Abstract
Background and the purpose of the study
Layer-by-layer (LbL) deposition of polyelectrolytes (PEs) has received a great attention in the area of drug delivery due to its simplicity and versatility. This research was aimed to develop multilayered microcapsules through LbL deposition of chitosan (CHI) and sodium alginate (NaALG) and utilize them as vehicle for controlled delivery of isoniazid (INH).
Methods
CaCO3 particles, prepared by colloidal crystallization of CaCl2 and Na2CO3 solutions, were used as micro-templates for LbL deposition of CHI and NaALG. Subsequent to the deposition, templates were decomposed to obtain hollow microcapsules. Prepared microcapsules were subjected to physicochemical evaluations, drug release and stability studies.
Results and major conclusion
Though CaCO3 particles possessed a rough and irregular surface, prepared hollow microcapsules were spherical in shape, having smooth surface and regular thickness. Following deposition of each layer, alternating values of zeta potential were observed, indicating the formation of multilayered films. Microcapsules with 5 bilayers, i.e. (CHI/NaALG)5 provided 39% entrapment efficiency and exhibited a controlled release behavior, lasting up to 24 hrs. An improvement in drug release rate and stability profile of the formulation was observed by increasing the number of deposition steps and performing the crosslinking of polyelectrolytes. This study showed that the prepared formulation could promisingly be utilized as controlled delivery vehicle for INH.
Keywords: Layer-by-layer, Polyelectrolyte, Multilayers microcapsules, Controlled delivery
INTRODUCTION
Past few decades have witnessed an upsurge in the microencapsulation of biomolecules using layer-by-layer (LbL) deposition. The technique, based on the electrostatic interaction between oppositely charged polyelectrolytes (PEs), has increasingly received a great recognition in the area of drug delivery due to its reproducibility, simplicity, versatility, lack of requirement of organic solvents and less polymer consumption (1–4). By this technique, polyanions and polycations are repeatedly allowed to adsorb on to the surface of a microtemplate, creating alternating regions of opposite charges and subsequent to the dissolution of template, hollow polyelectrolyte microcapsules (PEMs) are resulted. The multilayer film assembly is uniform and continuous, possesses a defined composition and reproducible thickness. They can deliver a high drug payload and improve the pharmacokinetic profile of loaded drug (4).
The present research was aimed to develop multilayered microcapsules based on two ionic PEs, chitosan (CHI) and sodium alginate (NaALG).
They were selected due to their lower cost, biocompatibility and versatility of pharmaceutical applications. Calcium carbonate (CaCO3) microparticles, prepared by colloidal crystallization (5), were used as sacrificial micro-templates. Clean hollow microcapsules (NaALG/CHI)n, containing no residual core, with a narrow size distribution, could be achieved with them (2). Microcapsules were finally utilized as controlled release microcarriers of isoniazid (INH), a first line anti-tubercular drug which is used for the treatment of all forms of tuberculosis.
MATERIAL AND METHODS
Material
Isoniazid (INH) was received as a gift sample from Macleod Pharmaceuticals, (Mumbai, India).Chitosan (CHI) was kindly gifted by Biologicals E. Ltd., (Hyderabad, India) and sodium alginate (NaALG) was purchased from Central Drug House, (New Delhi, India). Fluorescein sodium (FS) was purchased from Hi Media, Mumbai. Other solvents and chemicals were procured from manufacturers and used as received.
Methods
Formulation of multilayered polyelectrolyte microcapsules
Calcium carbonate microtemplates were prepared by colloidal crystallization induced by mixing equal volumes of calcium chloride (CaCl2) and sodium carbonate (Na2CO3) solutions (5). Solutions of CHI and NaALG (1 mg/ml each), prepared in 1% acetic acid and triple distilled water (TDW), respectively, and filtered through a 0.2 µm membrane syringe filter prior to use. Suspension (5% w/v) of the prepared CaCO3 particles in 0.5M NaCl was subjected to LbL deposition of PEs. Layer deposition was started with NaALG and pH was adjusted at each deposition step (6, 7). The mixture was incubated for 15 min at room temperature with gentle stirring. Non-adsorbed PE at each deposition was removed by two washings in TDW. Subsequent deposition cycles were performed in the identical way until desired numbers of PE bilayers were achieved. Hollow microcapsules were obtained by dissolving the core in 0.1M citric acid solution (pH 5.4) (4). Microcapsules were further washed with water, centrifuged to remove citric acid and finally re-dispersed in TDW. The pH of each formulation batch at the time of storage was 7.0 (±0.2).
Microscopic characterizations
Scanning electron microscopic analysis (SEM) of gold sputtered samples was performed at an operation voltage of 10 kV (Philips XL-20, Holland). Transmission electron microscopic (TEM) images were acquired after performing negative staining of samples with 1% phospho tungstic acid solution (Technai G2, Netherlands). For confocal laser scanning microscopic (CLSM) studies, equal amounts of microcapsule suspension and an aqueous solution of fluorescein sodium (FS; 0.1 mg/ml) were mixed one hour before imaging and scanning was performed with a 20× Plan Aprochromat objective. Images were taken at an excitation wavelength of 488 nm and band pass of 505–550 nm (Zeiss 510 META, Germany). Images were stored and visualized as JPEG image format.
Zeta potential measurements
Layer deposition was studied as a function of change in zeta potential of the core particles (Delsa™ Nano, USA). Suspension was centrifuged (2000 rpm/2 min), 100 µl supernatant was collected and diluted up to 2 ml with HPLC grade water (8). It was placed into the electrophoretic cell, where a potential of ±150 mV was applied. The mobility, µ was converted to the ζ-potential by Smoluchowski relation.
Entrapment of drug into the prepared PEMs
Microcapsule suspension was centrifuged at 5000 rpm for 5 min and the supernatant was removed. Microcapsule pellet was then dispersed in INH solution (2 mg/ml) at room temperature which resulted into spontaneous entrapment of drug into the formulation. After being incubated for 2 hrs, the mixture was centrifuged (2000 rpm/2 min) and INH-loaded microcapsules were washed twice with water. Cross-linking of the formulation was performed by overnight treatment with glutaraldehyde. Entrapment efficiency (EE) was estimated through the difference between theoretical amount (Mt) and the amount obtained in the supernatants during washing process (Ml) (9).
In vitro drug release studies
In vitro drug release studies of the formulations were performed using egg shell membrane. The latter was obtained from fresh chicken egg and prepared as per the method described by Philip et al. (10). The membrane, containing 2 ml of formulation, was assembled as a bag and immersed into 10 ml of release media, maintained at 37±0.5°C and stirred at 100 rpm (Decibel Instruments, India). Two different media were used: acidic buffer of pH 1.4 (simulated gastric fluid without enzyme for 2 hrs) and phosphate buffer of pH 6.8 (simulated intestinal fluid for the rest of the study period). Samples (1 ml) were withdrawn at pre-determined time intervals, replaced with equal volume of pre-warmed (37±0.5°C) fresh medium, filtered through 0.45 µm membrane filter and analyzed spectrophotometrically at 262 nm for drug content (Shimandzu 7800, Japan).
Accelerated stability studies
Batches F2 and F3 were stored for 3 months in ICH certified stability chamber (NSW-175, India). maintained at 40±2°C and 75±5%RH (11). Their release characteristics, drug content, size and integrity of shape were studied.
RESULTS AND DISCUSSION
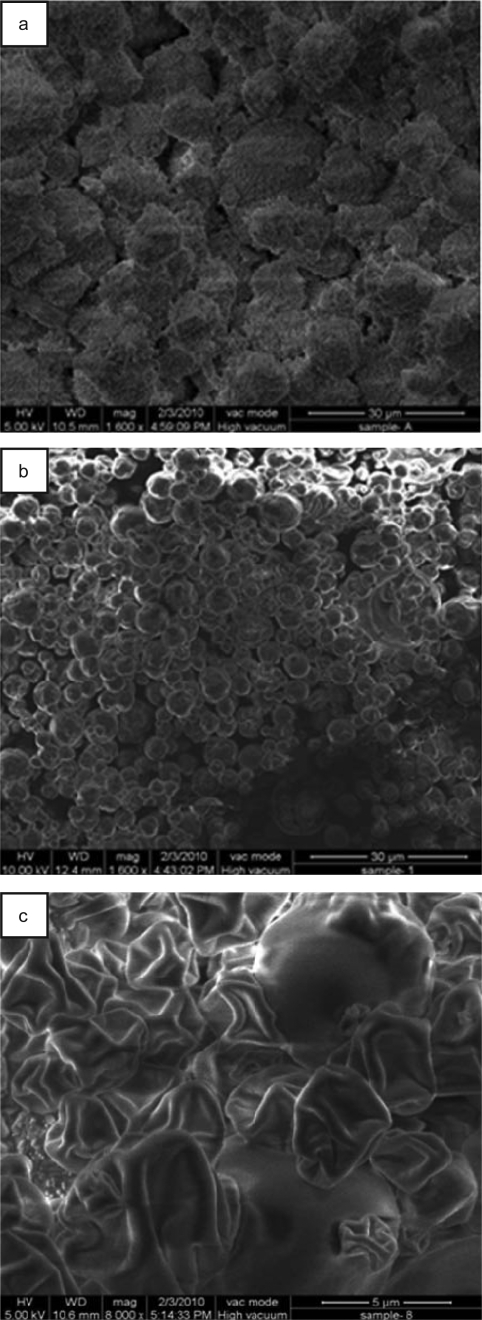
SEM images showed three populations, which differed with respect to their surface characteristics (Fig. 1a-c). Bare CaCO3 particles presented a rough, granular and irregular outline and porous structure (Fig. 1a), which could be due to inefficient stirring during their precipitation. Surface roughness of the template particles, was also desirable as it would help adsorption of deposited PEs to form a more stable multilayer shell (12). On the contrary, hollow (NaALG/CHI)5 microcapsules templated upon these particles were almost spherical. Their diameters typically ranged between 1 to 5 µm and exhibited entirely different surface profile (Fig. 1b), which could be explained as follows. Existence of surface charge resulted into inter-digitation among the neighboring PE chains. Assuming that excess of PEs were removed during washing, matching ratio of CHI and NaALG led to creation of a stable film Further, in the process of minimizing free surface energy, the overall composite structure oriented into a well formed spherical shape and thus, provided an even coverage to the template surface (13). However, upon drying, microcapsules collapsed, leading to creation of various indentations upon the surface (Fig. 1c). Also, some microcapsules deformed during drying which could be due to the sudden loss of water from their matrixes (14).
Figure 1.
Scanning electron microscopic image of: a) prepared CaCO3 particles (scale bar 30 µm), b) (CHI/NaALG)5 PEMs (scale bar 30 µm) and c) dried (CHI/NaALG)5 PEMs (scale bar 5 µm).
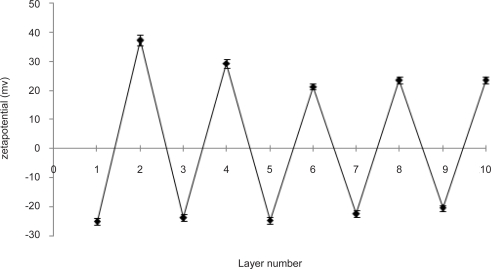
Alternating zeta potentials were observed up to 5 pair of deposited layers (Fig. 2), which were consistent to the literature reports (15). When NaALG formed the outermost layer, the ζ- value was negative (−20.53 to 25.17 mV). However, it was reversed to a positive value (ranging from 21.38 mV to 37.24 mV) when CHI layer was adsorbed. A significant decrease in the absolute value of zeta potential was observed after deposition of two initial bilayers; which remained constant thereafter. This could be attributed to the nature and density of residual charges borne by the respective PE chains (16). Thus, regular and stable charge reversal of ζ- value proved that deposition of NaALG/CHI multilayers onto CaCO3 microparticles was driven by the electrostatic interactions (17) and volume fractions of both PEs were proportionate.
Figure 2.
Change of zeta potential as a function of layer number during LbL adsorption.
Batches F1 (NaALG/CHI)2 (subscript indicates the number of deposition cycles), F2 (NaALG/CHI)5, F3 (cross-linked NaALG/CHI)5 provided EE of 34.63±1.31%, 39.24±1.9% and 47.19±1.04%, respectively. Difference in EE for each pair of formulations was significant (one way ANOVA, P<0.05). Among others, high aqueous solubility of INH could be a primary factor responsible for its lower EE. Additional studies were conducted upon supernatants to study the effect of washing upon EE of the formulations. It was observed that between first and second washing step, batches F1 and F2 suffered a drug loss of 7.1% and 3.9%, respectively. However, this effect remained insignificant in the subsequent washing steps. Batch F3 showed a drastic reduction in drug loss (1.2%). Crosslinking of PEs might have resulted into closing of pores and suppressed the drug loss which could presumably have otherwise occurred during washing of microcapsules.
In vitro drug release studies
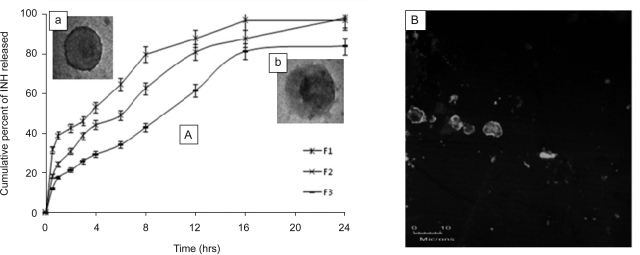
The release process of INH from the different PEMs in buffer systems of pH of 1.2 and 6.8 was studied as a function of time (Fig. 3). At the end of 24 hrs, more than 95% cumulative release was observed for F1 and F2; though it differed significantly at different time points. The pattern of drug release from the formulations could be explained on the basis of diffusion and erosion characteristics of the composite membrane. Batch F1 released more than 30% drug during first 30 min. High aqueous solubility of INH would have helped its free diffusion across the (NaALG/CHI)2 membrane and thereby, providing burst effect. However, release rate was significantly improved by increasing the number of bilayers and performing cross-linking of PEs. Formulation F2 showed a sustained release behavior up to 24 hrs, which was well in agreement with the findings of Li et al. (14). In the case of formulation F3, only 84% of the total drug was released in 24 hrs. It differed significantly from F1 and F2 (one way ANOVA; P<0.05) which could be due to the adsorption of remaining drug within the cross-linked PE complex.
Figure 3.
A) In vitro drug release of PEMs as a function of time: F1-un-crosslinked (CHI/NaALG)2, F2-un-crosslinked (CHI/NaALG)5, and F3-crosslinked (CHI/NaALG)5; B) confocal microscopic image of F2 after in vitro dissolution study.
To understand the mechanism of drug release, in vitro release data were fitted to Peppas equation (11, 18). The value of n for all the three formulations was found to be close to 0.7, which suggested that drug release occurred by both, Fickian diffusion and erosion. Further, TEM image of the formulation (F2), acquired after in vitro dissolution study, exhibited a distinct black shadow in the microcapsule center (inset b of Fig. 3). It signified that rather than being simply adhered onto the wall, drug was localized within the microcapsule interior and that the matrix was depleted to a greater extent than PE composite membrane. CLSM image also supplemented this observation (Fig. 3B). It demonstrated an alteration in the shape of microcapsules from spherical to irregularly shaped and broken micro-structures. The results were suggestive that core of microcapsules dissolved and eroded upon being subjected to in vitro dissolution.
Accelerated stability studies
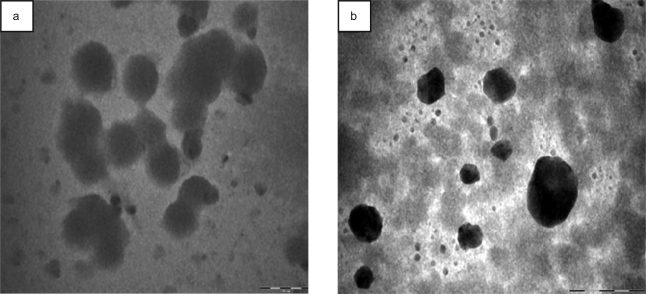
As it is revealed from TEM image, batch F2 exhibited considerable aggregation upon accelerated storage conditions (Fig. 4a). Besides a significant increase in the bulk pH of microcapsule suspension was observed. Deposition of PEs was performed within the pH range of 3.5 to 6. Formulation, however, was finally dispersed and stored in TDW (pH 7.0). Since the formulation was provided a hydrostatic immersion condition during storage, this change in microenvironment pH of microcapsules could be expected to influence the stability of PE complex as a function of time. Higher pH promoted the dissolution of NaALG containing carboxylate groups and consequently, overall charge distribution of PE complex was destabilized (19). As a result, there was an increased propensity of physical entanglement among the neighboring microcapsules. Besides, hydrogen bonding and other non specific interactive forces would also have contributed significantly to the aggregation and clustering. Batch F3, on the other hand, did not show any noticeable change either in release behavior or morphological characters (Fig. 4b). Their pH also remained unchanged. Stable ionic crosslinking of amino group of CHI with carboxylic acid group of NaALG restrained the swelling of PEMs. Thus, stability of crosslinked microcapsules under physiological pH conditions could open up a strong possibility of avoiding the use of stabilizer, which is a necessity in the conventional technique of formulating microcapsules.
Figure 4.
Transmission electron microscopic image of the formulations subjected to accelerated stability study: a) batch F2 and b) batch F3 (scale bar 2 µm).
CONCLUSION
This research demonstrated a successful formulation of multilayered microcapsules using LbL technique. The prepared microcapsules possessed defined size and morphology. Composite membrane constituted of biocompatible polyelectrolytes conferred them ability to carry a high payload of drug and release it in a controlled manner for a period of 24 hrs. Drug release rate and stability profile of the formulation could be improved by increasing the number of deposition steps and performing the cross-linking of PEs. Therefore, it could promisingly be utilized as a vehicle for controlled and efficacious delivery of isoniazid. Anchoring membrane specific components with PEs to achieve site specific targeting of the carrier system is the future plan of this study.
ACKNOWLEDGEMENTS
First author acknowledges the support of Dr. S.C. Lakhotia (Department of Zoology, Faculty of Science), Dr. O.N. Srivastava (Department of Physics, Faculty of Science) and Dr. Madhu Yashpal (Institute of Medical Sciences) of Banaras Hindu University for providing the facility of CLSM, SEM and TEM, respectively. Indian Council of Medical Research (ICMR), New Delhi is thankfully acknowledged for providing financial support in carrying out this research work.
REFERENCES
- 1.Volodkin DV, Balabushevitch NG, Sukhorukov GB, Larionova NI. Model system for controlled protein release: pH-sensitive polyelectrolyte microparticles. STP Pharm Sci. 2003;13:163–170. [Google Scholar]
- 2.Peyratout CS, Dahne L. Tailor-made polyelectrolyte microcapsules: from multilayers to smart containers. Angew Chem Int Ed. 2004;43:3743–3762. doi: 10.1002/anie.200300568. [DOI] [PubMed] [Google Scholar]
- 3.Antipov AA, Sukhorukov GB. Polyelectrolyte multilayer capsules as vehicles with tunable permeability. Adv Coll Inter Sci. 2004;111:49–61. doi: 10.1016/j.cis.2004.07.006. [DOI] [PubMed] [Google Scholar]
- 4.Szarpak A, Pignot-Paintrand I, Nicolas C, Picart C, Auzely-Velty R. Multilayer assembly of hyaluronic acid/poly(allylamine): control of the build up for the production of hollow capsules. Langmuir. 2008;24:9767–9774. doi: 10.1021/la801274z. [DOI] [PubMed] [Google Scholar]
- 5.Volodkin DV, Petrov AI, Prevot M, Sukhorukov GB. Matrix polyelectrolyte microcapsules: new system for macromolecule encapsulation. Langmuir. 2004;20:3398–3406. doi: 10.1021/la036177z. [DOI] [PubMed] [Google Scholar]
- 6.Tong W, Dong W, Gao C, Mohwald H. Charge-controlled permeability of polyelectrolyte microcapsules. J Phys Chem B. 2005;109:13159–13165. doi: 10.1021/jp0511092. [DOI] [PubMed] [Google Scholar]
- 7.Xu Y, Zhan C, Fan L, Wang L, Zheng H. Preparation of dual crosslinked alginate–chitosan blend gel beads and in vitro controlled release in oral site-specific drug delivery system. Int J Pharm. 2007;336:329–337. doi: 10.1016/j.ijpharm.2006.12.019. [DOI] [PubMed] [Google Scholar]
- 8.Geest BGD, Déjugnat C, Verhoeven E, Sukhorukov GB, Jonas AM, Plain J, Demeester J, Smedt SCD. Layer-by-layer coating of degradable microgels for pulsed drug delivery. J Control Rel. 2006;116:159–169. doi: 10.1016/j.jconrel.2006.06.016. [DOI] [PubMed] [Google Scholar]
- 9.Atyabi F, Majzoob S, Iman M, Salehi M, Dorkoosh F. In vitro evaluation and modification of pectinate gel beads containing trimethyl chitosan, as a multi-particulate system for delivery of water-soluble macromolecules to colon. Carbohyd Poly. 2005;61:39–51. [Google Scholar]
- 10.Philip AK, Singh N, Pathak K. Egg shell membrane as a substrate for optimizing in vitro transbuccal delivery of glipizide. Pharm Dev Tech. 2009;14:540–547. doi: 10.1080/10837450902832893. [DOI] [PubMed] [Google Scholar]
- 11.Mishra B, Arya N, Tiwari S. Investigation of formulation variables affecting the properties of lamotrigine nanosuspension using fractional factorial design. DARU. 2010;18:1–8. [PMC free article] [PubMed] [Google Scholar]
- 12.Ye S, Wang C, Liu X, Tong Z, Ren B, Zeng F. New loading process and release properties of insulin from polysaccharide microcapsules fabricated through layer-by-layer assembly. J Control Rel. 2006;112:79–87. doi: 10.1016/j.jconrel.2006.01.015. [DOI] [PubMed] [Google Scholar]
- 13.Dinsmore AD, Hsu MF, Nikolaides MG, Marquez M, Bausch AR, Weitz DA. Colloidosomes: selectively permeable capsules composed of colloidal particles. Science. 2002;298:1006–1009. doi: 10.1126/science.1074868. [DOI] [PubMed] [Google Scholar]
- 14.Li J, Mohwald H, An Z, Lu G. Molecular assembly of biomimetic microcapsules. Soft Matter. 2005;1:259–264. doi: 10.1039/b506092n. [DOI] [PubMed] [Google Scholar]
- 15.Sukhorukov GB, Donath E, Moya S, Susha AS, Voigt A, Hartmann J, Mohwald H. Microencapsulation by means of step-wise adsorption of polyelectrolytes. J Microencaps. 2000;17:177–185. doi: 10.1080/026520400288418. [DOI] [PubMed] [Google Scholar]
- 16.Chen Y, Lin X. Studies on the drug release properties nano-encapsulated indomethacin microparticles. J Microencap. 2005;22:47–55. doi: 10.1080/02652040500044972. [DOI] [PubMed] [Google Scholar]
- 17.An ZH, Tao C, Lu G, Mohwald H, Zheng SP, Cui Y, Li J. Fabrication and characterization of human serum albumin and l-(-dimyristoylphosphatidic acid microcapsules based on template technique. Chem Mater. 2005;17:2514–2519. [Google Scholar]
- 18.Costa P, Lobo JMS. Modeling and comparison of dissolution profiles. Eur J Pharm Sci. 2001;13:123–133. doi: 10.1016/s0928-0987(01)00095-1. [DOI] [PubMed] [Google Scholar]
- 19.Ribeiro AJ, Silva C, Ferreira D, Veiga F. Chitosan-reinforced alginate microspheres obtained through the emulsification/internal gelation technique. Eur J Pharm Sci. 2005;25:31–40. doi: 10.1016/j.ejps.2005.01.016. [DOI] [PubMed] [Google Scholar]