dev 15:2203–2208 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. O'Shaughnessy RF, Akgũl B, Storey A, Pfister H, Harwood CA, Byrne C. 2007. Cutaneous human papillomaviruses down-regulate AKT1, whereas AKT2 up-regulation and activation associates with tumors. Cancer Res 67:8207–8215 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Altomare DA, Lyons GE, Mitsuuchi Y, Cheng JQ, Testa JR. 1998. Akt2 mRNA is highly expressed in embryonic brown fat and the AKT2 kinase is activated by insulin. Oncogene 16:2407–2411 [DOI] [PubMed] [Google Scholar]
- 18. Dummler B, Tschopp O, Hynx D, Yang ZZ, Dirnhofer S, Hemmings BA. 2006. Life with a single isoform of Akt: mice lacking Akt2 and Akt3 are viable but display impaired glucose homeostasis and growth deficiencies. Mol Cell Biol 26:8042–8051 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Easton RM, Cho H, Roovers K, Shineman DW, Mizrahi M, Forman MS, Lee VM, Szabolcs M, de Jong R, Oltersdorf T, Ludwig T, Efstratiadis A, Birnbaum MJ. 2005. Role for Akt3/protein kinase Bγ in attainment of normal brain size. Mol Cell Biol 25:1869–1878 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Tschopp O, Yang ZZ, Brodbeck D, Dummler BA, Hemmings-Mieszczak M, Watanabe T, Michaelis T, Frahm J, Hemmings BA. 2005. Essential role of protein kinase B γ (PKBγ/Akt3) in postnatal brain development but not in glucose homeostasis. Development 132:2943–2954 [DOI] [PubMed] [Google Scholar]
- 21. Lang F, Cohen P. 2001. Regulation and physiological roles of serum- and glucocorticoid-induced protein kinase isoforms. Sci STKE 2001:re17. [DOI] [PubMed] [Google Scholar]
- 22. Kobayashi T, Deak M, Morrice N, Cohen P. 1999. Characterization of the structure and regulation of two novel isoforms of serum- and glucocorticoid-induced protein kinase. Biochem J 344:189–197 [PMC free article] [PubMed] [Google Scholar]
- 23. Dai F, Yu L, He H, Zhao Y, Yang J, Zhang X, Zhao S. 1999. Cloning and mapping of a novel human serum/glucocorticoid regulated kinase-like gene, SGKL, to chromosome 8q12.3-q13.1. Genomics 62:95–97 [DOI] [PubMed] [Google Scholar]
- 24. Brunet A, Bonni A, Zigmond MJ, Lin MZ, Juo P, Hu LS, Anderson MJ, Arden KC, Blenis J, Greenberg ME. 1999. Akt promotes cell survival by phosphorylating and inhibiting a Forkhead transcription factor. Cell 96:857–868 [DOI] [PubMed] [Google Scholar]
- 25. Brunet A, Park J, Tran H, Hu LS, Hemmings BA, Greenberg ME. 2001. Protein kinase SGK mediates survival signals by phosphorylating the forkhead transcription factor FKHRL1 (FOXO3a). Mol Cell Biol 21:952–965 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Dai F, Yu L, He H, Chen Y, Yu J, Yang Y, Xu Y, Ling W, Zhao S. 2002. Human serum and glucocorticoid-inducible kinase-like kinase (SGKL) phosphorylates glycogen syntheses kinase 3 β (GSK-3β) at serine-9 through direct interaction. Biochem Biophys Res Commun 293:1191–1196 [DOI] [PubMed] [Google Scholar]
- 27. Liu D, Yang X, Songyang Z. 2000. Identification of CISK, a new member of the SGK kinase family that promotes IL-3-dependent survival. Curr Biol 10:1233–1236 [DOI] [PubMed] [Google Scholar]
- 28. Zhou QL, Park JG, Jiang ZY, Holik JJ, Mitra P, Semiz S, Guilherme A, Powelka AM, Tang X, Virbasius J, Czech MP. 2004. Analysis of insulin signalling by RNAi-based gene silencing. Biochem Soc Trans 32:817–821 [DOI] [PubMed] [Google Scholar]
- 29. Mauro TM, McCormick JA, Wang J, Boini KM, Ray L, Monks B, Birnbaum MJ, Lang F, Pearce D. 2009. Akt2 and SGK3 are both determinants of postnatal hair follicle development. FASEB J 23:3193–3202 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. McCormick JA, Feng Y, Dawson K, Behne MJ, Yu B, Wang J, Wyatt AW, Henke G, Grahammer F, Mauro TM, Lang F, Pearce D. 2004. Targeted disruption of the protein kinase SGK3/CISK impairs postnatal hair follicle development. Mol Biol Cell 15:4278–4288 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Lang F, Böhmer C, Palmada M, Seebohm G, Strutz-Seebohm N, Vallon V. 2006. (Patho)physiological significance of the serum- and glucocorticoid-inducible kinase isoforms. Physiol Rev 86:1151–1178 [DOI] [PubMed] [Google Scholar]
- 32. Ullrich S, Berchtold S, Ranta F, Seebohm G, Henke G, Lupescu A, Mack AF, Chao CM, Su J, Nitschke R, Alexander D, Friedrich B, Wulff P, Kuhl D, Lang F. 2005. Serum- and glucocorticoid-inducible kinase 1 (SGK1) mediates glucocorticoid-induced inhibition of insulin secretion. Diabetes 54:1090–1099 [DOI] [PubMed] [Google Scholar]
- 33. Grahammer F, Henke G, Sandu C, Rexhepaj R, Hussain A, Friedrich B, Risler T, Metzger M, Just L, Skutella T, Wulff P, Kuhl D, Lang F. 2006. Intestinal function of gene-targeted mice lacking serum- and glucocorticoid-inducible kinase 1. Am J Physiol Gastrointest Liver Physiol 290:G1114–G1123 [DOI] [PubMed] [Google Scholar]
- 34. Huang DY, Wulff P, Völkl H, Loffing J, Richter K, Kuhl D, Lang F, Vallon V. 2004. Impaired regulation of renal K+ elimination in the sgk1-knockout mouse. J Am Soc Nephrol 15:885–891 [DOI] [PubMed] [Google Scholar]
- 35. Ackah E, Yu J, Zoellner S, Iwakiri Y, Skurk C, Shibata R, Ouchi N, Easton RM, Galasso G, Birnbaum MJ, Walsh K, Sessa WC. 2005. Akt1/protein kinase Bα is critical for ischemic and VEGF-mediated angiogenesis. J Clin Invest 115:2119–2127 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Peng XD, Xu PZ, Chen ML, Hahn-Windgassen A, Skeen J, Jacobs J, Sundararajan D, Chen WS, Crawford SE, Coleman KG, Hay N. 2003. Dwarfism, impaired skin development, skeletal muscle atrophy, delayed bone development, and impeded adipogenesis in mice lacking Akt1 and Akt2. Genes Dev 17:1352–1365 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Yang ZZ, Tschopp O, Di-Poö N, Bruder E, Baudry A, Dümmler B, Wahli W, Hemmings BA. 2005. Dosage-dependent effects of Akt1/protein kinase Bα (PKBα) and Akt3/PKBγ on thymus, skin, and cardiovascular and nervous system development in mice. Mol Cell Biol 25:10407–10418 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Grahammer F, Artunc F, Sandulache D, Rexhepaj R, Friedrich B, Risler T, McCormick JA, Dawson K, Wang J, Pearce D, Wulff P, Kuhl D, Lang F. 2006. Renal function of gene-targeted mice lacking both SGK1 and SGK3. Am J Physiol Regul Integr Comp Physiol 290:R945–R950 [DOI] [PubMed] [Google Scholar]
- 39. Dieter M, Palmada M, Rajamanickam J, Aydin A, Busjahn A, Boehmer C, Luft FC, Lang F. 2004. Regulation of glucose transporter SGLT1 by ubiquitin ligase Nedd4-2 and kinases SGK1, SGK3, and PKB. Obes Res 12:862–870 [DOI] [PubMed] [Google Scholar]
- 40. Sandu C, Rexhepaj R, Grahammer F, McCormick JA, Henke G, Palmada M, Nammi S, Lang U, Metzger M, Just L, Skutella T, Dawson K, Wang J, Pearce D, Lang F. 2005. Decreased intestinal glucose transport in the sgk3-knockout mouse. Pflugers Arch 451:437–444 [DOI] [PubMed] [Google Scholar]
- 41. Summers SA, Kao AW, Kohn AD, Backus GS, Roth RA, Pessin JE, Birnbaum MJ. 1999. The role of glycogen synthase kinase 3β in insulin-stimulated glucose metabolism. J Biol Chem 274:17934–17940 [DOI] [PubMed] [Google Scholar]
- 42. Khamzina L, Veilleux A, Bergeron S, Marette A. 2005. Increased activation of the mammalian target of rapamycin pathway in liver and skeletal muscle of obese rats: possible involvement in obesity-linked insulin resistance. Endocrinology 146:1473–1481 [DOI] [PubMed] [Google Scholar]
- 43. Pende M, Kozma SC, Jaquet M, Oorschot V, Burcelin R, Le Marchand-Brustel Y, Klumperman J, Thorens B, Thomas G. 2000. Hypoinsulinaemia, glucose intolerance and diminished β-cell size in S6K1-deficient mice. Nature 408:994–997 [DOI] [PubMed] [Google Scholar]
- 44. Tanabe K, Liu Z, Patel S, Doble BW, Li L, Cras-Méneur C, Martinez SC, Welling CM, White MF, Bernal-Mizrachi E, Woodgett JR, Permutt MA. 2008. Genetic deficiency of glycogen synthase kinase-3β corrects diabetes in mouse models of insulin resistance. PLoS Biol 6:e37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Gleason CE, Lu D, Witters LA, Newgard CB, Birnbaum MJ. 2007. The role of AMPK and mTOR in nutrient sensing in pancreatic β-cells. J Biol Chem 282:10341–10351 [DOI] [PubMed] [Google Scholar]
- 46. Rulifson IC, Karnik SK, Heiser PW, ten Berge D, Chen H, Gu X, Taketo MM, Nusse R, Hebrok M, Kim SK. 2007. Wnt signaling regulates pancreatic β cell proliferation. Proc Natl Acad Sci USA 104:6247–6252 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Dabernat S, Secrest P, Peuchant E, Moreau-Gaudry F, Dubus P, Sarvetnick N. 2009. Lack of β-catenin in early life induces abnormal glucose homeostasis in mice. Diabetologia 52:1608–1617 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Muller D, Huang GC, Amiel S, Jones PM, Persaud SJ. 2007. Gene expression heterogeneity in human islet endocrine cells in vitro: the insulin signalling cascade. Diabetologia 50:1239–1242 [DOI] [PubMed] [Google Scholar]
- 49. Gunton JE, Kulkarni RN, Yim S, Okada T, Hawthorne WJ, Tseng YH, Roberson RS, Ricordi C, O'Connell PJ, Gonzalez FJ, Kahn CR. 2005. Loss of ARNT/HIF1β mediates altered gene expression and pancreatic-islet dysfunction in human type 2 diabetes. Cell 122:337–349 [DOI] [PubMed] [Google Scholar]
- 50. Bernal-Mizrachi E, Fatrai S, Johnson JD, Ohsugi M, Otani K, Han Z, Polonsky KS, Permutt MA. 2004. Defective insulin secretion and increased susceptibility to experimental diabetes are induced by reduced Akt activity in pancreatic islet β cells. J Clin Invest 114:928–936 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Donath MY, Ehses JA, Maedler K, Schumann DM, Ellingsgaard H, Eppler E, Reinecke M. 2005. Mechanisms of β-cell death in type 2 diabetes. Diabetes 54(Suppl 2):S108–S113 [DOI] [PubMed] [Google Scholar]
- 52. Otani K, Kulkarni RN, Baldwin AC, Krutzfeldt J, Ueki K, Stoffel M, Kahn CR, Polonsky KS. 2004. Reduced β-cell mass and altered glucose sensing impair insulin-secretory function in βIRKO mice. Am J Physiol Endocrinol Metab 286:E41–E49 [DOI] [PubMed] [Google Scholar]
- 53. Gautier JF, Choukem SP, Girard J. 2008. Physiology of incretins (GIP and GLP-1) and abnormalities in type 2 diabetes. Diabetes Metab 34(Suppl 2):S65–S72 [DOI] [PubMed] [Google Scholar]
- 54. Zhang J, Gao Z, Yin J, Quon MJ, Ye J. 2008. S6K directly phosphorylates IRS-1 on Ser-270 to promote insulin resistance in response to TNF-(alpha) signaling through IKK2. J Biol Chem 283:35375–35382 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Um SH, D'Alessio D, Thomas G. 2006. Nutrient overload, insulin resistance, and ribosomal protein S6 kinase 1, S6K1. Cell Metab 3:393–402 [DOI] [PubMed] [Google Scholar]
- 56. Um SH, Frigerio F, Watanabe M, Picard F, Joaquin M, Sticker M, Fumagalli S, Allegrini PR, Kozma SC, Auwerx J, Thomas G. 2004. Absence of S6K1 protects against age- and diet-induced obesity while enhancing insulin sensitivity. Nature 431:200–205 [DOI] [PubMed] [Google Scholar]
- 57. Lenschow DJ, Zeng Y, Hathcock KS, Zuckerman LA, Freeman G, Thistlethwaite JR, Gray GS, Hodes RJ, Bluestone JA. 1995. Inhibition of transplant rejection following treatment with anti-B7–2 and anti-B7–1 antibodies. Transplantation 60:1171–1178 [DOI] [PubMed] [Google Scholar]
- 58. Wilson ME, Yang KY, Kalousova A, Lau J, Kosaka Y, Lynn FC, Wang J, Mrejen C, Episkopou V, Clevers HC, German MS. 2005. The HMG box transcription factor Sox4 contributes to the development of the endocrine pancreas. Diabetes 54:3402–3409 [DOI] [PubMed] [Google Scholar]