Abstract
Background:
Central sleep apnea (CSA) is frequent among patients with heart failure (HF) and associated with increased morbidity and mortality. Elevated cardiac filling pressures promote CSA and atrial natriuretic peptide (ANP) and brain natriuretic peptide (BNP) secretion. We hypothesized that circulating natriuretic peptide concentrations predict CSA.
Methods:
Consecutive patients with HF (n = 44) with left ventricular ejection fraction (LVEF) ≤ 35% underwent polysomnography for detection of CSA. CSA was defined as an apnea-hypopnea index ≥ 15 with ≥ 50% central apneic events. The relation of natriuretic peptide concentrations to CSA was evaluated by estimation of ORs and receiver operator characteristics (ROCs).
Results:
Twenty-seven subjects (61%) had CSA, with men more frequently affected than women (73% vs 27%; OR, 7.1; P = .01); given that only three women had CSA, further analysis was restricted to men. Subjects with CSA had higher mean ANP (4,336 pg/mL vs 2,510 pg/mL, P = .03) and BNP concentrations (746 pg/mL vs 379 pg/mL, P = .05). ANP and BNP concentrations were significantly related to CSA (OR, 3.7 per 3,000 pg/mL, P = .03 and OR, 1.5 per 200 pg/mL, P = .04, respectively), whereas age, LVEF, and New York Heart Association functional class were not. Concentrations of ANP and BNP were predictive of CSA as ROC demonstrated areas under the curve of 0.75 and 0.73, respectively.
Conclusions:
Risk of CSA is related to severity of HF. ANP and BNP concentrations performed similarly for detection of CSA; low concentrations appear associated with low risk for CSA in men.
Central sleep apnea (CSA) is frequent in patients with heart failure (HF), is promoted by elevated cardiac filling pressures,1,2 and has been associated with disease progression and increased mortality risk.3,4 In contrast to the general population, CSA is more prevalent than obstructive sleep apnea (OSA) in patients with HF.5 Recent technical advances in nocturnal assisted ventilation have enhanced the management of CSA.6‐10 Indeed, effective treatment of CSA improves sleep architecture, heart failure symptoms, cardiac function, and exercise capacity.11,12
Although evaluation for symptoms of sleep-disordered breathing has been advocated for all patients with HF, and formal sleep study has been suggested for patients who remain symptomatic despite optimal HF therapy,13 the diagnosis of CSA requires polysomnography (PSG), which is expensive, not widely available, and not recommended for all patients with HF.14,15 Moreover, clinical history, symptoms, and physical signs are of limited usefulness in the identification of patients for referral for PSG,16 and no methods have been endorsed for routine application in patients with HF as screening tools for CSA.17,18
Circulating brain natriuretic peptide (BNP) and atrial natriuretic peptide (ANP) are secreted in response to increased cardiac volume and pressure. BNP measurement has been advocated for the diagnosis of HF,19‐21 is related to disease severity and prognosis,22 and may be useful for guiding therapy.23 Whereas ANP measurement initially appeared promising for HF detection, it is not a widely used biomarker.24 Whereas BNP is primarily secreted from the ventricles, ANP is secreted predominantly from the atria, and therefore potentially closely related to pulmonary congestion and lung J-receptor stretch, which elicit the characteristic hyperventilation and periodic breathing of CSA.1,25 In addition, hypoxia downregulates the expression of the ANP clearance receptor leading to higher ANP concentration in animal models.26 Therefore, we hypothesized that natriuretic peptide elevations are predictive of the presence of CSA, and that ANP is superior to BNP for detection of CSA. Accordingly, the specific aims of this study were to evaluate the receiver-operator characteristics (ROCs) of ANP and BNP concentrations for detection of CSA.
Materials and Methods
This study was approved by the Mayo Clinic Institutional Review Board (IRB# 923-02). Consecutive ambulatory outpatients were prospectively enrolled from the Mayo Heart Failure Clinic. Consideration for enrollment required that patients have stable HF with no change of New York Heart Association (NYHA) class or medical therapy in the preceding 3 months and left ventricular ejection fraction (LVEF) ≤ 35% as measured by echocardiography. BMI was computed as weight in kilograms divided by body surface area in square meters. Estimated glomerular filtration rate (eGFR) was calculated by the Cockroft-Gault formula.27 All subjects underwent laboratory-based, overnight, attended PSG for the detection of OSA or CSA. Subjects found to have OSA or mixed apneas in which ≥ 50% of disordered breathing events were obstructive were excluded from analysis (n = 11).
Measurement of Natriuretic Peptides
ANP and BNP concentrations were measured from serum drawn on the same day as PSG. Measurement of ANP was performed by radioimmunoassay for the N-terminal ANP (Phoenix Pharmaceuticals; Burlingame, California). Measurement of BNP was evaluated by either the Shionogi immunoradiometric assay (Shionogi & Co, Ltd; Osaka, Japan) or DxI 800 immunoassay (Beckman Instruments; Chaska, Minnesota). The coefficient of variation of these latter two BNP assays was > 0.99.
Sleep Evaluation
Diagnostic PSG was performed in the Center for Translational Science Activity Sleep Core Facility of the Clinical Research Unit and digitally recorded on Network Concepts Incorporated Dimensions (Middleton, Wisconsin) or PSG Online2 E-Series (Compumedics; Abbotsford, Victoria, Australia) and scored using Uniquant or Profusion2 software. Recorded parameters included three-channel EEGs, two-channel electrooculograms, oronasal airflow by pressure transducer and thermocouple sensors, submental and limb electromyograms, one-channel ECG, transcutaneous pulse oximetry (Ohmeda 3740; Madison, Wisconsin, and Compumedics integrated pulse oximetry), thoracic and abdominal respiratory effort by inductance plethysmography, snoring by tracheal microphone or piezo crystal sensor, and body position by closed-circuit video monitoring. Disordered breathing events were classified as apneas or hypopneas and as either obstructive or central. Apneas were defined as a cessation of airflow or > 90% reduction in airflow from baseline for > 10 s with an oxygen desaturation of ≥ 4%. Hypopneas were defined as a reduction in airflow of ≥ 50% with an oxygen desaturation of ≥ 4%. Events were classified as central when the airflow criteria were met in the absence of respiratory effort as recorded by thoracic and abdominal inductance plethysmography and as obstructive when airflow criteria were met despite continued or increased respiratory effort. As per published guidelines, patients were considered to have CSA if the total apnea-hypopnea index (AHI) (events/h) was ≥ 15 with ≥ 50% disordered breathing events of central origin, regardless of the presence or absence of respiratory periodism.28
Statistical Analysis
Group means were tested for differences by two-sided t tests or Wilcoxon rank sum tests depending on distribution. Differences in proportions were tested by Fisher exact test and continuous variables were compared by linear least squares regression. The primary analysis was logistic regression comparing clinical variables to ANP and BNP concentrations for association with CSA, with results expressed as the OR with 95% CI. Variables that predicted CSA were evaluated by ROC analysis with results presented as area under the curve (AUC) and 95% CI derived by the Mann-Whitney U statistic. Sensitivity, specificity, positive and negative predictive values, and positive and negative likelihood ratios were calculated for several cutoff values of ANP and BNP. Accuracy was defined as the ratio of the sum of true positives plus true negatives to the total number of subjects. Analyses were performed with JMP, version 7 (SAS Institute; Cary, North Carolina). For all comparisons, a two-tailed P value < .05 was considered significant.
Results
Of the 44 consecutive subjects with HF studied by PSG who did not have OSA, 27 (61%) met criteria for CSA. Men were significantly more likely to have CSA than women (24 of 33 vs three of 11; OR, 7.1; 95% CI, 1.5-32.9; P < .01). Given that only three women were found to have CSA, and because CSA may be a disorder that affects primarily men,16,29 further analyses were restricted to the 33 men. Patients with HF with CSA had lower LVEF, whereas age, BMI, NYHA class, and eGFR were similar (Table 1). Subjects with CSA had significantly higher mean AHI and greater time with arterial oxygen saturation < 90% (T90%), although mean oxygen saturation was similar (Table 2). No statistical differences in the proportion of patients with ischemic etiology, atrial fibrillation, or renal dysfunction as measured by eGFR were observed (Table 1).
Table 1.
—Subject Characteristics
| Characteristic | HF Without CSA | HF With CSA | P Value |
| No. | 9 | 24 | … |
| Age, y | 61.1 ± 12.4 | 66.7 ± 9.9 | .20 |
| BMI, kg/m2 | 28.2 ± 4.0 | 27.7 ± 4.6 | .89 |
| Ischemic etiology | 5 | 15 | .72 |
| Atrial fibrillation | 0 | 5 | .29 |
| NYHA I-II, No. | 5 | 10 | .70 |
| NYHA III-IV, No. | 4 | 14 | |
| LVEF, % | 26.7 ± 5.5 | 21.8 ± 8.0 | .04 |
| eGFR, mL/min/1.73 m2 | 76.1 ± 25.5 | 66.8 ± 20.4 | .35 |
| β-Blocker, % | 78 | 88 | .52 |
| ACE/ARB, % | 100 | 96 | .59 |
| Aldosterone antagonist, % | 33 | 25 | .66 |
| Diuretics, % | 78 | 79 | .95 |
| Digitalis, % | 77 | 75 | .89 |
| Nitrates, % | 22 | 16 | .74 |
Values are from the 33 male subjects only and presented as mean ± SD unless otherwise noted. ACE/ARB = angiotensin converting enzyme inhibitor or angiotensin II receptor blocker; CSA = central sleep apnea; eGFR = estimated glomerular filtration rate; HF = heart failure; LVEF = left ventricular ejection fraction; NYHA = New York Heart Association.
Table 2.
—Polysomnographic Findings
| Characteristic | HF Without CSA | HF With CSA | P Value |
| AHI, events/h | 4.5 ± 4.4 | 44.6 ± 18.8 | < .001 |
| Sleep Sao2, % | 93.0 ± 5.1 | 93.0 ± 3.0 | .46 |
| Awake Sao2, % | 95.7 ± 2.0 | 94.8 ± 3.2 | .62 |
| T90% | 1.3 ± 2.2 | 18.2 ± 20.9 | .006 |
Values are presented as mean ± SD. AHI = apnea-hypopnea index; Sao2 = arterial oxygen saturation by transcutaneous oxygen sensor while awake or during the entire sleep period; T90% = time with arterial oxygen saturation < 90%. See Table 1 legend for expansion of other abbreviations.
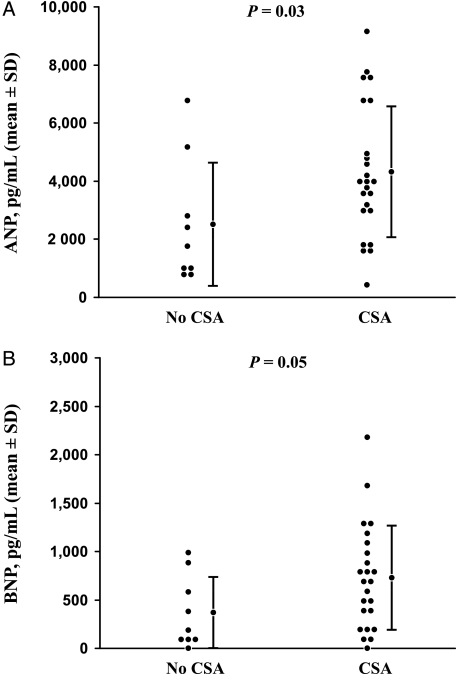
There was significant covariation between ANP and BNP (r = 0.71, P < .01). ANP and BNP concentrations were higher among subjects with NYHA class III or IV HF compared with NYHA class I-II HF (4,784 pg/mL vs 2,703 pg/mL, P < .01 and 945 pg/mL vs 586 pg/mL, P < .01, respectively) and inversely correlated to LVEF (r = −0.56, P < .01 and r = −0.64, P < 0.01, respectively). Concentrations of ANP and BNP were higher among subjects with CSA compared with subjects without CSA (4,336 pg/mL vs 2,510 pg/mL, P = .03 and 746 pg/mL vs 379 pg/mL, P = .05, respectively) (Fig 1). However, the AHI was not related to either ANP (r = 0.11, P = .55) or BNP (r = 0.04, P = .84) by linear regression. Similarly, T90% was not related to either ANP (r = 0.19, P = .29) or BNP concentration (r = 0.26, P = .14).
Figure 1.
A, Men with heart failure (HF) and CSA had significantly higher mean ANP concentrations (4,336 pg/mL vs 2,510 pg/mL, P = .03) compared with patients with HF without CSA, although considerable overlap between patient groups is present. B, Men with HF and CSA had significantly higher mean BNP concentrations (746 pg/mL vs 379 pg/mL, P = .05) compared with patients with HF without CSA, although considerable overlap between patient groups is present. ANP = atrial natriuretic peptide; BNP = brain natriuretic peptide; CSA = central sleep apnea.
Among men, age, LVEF, and NYHA class were not related to CSA. However, both ANP and BNP concentration were significantly related to the presence of CSA (OR, 3.74 per 3,000 pg/mL; P = .03 and OR, 1.48 per 200 pg/mL; P = .04, respectively, Table 3). The optimal cutoff for ANP concentration was 3,024 pg/mL, with an OR of 10.50 (95% CI, 1.70-65.00), an AUC of 0.75 (95% CI, 0.52-0.97), sensitivity of 79%, and specificity of 78%. The optimal cutoff for BNP concentration was 210 pg/mL, with an OR of 6.25 (95% CI, 1.14-34.12), an AUC of 0.73 (95% CI, 0.52-0.94), sensitivity of 83%, and specificity of 56% (Table 4).
Table 3.
—Predictors of CSA
| Parameter | OR | 95% CI | P Value |
| Age, per y | 0.95 | 0.88-1.02 | .18 |
| LVEF, per % | 1.09 | 0.98-1.23 | .10 |
| NYHA class, I-II vs III-IV | 0.57 | 0.11-2.69 | .48 |
| ANP, per 3,000 pg/mL | 3.74 | 1.12-18.76 | .03 |
| BNP, per 200 pg/mL | 1.48 | 1.03-2.46 | .04 |
ANP = atrial natriuretic peptide; BNP = brain natriuretic peptide. See Table 1 legend for expansion of other abbreviations.
Table 4.
—Natriuretic Peptide Cutoff Values for Detection of CSA
| Peptide Cutoff | Sensitivity | Specificity | PPV | NPV | Accuracy | LR+ | LR− |
| ANP cutoff, pg/mL | |||||||
| 1,638 | 92 | 44 | 81 | 67 | 79 | 1.64 | 0.18 |
| 3,024 | 79 | 78 | 90 | 58 | 79 | 3.59 | 0.27 |
| 6,709 | 25 | 89 | 86 | 31 | 42 | 2.27 | 0.84 |
| BNP cutoff, pg/mL | |||||||
| 142 | 88 | 44 | 81 | 57 | 76 | 1.57 | 0.27 |
| 210 | 83 | 56 | 83 | 56 | 76 | 1.88 | 0.30 |
| 1,016 | 29 | 89 | 88 | 32 | 45 | 2.64 | 0.80 |
Discussion
The novel observations of this study are that ANP and BNP concentrations are increased among patients with HF with CSA compared with those without CSA and that ANP and BNP concentrations perform similarly for the detection of CSA. In patients with CSA, the magnitude of the AHI appears related to the severity of left atrial hypertension.2 Because circulating ANP and BNP concentrations are related to the hemodynamic severity of HF, it follows that these biomarkers may be related to CSA, which our data support. However, ROC analysis did not support the hypothesis that ANP is superior to BNP concentration for the detection of CSA. Whether this reflects the disassociation of ANP secretion from the atria and BNP secretion from the ventricles as can be seen in HF,30 secretion of both ANP31‐37 and BNP36‐41 in response to hypoxia, biologic variability of ANP and BNP concentration, or the pathophysiology underlying ventilatory control instability in CSA42 is unknown.
In contrast to a prior report showing a modest correlation between BNP and the AHI (r = 0.50, P < .01),43 we did not see a significant correlation of either ANP or BNP to the AHI. The reasons for these results are unclear and may be related to different methodologies used to obtain PSG data (attended vs unattended PSG), differences in scoring classification, the apparent weak association between pulmonary capillary wedge pressure and CSA,1,2 variation in the correlation between elevated cardiac filling pressures and natriuretic peptide concentrations, a nonlinear relationship between markers of elevated cardiac filling pressures and AHI, or lack of statistical power.
Two prior reports described measurement of circulating BNP concentration for detection of CSA in patients with HF. The first study reported that BNP was higher among patients with CSA and that a BNP cutoff of 116 pg/mL yielded sensitivity of 62% with specificity of 92% for detection of CSA.43 However, in this study the mean age was 55 years, 77% of patients had NYHA class I-II HF, the frequency of CSA was low, a nonstandard definition of CSA was used, and only 35% of patients were treated with β-blockers, raising concerns regarding the generalizability of the findings. A more recent report44 described similar observations, although this latter study relied on unattended polygraphy for diagnosis and also used a nonstandard definition of CSA. In contrast, our study used a subject cohort receiving contemporary medical therapy studied by full, in-laboratory, attended PSG with CSA defined by consensus guidelines.
In the absence of large screening trials, the optimal methods for identification of patients appropriate for referral for PSG remains poorly defined. Unlike OSA, in which certain features may suggest the disease, no signs or symptoms reliably suggest the presence of CSA.16,18 Published guidelines endorse selected use of natriuretic peptides for the evaluation of patients with suspected or established HF.15,45 Based on our observations, natriuretic peptides do not appear to have optimal characteristics as a screening tool for the presence (detection) of CSA. However, low concentration of either ANP or BNP appears to identify a patient population at low risk for CSA. The interpretation of natriuretic peptide concentrations with respect to the presence of CSA will depend on the prevalence of CSA in the population or the clinician’s estimate of the pretest probability for CSA in a specific patient. Based on recently published data from men with HF on optimal contemporary pharmacotherapy showing a prevalence of CSA of 24%,29 an ANP concentration > 3,024 pg/mL or a BNP concentration > 210 pg/mL would imply a posttest likelihood of CSA of 53% and 39%, respectively. Whether this would justify the widespread application of PSG as has been suggested when the likelihood is > 50%18 is not yet established. Conversely, an ANP concentration < 3,024 pg/mL or a BNP concentration < 210 pg/mL would suggest a posttest probability of CSA of just 8% and 6%. Accordingly, our data support the concept that the main usefulness of natriuretic peptide testing may be the exclusion of CSA as has been previously suggested.43,44 Larger studies are needed to confirm these findings in a broader population of patients with HF, including larger numbers of women.
Limitations
Our sample size was modest and included only patients with systolic HF with LVEF ≤ 35%; the sample size limits the precision of our point estimates. Whether natriuretic peptides or other factors are predictive of CSA among patients with HF with preserved LVEF has not been reported, although BNP concentrations appear higher in patients with HF with preserved LVEF who have CSA and correlates with the magnitude of cardiac pressure elevation.46 We observed a high frequency of CSA in our study cohort, perhaps because the majority of the subjects had advanced HF. Hence, these findings should not be generalized to patients with less severe HF.
Although prior studies evaluating BNP concentrations from patients with CSA did not present sex-specific results, we chose to restrict our analyses to men because we observed so few women with CSA. Furthermore, the prevalence of CSA in women appears much lower than men,16,29 risk factors for CSA may differ between men and women,16 and sex may affect BNP concentration.47 Although the exclusion of women limited our ability to generalize our results, it also decreased the chance that our findings were confounded by sex and increases the precision with which the results can be applied to men.
We also excluded subjects with HF with OSA or mixed apnea with a significant obstructive component. Although this may have avoided the confounding effects of intrathoracic pressure changes on natriuretic peptide concentrations48‐50 and facilitated comparison with prior studies, the results are less applicable to the general HF population, in which detection of both CSA and OSA may be useful.
The diagnosis of CSA depends on the interpretation of PSG findings; abnormal sleep breathing events may be open to some variation of interpretation. It is possible that subjects with a significant obstructive burden (up to 50% of apneas) were included, raising the possibility that intrathoracic pressure changes contributed to natriuretic peptide elevation as has been previously reported for individuals with OSA.50 Finally, differences in PSG methodology may result in different point estimates that meaningfully affect the potential usefulness of natriuretic peptide testing.
In summary, these data confirm that CSA is related to the hemodynamic severity of HF and that it appears to be much more common in men than women with HF on contemporary pharmacotherapy; the data also demonstrate that the natriuretic peptides ANP and BNP are significantly higher among men with HF and CSA. Because so few women had CSA, it is not possible to comment on the relation of ANP or BNP concentrations to CSA in women with HF. The potential usefulness of ANP and BNP measurement appears to be for identification of patients with low concentrations of natriuretic peptides who are at low risk for CSA.
Acknowledgments
Author contributions: Dr Olson had full access to the data and will vouch for the integrity of the data analysis.
Dr Calvin: contributed to analysis and interpretation of the data and writing of the manuscript.
Dr Somers: contributed to the interpretation of the data and revision of the manuscript.
Ms van der Walt: contributed as the lead sleep technologist and contributed to acquisition of the data and writing the manuscript.
Mr Scott: contributed to the statistical analysis, reporting of the data, and writing of the manuscript.
Dr Olson: contributed to the planning of the study, interpretation of the data, and writing of the manuscript.
Financial/nonfinancial disclosures: The authors have reported to CHEST the following conflicts of interest: Dr Somers has served as a Consultant for Respironics, ResMed, Cardiac Concepts, GlaxoSmithKline, Sunovion Pharmaceuticals, and Medtronic Corporation and has been a principal investigator or coinvestigator on research grants funded by the Respironics Foundation, the ResMed Foundation, and the Sorin Corporation. Dr Olson has received research grants from the Medtronic Corporation and the Sorin Corporation. Dr Calvin, Ms van der Walt, and Mr Scott have reported to CHEST that no potential conflicts of interest exist with any companies/organizations whose products or services may be discussed in this article.
Role of sponsors: The contents of this work are solely the responsibility of the authors and do not necessarily represent the official view of National Center for Research Resources or National Institutes of Health. The sponsors had no role in the design of the study, the collection and analysis of the data, or in the preparation of the manuscript.
Other contributions: We thank Michelle M. Small for secretarial support. This work was performed at Mayo Clinic, Rochester, Minnesota.
Abbreviations
- ANP
atrial natriuretic peptide
- AUC
area under the curve
- BNP
brain natriuretic peptide
- CSA
central sleep apnea
- eGFR
estimated glomerular filtration rate
- HF
heart failure
- LVEF
left ventricular ejection fraction
- NYHA
New York Heart Association
- OSA
obstructive sleep apnea
- PSG
polysomnography
- ROC
receiver-operator characteristic
- T90%
time with arterial oxygen saturation < 90%
Footnotes
Funding/Support: This work was supported by the Mayo Clinic Clinician-Investigator Training Program (A. D. C.); Mayo Foundation; American Heart Association [Grant 04-50103Z]; National Heart Lung and Blood Institute [Grants HL65176, HL70302, and HL73211]; and the National Center for Research Resources (NCRR) [Grant 1ULI RR024150], a component of the National Institutes of Health and the National Institutes of Health Roadmap for Medical Research.
Reproduction of this article is prohibited without written permission from the American College of Chest Physicians (http://www.chestpubs.org/site/misc/reprints.xhtml).
References
- 1.Oldenburg O, Bitter T, Wiemer M, Langer C, Horstkotte D, Piper C. Pulmonary capillary wedge pressure and pulmonary arterial pressure in heart failure patients with sleep-disordered breathing. Sleep Med. 2009;10(7):726–730. doi: 10.1016/j.sleep.2008.08.004. [DOI] [PubMed] [Google Scholar]
- 2.Solin P, Bergin P, Richardson M, Kaye DM, Walters EH, Naughton MT. Influence of pulmonary capillary wedge pressure on central apnea in heart failure. Circulation. 1999;99(12):1574–1579. doi: 10.1161/01.cir.99.12.1574. [DOI] [PubMed] [Google Scholar]
- 3.Lanfranchi PA, Braghiroli A, Bosimini E, et al. Prognostic value of nocturnal Cheyne-Stokes respiration in chronic heart failure. Circulation. 1999;99(11):1435–1440. doi: 10.1161/01.cir.99.11.1435. [DOI] [PubMed] [Google Scholar]
- 4.Hanly PJ, Zuberi-Khokhar NS. Increased mortality associated with Cheyne-Stokes respiration in patients with congestive heart failure. Am J Respir Crit Care Med. 1996;153(1):272–276. doi: 10.1164/ajrccm.153.1.8542128. [DOI] [PubMed] [Google Scholar]
- 5.Javaheri S, Parker TJ, Liming JD, et al. Sleep apnea in 81 ambulatory male patients with stable heart failure. Types and their prevalences, consequences, and presentations. Circulation. 1998;97(21):2154–2159. doi: 10.1161/01.cir.97.21.2154. [DOI] [PubMed] [Google Scholar]
- 6.Philippe C, Stoïca-Herman M, Drouot X, et al. Compliance with and effectiveness of adaptive servoventilation versus continuous positive airway pressure in the treatment of Cheyne-Stokes respiration in heart failure over a six month period. Heart. 2006;92(3):337–342. doi: 10.1136/hrt.2005.060038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Szollosi I, O’Driscoll DM, Dayer MJ, Coats AJ, Morrell MJ, Simonds AK. Adaptive servo-ventilation and deadspace: effects on central sleep apnoea. J Sleep Res. 2006;15(2):199–205. doi: 10.1111/j.1365-2869.2006.00515.x. [DOI] [PubMed] [Google Scholar]
- 8.Kasai T, Narui K, Dohi T, et al. First experience of using new adaptive servo-ventilation device for Cheyne-Stokes respiration with central sleep apnea among Japanese patients with congestive heart failure: report of 4 clinical cases. Circ J. 2006;70(9):1148–1154. doi: 10.1253/circj.70.1148. [DOI] [PubMed] [Google Scholar]
- 9.Teschler H, Döhring J, Wang YM, Berthon-Jones M. Adaptive pressure support servo-ventilation: a novel treatment for Cheyne-Stokes respiration in heart failure. Am J Respir Crit Care Med. 2001;164(4):614–619. doi: 10.1164/ajrccm.164.4.9908114. [DOI] [PubMed] [Google Scholar]
- 10.Noda A, Izawa H, Asano H, et al. Beneficial effect of bilevel positive airway pressure on left ventricular function in ambulatory patients with idiopathic dilated cardiomyopathy and central sleep apnea-hypopnea: a preliminary study. Chest. 2007;131(6):1694–1701. doi: 10.1378/chest.06-2271. [DOI] [PubMed] [Google Scholar]
- 11.Naughton MT, Benard DC, Liu PP, Rutherford R, Rankin F, Bradley TD. Effects of nasal CPAP on sympathetic activity in patients with heart failure and central sleep apnea. Am J Respir Crit Care Med. 1995;152(2):473–479. doi: 10.1164/ajrccm.152.2.7633695. [DOI] [PubMed] [Google Scholar]
- 12.Arzt M, Floras JS, Logan AG, et al. Suppression of central sleep apnea by continuous positive airway pressure and transplant-free survival in heart failure - a post hoc analysis of CANPAP. Circulation. 2007;115(25):3140–3142. doi: 10.1161/CIRCULATIONAHA.106.683482. [DOI] [PubMed] [Google Scholar]
- 13.Heart Failure Society of America Executive Summary: HFSA 2010 Comprehensive Heart Failure Practice Guideline. J Card Fail. 2010;16(6):475–539. [Google Scholar]
- 14.Jessup M, Abraham WT, Casey DE, et al. 2009 focused update: ACCF/AHA Guidelines for the Diagnosis and Management of Heart Failure in Adults: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines: developed in collaboration with the International Society for Heart and Lung Transplantation. Circulation. 2009;119(14):1977–2016. doi: 10.1161/CIRCULATIONAHA.109.192064. [DOI] [PubMed] [Google Scholar]
- 15.Dickstein K, Cohen-Solal A, Filippatos G, et al. Task Force for Diagnosis and Treatment of Acute and Chronic Heart Failure 2008 of European Society of Cardiology ESC Committee for Practice Guidelines ESC guidelines for the diagnosis and treatment of acute and chronic heart failure 2008: the Task Force for the diagnosis and treatment of acute and chronic heart failure 2008 of the European Society of Cardiology. Developed in collaboration with the Heart Failure Association of the ESC (HFA) and endorsed by the European Society of Intensive Care Medicine (ESICM) [published corrections appear in Eur Heart J. 2010;12(4):416 and Eur Heart J. 2010;31(5):624] Eur Heart J. 2008;29(19):2388–2442. doi: 10.1093/eurheartj/ehn309. [DOI] [PubMed] [Google Scholar]
- 16.Sin DD, Fitzgerald FS, Parker JD, Newton G, Floras JS, Bradley TD. Risk factors for central and obstructive sleep apnea in 450 men and women with congestive heart failure. Am J Respir Crit Care Med. 1999;160(4):1101–1106. doi: 10.1164/ajrccm.160.4.9903020. [DOI] [PubMed] [Google Scholar]
- 17.Garcia-Touchard A, Somers VK, Olson LJ, Caples SM. Central sleep apnea: implications for congestive heart failure. Chest. 2008;133(6):1495–1504. doi: 10.1378/chest.07-0871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bradley TD, Floras JS. Sleep apnea and heart failure: part II: central sleep apnea. Circulation. 2003;107(13):1822–1826. doi: 10.1161/01.CIR.0000061758.05044.64. [DOI] [PubMed] [Google Scholar]
- 19.McCullough PA, Nowak RM, McCord J, et al. B-type natriuretic peptide and clinical judgment in emergency diagnosis of heart failure: analysis from Breathing Not Properly (BNP) Multinational Study. Circulation. 2002;106(4):416–422. doi: 10.1161/01.cir.0000025242.79963.4c. [DOI] [PubMed] [Google Scholar]
- 20.Lainchbury JG, Campbell E, Frampton CM, Yandle TG, Nicholls MG, Richards AM. Brain natriuretic peptide and n-terminal brain natriuretic peptide in the diagnosis of heart failure in patients with acute shortness of breath. J Am Coll Cardiol. 2003;42(4):728–735. doi: 10.1016/s0735-1097(03)00787-3. [DOI] [PubMed] [Google Scholar]
- 21.Maisel AS, Krishnaswamy P, Nowak RM, et al. Breathing Not Properly Multinational Study Investigators Rapid measurement of B-type natriuretic peptide in the emergency diagnosis of heart failure. N Engl J Med. 2002;347(3):161–167. doi: 10.1056/NEJMoa020233. [DOI] [PubMed] [Google Scholar]
- 22.Doust JA, Pietrzak E, Dobson A, Glasziou P. How well does B-type natriuretic peptide predict death and cardiac events in patients with heart failure: systematic review. BMJ. 2005;330(7492):625–634. doi: 10.1136/bmj.330.7492.625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Porapakkham P, Porapakkham P, Zimmet H, Billah B, Krum H. B-type natriuretic peptide-guided heart failure therapy: a meta-analysis. Arch Intern Med. 2010;170(6):507–514. doi: 10.1001/archinternmed.2010.35. [DOI] [PubMed] [Google Scholar]
- 24.Jarolim P. Serum biomarkers for heart failure. Cardiovasc Pathol. 2006;15(3):144–149. doi: 10.1016/j.carpath.2006.01.001. [DOI] [PubMed] [Google Scholar]
- 25.Lorenzi-Filho G, Azevedo ER, Parker JD, Bradley TD. Relationship of carbon dioxide tension in arterial blood to pulmonary wedge pressure in heart failure. Eur Respir J. 2002;19(1):37–40. doi: 10.1183/09031936.02.00214502. [DOI] [PubMed] [Google Scholar]
- 26.Li H, Oparil S, Meng QC, Elton TS, Chen YF. Selective downregulation of ANP-clearance-receptor gene expression in lung of rats adapted to hypoxia. Am J Physiol. 1995;268(2 pt 1):L328–L335. doi: 10.1152/ajplung.1995.268.2.L328. [DOI] [PubMed] [Google Scholar]
- 27.Cockcroft DW, Gault MH. Prediction of creatinine clearance from serum creatinine. Nephron. 1976;16(1):31–41. doi: 10.1159/000180580. [DOI] [PubMed] [Google Scholar]
- 28.Iber C, Ancoli-Israel S, Chesson A, Quan SF. for the American Academy of Sleep Medicine . The AASM Manual for the Scoring of Sleep and Associated Events: Rules, Terminology, and Technical Specifications. 1st ed. Westchester, Illinois: American Academy of Sleep Medicine; 2007. [Google Scholar]
- 29.Yumino D, Wang H, Floras JS, et al. Prevalence and physiological predictors of sleep apnea in patients with heart failure and systolic dysfunction. J Card Fail. 2009;15(4):279–285. doi: 10.1016/j.cardfail.2008.11.015. [DOI] [PubMed] [Google Scholar]
- 30.Yasue H, Yoshimura M, Sumida H, et al. Localization and mechanism of secretion of B-type natriuretic peptide in comparison with those of A-type natriuretic peptide in normal subjects and patients with heart failure. Circulation. 1994;90(1):195–203. doi: 10.1161/01.cir.90.1.195. [DOI] [PubMed] [Google Scholar]
- 31.Chun Y-S, Hyun J-Y, Kwak Y-G, et al. Hypoxic activation of the atrial natriuretic peptide gene promoter through direct and indirect actions of hypoxia-inducible factor-1. Biochem J. 2003;370(pt 1):149–157. doi: 10.1042/BJ20021087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Chen Y-F, Durand J, Claycomb WC. Hypoxia stimulates atrial natriuretic peptide gene expression in cultured atrial cardiocytes. Hypertension. 1997;29(1 pt 1):75–82. doi: 10.1161/01.hyp.29.1.75. [DOI] [PubMed] [Google Scholar]
- 33.Baertschi AJ, Hausmaninger C, Walsh RS, Mentzer RM, Jr, Wyatt DA, Pence RA. Hypoxia-induced release of atrial natriuretic factor (ANF) from the isolated rat and rabbit heart. Biochem Biophys Res Commun. 1986;140(1):427–433. doi: 10.1016/0006-291x(86)91108-3. [DOI] [PubMed] [Google Scholar]
- 34.Baertschi AJ, Adams JM, Sullivan MP. Acute hypoxemia stimulates atrial natriuretic factor secretion in vivo. Am J Physiol. 1988;255(2 pt 2):H295–H300. doi: 10.1152/ajpheart.1988.255.2.H295. [DOI] [PubMed] [Google Scholar]
- 35.Baertschi AJ, Teague WG. Alveolar hypoxia is a powerful stimulus for ANF release in conscious lambs. Am J Physiol. 1989;256(4 pt 2):H990–H998. doi: 10.1152/ajpheart.1989.256.4.H990. [DOI] [PubMed] [Google Scholar]
- 36.Tóth M, Vuorinen KH, Vuolteenaho O, et al. Hypoxia stimulates release of ANP and BNP from perfused rat ventricular myocardium. Am J Physiol. 1994;266(4 pt 2):H1572–H1580. doi: 10.1152/ajpheart.1994.266.4.H1572. [DOI] [PubMed] [Google Scholar]
- 37.Perhonen M, Takala TE, Vuolteenaho O, Mantymaa P, Leppaluoto J, Ruskoaho H. Induction of cardiac natriuretic peptide gene expression in rats trained in hypobaric hypoxic conditions. Am J Physiol. 1997;273(1 pt 2):R344–R352. doi: 10.1152/ajpregu.1997.273.1.R344. [DOI] [PubMed] [Google Scholar]
- 38.Weidemann A, Klanke B, Wagner M, et al. Hypoxia, via stabilization of the hypoxia-inducible factor HIF-1alpha, is a direct and sufficient stimulus for brain-type natriuretic peptide induction. Biochem J. 2008;409(1):233–242. doi: 10.1042/BJ20070629. [DOI] [PubMed] [Google Scholar]
- 39.Hama N, Itoh H, Shirakami G, et al. Rapid ventricular induction of brain natriuretic peptide gene expression in experimental acute myocardial infarction. Circulation. 1995;92(6):1558–1564. doi: 10.1161/01.cir.92.6.1558. [DOI] [PubMed] [Google Scholar]
- 40.He Q, Wang D, Yang X-P, Carretero OA, LaPointe MC. Inducible regulation of human brain natriuretic peptide promoter in transgenic mice. Am J Physiol Heart Circ Physiol. 2001;280(1):H368–H376. doi: 10.1152/ajpheart.2001.280.1.H368. [DOI] [PubMed] [Google Scholar]
- 41.Hill NS, Klinger JR, Warburton RR, Pietras L, Wrenn DS. Brain natriuretic peptide: possible role in the modulation of hypoxic pulmonary hypertension. Am J Physiol. 1994;266(3 pt 1):L308–L315. doi: 10.1152/ajplung.1994.266.3.L308. [DOI] [PubMed] [Google Scholar]
- 42.Javaheri S. A mechanism of central sleep apnea in patients with heart failure. N Engl J Med. 1999;341(13):949–954. doi: 10.1056/NEJM199909233411304. [DOI] [PubMed] [Google Scholar]
- 43.Carmona-Bernal C, Quintana-Gallego E, Villa-Gil M, Sánchez-Armengol A, Martínez-Martínez A, Capote F. Brain natriuretic peptide in patients with congestive heart failure and central sleep apnea. Chest. 2005;127(5):1667–1673. doi: 10.1378/chest.127.5.1667. [DOI] [PubMed] [Google Scholar]
- 44.Christ M, Sharkova Y, Fenske H, et al. Brain natriuretic peptide for prediction of Cheyne-Stokes respiration in heart failure patients. Int J Cardiol. 2007;116(1):62–69. doi: 10.1016/j.ijcard.2006.03.031. [DOI] [PubMed] [Google Scholar]
- 45.Hunt SA, Abraham WT, Chin MH, et al. American College of Cardiology American Heart Association Task Force on Practice Guidelines American College of Chest Physicians International Society for Heart and Lung Transplantation Heart Rhythm Society ACC/AHA 2005 guideline update for the diagnosis and management of chronic heart failure in the adult: a report f the American College of Cardiology/American Heart Association Task Force on practice guidelines (writing committee to update the 2001 guidelines for the evaluation and management of heart failure): developed in collaboration with the American College of Chest Physicians and the International Society for Heart and Lung Transplantation: endorsed by the Heart Rhythm Society. Circulation. 2005;112(12):e154–e235. doi: 10.1161/CIRCULATIONAHA.105.167586. [DOI] [PubMed] [Google Scholar]
- 46.Bitter T, Faber L, Hering D, Langer C, Horstkotte D, Oldenburg O. Sleep-disordered breathing in heart failure with normal left ventricular ejection fraction. Eur J Heart Fail. 2009;11(6):602–608. doi: 10.1093/eurjhf/hfp057. [DOI] [PubMed] [Google Scholar]
- 47.Redfield MM, Rodeheffer RJ, Jacobsen SJ, Mahoney DW, Bailey KR, Burnett JC., Jr Plasma brain natriuretic peptide concentration: impact of age and gender. J Am Coll Cardiol. 2002;40(5):976–982. doi: 10.1016/s0735-1097(02)02059-4. [DOI] [PubMed] [Google Scholar]
- 48.Koshino Y, Villarraga HR, Orban M, et al. Changes in left and right ventricular mechanics during the Mueller maneuver in healthy adults: a possible mechanism for abnormal cardiac function in patients with obstructive sleep apnea. Circ Cardiovasc Imaging. 2010;3(3):282–289. doi: 10.1161/CIRCIMAGING.109.901561. [DOI] [PubMed] [Google Scholar]
- 49.Orban M, Bruce CJ, Pressman GS, et al. Dynamic changes of left ventricular performance and left atrial volume induced by the mueller maneuver in healthy young adults and implications for obstructive sleep apnea, atrial fibrillation, and heart failure. Am J Cardiol. 2008;102(11):1557–1561. doi: 10.1016/j.amjcard.2008.07.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Svatikova A, Shamsuzzaman AS, Wolk R, Phillips BG, Olson LJ, Somers VK. Plasma brain natriuretic peptide in obstructive sleep apnea. Am J Cardiol. 2004;94(4):529–532. doi: 10.1016/j.amjcard.2004.05.010. [DOI] [PubMed] [Google Scholar]