Abstract
Background and the purpose of the study
Domperidone (DOM) is a dopamine- receptor (D2) antagonist, widely used in the treatment of motion-sickness. The pharmacokinetic parameters of DOM make it a suitable candidate for development of Solid Lipid Nanoparticle (SLN) and Nanostructured Lipide Carrier (NLC). The purpose of the present investigation was to prepare and evaluate DOM loaded solid lipid nanoparticles (DOM-SLN) and DOM loaded nanostructured lipid carriers (DOM-NLC).
Methods
DOM loaded SLN and NLC were prepared by hot homogenization followed by ultrasonication technique, using trimyristin as solid lipid, cetyl recinoleate as liquid lipid and a mixture of soy phosphatidylcholine (99%) and tween 80 as surfactant. SLN and NLC were characterized for particle size, polydispersity index (PDI), zeta potential and entrapment efficiency. The effects of composition of lipid materials and surfactant mixture on the particle size, PDI, zeta potential, drug entrapment efficiency, and in vitro drug release behavior were investigated. DSC analysis was performed to characterize the state of drug and lipid modification. Shape and surface morphology were determined by transmission electron microscopy (TEM). SLN and NLC formulations were subjected to stability study over a period of 40 days.
Results
The mean particle size, PDI, zeta potential and entrapment efficiency of optimized SLN (SLN1) and NLC were found to be 30.45 nm, 0.156, 12.40 mV, 87.84% and 32.23 nm, 0.160, 10.47 mV, 90.49% respectively. DSC studies revealed that DOM was in an amorphous state and triglycerides were in the β prime form in SLN and NLC. Shape and surface morphology was determined by TEM revealed fairly spherical shape of nanoparticles. In vitro release studies demonstrated that both the SLN and NLC formulations possessed a controlled release over a period of 24 hrs. SLN and NLC formulations were subjected to stability over a period of 40 days. There was no significant (P<0.05) change in particle size, zeta potential, PDI and entrapment efficiency indicating the developed SLN and NLC were fairly stable.
Conclusion
Fairly spherical shaped, stable and controlled release DOM-SLN and DOM-NLC could be prepared by hot homogenization followed by ultrasonication technique.
Keywords: Entrapment efficiency, Polydispersity index, DSC, TEM
INTRODUCTION
Nanoparticles can be prepared using different kinds of materials, such as, biodegradable and biocompatible polymers, phospholipids, surfactants and lipids (1, 2). Several advantages of nanoparticles prepared from lipid materials including biocompatibility, drug targeting, modified release and ease of large scale production have been demonstrated (3). SLN have more advantages for drug delivery system, because of a good tolerability due to the use of physiological lipids (4), larger scale production and also a targeting effect on brain (5). However, depending on the drug, some potential problems can occur, such as drug leakage during storage and insufficient total drug load. To overcome the limitations of SLN, nanostructured lipid carriers (NLC) have been developed (2).
SLN and NLC are colloidal lipid systems, which have been proposed for several administration routes, such as parenteral (6), oral (7) and topical (8) routes, providing controlled release profiles for many substances. SLN consist of pure solid lipids and NLC contain a certain percentage of additional liquid lipids leading to imperfections in the crystal lattice. These nanoparticles are produced by one of the following techniques, namely, high pressure homogenization (9), microemulsion template (10), cold homogenization, solvent emulsification (11), solvent diffusion (12), reverse micelle-double emulsion (13), homogenization followed by ultrasonication (8), solvent injection (14), and a very recently introduced membrane contractor techniques (15).
Domperidone is a dopamine-receptor (D2) anta-gonist, widely used in the treatment of motion sickness. It is poorly water soluble (log p, 3.1) and has low absorbability after oral administration, and undergoes extensive first pass metabolism; leading to poor bioavailability of 15% (16, 17). In addition, it is supplied at low dose (10 mg) and has low molecular weight (425.9), require long term treatment and repetitive dosing which make this drug an interesting candidate for development of SLN and NLC.
The objective of the present research was to study the preparation of SLN and NLC loaded DOM by homogenization followed by ultrasonication technique and to study the effects of composition of lipid materials and surfactant mixture on the particle size, zeta potential, drug entrapment efficiency and in vitro drug release behavior in detail.
MATERIAL AND METHODS
Material
Domperidone (Torrent pharmaceuticals, Ahmadabad, India); Trimyristin (Dynasan 114) (Sasol, Witten, Germany); Cetyl resinoleate (IICT, Hyderabad, India); Soy phosphatidylcholine 99% (Epikuron 200, Degussa Texturant Systems, Hamburg, Deutschland); Tween 80 and dialysis membrane-70 (Hi-media, Mumbai, India); Centrisart filters (molecular weight cutoff 20,000) (Sartorius, Goettingen, Germany) were used in this study. All other chemicals were of analytical reagent grade.
Preparation of Domperidone loaded SLN and NLC
Domperidone, trimyristin, and soy phosphatidyl-choline 99% were dissolved in 10 ml mixture of chloroform and methanol (1:1). Organic solvents were completely removed using a rotaevaporator (Laborota 4000, Heidolph, Germany). Drug-embedded lipid layer was melted by heating at 5°C above melting point of the lipid. An aqueous phase was prepared by dissolving polysorbate 80 in double distilled water (sufficient to produce 10 ml of the preparation) and heated to the same temperature of oil phase. Hot aqueous phase was added to the oil phase, and homogenization was carried out at 12,000 rpm using a Diax 900 homogenizer (Heidolph, Germany) for 3 min. Coarse hot oil in water emulsion was ultrasonicated (12T-probe) using a sonoplus ultrahomogenizer (Bandelin, Germany) for 20 min. DOM-SLN were obtained by allowing hot nanoemulsion to cool to room temperature. Domperidone loaded NLC (DOM-NLC) was prepared in exactly the same manner as the SLN dispersions, but replacing 30% of the solid lipid by liquid lipid (cetyl resinoleate). The final composition of the investigated SLN and NLC dispersions are shown in table 1.
Table 1.
Composition of DOM-SLN and DOM-NLC formulations.
| Ingredients | Formulations | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| SLN1 | SLN2 | SLN3 | SLN4 | SLN5 | SLN6 | SLN7 | SLN8 | NLC | |
| DOM (mg) | 10 | 10 | 10 | 10 | 10 | 10 | 10 | 10 | 10 |
| TM (mg)a | 300 | 300 | 300 | 300 | 250 | 250 | 250 | 250 | 210 |
| SP 99% (mg)b | 100 | 100 | 100 | 100 | 100 | 100 | 100 | 100 | – |
| CR (mg)c | – | – | – | – | – | – | – | – | 90 |
| SS (mL)d | 10 | 10 | 10 | 10 | 10 | 10 | 10 | 10 | 10 |
| Tween 80 (mge | 300 | 250 | 200 | 150 | 300 | 250 | 200 | 150 | 300 |
TM: trimyristin
SP 99%: Soy Phosphatidyl choline
CR: Cetyl resinoleate
SS: solvent system (1:1 ratio of chloroform and methanol)
Tween 80: used in 20 ml of double distilled water
Measurement of particle size and zeta potential of SLN and NLC
The size and zeta potential of SLN were measured by photon correlation spectroscopy using a Zetasizer 3000 HSA (Malvern, UK). Samples were diluted appropriately with the aqueous phase of the formulation to get optimum kilo counts per second (Kcps) of 50-200 for measurements, and the pH of diluted samples ranged from 6.9 to 7.2. Zeta potential measurements were carried out at 25°C, and the electric field strength was around 23.2 V/cm.
Determination of entrapment efficiency
Entrapment efficiency (EE%) was determined by measurement of the concentration of free drug (unentrapped) in aqueous medium using centrisart tubes (Sartorius, Germany) (18). The entrapment efficiency was calculated by the following equation.
HPLC methodology for domperidone
HPLC determination of DOM was performed using Shimadzu LC 20AT solvent delivery pump equipped with a 20 µl loop and rheodyne sample injector and UV-Visible detector operating at 284 nm. Samples were chromatographed on a stainless steel C18 reverse phase column (250×4.25 mm) packed with 5 µm particles (phenomenex; Gemini column). Elution was conducted with a mobile phase of acetonitrile: double distilled water: triethylamine (31: 69: 0.25) of pH 2.5 at a flow rate of 1 ml.min−1 and the entire analysis was carried out at room temperature. A calibration curve was plotted for DOM in the concentration range of 0.5–10 µg.ml−1. A good linear relationship was observed between the concentration of DOM and the peak area of DOM with a correlation coefficient (r2=0.999). The required studies were carried out to estimate the precision and accuracy of the HPLC method (17).
In vitro release Study
In vitro release studies were performed using dialysis membrane (Hi-media, Mumbai, India) having pore size of 2.4 nm and molecular weight cutoff between 12,000–14,000 Dalton. Membrane was soaked in double distilled water for 12 hrs before mounting in a Franz diffusion cell. A volume of 2 ml of DOM loaded SLN/NLC was placed in the donor compartment and the receptor compartment was filled with 50 ml of 0.1N HCl and phosphate buffer of pH 6.8 and mixture at 37±0.5°C was stirred at 800 rpm. An aliquot of 1ml of the sample was withdrawn from receiver compartment at pre-determined time intervals and replenished with fresh medium. Samples were analyzed by HPLC. Data obtained from in vitro release studies were fitted to various kinetic equations to find out the mechanism of DOM release from SLN and NLC. The Kinetic models of zero order, first order and Higuchi were used in this study.
Differential scanning calorimetry (DSC)
Thermal characteristics of the drug, trimyristin (TM), physical mixture (PM), lyophilized DOM-SLN and lyophilized DOM-NLC were studied by Mettler-Toledo DSC (Mettler-Toledo, Viroflay, France). Ultra high pure nitrogen was used at a flow rate of 50 ml min−1. Samples were analyzed in crimped aluminum pans and heated at 30–250°C at a linear heating rate of 10°C min−1. The sample size was 3–5 mg for each measurement.
Transmission electron microscopic studies
Images were recorded using Transmission Electron Microscope (Hitachi, Japan, Model H 7500 ID) with magnification: 179000×. DOM loaded SLN and NLC was diluted appropriately with 0.1M phosphate buffer and centrifuged at 4000 rpm for 5 min. Few drops of the diluted emulsion was placed on grid and stained with 2% uranyl acetate, and then the image was captured.
Stability studies
DOM loaded SLN and NLC were stored at room and refrigerator temperature for 40 days. At different time intervals of the days of 1, 20 and 40, the average size, zeta potential, PDI and entrapment efficiency were determined. Samples were estimated in triplicates. Statistical analysis of the data was performed using the Student's t–test. A probability, P, of less than 0.05 (P<0.05) was considered significant in this study.
RESULTS AND DISCUSSIONS
Preparation of DOM loaded SLN and NLC
Homogenization followed by ultrasonication is a reliable, simple and reproducible method for preparation of SLN and NLC (18). It is known that employment of two surfactants of lipophilic and hydrophilic nature yields a better stabilization of the disperse system. The solvent mixture of chloroform and methanol (1:1) was found to be effective in homogenously dispersing of the drug in the lipid phase. Homogenization of the lipid phase with hot aqueous tween 80 for 3 min was sufficient to produce a coarse emulsion with average particle size between 2.52 and 2.83 µm. Further increase in homogenization time did not show any significant decrease in the particle size. Thus a homogenization time of 3 min was selected for all formulations and further reduction of size was preceded with sonication. Sonicating the coarse emulsion for 20 min resulted in particle size between 30 and 38 nm with narrow size distribution. In order to optimize the lipid to drug ratio, two different amounts of trimyristin (250 and 300 mg) were tried with fixed amount of DOM (10 mg) and soy phosphatidylcholine 99% (100 mg).
Particle size distribution and zeta potential of formulations
In all formulations, the particle sizes ranged from 30.4±6.12 to 38.1±5.12 nm (Table 2). Increase in the concentration of surfactant in SLN formulations could reduce the interfacial tension between lipid matrix and dispersion medium (aqueous phase), consequently favor the formation of SLN with smaller particle size. Tween 80 also maintain the stability of SLN (18). To obtain stable and smaller SLN, tween 80 concentrations were varied from 0.75 to 1.5% (150 to 300 mg). From the results it is evident that the concentration of 1.5% was effective in producing smaller particle size. Further increase in Tween 80 concentration to 2% had no effect in reduction of the particle size. These results clearly suggest that an optimum concentration of 1.5% of tween 80 was sufficient to cover the surface of nanoparticles effectively and prevent agglomeration during the homogenization process.
Table 2.
PDI, zeta size, zeta potential and entrapment efficiency (EE%) of SLN and NLC formulations (mean±SD, n=3).
| Formulation | aPDI | Particle size (nm) | Zeta potential (mV) | bEE% |
|---|---|---|---|---|
| SLN 1 | 0.15±0.091 | 30.4±6.12 | 12.4±4.72 | 87.8±7.07 |
| SLN 2 | 0.14±0.012 | 31.3±5.55 | 10.4±4.68 | 84.1±6.33 |
| SLN 3 | 0.20±0.008 | 34.8±9.91 | 7.26±3.11 | 83.1±4.99 |
| SLN 4 | 0.18±0.021 | 33.4±4.89 | 6.27±2.58 | 81.7±8.22 |
| SLN 5 | 0.19±0.011 | 33.6±2.33 | 7.76±3.61 | 85.1±5.48 |
| SLN 6 | 0.22±0.016 | 35.6±6.47 | 6.44±2.48 | 84.8±6.78 |
| SLN 7 | 0.21±0.014 | 38.1±5.12 | 6.94±2.15 | 82.9±7.66 |
| SLN 8 | 0.19±0.025 | 37.8±6.66 | 5.82±1.22 | 80.9±8.71 |
| NLC | 0.16±0.015 | 32.2±6.61 | 10.4±3.16 | 90.5±7.01 |
PDI: polydispersity index
EE%: entrapment efficiency
Zeta potential is a key factor for evaluation of the stability of colloidal dispersion. It was currently admitted that zeta potentials above 30 mV were required for full electrostatic stabilization. The values of zeta potential of DOM loaded SLN and NLC ranged from 5.82±1.22 to 12.4±4.72 (Table 2) were not sufficient to keep the particles stable. However, many experiments demonstrated that not only electrostatic repulsion dominated the stability of nanoparticles but the use of steric stabilizer also favored the formation of stable nanoparticle dispersion. High surfactant mixture can easily compensate missing electrostatic repulsion to stabilize the dispersion for long time. Tween 80 provides a steric stability for maintaining the stability of SLN and NLC.
Entrapment efficiency
Entrapment efficiency of SLN and NLC formulations are shown in table 2. Among the SLN formulations highest entrapment efficiency (87.8±7.07%) was observed with formulation of SLN1, whereas formulation SLN4 showed lowest entrapment efficiency (81.7±8.22%). As the lipid concentration decreased there was a decline in entrapment efficiency of the SLN formulations. NLC formulation showed highest entrapment efficiency (90.49±7.01%). In general, drug solubility is higher in liquid lipid than in solid lipid, which increases the entrapment efficiency (19). Incorporation of liquid lipids to solid lipids leads to reduced crystallinity and increased imperfections in the crystal lattice which leaves enough space to accommodate drug molecules, thus, leading to improved drug loading capacity and drug entrapment efficiency.
Effect of surfactant mixture on particle size, zeta potential and PDI
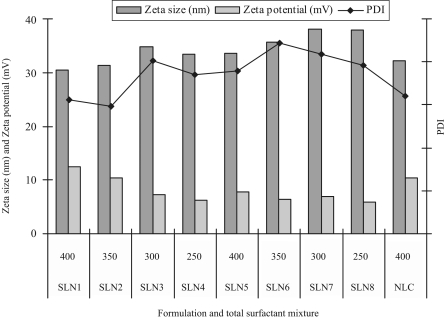
The effect of surfactant composition on the size, zeta potential and PDI of SLN and NLC are presented in figure 1. Amount of the surfactant in SLN and NLC is an important factor for determination of the physicochemical characteristics due to the surface active properties. The zeta potential of SLN formulations (SLN1 to SLN8) was decreased by decrease in the total amount of surfactant mixture (soy phosphatidylcholine & tween 80) from 400 to 250 mg. As the amount of surfactant mixture was increased, the particle size was decreased. The amount and type of emulsifier affects the particle size and the stability of the formulation. The amount of emulsifier should be optimum to cover the surface of the nanoparticles and combination of hydrophilic and lipophilic surfactants were used in the formulation to improve the stability of the formulation.
Figure 1.
Effect of surfactant composition on the size, zeta potential and PDI of SLN and NLC formulations.
In vitro release study
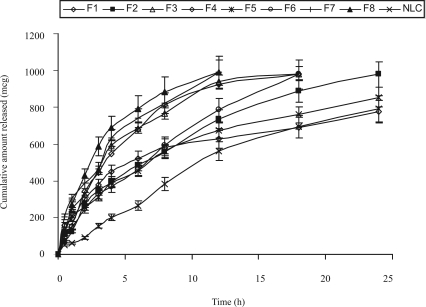
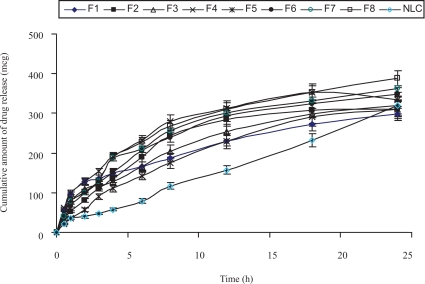
The in vitro drug release profiles of DOM-loaded SLNs with different formulations and DOM-loaded NLC are shown in figure. 2a and figure 2b. In order to evaluate the controlled release potential of the investigated formulations, the release of DOM from the lipid particles was investigated for 24 hrs. Maximum amount of drug release was found from the formulations SLN 5 to SLN 8 compared to the formulations SLN 1 to 4 in 0.1N HCl and phosphate buffer of pH 6.8; which may be due to low concentration of lipid in the formulations. Formulations SLN 4 and 8 showed maximum drug release in 12 hrs, whereas for SLN 3 and 7 were 18 hrs. As the surfactant concentration decreased from 1.5% to 0.75% there was a decrease in controlled release properties of the SLN formulations. In the NLC formulation 30% of the solid lipid was replaced by liquid lipid. Due to the addition of liquid lipid in the NLC formulation there was an increase in the amount of drug release in comparison to the SLN 1, since the liquid lipid adheres to the lipid matrix and decreases the diffusion path length of the lipid matrix. The data for drug release of most of the SLN & NLC formulations fitted well into the Higuchi square root release kinetics (r2 values ranging from 0.92 to 0.99). This indicates that the test product follows matrix diffusion based release kinetics.
Figure 2a.
In vitro drug release profile of SLN and NLC formulations in 0.1 N HCl.
Figure 2b.
In vitro drug release profile of SLN and NLC formulations in phosphate buffer of pH 6.8.
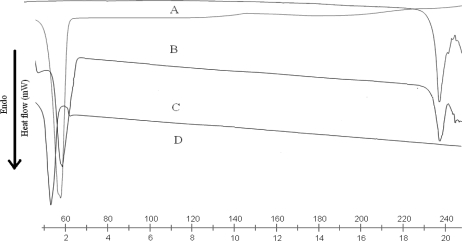
DSC analysis
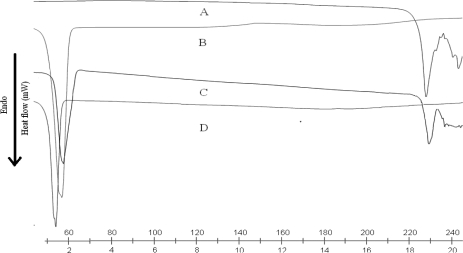
DSC thermograms of DOM, trimyristin, physical mixture of drug and lipid, SLN and NLC are shown in figure. 3a and figure 3b. DSC studies revealed that DOM was in an amorphous state and triglycerides are in the β prime form in SLN and NLC. The melting endotherm of the drug was completely absent in the thermograms of DOM loaded SLN and NLC, which indicates that DOM was completely solubilized inside the lipid matrix of the SLN and NLC. Incorporation of DOM inside the lipid matrix results in an increase in the number of defects in the lipid crystal lattice, and hence causes a decrease in the melting point of the lipid in the final SLN formulations. Bulk trimyristin showed a sharp endothermic event, around 58.68°C (minimum) with an extrapolated onset of the melting peak at 53.28°C. When the trimyristin was formulated as nanoparticles, the endothermic happened at a slightly lower temperature and in the form of beta prime polymorph. These differences are generally ascribed to the nanometric size of the particles, having then a high specific surface area (20). A certain effect due to the surfactant should also be considered. The addition of oil (i.e., cetyl resinoleate) into the matrix provoked an additional shift of the melting point to lower temperatures in NLC.
Figure 3a.
DSC thermograms of DOM (A), trimyristin (B), physical mixture of DOM and TM (C), and lyophilized DOM-SLN (D).
Figure 3b.
DSC thermograms of DOM (A), trimyristin (B), physical mixture of DOM and TM (C), and lyophilized DOM-NLC (D).
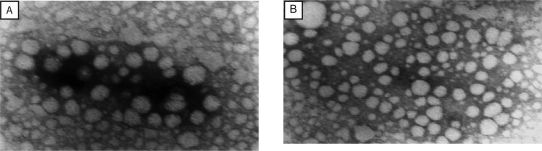
Visualization by Transmission Electron Microscopy (TEM)
The result of TEM imaging of DOM loaded SLN and NLC, which are shown in figure 4, indicate that the particles had nanometer-size spherical shapes and no drug crystal (irregular crystallization with the vast majority of needle or rod crystal in the length range from 10 micron to a few dozen microns) was visible. The average particle size of optimized DOM loaded SLN and DOM loaded NLC were found to be 30.45 nm and 32.23 nm respectively.
Figure 4.
Transmission electron micrograph of (A) DOM loaded SLN and (B) DOM loaded NLC.
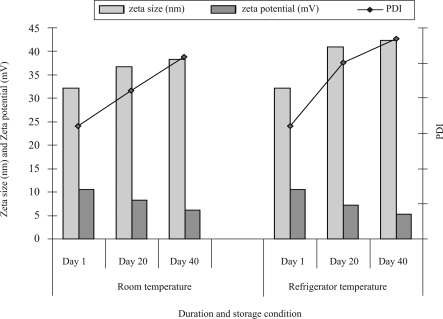
Stability study
All formulations were stored in amber colored bottles at room and refrigerator temperature. As SLN 3, 4, 7 and 8 formulations are not stable, only formulations SLN 1, 2, 5, 6 and NLC were analyzed for particle size, zeta potential and PDI after stored at room and refrigerator temperature for the days of 1, 20 and 40. SLN 1, 2, 5, 6 and NLC were analyzed for entrapment efficiency on the different time intervals (day 1, 20 and 40) after stored at room temperature. The effect of duration of storage condition on particle size, zeta potential and PDI of SLNs and NLC are shown in table 3 and figure 5 respectively. There was no significant difference (P<0.05) in particle size, zeta potential, and PDI between SLN and NLC on the day of preparation but as the duration of storage increased the NLC formulation found to be more stable than SLN. The good stability might derive from the slow transition of lipid in nanoformulations and the steric effect of tween 80. These results clearly suggest that an optimum tween 80 concentration of 1.5% was sufficient to cover the surface of nanoparticles effectively and prevent agglomeration during the homogenization process.
Table 3.
Influence of storage condition and duration of storage on size, zeta potential and PDI of SLN.
| Storage condition | Duration | Formulation | Particle Size (nm) | Zeta Potential (mV) | PDI |
|---|---|---|---|---|---|
| Room temperature | Day 1 | SLN1 | 30.45±2.4 | 12.43±3.1 | 0.156±0.043 |
| SLN2 | 31.37±5.3 | 7.26±2.2 | 0.148±0.088 | ||
| Day 20 | SLN1 | 36.47±2.5 | 8.14±2.3 | 0.215±0.056 | |
| SLN2 | 39.43±3.8 | 5.31±4.1 | 0.213±0.066 | ||
| Day 40 | SLN1 | 38.37±6.2 | 3.64±2.2 | 0.303±0.073 | |
| SLN2 | 41.29±8.4 | 3.31±2.1 | 0.377±0.028 | ||
| Refrigerator temperature | Day 1 | SLN1 | 30.45±2.6 | 12.43±4.6 | 0.156±0.097 |
| SLN2 | 34.88±6.6 | 7.26±4.3 | 0.201±0.066 | ||
| Day 20 | SLN1 | 41.08±4.9 | 6.56±2.4 | 0.268±0.058 | |
| SLN2 | 46.59±5.5 | 5.74±2.2 | 0.307±0.043 | ||
| Day 40 | SLN1 | 56.17±7.7 | 4.95±1.1 | 0.325±0.078 | |
| SLN2 | 58.25±7.5 | 3.26±2.4 | 0.322±0.054 | ||
| Room temperature | Day 1 | SLN5 | 33.61±4.8 | 10.43±5.4 | 0.189±0.066 |
| SLN6 | 35.66±3.6 | 6.27±3.6 | 0.222±0.073 | ||
| Day 20 | SLN5 | 34.16±4.5 | 7.59±4.2 | 0.225±0.044 | |
| SLN6 | 38.51±6.3 | 5.23±2.4 | 0.401±0.057 | ||
| Day 40 | SLN5 | 36.54±7.9 | 5.05±1.4 | 0.315±0.085 | |
| SLN6 | 39.96±8.8 | 1.61±1.1 | 0.420±0.077 | ||
| Refrigerator temperature | Day 1 | SLN5 | 31.37±3.4 | 10.43±5.8 | 0.148±0.089 |
| SLN6 | 33.45±5.3 | 6.27±3.4 | 0.185±0.099 | ||
| Day 20 | SLN5 | 40.11±4.4 | 7.11±4.3 | 0.239±0.048 | |
| SLN6 | 42.89±2.8 | 5.88±4.1 | 0.260±0.088 | ||
| Day 40 | SLN5 | 53.51±8.8 | 5.33±2.4 | 0.44±0.092 | |
| SLN6 | 53.64±6.9 | 4.11±2.8 | 0.419±0.055 | ||
Figure 5.
Influence of storage condition and duration of storage on size and zeta potential and PDI of NLC formulation.
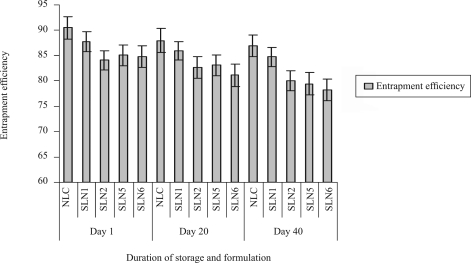
During long-term storage, triglycerides undergo degradation to fatty acids and mono- and diglycerides, which could compete with formulation surfactants for positioning on the surface. Fatty acids and monoglycerides can form mixed micelles that might enhance partitioning of hydrophobic drug out of the nanoparticle. Therefore, the concentration of excipients and possible degradation of products need to be determined to understand the stability of nanoparticle (21). The effect of duration of storage condition at room temperature on entrapment efficiency of DOM-SLN and DOM-NLC are presented in figure 6. As the duration of storage increased, the entrapment efficiency decreased slightly. There was no significant (P<0.05) difference in the entrapment efficiency during storage for 40 days.
Figure 6.
Influence of duration of storage and Storage condition at room temperature on entrapment efficiency of SLN and NLC formulations.
CONCLUSION
This study indicates that both SLN and NLC are potential carriers systems for DOM. The entrapment efficiency and the drug release profile depend on the concentration of lipid and surfactant mixture employed. NLC showed higher entrapment efficiency due to their liquid lipid parts. Consistent with these results NLC also show a faster release profile in comparison to SLN 1 with the same lipid concentration. The release rate decreases for SLN with a higher lipid concentration, which is explained by the physical morphology of the lipid particles. Stability studies revealed that after 40 days of storage at different temperatures the mean diameters of NLC remain practically the same, which emphasizes the physical stability of lipid particles. These data collectively support that SLN and NLC are the promising delivery systems for poorly water soluble drugs, such as domperidone.
ACKNOWLEDGMENTS
The authors acknowledge the financial support from AICTE, (New Delhi, India). The author acknowledges M/s Torrent pharmaceuticals, (India) for providing domperidone, M/s.Sasol, (Witten Germany) for providing trimyristin and M/s Degussa Texturant Systems (Hamburg, Deutschland) for providing soy phosphatidylcholine 99%.
REFERENCES
- 1.Muller RH, Mader K, Gohla S. Solid lipid nanoparticles (SLN) for controlled drug delivery-a review of the state of the art. Eur. J. Pharm. Biopharm. 2000;50:161–177. doi: 10.1016/s0939-6411(00)00087-4. [DOI] [PubMed] [Google Scholar]
- 2.Muller RH, Radtke M, Wissing SA. Solid lipid nanoparticles (SLN) and nanostructured lipid carriers (NLC) in cosmetic and dermatological preparations. Adv. Drug Deliv. Rev. 2002b;54:S131–S155. doi: 10.1016/s0169-409x(02)00118-7. [DOI] [PubMed] [Google Scholar]
- 3.Mehnert W, Mader K. Solid lipid nanoparticles: production, characterization and applications. Adv. Drug Deliv. Rev. 2001;47:165–196. doi: 10.1016/s0169-409x(01)00105-3. [DOI] [PubMed] [Google Scholar]
- 4.Muller RH, MaaBen S, Weyhers H, Specht F, Lucks JS. Cytotoxicity of magnetite loaded polylactide, polylactide/glycolide particles and solid lipid nanoparticles (SLN) Int. J. Pharm. 1996a;138:85–94. [Google Scholar]
- 5.Yang SC, Lu LF, Cai Y, Zhu JB, Liang BW, Yang CZ. Body distribution in mice of intravenously injected camptothecin solid lipid nanoparticles and targeting effect on brain. J. Control. Rel. 1999;59:299–307. doi: 10.1016/s0168-3659(99)00007-3. [DOI] [PubMed] [Google Scholar]
- 6.Liu J, Zhu J, Du , Bin Qin Z. Preparation and Pharmacokinetic Evaluation of Tashinone IIA Solid Lipid Nanoparticles. Drug Dev. Ind. Pharm. 2005;31:551–556. doi: 10.1080/03639040500214761. [DOI] [PubMed] [Google Scholar]
- 7.Zhang N, Ping Q, Huang G, Xu W, Cheng Y, Xiuzhen H. Lectin-modified solid lipid nanoparticles as carriers for oral administration of insulin. Int. J. Pharm. 2006;327:153–159. doi: 10.1016/j.ijpharm.2006.07.026. [DOI] [PubMed] [Google Scholar]
- 8.Bhaskar K, Krishna Mohan C, Lingam M, Jagan Mohan S, Venkateswarlu V, Madhusudan Rao Y, Jayaraman A, Ravichandran V. Development of SLN and NLC Enriched Hydrogels for Transdermal Delivery of Nitrendipine: In Vitro and In Vivo Characteristics. Drug Dev. Ind. Pharm. 2008;34:719–725. doi: 10.1080/03639040802192822. [DOI] [PubMed] [Google Scholar]
- 9.Yang S, Lu LF, Cai Y, Zhu JB, Liang BW, Yang CZ. Body distribution in mice of intravenously injected camptothecin solid lipid nanoparticles and targeting effect on brain. J. Control Rel. 1999;59:299–307. doi: 10.1016/s0168-3659(99)00007-3. [DOI] [PubMed] [Google Scholar]
- 10.Cavalli R, Alessandro B, Valerio P, Muntoni E, Zara G, Gasco MR. Duodenal Administration of Solid Lipid Nanoparticles Loaded with Different Percentages of Tobramycin. J. Pharm. Sci. 2003;92:1085–1094. doi: 10.1002/jps.10368. [DOI] [PubMed] [Google Scholar]
- 11.Luo Y, Chen D, Ren L, Zhao X, Jing Q. Solid lipid nanoparticles for enhancing vinpocetine's oral bioavailability. J. Control Rel. 2006;114:53–59. doi: 10.1016/j.jconrel.2006.05.010. [DOI] [PubMed] [Google Scholar]
- 12.Yuan H, Miao J, Du YZ, You J, Hu FQ, Zeng S. Cellular uptake of solid lipid nanoparticles and cytotoxicity of encapsulated paclitaxel in A549 cancer cells. Int. J. Pharm. 2008;348:137–145. doi: 10.1016/j.ijpharm.2007.07.012. [DOI] [PubMed] [Google Scholar]
- 13.Jores K, Mehenert W, Mader K. Physicochemical investigations on solid lipid nanoparticles and on oil-loaded solid lipid nanoparticles. A nuclear magnetic resonance study. 2003;20:1274–1283. doi: 10.1023/a:1025065418309. [DOI] [PubMed] [Google Scholar]
- 14.Schubert MA, Muller-Goymann CC. Solvent injection as a new approach for manufacturing lipid Nanoparticles-Evaluation of the method and process parameters. Eur. J. Pharm Biopharm. 2003;55:125–131. doi: 10.1016/s0939-6411(02)00130-3. [DOI] [PubMed] [Google Scholar]
- 15.Charcosset C, El-Harati A, Fessi H. Preparation of solid lipid nanoparticles using a membrane contactor. J. Control. Rel. 2005;108:112–120. doi: 10.1016/j.jconrel.2005.07.023. [DOI] [PubMed] [Google Scholar]
- 16.Sweetman SC. Martindale-The complete drug reference. London. (U.K): Pharmaceutical Press; 2007. pp. 1556–1557. [Google Scholar]
- 17.Madishetti SK, Palem CR, Gannu R, Thatipamula RP, Panakanti PK, Yamsani MR. Development of domperidone bilayered matrix type transdermal patches: physicochemical, in vitro and ex vivo characterization. DARU. 2010;18:221–229. [PMC free article] [PubMed] [Google Scholar]
- 18.Venkateswarlu V, Manjunath K. Preparation, characterization and in vitro release kinetics of clozapine solid lipid nanoparticles. J. Control. Rel. 2004;95:627–638. doi: 10.1016/j.jconrel.2004.01.005. [DOI] [PubMed] [Google Scholar]
- 19.Radtke M, Eliana Souto B, Muller RH. Nanostructured Lipid Carriers: A Novel Generation of Solid Lipid Drug Carriers, Pharm. Tech. Europe. 2005;17:45–50. [Google Scholar]
- 20.Westesen K, Bunjes H. Do nanoparticles prepared from lipids solid at room temperature always possess a solid lipid matrix? Int. J. Pharm. 1995;115:129–131. [Google Scholar]
- 21.Staggers JE, Hernell O, Stafford RJ, Carey MC. Physical-chemical behaviour of dietary and biliary lipids during intestinal digestion and absorption, I: phase behavior and aggregation states of model lipid systems patterned after aqueous duodenal contents of healthy adult human beings. Biochemistry. 1990;29:2028–2040. doi: 10.1021/bi00460a011. [DOI] [PubMed] [Google Scholar]