Abstract
Back ground and the purpose of study
Sumatriptan succinate is a Serotonin 5- HT1 receptor agonist, used in treatment of migraine. It is absorbed rapidly but incompletely when given orally and undergoes first-pass metabolism, resulting in a low absolute bioavailability of about 15%. The aim of this work was to design mucoadhesive bilayered buccal tablets of sumatriptan succinate to improve its bioavailability.
Methods
Mucoadhesive polymers carbopol 934 (Carbopol), HPMC K4M, HPMC K15M along with ethyl cellulose as an impermeable backing layer were used for the preparation of mucoadhesive bilayered tablets. In vivo bioavailability studies was also conducted in rabbits for optimized formulation using oral solution of sumatriptan succinate as standard.
Results
Bilayered buccal tablets (BBT) containing the mixture of Carbopol and HPMC K4M in the ratio 1:1 (T1) had the maximum percentage of in vitro drug release within 6 hrs. The optimized formulation (T1) followed non-Fickian release mechanism. The percentage relative bioavailability of sumatriptan succinate from selected bilayered buccal tablets (T1) was found to be 140.78%.
Conclusions
Bilayered buccal tablets of sumatriptan succinate was successfully prepared with improved bioavailability.
Keywords: Bilayered Buccal Tablets (BBT), Carbopol, HPMC K4M, HPMC K15M.
INTRODUCTION
Buccal delivery of drugs which exhibit a low oral bioavailability is a useful method for increasing bioavailability (1) and provides an attractive alternative to the oral route of drug administration, particularly in overcoming deficiencies associated with the latter mode of dosing. Problems such as high first-pass metabolism, and drug degradation in the harsh gastrointestinal environment, can be circumvented by administration of the drug via the buccal route (2). This route has been used successfully for the systemic delivery of a number of drug candidates (3). Moreover, buccal drug delivery offers a safe and easy method of drug utilization, because drug absorption can be promptly terminated in the case of toxicity by removing the dosage form from the buccal cavity. It is also an alternative route to administer drugs to patients who are unable to be dosed orally. Therefore, adhesive mucosal dosage forms are suggested for buccal delivery, including adhesive tablets (4), adhesive gels (5) and adhesive patches (3).
Sumatriptan succinate is 5-HT1receptor agonist used in the treatment of migraine. However, since a substantial proportion of patients suffer from severe nausea or vomiting during their migraine attacks, and low oral bioavailability (15%) because of high first-pass metabolism, may be oral treatment unsatisfactory. Nasal and subcutaneous routes have their own limitations, like lower retention time for nasal solution and inability of self administration for injectables respectively (6).
In the treatment migraine, therapy requires constant levels of the drug in the blood for an extended period, which can be achieved by design of buccal drug delivery system to deliver the drug via oral buccal mucosa.
The aim of this investigation was to determine pharmacokinetics of sumatriptan succinate from administered as BBT formulation of a dose of 10 mg.
MATERIAL AND METHODS
Sumatriptan succinate (Aurobindo Pharma Ltd, Medak) aspartame (Strides Arco Labs, Bangalore India) HPMC K4M and HPMC K15M (Colorcon Pvt limited, Goa) and Carbopol 934P (Mumbai India) were obtained as gift sample. All other chemicals and reagents used in the work were of analytical grades.
Compatibility studies
Compatibility studies of drug and polymers were studied using Fourier Transform Infrared (FTIR) spectroscopy and Differential scanning calorimetry (DSC) techniques. FTIR Spectrum was recorded between 600–4000 cm−1 using Bruker Tensor(ATR). DSC thermograms were recorded at a standard heating rate of 10°C/min over a temperature range 50–400°C using DSC-60 Shimadzu (Japan). Samples were treated under nitrogen atmosphere in order to eliminate oxidative and pyrolytic effects.
Preparation of BBT of sumatriptan succinate
Various batches of BBT were prepared by changing the ratio of carbopol, HPMC K4M, and HPMC K15M. The drug-polymer combination was mixed and triturated for 15min (Table 1) in a glass mortar to obtain homogeneous mixture. The powder mixture equivalent to 131mg was then compressed directly using an 8mm diameter die in a single-stroke multistation tablet machine (Karnavati mini press, India). Upper punch was raised and the backing layer of ethyl cellulose was placed on the above compact. Then 2 layers were compressed into a mucoadhesive bilayer tablet with a total weight of 151 mg/tablet (7).
Table 1.
Ingredients of bilayered buccal tablets of sumatriptan succinate.
| Formulationcode | Sumatriptan succinate (mg) | HPMC K4M (mg) | HPMC K15M (mg) | Carbopol 934P(mg) | Aspartame (mg) | MCC (mg) | Magnesium stearate (mg) | Ethyl cellulose (mg) |
|---|---|---|---|---|---|---|---|---|
| T1 | 10 | 25 | 75 | 1 | 18 | 2 | 20 | |
| T2 | 10 | 50 | 50 | 1 | 18 | 2 | 20 | |
| T3 | 10 | 75 | 25 | 1 | 18 | 2 | 20 | |
| T4 | 10 | 25 | 75 | 1 | 18 | 2 | 20 | |
| T5 | 10 | 50 | 50 | 1 | 18 | 2 | 20 | |
| T6 | 10 | 75 | 25 | 1 | 18 | 2 | 20 |
Evaluation of physical properties of BBT
The thickness, hardness, friability, weight uniformity and drug content uniformity were determined as per the procedure of Indian Pharmacopoeia (8).
Surface pH
The BBT were allowed to swell at 37±0.5°C for 2hrs in 40ml phosphate buffer (pH of 6.8) and surface pH of swollen BBT's was measured using pH paper (9).
Swelling study
Swelling properties of tablets were evaluated by determination of the percentage of hydration (10) and calculated according to the following equation:
| 1 |
Ex Vivo mucoadhesive strength and mucoadhesion Time
Ex vivo adhesion strength is the force in grams required to pull out a tablet from sheep buccal mucosa (11). Time required to detach or erode BBT from the sheep buccal mucosa was taken as ex-vivo mucoadhesion time in hours (12).
In vitro dissolution studies
The United States Pharmacopoeia (USP) XXIII method was used to study the drug release from the tablets (13). Each BBT tablet was attached on a glass plate with cyanoacrylate adhesive. The glass plate was then placed in a dissolution tester. The experiments were performed at 37±0.5°C using the paddle method at 50 rpm with 500 ml of phosphate buffer of pH 6.8 as a dissolution medium. Samples equivalent to 5 ml was withdrawn for every one hour, filtered and analyzed at 227 nm using UV-Visible spectrophotometer (Shimadzu, Kyoto-1700, Japan).
Release data were fitted to various mathematical models Korsmeyer-Peppa's [Eq 1]14, zero order [Eq 2] (15) and Higuchi release models [Eq 3]16 in order determine the release mechanism from BBT.
In vivo study
The experimental protocol for all In vivo studies was approved by institutional animal ethical committee (997/c/06/ CPCSEA).
White male rabbits were fasted for 24 hrs before drug administration and sedated using ketamine: lignocaine (1:5) mixture. A bioadhesive tablet was fixed in the buccal position of the oral cavity. Blood samples were withdrawn from the ear vein in the eppendorf tubes containing sodium EDTA of 10l (10% w/v) at time interval of 0.5–12 and 24hrs, analysed by LC-MS/MS. For oral administration, 10mg doses in 25ml of aqueous solution were administered by a stomach tube (17).
Estimation of sumatriptan succinate from plasma samples
Centrifuge a mixture of 100 l plasma sample and 600 l of acetonitrile at 13000 rpm for 4min and inject 10l supernatant solution at a flow rate of 1.2 ml/min and at 25°C with mobile phase consists of 60% formic acid in water (0.05%): formic acid in acetonitrile (0.05%) and telmisartan as internal standard. The ions monitored using multiple reactions monitoring (MRM), were m/z 296.1→251.1 for sumatriptan succinate and m/z 515.2→497.3 for telmisartan. Pharmacokinetic data were prepared by using software Sciex Analyst version 1.4.2.
RESULTS AND DISCUSSIONS
Drug polymer compatibility studies using FTIR and DSC
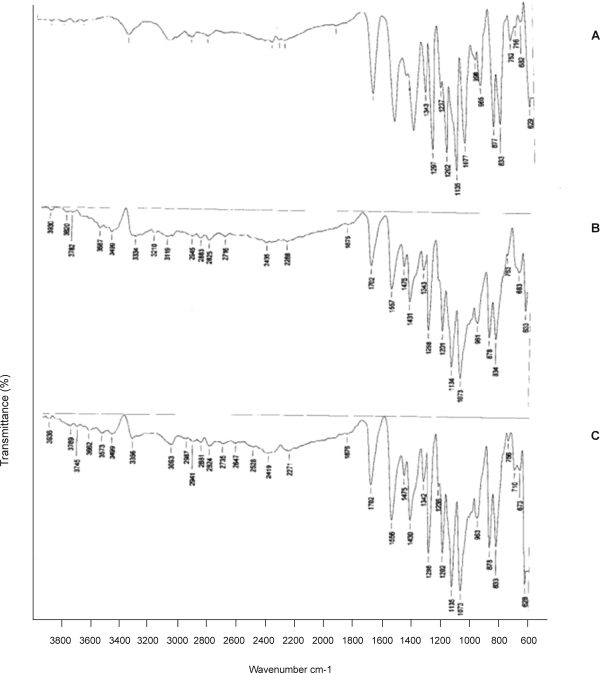
FTIR studies revealed that the characteristic absorbance bands of various functional groups of sumatriptan succinate were found in the vicinity of standard absorbance range (Fig. 1). Hence the FTIR studies indicated that there was no interaction between drugs and polymers under study.
Figure 1.
FTIR Spectra of formulations of Sumatriptan succinate A. Sumatriptan succinate, B. Sumatriptan succinate+HPMCK4M+Carbopol 394, C. Sumatriptan succinate+HPMCK15M+Carbopol 394
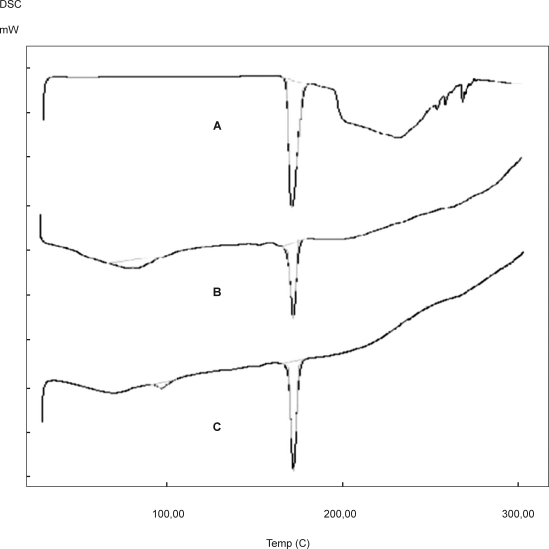
DSC studies revealed that the drug exhibit sharp melting endotherm at 170.30°C and thermograms of the physical mixture of sumatriptan succinate with polymers exhibited endothermic peak in the vicinity of its melting point range, indicates absence of any drug-polymer interactions (Fig. 2).
Figure 2.
DSC Thermograms of formulations of Sumatriptan succinate. A. Sumatriptan succinate, B. Sumatriptan succinate+HPMCK4M+Carbopol 394, C. Sumatriptan succinate+HPMCK15M+Carbopol 394.
Physical properties of BBT
The average weight, thickness, hardness and friability were found within the limits of IP (8). Whereas drug content (%) of all formulations were in the range of 99.24–97.89%. The surface pH of BBT was found to be 6–7 which indicates that there will not be any local irritation to the buccal mucosa.
Swelling index decreased by decrease in the concentration of carbopol which is evident from the determined mean swelling values of 95%, 80% and 71% after 2 hrs for the formulation T1-T3. In the case of formulations T4-T6 the mean swelling values were 73%, 61%, and 62% after 2 hrs, the swelling indices of the tablets with carbopol and HPMC increased by increase in the amounts of carbopol.
Ex-Vivo bioadhesive strength and Mucoadhesion Time
The tablets with the HPMC K4M, carbopol had bioadhesive strength between 17.5–21g and with HPMC K15M, carbopol had bioadhesive strength 15.6–20.4g (Table 2). The bioadhesive strength exhibited by the HPMC K4M tablets can be considered satisfactory for their maintainance in the oral cavity.
Table 2.
Physicochemical properties of bilayered buccal tablets of sumatriptan succinate.
| Formulation code | Thickness (mm)* | Average weight of tablet (mg)±SD | Hardness(kg/cm2)±SD | Friability (%)* | Drug content (%)±SD | Surface pH* | Mucoadhesion time (h)±SD | Mucoadhesion strength (g)±SD |
|---|---|---|---|---|---|---|---|---|
| T1 | 3.12 | 151±1.43 | 5.2±0.14 | 0.463 | 98.74±1.74 | 6 | 12.0±1.25 | 21.0±1.32 |
| T2 | 3.12 | 151±1.18 | 5.2±0.17 | 0.453 | 97.85±1.35 | 6 | 10.0±0.50 | 19.0±0.20 |
| T3 | 3.11 | 150±1.72 | 5.4±0.18 | 0.411 | 98.00±2.20 | 7 | 09.0 ±1.30 | 17.5±0.97 |
| T4 | 3.05 | 151±1.26 | 5.4±0.23 | 0.372 | 98.86±3.40 | 6 | 12.0±1.35 | 20.4±0.60 |
| T5 | 3.05 | 149±1.56 | 5.4±0.11 | 0.449 | 99.01±2.01 | 6 | 10.0±0.30 | 17.5±1.45 |
| T6 | 3.04 | 150±1.21 | 5.6±0.16 | 0.403 | 98.79±1.92 | 7 | 09.0±0.40 | 15.6±1.90 |
Average of three determinations±SD
Average of three determinations
Table 3.
Kinetic analyse of the release of bilayered buccal tablets of sumatriptan succinate.
| Formulation code | Korsmeyer Peppa's | Zero order | First order | Higuchi | t50% (h) | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| K | R 2 | n | K | R 2 | K | R 2 | K | R 2 | ||
| T1 | 15.24 | 0.997 | 0.817 | 10.86 | 0.992 | −0.17733 | 0.986 | 27.30 | 0.949 | 4.20 |
| T2 | 20.23 | 0.984 | 0.606 | 9.726 | 0.964 | −0.15430 | 0.980 | 25.15 | 0.976 | 4.60 |
| T3 | 17.90 | 0.993 | 0.644 | 9.187 | 0.970 | −0.13818 | 0.991 | 23.71 | 0.978 | 4.70 |
| T4 | 15.24 | 0.994 | 0.788 | 10.44 | 0.993 | −0.16582 | 0.990 | 26.23 | 0.948 | 4.45 |
| T5 | 16.33 | 0.964 | 0.681 | 9.409 | 0.984 | −0.14279 | 0.979 | 23.75 | 0.948 | 4.90 |
| T6 | 16.40 | 0.992 | 0.638 | 8.086 | 0.957 | −0.11515 | 0.987 | 21.10 | 0.987 | 5.80 |
Table 4.
Comparative pharmacokinetics parameters of sumatriptan succinate after oral and buccal administration in rabbits.
| Pharmacokinetic parameter | Pure drug | T1 |
|---|---|---|
| Ke (h−1) | 1.91 | 1.46 |
| Cmax (ng/ml) | 482.20±22.5 | 386.00±15.80 |
| Tmax (h) | 2.0 | 2.0 |
| AUC(0-24) (ng.h/ml) | 1199.64±150.60 | 1690.69±90.16 |
| AUC(0-8) (ng.h/ml) | 1200.90±150.60 | 1693.90±91.50 |
The mucoadhesive times on sheep buccal mucosa were 7–12 hrs. The increase in concentration of carbopol in series from formulation T1-T6, showed a gradual rise in mucoadhesion time, while HPMC K4M, HPMC K15M were also a good mucoadhesive polymers, showed a decrease in mucoadhesion time (Table 2).
In vitro dissolution studies
Release of drug from the BBT varied according to the type and ratio of matrix-forming polymer. Carbopol has excellent mucoadhesive, gelling properties and also helps in sustaining effect. Combination of carbopol and HPMC are hydrophilic swellable polymer matrices, which are able to form a viscous gel layer which controls the drug release via diffusion through the gel and erosion of gel barrier (18).
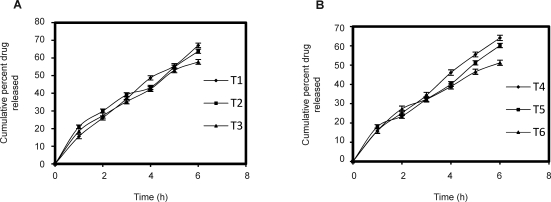
The cumulative drug release at the end of 6th hour for formulations T1 and T4 were 67.73%, 64.11%, respectively (Fig. 3). The results indicate that the rate of drug release was higher for T1 formulation which may be due to rapid ionization of carbopol (19). The rate of drug release decreased by increase in the concentration of HPMC K4M which may be due to the increase in viscosity produced by the gelling of the hydrophilic polymer HPMC K4M.
Figure 3.
Dissolution profiles of bilayered buccal tablets of sumatriptan succinate. (A) HPMC K4M: Carbopol 934P, (B) HPMC K15M: Carbopol 934P (Mean±SD of three determinations).
For non-Fickian release, the value of n falls between 0.5 and 1.0, while in the case of Fickian diffusion, n=0.5; for zero order release (case II transport), n=1; and for supercase II transport, n is greater than 1. All of these formulations exerted non-fickian diffusion mechanism with n value varied from 0.604–0.817. This is an indicative of both diffusion and chain relaxation mechanism which is a prerequisite for sustained drug release effect. Based on these results T1 formulation was selected for further studies.
In vivo bioavailability studies in rabbits
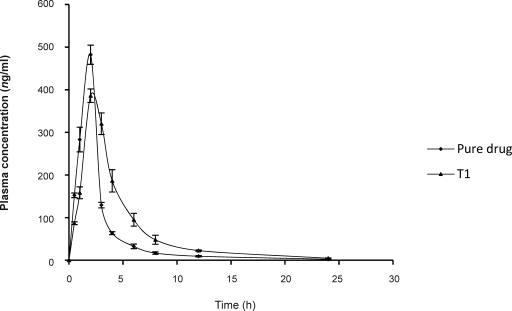
The mean plasma concentration of sumatriptan succinate at different time intervals following the application of BBT and after oral administration of solution in rabbits is shown in (Fig. 4).
Figure 4.
Plasma concentration time profile of sumatriptan succinate after oral and buccal administration in rabbits (Mean±SD of three determinations).
Following oral administration of sumatriptan succinate (10 mg) in solution form, average maximum serum concentration (Cmax) 482.20±22.5 ng/ml was achieved after 2 hrs and the area under the serum concentration-time curve (AUC(0-8)) after oral dosing was found to be 1200.90±150.60 ng/ml. After administration of T1 formulation the drug levels in serum were detectable till 12 hrs with Cmax 386.00±15.80 ng/ml achieved 2 hrs after dosing and the AUC(0-8) following buccal administration of sumatriptan succinate 1693.90±91.50 ng/ml. Relative bioavailability of sumatriptan succinate following buccal administration was found to be 140.78% which could be due to reduced first pass metabolism, when it is administered via buccal route.
CONCLUSIONS
Developed BBT of sumatriptan succinate may overcome the disadvantage of poor and erratic oral bioavailability of sumatriptan succinate associated with marketed formulations. This increased predictable availability of sumatriptan succinate from designed formulation may result in substantial dose reduction.
ACKNOWLEDGEMENT
The authors are thankful to Dr Goli Divakar Principal Acharya B.M Reddy College of Pharmacy, Bangalore for providing the facility to carry out the research work. The authors are thankful to Aurobindo Pharma ltd, Medak, Andhra Pradesh for providing the gift sample of sumatriptan succinate.
REFERENCES
- 1.Ikinci G, Senel S, Wilson CG, Sumnu M. Development of a buccal bioadhesive nicotine tablet formulation for smoking cessation. Int J Pharm. 2004;277:173–178. doi: 10.1016/j.ijpharm.2003.10.040. [DOI] [PubMed] [Google Scholar]
- 2.Wong CF, Yuen KH, Peh KK. Formulation and evaluation of controlled release Eudragit buccal patches. Int J Pharm. 1999;178:11–22. doi: 10.1016/s0378-5173(98)00342-1. [DOI] [PubMed] [Google Scholar]
- 3.Anders R, Merkle HP. Evaluation of laminated mucoadhesive patches for buccal drug delivery. Int J Pharm. 1989;49:231–240. [Google Scholar]
- 4.Dortunc B, Ozer L, Uyanik N. Development and in vitro evaluation of a buccoadhesive pindolol tablet formulation. Drug Dev Ind Pharm. 1998;24:281–288. doi: 10.3109/03639049809085621. [DOI] [PubMed] [Google Scholar]
- 5.Ishida M, Nambu N, Nagai T. Highly viscous gel ointment containing Carbopol for application to the oral mucosa. Chem Pharm Bull (Tokyo) 1983;31:4561–4564. doi: 10.1248/cpb.31.4561. [DOI] [PubMed] [Google Scholar]
- 6.Brunton LL, Parker KL, Blumenthal DK, Buxton ILO, editors. New Delhi: McGraw-Hill; 2008. Goodman and Gilaman's Manual of Pharmacology and Therapeutics 11th ed; pp. 164pp. 194–208.–347. [Google Scholar]
- 7.Dhiman MK, Yedurkar PD, Sawant KK. Buccal bioadhesive system of 5-fluorouracil: Optimization and characterization. Drug Dev Ind Pharm. 2008;34:761–70. doi: 10.1080/03639040801926337. [DOI] [PubMed] [Google Scholar]
- 8.Pharmacopoeia of India. New Delhi. Controller of Publications: Government of India; 1996. India: Ministry of Health and Welfare. [Google Scholar]
- 9.El-Samaligy MS, Yahia SA, Basalious EB. Formulation and evaluation of diclofenac sodium buccoadhesive discs. Int J Pharm. 2004;286:27–39. doi: 10.1016/j.ijpharm.2004.07.033. [DOI] [PubMed] [Google Scholar]
- 10.Perioli L, Ambrogi V, Giovagnoli S, Ricci M, Blasi P, Rossi C. Mucoadhesive bilayered tablets for buccal sustained release of flurbiprofen. AAPS PharmSciTech. 2007;8:E54. doi: 10.1208/pt0802034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Khanna R, Agarwal SP, Ahuja A. Preparation and evaluation of bioerodible buccal tablets containing clotrimazole. Int J Pharm. 1996;138:67–73. [Google Scholar]
- 12.Shidhaye SS, Thakkar PV, Dand NM, Kadam VJ. Buccal drug delivery of pravastatin sodium. AAPS PharmSciTech. 2010;11:416–424. doi: 10.1208/s12249-010-9381-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Khanna R, Agarwal SP. Ahuja A. Preparation and evaluation of bioerodible buccal tablets containing clotrimazole. Int J Pharm. 1996;138:67–73. [Google Scholar]
- 14.Korsmeyer RW, Gurny R, Doelker E, Buri P, Peppas NA. Mechanisms of potassium chloride from compressed, hydrophilic, polymeric matrices: effect of entrapped air. J Pharm Sci. 1983;72:1189–1191. doi: 10.1002/jps.2600721021. [DOI] [PubMed] [Google Scholar]
- 15.Lee PI. Novel approach to zero-order drug delivery via immobilized nonuniform drug distribution in glassy hydrogels. J Pharm Sci. 1984;73:1344–1347. doi: 10.1002/jps.2600731004. [DOI] [PubMed] [Google Scholar]
- 16.Higuchi T. Mechanism of Sustained- Action Medication Theoretical Analysis of Rate of Release of Solid Drugs Dispersed in Solid Matrices. J Pharm Sci. 1963;52:1145–1149. doi: 10.1002/jps.2600521210. [DOI] [PubMed] [Google Scholar]
- 17.Charde S, Mudgal M, Kumar L, Saha R. Development and evaluation of bucco adhesive controlled release tablets of lercanidipine. AAPS PharmSciTech. 2008;9:182–190. doi: 10.1208/s12249-007-9031-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Dhiman MK, Yedurkar PD, Sawant KK. Buccal bioadhesive system of 5-fluorouracil: Optimization and characterization. Drug Dev Ind Pharm. 2008;34:761–770. doi: 10.1080/03639040801926337. [DOI] [PubMed] [Google Scholar]
- 19.Kumar TM, Shivakumar HG. Novel core in cup buccoadhesive systems and films of terbutaline sulphate-development and in vitro evaluation. Asian J Pharm Sci. 2006;1(3-4):175–187. [Google Scholar]