Abstract
Recent new findings indicate significant biological roles of cysteine cathepsin proteases in secretory vesicles for production of biologically active peptides. Notably, cathepsin L in secretory vesicles has been demonstrated as a key protease for proteolytic processing of proneuropeptides (and prohormones) into active neuropeptides that are released to mediate cell-cell communication in the nervous system for neurotransmission. Moreover, cathepsin B in secretory vesicles has been recently identified as a β-secretase for production of neurotoxic β-amyloid (Aβ) peptides that accumulate in Alzheimer’s disease (AD), participating as a notable factor in the severe memory loss in AD. These secretory vesicle functions of cathepsins L and B for production of biologically active peptides contrasts with the well-known role of cathepsin proteases in lysosomes for the degradation of proteins to result in their inactivation. The unique secretory vesicle proteome indicates proteins of distinct functional categories that provide the intravesicular environment for support of cysteine cathepsin function. Features of the secretory vesicle protein systems insure optimized intravesicular conditions that support the proteolytic activity of cathepsins. These new findings of recently discovered biological roles of cathepsins L and B indicate their significance in human health and disease.
Keywords: cathepsin L, cathepsin B, secretory vesicle, peptide neurotransmitters, β-amyloid, Alzheimer’s disease, proteome
1. Introduction - Cysteine cathepsins in secretory vesicles for production of active peptides for neurotransmission and in Alzheimer’s disease
Cathepsin proteases participate in protein degradation in lysosomes, resulting in inactivation of protein functions. However, new research findings indicate novel biological functions of cathepsins in secretory vesicles. The novel secretory vesicle role of the cysteine protease cathepsin L has been demonstrated for production of active peptides required for cell-cell communication in the nervous and endocrine systems [1–8]. Significantly, cathepsin B in secretory vesicles produces neurotoxic β-amyloid (Aβ) [9–16] that is known to be a major factor involved in the development of Alzheimer’s disease (AD) that results in severe memory loss [17–19]. Inhibitors of cathepsin B result in reduced brain levels of Aβ peptides [11–16] and substantial improvement in memory deficits in an Alzheimer’s mouse model [13, 15], indicating cathepsin B as a logical drug target for AD. This review article explains the interdisciplinary strategies in cell biology, chemical biology, and genetics utilized for discovery of new biological functions of secretory vesicle cathepsins L and B for the biosynthesis of active peptides. Furthermore, the secretory vesicle proteome that supports these functions of cathepsins L and B have been investigated to gain insight into the unique intravesicular environment for production of active peptides by these cysteine cathepsin proteases.
2. Cathepsin L in secretory vesicles for the biosynthesis of peptide neurotransmitters and hormones
2.1. Summary
Secretory vesicles in neurons and endocrine cells provide regulated secretion of biologically active small peptides for cell-cell communication. Peptides comprise the majority of neurotransmitters and hormones among physiological systems. These peptide neurotransmitters and hormones are first synthesized as proprotein precursors that must undergo limited proteolysis to generate active peptides. Proteolytic processing of the proproteins occurs primarily within the secretory vesicle organelle and, thus, the search for such proteases has recently identified the cysteine protease cathepsin L as a key processing enzyme for production of numerous peptide neurotransmitters [1–8]. Cathepsin L produces substantial portions of brain peptide neurotransmitters, known as neuropeptides, that include enkephalin, NPY (neuropeptide Y), dynorphins, cholecystokinin, and others as demonstrated by in vivo cathepsin L gene knockout and cellular cathepsin L gene expression studies. The chemical biological and genetic strategies utilized to identify cathepsin L as a proneuropeptide processing enzyme in secretory vesicles is covered by this review.
2.2. Neuropeptides mediate cell-cell communication in the nervous and endocrine systems
Peptide neurotransmitters are essential for activity-dependent neurotransmission in the nervous system. Many neuropeptides also function in peripheral systems for endocrine regulation of physiological functions. Moreover, the nervous and endocrine systems communicate with one another via these neuropeptides.
Production of neuropeptides requires proteolytic processing of their precursor proteins to result in a multitude of distinct peptides with diverse physiological actions such as enkephalin for opioid peptide regulation of analgesia [20, 21], ACTH induction of steroid synthesis [22], galanin regulation of cognition [23], neuropeptide Y regulation of feeding behavior [24, 25], and numerous other functions (Table 1). The primary structures for proneuropeptides indicate that neuropeptides are typically flanked at their NH2- and COOH-termini by pairs of basic residues, and sometimes by monobasic residues [5, 26, 27] (Figure 1). These multi-basic and monobasic residues are cleaved to generate the active neuropeptides. Clearly, proteolytic pathways represent key steps for the biosynthesis of essential peptide neurotransmitters and hormones.
Table 1.
Neuropeptides in the Nervous and Endocrine Systems
| Neuropeptide | Regulatory Function |
|---|---|
| enkephalin | analgesia |
| β-endorphin | analgesia |
| dynorphin | analgesia |
| ACTH | steroid production |
| α-MSH | skin pigmentation |
| insulin | glucose metabolism |
| glucagon | glucose metabolism |
| galanin | cognition |
| NPY | blood pressure (peripheral) and obesity (CNS) |
| cholecystokinin | anxiety, cognition, analgesia |
| somatostatin | growth regulation |
| vasopressin | water balance |
Peptide neurotransmitters and hormones are collectively termed neuropeptides.
Examples of several neuropeptides and their primary regulatory functions are listed.
Abbrevations are adrenocorticotropin hormone (ACTH), α-melanocyte stimulating hormone (α-MSH), and neuropeptide Y (NPY).
Figure 1. Structural Features of Proneuropeptides.
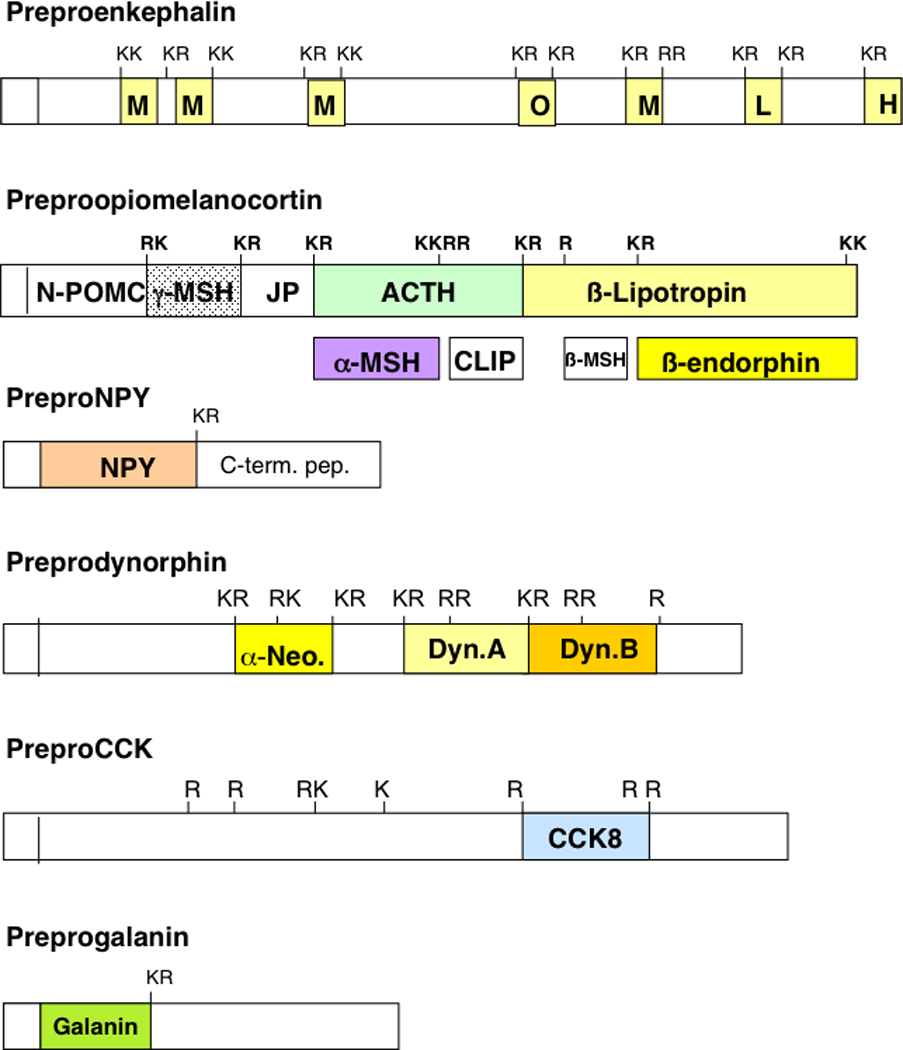
Prohormone precursor protein structures indicate that active peptide neurotransmitters and hormones are flanked by multi-basic residues that represent sites of proteolytic processing to generate active neuropeptides. The precursor proteins are shown for preproenkephalin, preproopiomelanocortin, preproNPY (NPY, neuropeptide Y), preprodynorphin, preproCCK (CCK, cholecystokinin), and preprogalanin. The NH2-terminal signal sequence is known to be cleaved by signal peptidases at the RER (rough endoplasmic reticulum) and the resultant prohormone undergoes trafficking to Golgi apparatus and packaging into secretory vesicles where prohormone processing occurs.
Proteolytic processing of proneuropeptides may occur at one of three positions at paired basic processing sites. These cleavages may consist of processing at the COOH- and NH2-termini of the dibasic residues, or between the dibasic residues (Figure 2). Processing at the dibasic residue sites is known to be achieved by two distinct pathways consisting of the recently discovery cathepsin L cysteine protease pathway combined with the well known subtilisin-like prohormone convertase pathway utilizing primarily PC/13 and PC2 prohormone convertase members of the PC family [5, 26, 27] Resultant peptide intermediates require removal of basic residues from COOH- and/or NH2-termini by carboxypeptidase and aminopeptidase enzymes, respectively.
Figure 2. Cathepsin L and Proprotein Convertase Pathways for Neuropeptide Production.
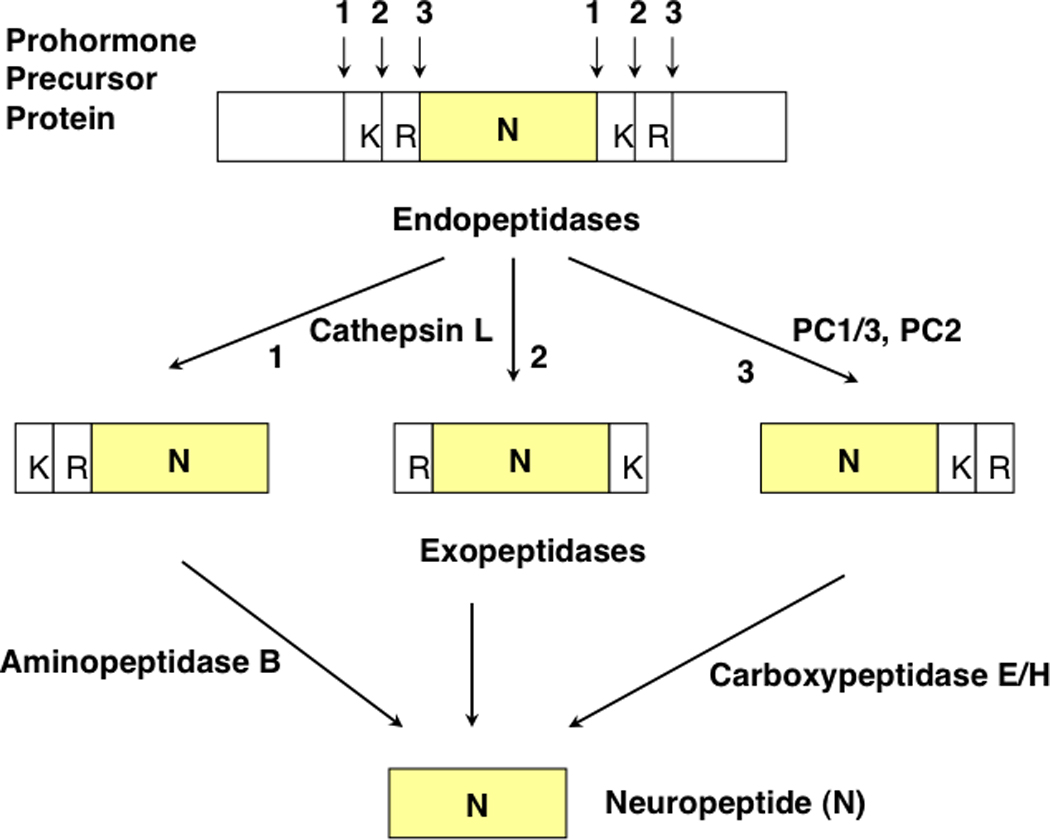
Proneuropeptides typically contain active peptides flanked by paired basic residues. The dibasic processing sites undergo proteolytic cleavage at one of three sites (numbered 1, 2, and 3) which consist of cleavage at the NH2- or COOH-terminal sides of the dibasic residues, or between the dibasic residues. Peptide intermediates generated by cleavage at the NH2-terminal side of the dibasic site, or between the dibasic residues, will then require Arg/Lys aminopeptidase, represented by aminopeptidase B, to remove basic residues at the NH2-termini. Cleavage of proneuropeptides at the COOH-terminal side of dibasic residues then requires carboxypeptidase E to remove NH2-terminal basic residues [1, 5].
2.3. Strategy for identification of cathepsin L for processing proenkephalin by activity-based profiling and mass spectrometry
The strategy to elucidate the major proneuropeptide-cleaving activity in secretory vesicles was to determine the protease subclass for activity cleaving proenkephalin and then to identify the responsible protease(s) by activity-probe labeling and mass spectrometry [2]. Neuropeptide secretory vesicles of the sympathetic nervous system in chromaffin cells were utilized for purification of the proenkephalin cleaving activity. The activity was substantially inhibited by selective inhibitors of cysteine proteases [1, 2].
Chemical biology has developed sophisticated activity-based probes for identification of protease and enzyme families [28]. Activity-based profiling of active cysteine proteases was instrumental for identification of the protease responsible for proenkephalin cleaving activity in chromaffin granules. The activity probe DCG-04, the biotinylated form of E64c that inhibits cysteine proteases, was utilized for specific affinity labeling of the the proenkephalin cleaving enzyme activity (Figure 3a–c) [2]. Two-dimensional gels resolved DCG-04 labeled proteins which were identified as cathepsin L by mass spectrometry (Figure 3d). These findings suggested a new biological function for cathepsin L in secretory vesicles for producing neuropeptides.
Figure 3. Cathepsin L is Identified as the Proenkephalin-Cleaving Activity in Secretory Vesicles.
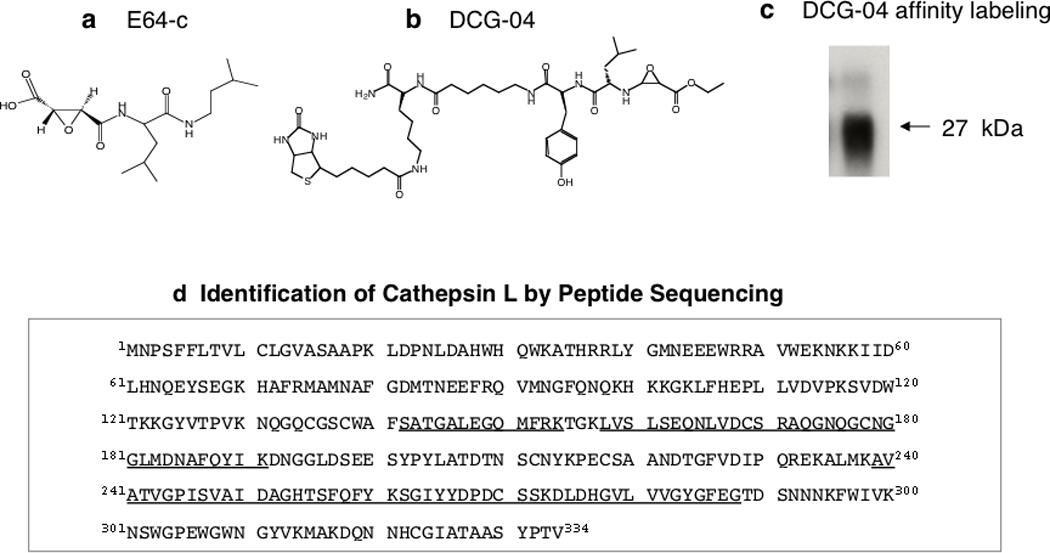
(a) E64-c cysteine protease inhibitor. The cysteine protease inhibitor E64-c was found to be a potent inhibitor of the proenkephalin (PE) – cleaving activity in secretory vesicles isolated from adrenal medullary chromaffin cells of the sympathetic nervous system [2].
(b) Structure of DCG-04, an activity-based probe for cysteine proteases. The modified cysteine protease inhibitor DCG-04 [2, 93], resulting from biotinylation of E64-c, was utilized for affinity-labeling of PE-cleaving activity in secretory vesicles.
(c) DCG-04 affinity labeling of cysteine protease activity in secretory vesicles. DCG-04 affinity labeling of purified PE-cleaving activity reveals a 27 kDa protein band. This band was subjected to peptide sequencing by tryptic digestion and tandem mass spectrometry.
(d) Identification of purified PE-cleaving activity as cathepsin L by mass spectrometry for peptide sequencing. Peptides derived from tryptic digests of DCG-04 affinity labeled 27 kDa proteins, sequenced by CID (MS/MS) mass spectrometry, are illustrated as the underlined amino acid sequences of bovine cathepsin L.
2.4. Secretory vesicle localization of cathepsin L with neuropeptides
The identification of cathepsin L in secretory vesicles indicated the novel localization of cathepsin L in this organelle. Confirmation of the localization of cathepsin L within secretory vesicles was demonstrated by immunofluorescence confocal microscopy and immunoelectron microscopy (Figure 4) [2, 3]. Furthermore, cathepsin L undergoes cosecretion with enkephalin from regulated secretory pathway [2]. Cellular routing and trafficking of cathepsin L was demonstrated by coexpression of cathepsin L with proenkephalin in neuroendocrine PC12 cells (derived from rat adrenal medulla). Expression of cathepsin L resulted in its trafficking to secretory vesicles that contain enkephalin and neuropeptides [29]. These findings indicated the novel location of cathepsin L in neuropeptide-containing secretory vesicles.
Figure 4. Localization of cathepsin L within enkephalin-containing secretory vesicles.
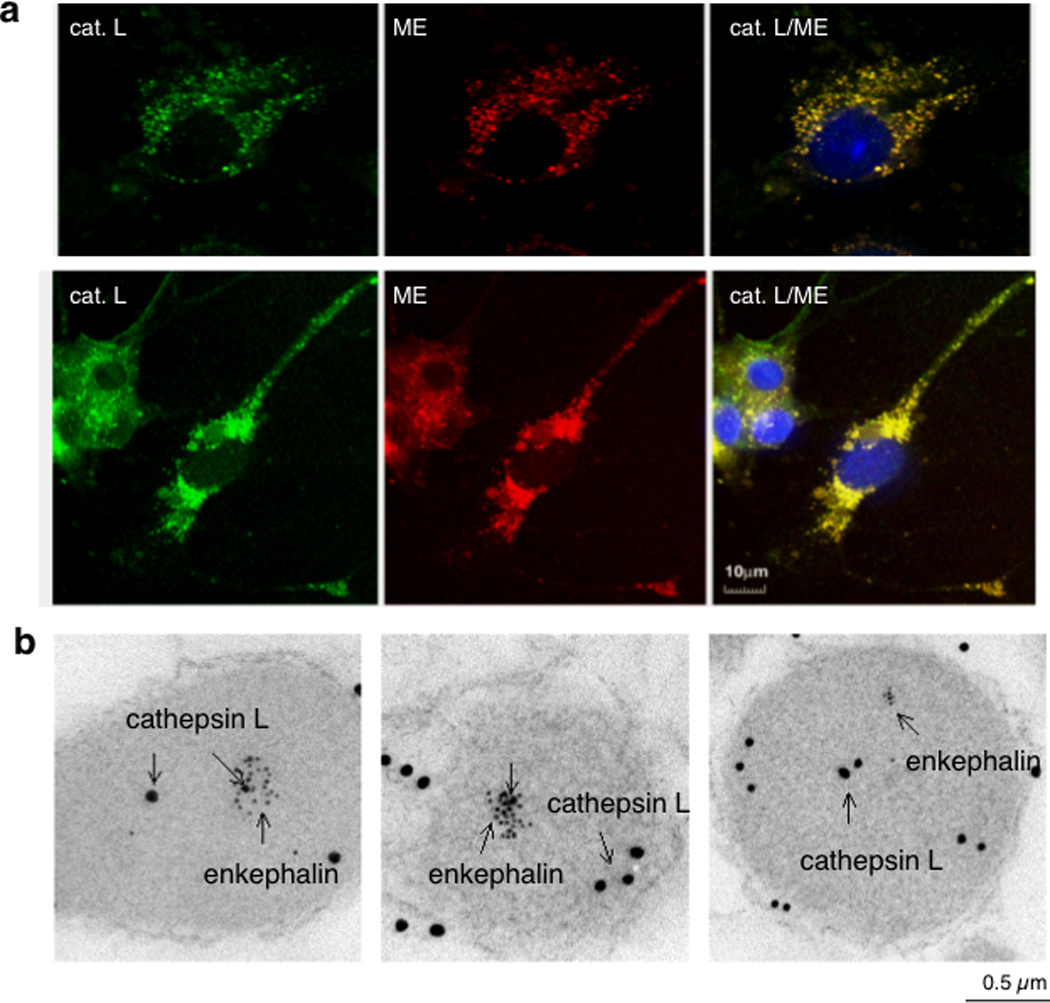
(a) Cathepsin L colocalization with (Met)enkephalin (ME) by immunofluorescence confocal microscopy. Immunofluorescence localization of cathepsin L (cat. L) was assessed by anti-cathepsin L detected with anti-rabbit IgG-Alexa 488 (green fluorescence), and ME was detected with anti-ME and anti-mouse IgG-Alexa 594 (red) [2]. Colocalization is illustrated by overlay of the images, illustrated by yellow fluorescence. Nuclei are illustrated by DAPI blue staining.
(b) Immunoelectron microscopy demonstrates colocalization of cathepsin L and (Met)enkephalin in secretory vesicles. Cathepsin L in secretory vesicles was indicated by anti-cathepsin L detected with 15 nm colloidal gold conjugated anti-rabbit IgG, and ME was detected with anti-ME and 6 nm colloidal gold conjugated to anti-mouse IgG. The presence of both 15 and 6 nm gold particles within these vesicles demonstrated the in vivo colocalization of cathepsin L and ME.
2.5. Cleavage specificity of cathepsin L for dibasic residue sites of proneuropeptides
Cathepsin L cleaves at dibasic and monobasic residue sites of proneuropeptides. Endogenous secretory vesicle cathepsin L cleaves proenkephalin (PE) at such dibasic residue sites. Cathepsin L generated (Met)enkephalin from the PE-derived intermediate BAM-22P via cleavage at the dibasic ↓Arg-↓Arg and monobasic ↓Arg sites, and the PE-derived peptide F intermediate was cleaved by cathepsin L at dibasic ↓Lys-↓Lys and ↓Lys-Arg sites [2, 30]. Further cleavage studies with peptide-MCA substrates showed that cathepsin L cleaves at the COOH-terminal side of the dibasic sites, as well as at the N-terminal side of basic residues [31]. Cathepsin L generates peptide intermediates with basic residue extensions at NH2- and COOH-termini, which are then removed by aminopeptidase B and carboxypeptidase E exopeptidases, respectively (Figure 2). These exopeptidases have been shown to participate in neuropeptide biosynthesis in secretory vesicles [5, 32–34]. These basic residue cleavage specificities of cathepsin L are appropriate for processing proprotein precursors into active neuropeptides.
2.6. Cathepsin L gene knockout and expression demonstrate its role in producing neuropeptides
2.6.1. Production of the enkephalin opioid neuropeptide by cathepsin L
The in vivo role of cathepsin L in enkephalin peptide production was assessed in cathepsin L gene knockout mice. Brain levels of (Met)enkephalin (ME) were reduced by ~ 50% in cathepsin L knockou mice compared to wild-type controls [2]. Brains contained a higher ratio of proenkephalin/ME in brain, indicating retarded proenkephalin processing. Thus, the knockout results demonstrate the in vivo function of cathepsin L for enkephalin neuropeptide production.
Studies of cathepsin L expression showed that cathepsin L participates in cellular processing of proenkephalin into (Met)enkephalin [29]. Cathepsin L generated high molecular weight PE-derived intermediates (of ~23, 18–19, 8–9, and 4.5 kDa) that were similar to those in vivo in chromaffin granules [29]. Such results demonstrated a cellular role for cathepsin L in the production of (Met)enkephalin in secretory vesicles.
Continued investigation of cathepsin L in secretory vesicles demonstrated its prominent role in the biosynthesis of numerous neuropeptides represented by neuropeptide Y (NPY), POMC-derived ACTH, β-endorphin, and α-MSH, as well as dynorphin, CCK, and catestatin neuropeptides [3, 4, 6–8]. Illustration of the function of cathepsin L for producing neuropeptides has been demonstrated by studies of cathepsin L protease gene knockout (summarized in Table 2), cathepsin L expression, and small molecule inhibition of cathepsin L.
Table 2.
Reduced Levels of Neuropeptides in Cathepsin L Knockout Mice
| Neuropeptide | Tissue | Wild-Type | Cathepsin L Knockout |
|---|---|---|---|
| Met-Enkephalin | Brain Cortex | 100% | 44% |
| NPY | Brain Cortex | 100% | 22% |
| CCK8 | Brain Cortex | 100% | 75% |
| Dynorphin A | Brain Cortex | 100% | 25% |
| Dynorphin B | Brain Cortex | 100% | 17% |
| α-neoendorphin | Brain Cortex | 100% | 10% |
| ACTH | Pituitary | 100% | 23% |
| β-Endorphin | Pituitary | 100% | 18% |
| α-MSH | Pituitary | 100% | 7% |
Results of tissue levels of several neuropeptides in brain cortex and pituitary in cathepsin L knockout mice compared to wild-type controls (100%) are illustrated [1–8]. Substantial decreases in levels of these neuropeptides occur in cathepsin L knockout mice compared to control wild-type mice. These data indicate a role of cathepsin L in neuropeptide production.
2.6.2. NPY
NPY in the brain functions as a peptide neurotransmitter for the regulation of feeding behavior [24, 25]. In the peripheral sympathetic nervous system, NPY regulates blood pressure [35]. Novel findings show that cathepsin L participates as a key proteolytic enzyme for NPY production [3]. NPY in cathepsin L knockout (KO) mice was significantly reduced in brain and adrenal medulla by 80% and 90%, respectively. Cathepsin L is colocalized with NPY in secretory vesicles of brain cortical neurons and in chromaffin cells of adrenal medulla. Coexpression of proNPY with cathepsin L in neuroendocrine PC12 cells resulted in increased production of NPY via cleavage of proNPY at paired basic residues. In contrast, PC2 gene knockout mice show no change in NPY brain levels [36] but a peptidomic study showed a partial decrease in NPY [37]. The field has not yet reported studies of NPY in PC1/3 knockout mice. These studies of demonstrate an important role for cathepsin L in the production of NPY.
2.6.3. Pituitary hormones derived from POMC
Production of the pituitary hormones ACTH, β-endorphin, and αMSH utilize cathepsin L for processing POMC (proopiomelanocortin) [4]. ACTH stimulates glucocorticoid synthesis in adrenal cortex, β-endorphin is an endogenous opioid for analgesia, and α-MSH is involved in pigmentation of the skin. Cathepsin L knockout (KO) mice have major decreases in ACTH, β-endorphin, and α-MSH that are reduced to 23%, 18%, and 7%, respectively, of wild-type controls (100%) in pituitary. Increased levels of POMC in these knockout mice are consistent with cathepsin L processing of POMC. Cathepsin L is colocalized with β-endorphin, α-MSH, and ACTH in pituitary secretory vesicles. Cathepsin L is also partially colocalized with the lysosomal marker lamp-1 in pituitary. Expression of cathepsin L in pituitary AtT-20 cells increased ACTH and β-endorphin production. Treatment of AtT-20 cells with CLIK-148, a specific inhibitor of cathepsin L, resulted in reduced ACTH and accumulation of POMC. For comparison, PC2 knockout (KO) mice showed substantial reduction in α-MSH levels in pituitary and brain [38], indicating that cathepsin L and PC2 function jointly for α-MSH production. ACTH and β-endorphin were elevated in pituitaries of PC2 KO mice suggesting that ACTH and β-endorphin serve as substrates for PC2. These studies demonstrate a prominent role for cathepsin L, combined with PC2, in the production of ACTH, β-endorphin, and α-MSH peptide hormones in secretory vesicles.
2.6.4. Dynorphins
Dynorphin opioid neuropeptides mediate neurotransmission for analgesia and behavioral functions [39–41]. Dynorphin A, dynorphin B, and α-neoendorphin are generated from prodynorphin. Cathepsin L participates in producing dynorphins from prodynorphin [8]. Cathepsin L KO mouse brains have decreased levels of dynorphin A, dynorphin B, and α-neoendorphin that are reduced by 75%, 83%, and 90%, respectively, compared to controls. Cathepsin L in brain cortical neurons is colocalized with dynorphins in secretory vesicles. Cellular coexpression of cathepsin L with prodynorphin in PC12 cells results in production of dynorphins A and B. Comparative studies of PC1/3 and PC2 convertases show that PC1/3 KO mouse brains had a modest decrease in dynorphin A, and PC2 knockout mice showed a minor decrease in α-neoendorphin [8]. These results demonstrate a prominent role for cathepsin L, jointly with PC1/3 and PC2, for production of dynorphin neuropeptides.
2.6.5. Cholecystokinin (CCK)
CCK is a neurotransmitter whose production utilizes cathepsin L for processing the proCCK precursor to generate active CCK8 neuropeptide in brain [6]. In cathepsin L knockout (KO) mice, CCK8 levels are reduced in brain cortex by ~75%. PC1/3 or PC2 KO mice showed more modest decreases of CCK8 [42–44]. Therefore, data from the cathepsin L KO results provide strong evidence for cathepsin L as a key protease responsible for CCK8 production in brain.
2.6.6. Catestatin
The catestatin peptide secreted in the adrenal medulla of the sympathetic nervous system regulates blood pressure and stress [45, 46]. Catestatin is generated from the precursor chromogranin A (CgA) by cathepsin L [7]. Cathepsin L colocalizes with CgA in the secretory vesicles. Cathepsin L cleaves CgA in vitro to generate catestatin-related peptides. Thus far, PC1/3 KO mice show no change in CgA-derived peptides [47]; several peptides derived from CgA were reduced in PC2 KO mouse brains [37]. These investigations demonstrate a novel role for cathepsin L in the production of active catestatin peptide from CgA.
2.6.7. Implications of secretory vesicle cathepsin L in neurotransmission and behavioral effects
It is of interest that cathepsin L participates in the production of multiple neurotransmitters, as discussed in this section for the production of enkephalin, NPY, β-endorphin, ACTH, α-MSH, CCK, dynorphins, and catestatin. It will be noteworthy in future studies to evaluate the effects of cathepsin L gene knockout mice on behavioral features.
2.7. Novel Secretory Vesicle Function of Cathepsin L in Contrast to its Lysosomal Function
The novel secretory vesicle function of cathepsin L contrasts with its known role in lysosomes for protein degradation. Cathepsin L was first identified as a degrading protease in lysosomes of rat liver and other types of cells and species [48–50]. However, new findings indicate that cathepsin L is present in secretory vesicles of neuroendocrine cells [1–8, 51, 52]. Comparison of the secretory vesicle location of cathepsin L compared to lysosomes reveals differences in the relative portion of cathepsin L in these two organelles in different cell types. In bovine chromaffin cells of the sympathetic nervous system, cathepsin L is primarily colocalized with NPY and enkephalin [2, 3], with essentially no colocalization with the lysosomal marker lamp-1. In pituitary, cathepsin L is present in secretory vesicles with β-endorphin and α-MSH, as well as in lysosomes [4, 53].
Cathepsin L is also present in cell nuclei and extracellularly for biological functions. Nuclear cathepsin L participates in proteolysis of histone H3 in the regulation of gene expression [54, 55]. A truncated form of cathepsin B that lacks the signal peptide is present in the nucleus of several tumor type of cells [56]. Furthermore, a cathepsin L isoform devoid of a signal peptide localizes to the nucleus in S phase and processes the CDP/Cux transcription factor [57]. In addition, extracellular functions of cysteine cathepsins have been demonstrated in matrix degradation and cell signaling and atherosclerosis [58–60].
New findings indicate that cathepsin L is located in several organelles, with notable function in secretory vesicles for neuropeptide production. These recent studies indicate the secretory vesicle as a significant organelle site for cathepsin L production of active peptide neurotransmitters and hormones.
3. Cathepsin B produces neurotoxic β-amyloid in secretory vesicles and represents a new drug target for Alzheimer’s disease
3.1. Summary
Alzheimer’s disease (AD) is an age-related neurodegenerative disease that results in severe memory deficits. Neurotoxic β-amyloid (Aβ) peptides have been demonstrated as a major factor involved in the development of memory loss of AD, characterized by the accumulation of Aβ in amyloid plaques in AD brains [17–19, 61–63]. Aβ peptides (composed primarily of Aβ(1–40) and Aβ(1–42)) are generated from the amyloid precursor protein (APP) by protease processing by β- and γ-secretase sites located at the N- and C-termini of Aβ within APP. Previously, β- and γ-secretases have been identified as BACE1 and the presenilin complex, respectively [17–19, 61–63]. Recent studies of β-secretase in regulated secretory vesicles show that cathepsin B also participates as β-secretase for Aβ production [9–16], the topic of this section. The AD field has high interest in finding inhibitors of APP processing for reduction of Aβ and improvement in memory deficit.
Investigation of β-secretase in regulated secretory vesicles led to identification of cathepsin B in secretory vesicles as a β-secretase for production of Aβ [9–16]. Inhibition of cathepsin B results in reduction of brain Aβ peptides and significant improvement in memory in a mouse model of AD [13, 15], and cathepsin B gene knockout results in reduced Aβ production in brain [14]. The cathepsin B data combined with that of the previously known BACE1 β-secretase, an aspartyl protease, indicate joint roles of these two proteases for Aβ production [16]. These findings indicate cathepsin B as an exciting new target for development of effective therapeutic agent(s) for AD. The rationale and steps involved in discovery of cathepsin B as a drug target for AD are discussed in this review.
3.2. Neurotoxic β-amyloid of Alzheimer’s disease (AD)
Alzheimer’s disease (AD) results in severe memory loss resulting from neurodegeneration in the brain, caused in large part by accumulation of neurotoxic β-amyloid (Aβ) peptides. The majority of AD patients are afflicted with sporadic AD that is not linked to genetic mutations [64, 65]. A smaller portion of AD patients possess familial forms of AD with inherited genetic mutations, especially mutations of APP and presenilins that result in increased Aβ production and memory deficit when overexpressed in transgenic mouse models of AD [17, 66, 67]. Development of effective therapeutic agents to improve memory in AD is essential for the increasing numbers of AD patients of the growing aged population.
A logical therapeutic approach for AD is to develop drugs which reduce the production of brain Aβ peptides because their accumulation has been demonstrated to be a major factor in causing the disease [17–19, 61–63, 66, 67]. Aβ peptides are produced from the amyloid precursor protein (APP) by proteolytic cleavage at the β-secretase and γ-secretase sites. The majority of AD patients express wild-type (WT) APP of sporadic AD that is not linked to genetic mutations [64, 65], but a large extended family expresses APP containing mutations at these sites [68] which results in Aβ accumulation and development of the disease [66, 67, 69]. Because the mutant APP produces more Aβ, expression of mutant APP forms has been useful in developing animal models to identify potential AD therapeutics. However, since most AD patients express WT APP, it is essential to investigate drug candidates in models expressing WT APP because different proteases may processing WT compared to mutant APP substrates.
3.3. Regulated secretory vesicles produce the majority of extracellular β-amyloid of AD
Accumulation of extracellular Aβ results from neuronal production and secretion of Aβ that results in neurodegeneration of neurons in brain regions (hippocampus and cortex) responsible for memory function (Figure 5). Neurons possess the regulated secretory pathway that is utilized for activity-dependent secretion of neurotransmitters, and the constitutive secretory pathway for basal secretion [5, 70–73]. It is important to understand the neurobiology of Aβ production with respect the primary secretory pathway that provides secretion of extracellular Aβ. Evaluation of data of in vivo secretion of Aβ indicates its secretion primarily from the regulated secretory pathway of neurons (summarized in next paragraph). The location of Aβ and its amyloid precursor protein (APP) in regulated secretory vesicles [9, 10, 74–76] indicates that the proteases that convert APP to Aβ are present in the secretory vesicle organelle.
Figure 5. Aβ production in the regulated secretory pathway of neurons provides the majority of extracellular Aβ that causes memory loss.
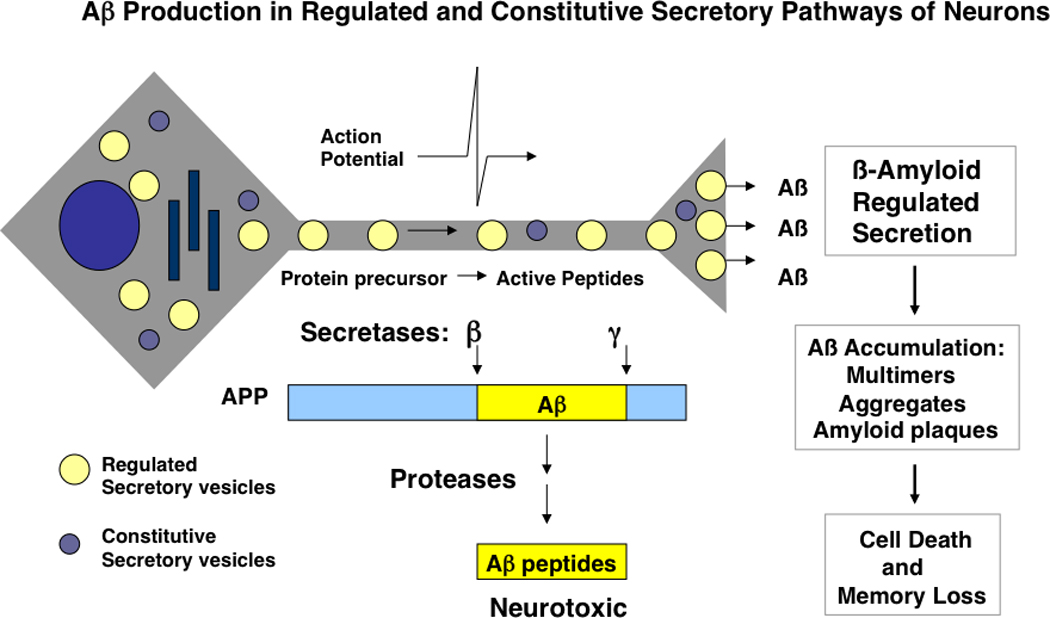
Aβ peptides are generated by proteolytic processing of the amyloid precursor protein (APP) in secretory vesicles that undergo axonal transport from the neuronal cell body to nerve terminals, where Aβ is secreted. Secretory vesicles of the regulated secretory pathway (yellow circles) provide the majority of secreted, extracellular Aβ peptides [9, 10, 16, 74–76]. Some Aβ is also provided by the basal, constitutive secretory pathway (constitutive secretory vesicles shown as blue circles). Intracellular production of Aβ within secretory vesicles occurs by cleavage at the N-terminus of Aβ within APP, achieved by proteases known as β-secretases, and cleavage at the C-termini of Aβ within APP which is achieved by γ-secretases. Proteolytic processing by β- and γ-secretases results in Aβ peptides of 40 and 42 residues, known as Aβ(1–40) and Aβ(1–42). Extracellular Aβ peptides in brain accumulate as oligomers and aggregates in amyloid plaques, and cause loss of memory in Alzheimer’s disease.
Studies in the field indicate that the majority of extracellular Aβ in brain is provided by activity-dependent secretion from neurons via the regulated secretory pathway. Neurons undergo high frequency, receptor-mediated, electrical activity-dependent secretion of Aβ [77–80]. Neurotransmitters also undergo regulated secretion [5, 70–73]. In parallel to the main regulated secretory pathway for Aβ secretion, a minor portion of Aβ undergoes basal secretion from the constitutive secretory pathway [9, 10]. Studies of peptide neurotransmitter biosynthesis show that different proteases are present in regulated compared to constitutive secretory pathways [5, 73, 81–83]. Therefore, it is important to identify the proteases for β-secretase in the regulated secretory vesicles that provides the majority of secreted, extracellular Aβ.
Studies of neuronal chromaffin cells (in primary culture) from in vivo nervous tissue finds that they naturally produce Aβ in the regulated secretory pathway that provides the majority of secreted Aβ [9, 10]. These regulated secretory vesicles can be purified in high quantity which allows biochemical characterization and purification of proteolytic activity for Aβ production. These secretory vesicles have been used as a model for studies of neurotransmitter synthesizing enzymes that function in brain [5, 84]. These secretory vesicles contain Aβ40 and Aβ42 with full-length APP (with wild-type β-secretase site), indicating the presence of secretases for production of Aβ peptides from APP. Therefore, these model Aβ-producing secretory vesicles were utilized for identification of β-secretase protease(s).
3.4. Strategy to identify protease(s) cleaving at the wild-type β-secretase site of APP for Aβ production in secretory vesicles
The wild-type β-secretase site of APP is cleaved between Met-↓Asp at the N-terminal side of the Aβ peptide sequence (fig. 6a). The amino acid residues adjacent to the cleavage site are important for recognition of the peptide cleavage site by the protease, according the model of substrate and protease interactions of Schechter and Berger [85, 86]. Cleavage of the wild-type β-secretase site of APP is relevant to the majority of AD patients because they express wild-type APP.
Figure 6. Model of APP and protease interactions at the β-secretase site.
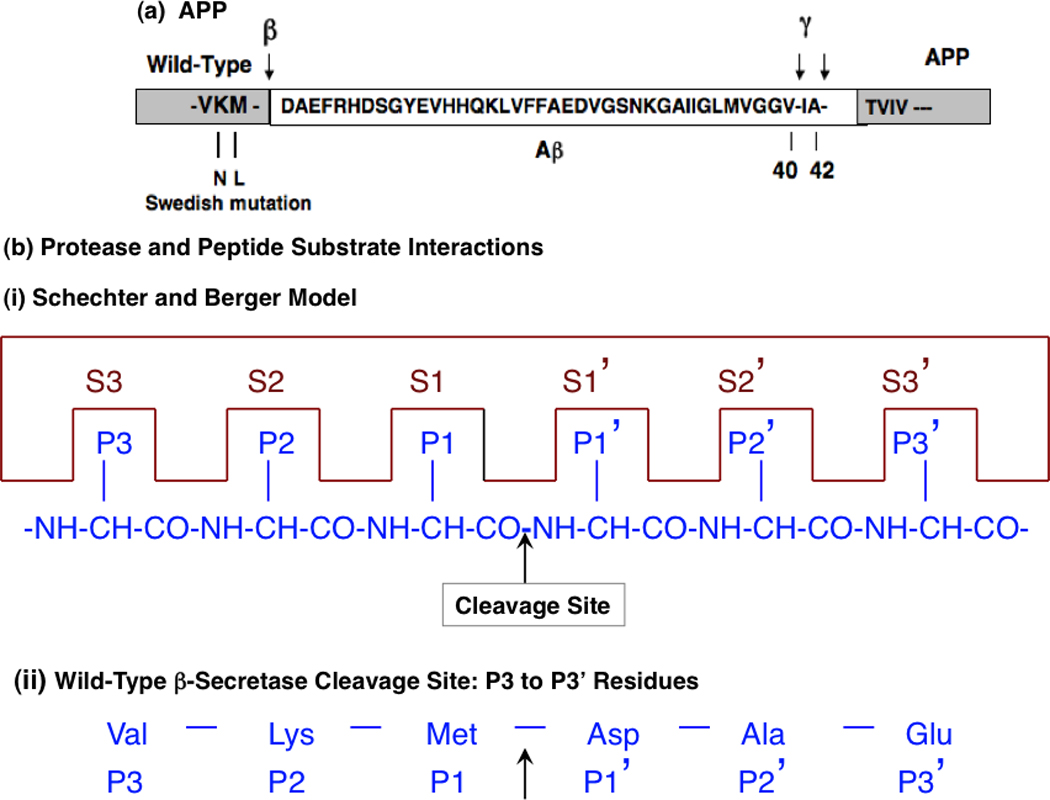
a. Interactions of amyloid precursor protein (APP) with proteases at the β-secretase site for production of neurotroxic β-amyloid peptides (Aβ). The APP precursor protein undergoes proteolytic cleavages at the β-secretase site and the γ-secretase sites to generate Aβ peptides, consisting primarily of Aβ(1–40) and Aβ(1–42) that contain the same N-terminus with differences in their C-termini. The primary sequence of Aβ peptides and flanking residues at the secretase cleavage sites are illustrated.
b. Protease active site interactions with APP at the β-secretase site: Schechter and Berger model. (i) The active site of the enzyme is composed of several subsites. The scheme shows an active site of six subsites, termed S1 to S3 and S1' to S3'. Subsites are located on both sides of the catalytic site and are numbered from this point in either direction. The positions of amino acid residues of the hexapeptide substrate are counted from the point of cleavage and thus have the same numbering as the subsites they occupy (P1 to P3 and P1' to P3'). Cleavage occurs between P1 and P1' [85, 86].
(ii) Wild-type β-secretase cleavage site: P3 to P3’ residues. Cleavage of the wild-type site β-secretase site of APP occurs between Met-↓Asp which represent the P1-P1’ residues. The Val-Lys-Met residues represent the P3, P2, and P1 residues, respectively; the Asp-Ala-Glu residues are the P1’, P2’, and P3’ residues, respectively.
3.4.1. Specificity of peptide substrate cleavage by proteases
The specificity for peptide substrate cleavage by proteases involves the active site divided into subsites, using the model and nomenclature of Schechter & Berger [85, 86]. This model describes interactions of the protease with the substrate amino acids flanking the cleavage site (figure 6b.i). The amino acids forming the cleaved peptide bond are termed P1-↓P1', with residues at the N-terminal and C-terminal sides of the cleavage site indicated as P1, P2…․ and P1', P2'…․, respectively. The protease active site is viewed as a series of subsites (S1, S2… and S1', S2'…) each accommodating one amino acid residue of the substrate. Protease subsites interact with the polypeptide backbone and with side chains of amino acids of the substrate.
Multiple points of interactions in several subsites are essential for obtaining the high association constants necessary for efficient biological function. Within the active site certain subsites (close or remote from the catalytic site) participate in determining specificity [85, 87–90]. For example, for papain and the cysteine cathepsins [89], specificity is determined mainly by the hydrophobic S2 subsite that prefers binding to hydrophobic P2 residues. The specificity and activity of caspases, cysteine proteases, is determined by both S1 and S4 remote from the catalytic site [90]. Generally, the P1 to P3 residues are important for protease selectivity for cleavage sites, based on interaction with S1 to S3 subsites of the protease. Therefore, cleavage site-specific assays often incorporate the native P1 to P3 residues.
3.4.2. Design of wild-type β-secretase peptide substrate based on P2-P1 residues at the wild-type β-secretase site of APP
At the wild-type β-secretase site of APP, the -P3-P2-P1-↓P1’-P2’-P3’- residues are Val-Lys-Met-↓Asp-Ala-Glu- (figure 6b.ii). This wild-type β-secretase site is expressed by the majority of AD patients. However, a mutant β-secretase site is expressed in an extended Swedish family [68], consisting of mutant Asn-Leu that substitutes for the wild-type P2-P1 residues of Lys-Met. Because the amino acids at the Swedish mutant site differ from the wild-type β-secretase site, proteases that selectively cleave these cleavage sites may exist.
It is desirable to identify the protease(s) cleaving at the wild-type β-secretase site, since it is expressed in >99% of the AD population. Therefore, consideration of P1 to P3 residues that may confer cleavage specificity can be designed as the peptide-MCA substrate Z-Val-Lys-Met-↓MCA representing a substrate mimicking the wild-type β-secretase site.
3.5. The wild-type β-secretase substrate identifies cathepsin B for production of Aβ in secretory vesicles
Processing of the wild-type β-secretase site substrate, Z-Val-Lys-Met-↓MCA, in Aβ-containing secretory vesicles was identified as activity belonging to the cysteine protease family [9, 10]. The wild-type β-secretase activity in Aβ-producing secretory vesicles was labeled by the activity probe DCG-04, a biotinylated form of the cysteine protease inhibitor E64c (fig. 7a). Inhibition by E64c and CA074 (selective inhibitor of cathepsin B) (fig. 7b) [10], purification, and peptide sequencing of the affinity-labeled protease by mass spectrometry indicated its sequence to be that of cathepsin B (fig. 7c). The colocalization of cathepsin B with Aβ peptides in secretory vesicles was demonstrated by immunoelectron microscopy (fig. 7d) [10]. This finding indicates the secretory vesicle as a new subcellular compartment for cathepsin B function.
Figure 7. Cathepsin B is identified as a β-secretase in regulated secretory vesicles.
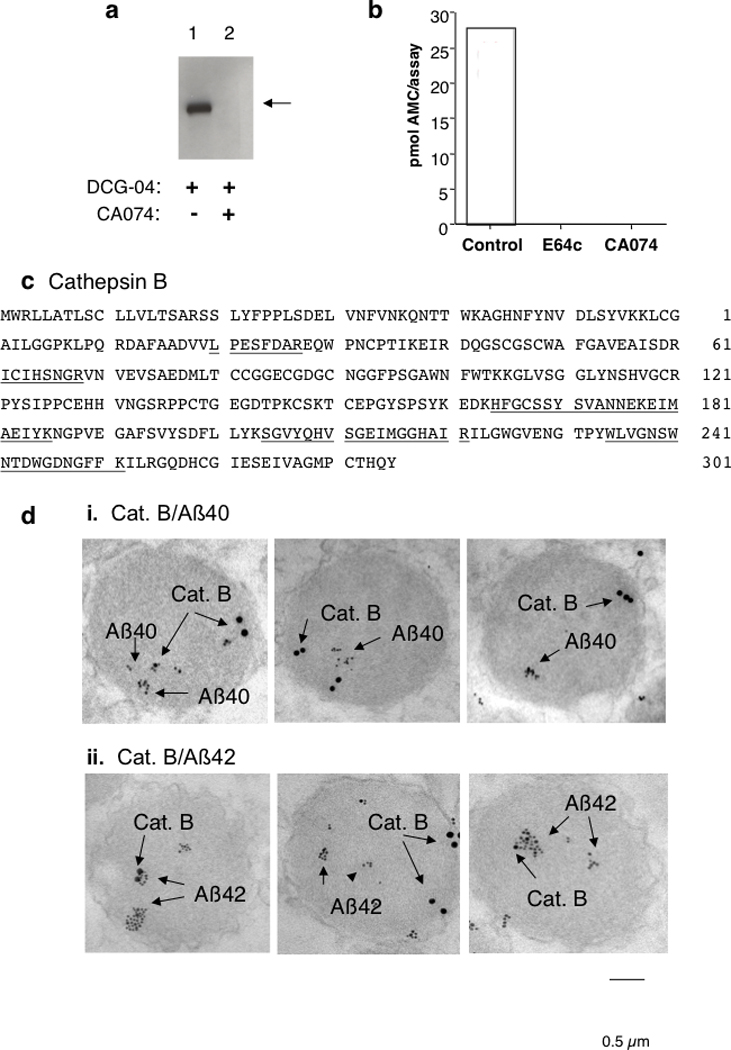
a. Purification of proteolytic activity cleaving the wild-type β-secretase cleavage site substrate Z-Val-Lys-Met-MCA: active-site directed probe labeling. Regulated secretory vesicles of neuronal-like chromaffin cells (from the sympathetic nervous system) produce and secrete Aβ peptides [9, 10]. Purification of proteolytic activity that cleaves the wild-type β-secretase site, present in the majority of Alzheimer’s patients, was conducted using isolated secretory vesicles of the regulated secretory pathway. The β-site cleaving protease was labeled with the active-site directed affinity probe DCG-04 (shown by the arrow) as a protein band of ~ 31 kDa (lane 1). The selective inhibitor of cathepsin B, CA074, blocked the DCG-04 probe labeling, suggesting that the protease may be represented by cathepsin B (lane 2) [10]
b. Inhibition of Z-Val-Lys-Met-MCA cleaving activity by E64c and CA074. The Z-Val-Lys-Met-MCA cleaving activity purified from Aβ-containing regulated secretory vesicles (chromaffin secretory vesicles) was inhibited by the cysteine protease inhibitor E64c and by the selective inhibitor of cathepsin B, CA074 [10].
c. Peptide sequencing identifies cathepsin B as β-secretase. The 31 kDa band (shown in part ‘a.ii) was indicated as the responsible enzyme for β-secretase activity in regulated secretory vesicles. The 31 kDa band was subjected to peptide sequencing by tryptic digestion and tandem mass spectrometry (MS/MS) of peptide fragments. The determined sequences of tryptic peptides are underlined within the complete primary sequence of cathepsin B [10]
d. Colocalization of cathepsin B with Aβ peptides in regulated secretory vesicles: analyses by immunoelectron microscopy. Cathepsin B (Cat. B, 15 nm gold particles) was colocalized with Aβ40 (6 nm gold particles) within regulated secretory vesicles isolated from neuronal chromaffin cells (panel i). Cathepsin B (15 nm gold particles) was also colocalized with Aβ42 (6 nm gold particles) in regulated secretory vesicles (panel ii) [10].
3.6. Distinct cleavage specificity of cathepsin B for the wild-type β-secretase site of APP, rather than the Swedish mutant β-secretase site
Cathepsin B cleavage of the wild-type β-secretase site of the model substrate Z–Val–Lys–Met–↓MCA was compared to the Swedish mutant β-secretase site substrate Z-Val-Asn-Leu-↓MCA (mutant residues are underlined). Cathepsin B shows clear preference for cleaving the WT β-secretase substrate, with essentially no activity for the Swedish mutant β-secretase substrate (Table 3). The distinct preference of cathepsin B for the WT β-secretase site is indicated by its high catalytic efficiency represented by its kcat/Km value of 3.17 × 105 M−1sec1 [13], indicating an active enzyme.
Table 3.
Selectivity of Cathepsin B for Cleaving the Wild-Type β-Secretase Site, Rather than the Swedish Mutant β-Secretase Site of APP.
| Substrate | Cathepsin B: β-Secretase Activity pmol AMC/min/µg enzyme |
|---|---|
| Wild-type β-secretase site: Z-Val-Lys-Met-↓MCA | 547 |
| Swedish mutant β-secretase site: Z-Val-Asn-Leu-↓MCA | 0.2 |
A cleavage site specific protease assay was designed to detect cleavage at the wild-type β-secretase site utilizing the Z-Val-Lys-Met-↓MCA (Z-V-K-M-MCA) substrate that mimics the wild-type cleavage site. In parallel, the substrate Z-Val-Asn-Leu-↓MCA was utilized to assay protease cleavage at the Swedish mutant site (mutant residues are underlined). These substrates provided cleavage site specific protease assays for cleavage of the wild-type or Swedish mutant sites. Assay of cathepsin B with these substrates provided comparison of its high specific activity (pmol AMC/min/µg enzyme) for the wild-type substrate, and essentially no activity with the Swedish mutant substrate [13].
Analyses with longer peptide substrates also show that cathepsin B cleaves the wild-type β-secretase site [9, 91]. The endogenous cathepsin B purified from Aβ-containing secretory vesicles cleaves the wild-type β-secretase site of the peptide SVKM↓DAEF (arrow shows β-secretase site) [9]. Furthermore, cathepsin B cleaves the internally quenched fluorescent peptide substrates containing the wild-type β-secretase site within RE(Edans)EVKM↓DAEFK(Dabcl)R-NH2 substrate [91]. These long peptide substrates are often used to evaluate protease cleavage of the β-secretase site [9, 91–93].
3.7. In vitro inhibition of cathepsin B reduces Aβ in regulated secretory vesicles of neurons
Aβ is produced in APP- and Aβ-containing secretory vesicles (isolated from neuronal chromaffin cells) after incubation at 37° C [10]. Notably, the specific cathepsin B inhibitor CA074, as well as with the methylated CA074Me form, blocked Aβ production (fig. 8) [10]. Also, E64c, which inhibits cathepsin B and cysteine proteases, blocked Aβ production in the purified secretory vesicles. These data clearly illustrate a role for cathepsin B in Aβ production.
Figure 8. Inhibitors of cathepsin B reduce Aβ production in regulated secretory vesicles.
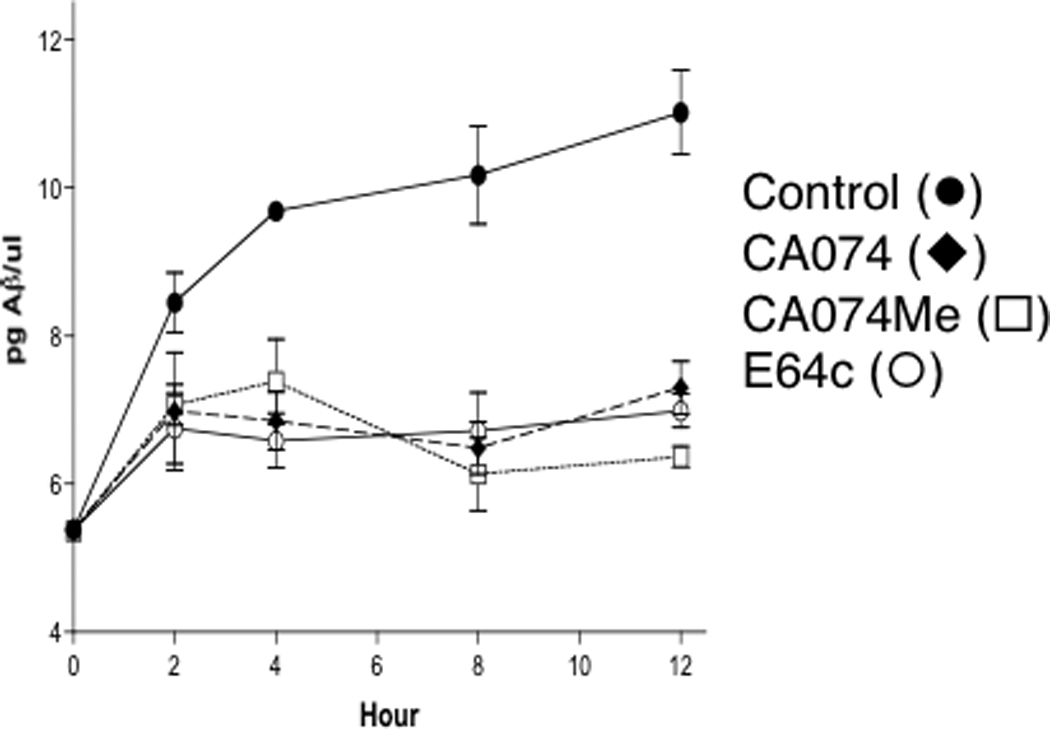
The effects of cathepsin B inhibitors, CA074 (10 µM, ◆) and CA074Me (10 µM, □), on the production of Aβ40 from endogenous APP in regulated secretory vesicles isolated from chromaffin cells was evaluated in time course studies [10]. Controls without inhibitors (●), or with the cysteine protease inhibitor E64c (10 µM, ○) were included. Each inhibitor was tested in triplicate; values represent×± sem.
The effect of the cathepsin B inhibitor was also evaluated in neuronal chromaffin cells. CA074Me reduced the amount of Aβ (Aβ40) in regulated secretory vesicles, whose content of Aβ was measured in the culture media after KCl stimulation of the regulated secretory pathway [10]. The CA074Me inhibitor, however, had no effect on the amount of Aβ secreted from the constitutive secretory pathway. These results indicate the cellular role of cathepsin B for production of Aβ in the regulated secretory pathway.
The E64c molecule inhibits cysteine proteases including cathepsin B [93]. CA074 is a selective inhibitor of cathepsin B [94, 95]. The methylated CA074Me form is more cell permeable, and is converted intracellularly by esterases to the selective CA074 inhibitor of cathepsin B [94–96]. Although some evidence shows that CA074Me is less selective than CA074 [97], cellular esterases convert CA074Me to CA074 [96]. Therefore, the effects of CA074Me are most likely due to the effects of CA074. The experiments with E64c, CA074, and CA074Me together support a role for cathepsin B in Aβ production in regulated secreory vesicles of neuronal-like chromaffin cells.
3.8. In vivo inhibition of cathepsin B reduces brain Aβ and improves memory deficits in a mouse model expressing human APP with the wild-type β-secretase site, but not in Swedish mutant APP mice
3.8.1. Cathepsin B inhibitor treatment of AD mice expressing human APP with the wild-type β-secretase site results in improved memory and reduced brain Aβ
Inhibitors of cathepsin B improve memory and reduce Aβ in transgenic mice expressing human APP with the wild-type β-secretase site (and the mutation V717 near the γ-secretase site of APP which promotes Aβ production [13. 16]], known as the London APP mouse model of AD [13]. Administration of the CA074Me and E64d inhibitors (via icv route into brain) resulted in substantial improvement in memory deficit in the London AD mice, assessed by the Morris water maze memory test that measures latency time to swim to a hidden platform (Figure 9). Importantly, the reduced latency time after inhibitor treatment indicates substantial improvement in memory, which approached that of normal mice.
Figure 9. The inhibitors CA074Me or E64d improve memory deficit in London APP mice assessed by the Morris water maze test.
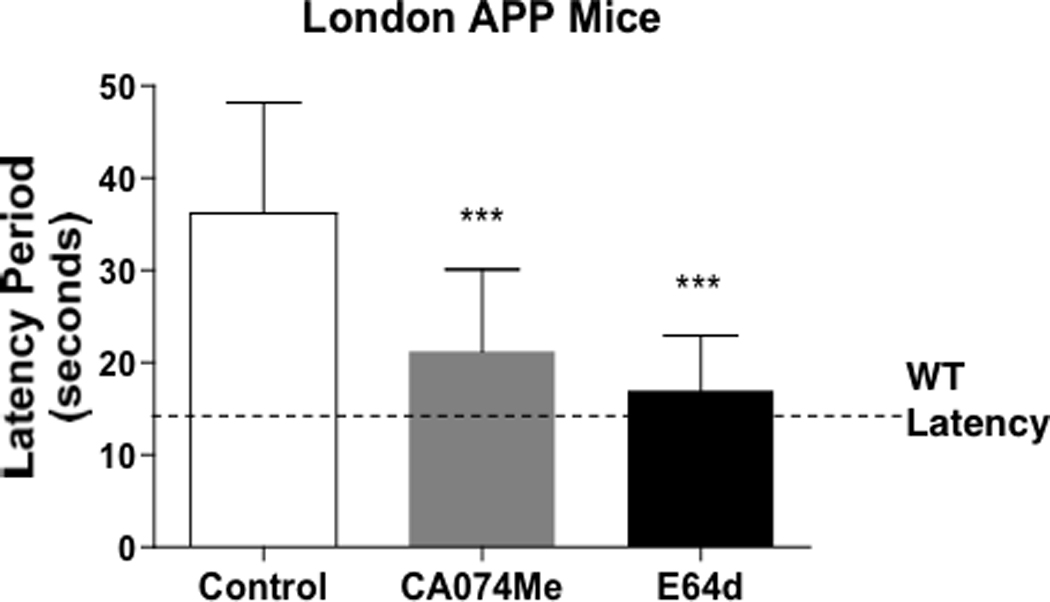
Memory function was assessed in the Morris water maze test after administration of CA074Me or E64d by icv administration [13]. CA074Me is a prodrug form of CA074 (conversion to CA074 by cellular esterases), a specific inhibitor of cathepsin B. E64d is a prodrug form of E64c (conversion to E64c by cellular esterases). The latency period measures the time it takes the animal to swim to a submerged platform after training, with shorter times reflecting improved memory. Results are displayed as the mean latency period (seconds) ± standard deviation (SD), with statistical significance indicated (*** p < 0.0001, student’s t-test). The latency time for wild-type, normal mice is illustrated by the dotted line. Results show that treatment with the inhibitors improves the memory deficits towards normal memory function of wild-type mice.
Treatment with the CA074Me and E64d inhibitors reduced amyloid plaque and substantially reduced Aβ40 and Aβ42 levels in brain (Figure 10a,b). The CA074Me and E64d inhibitors also reduced brain levels of CTFβ (Figure 10c) derived from APP by β-secretase (Figure 10d). Importantly, mice remained healthy after inhibitor treatment. It will be of interest to conduct pharmacological studies to assess the effective range of inhibitor doses for improving memory and brain Aβ. Most recently, oral administration of E64d resulted in improved memory and reduced brain Aβ with reduce amyloid plaque in AD mice, indicating he feasibility of E64d as a practical oral drug therapeutic for AD [15]. These novel results demonstrate the in vivo effectiveness of cathepsin B inhibitors to improve memory deficit and to reduce in brain Aβ peptides and amyloid plaque load.
Figure 10. The inhibitors CA074Me or E64d reduce brain Aβ peptides and CTFβ derived from APP by β-secretase.
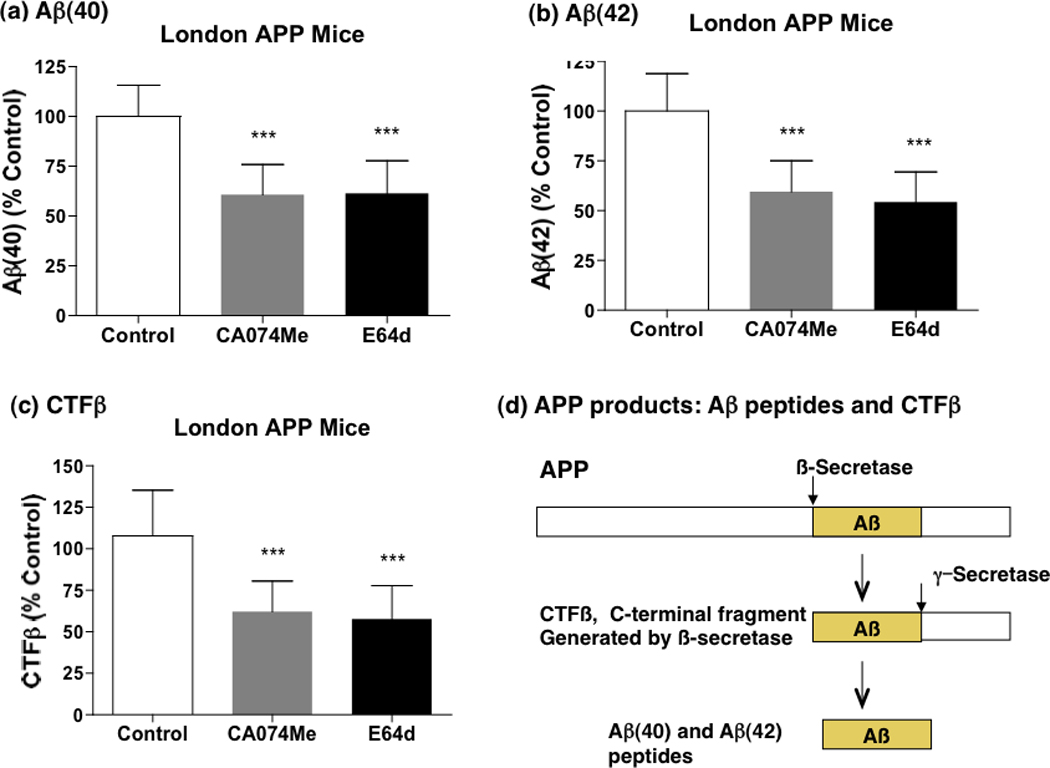
a. Reduction of brain Aβ40 after treatment of London AD mice with CA074Me or E64d. London APP mice were treated with CA074Me or E64d, and Aβ40 levels in brain were measured by ELISA assays and expressed as percent of the control Aβ40 levels. Results are expressed as the mean (% control) ± SD, with statistical significance indicated (*** p < 0.0001, student’s t-test). Brain levels of Aβ40 are substantially reduced after treatment with the inhibitors [13].
b. Reduction of brain Aβ42 after treatment of London AD mice with the inhibitors. After treatment of London APP mice with CA074Me or E64d, Aβ42 levels in brain were measured by ELISA assays and expressed as the percent of the control Aβ42. Results are expressed as the mean (% control) ± SD, with statistical significance indicated (*** p < 0.0001, student’s t-test). Brain levels of Aβ42 are decreased after treatment with the inhibitors [13].
c. Reduced CTFβ in brain after inhibitor treatment. Analyses of CTFβ derived from APP by β-secretase in London APP mouse brains were performed with brain extracts, and the CTFβ band (12 kDa) was quantitated by densitometry. Results show a decrease in CTFβ after inhibitor treatment [13]. Results are expressed as the mean (% control) ± SD, with statistical significance indicated (*** p < 0.0001, student’s t-test).
d. Proteolytic processing of APP into CTFβ and Aβ peptides by β- and γ-secretases. Cleavage of APP at the β-secretase site generates the C-terminal β-secretase fragment (CTFβ). Processing of CTFβ by γ-secretase results in the generation of Aβ peptides.
3.8.2. No effect of cathepsin B inhibitor treatment in AD mice expressing the Swedish mutant β-secretase site of APP
Transgenic mice expressing human Swedish mutant APP have been utilized as a mouse model of AD [17, 62, 66, 69]. The Swedish APP possesses the mutant Asn-Leu residues at the β-secretase cleavage that differs from the wild-type sequence of Lys-Met at that site. Most interestingly, administration of the inhibitors of cathepsin B, CA074Me and E64d to Swedish mutant mice (Swedish mutation in the London APP mice, ie., Swe/London APP mice) resulted in no effect on memory deficit in the Swedish mutant APP mice [13, 15]. Furthermore, inhibitors resulted in no change in brain levels of Aβ40 and Aβ42, or CTFβ in mice with the Swedish mutation of APP (Swe/London APP mice).
3.9. Knockout of the cathepsin B gene results in reduction of brain Aβ in mice expressing APP with the wild-type β-secretase site, but not in mice expressing the Swedish mutant site of APP
Notably, knockout of the cathepsin B gene in mice expressing human wild-type APP results in decreased Aβ40 and Aβ42 by 67% in brain (Figure 11), and decreased levels of the C-terminal β-secretase fragment (CTFβ) derived from APP by β-secretase [14]. These data suggest that deletion of the cathepsin B gene decreases β–secretase activity to reduce Aβ production.
Figure 11. Knockout of the cathepsin B gene in transgenic mice expressing human wild-type APP (hAPPwt) reduces brain Aβ40 and Aβ42.
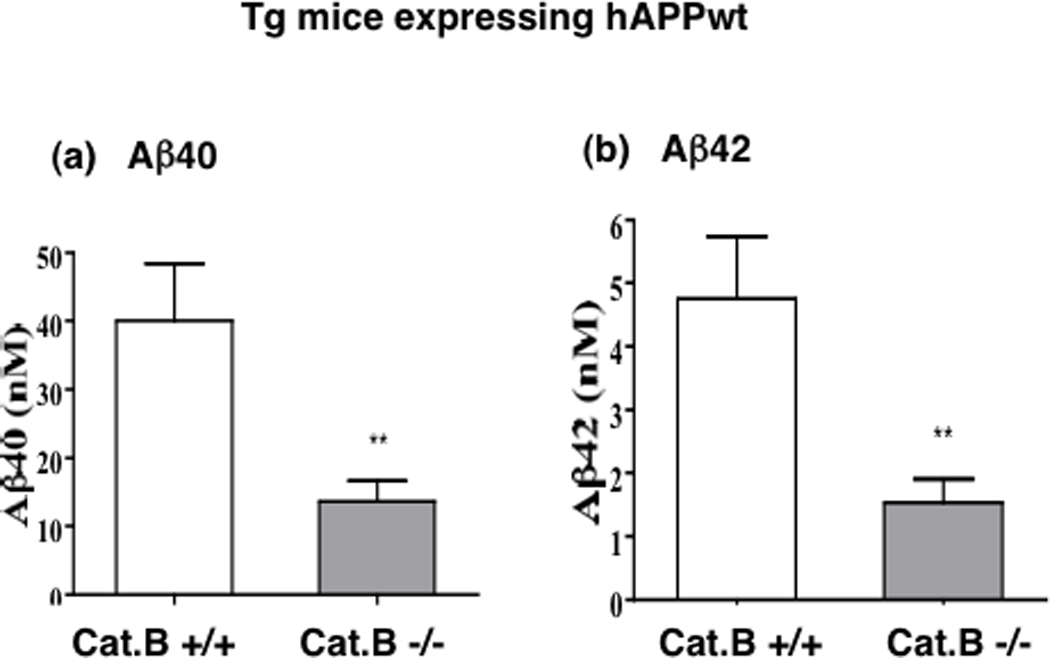
a. Aβ40 levels in brain. The wild-type Cat B+/+ and knockout Cat B−/− mice expressing human wild-type APP (hAPPwt) contained brain Aβ40 levels of 40.0 ± 8.4 and 13.6 ± 3.0 nM, respectively (significant, **p < 0.007). Knockout of the cathepsin B gene resulted in a 66% reduction in brain Aβ40 in mice expressing hAPPwt [14].
b. Aβ42 levels in brain. The Cat B+/+ and Cat B−/− mice expressing hAPPwt contained brain Aβ42 levels of 4.8 ± 1.0 and 1.5 ± 8.4 nM, respectively (significant, **p < 0.007). Knockout of the cathepsin B gene resulted in a 68% reduction in brain Aβ42 in animals expressing hAPPwt [14].
In contrast, knockout of cathepsin B in mice expressing human APP with the rare Swedish (Swe) and Indiana (Ind) mutations had no effect on Aβ [14] The difference in reduction of Aβ in mice expressing human wild-type APP mice, but not in mice expressing human Swedish mutant APP, shows that the transgenic model can affect cathepsin B gene knockout results. Since most AD patients express wild-type APP, these data validate cathepsin B as a target for development of inhibitors to lower Aβ in the majority of the AD population.
Other studies have confirmed the lack of effect of cathepsin B knockout on Aβ in mice expressing APP with the Swe mutation [98]. However, the Mueller-Steiner studies did not examine effects of cathepsin B knockout in mice expressing wild-type APP.
Furthermore, gene silencing of cathepsin B by siRNA in normal wild-type hippocampal brain neurons (in primary culture) reduces the amount of Aβ in the regulated secretory pathway [74]. These studies also showed that the CA074Me inhibitor reduces Aβ in the regulated secretory pathway of rat hippocampal neurons, indicating a role for cathepsin B in Aβ production.
Cathepsin B knockout mice appear healthy, showing normal eating, body weight, grooming, and appearance [14]. These results indicate that drug inhibition of cathepsin B will likely have minimal side effects and will be therapeutically safe.
These data from multiple groups validate cathepsin B as a target for development of drug inhibitors to lower Aβ in the majority of AD patients expressing wild-type APP.
3.10. BACE1 and cathepsin B function jointly as β-secretases for Aβ production
There has been much investigation in the field on the aspartyl protease BACE1 that functions as β-secretase [92, 93, 99–104]. While both cathepsin B and BACE1 are present in Aβ secretory vesicles [9, 10], cathepsin B is the primary β-secretase activity in the regulated secretory vesicle that provides the majority of extracellular Aβ. BACE1 has been demonstrated to provide Aβ released from the constitutive secretory pathway, measured in conditioned media from cells [92, 93, 99–104]. Analyses of the BACE1 studies together with that of cathepsin B support the hypothesis for joint roles of these proteases for Aβ production. Thus, the BACE1 results do not preclude cathepsin B as another β-secretase, explained in this section.
3.10.1. BACE1 prefers to cleave the Swedish mutant β-secretase site, rather than the wild-type β-secretase site of APP
The Swe mutant APP was utilized in the field to search for β-secretase, leading to identification of BACE1 [92, 93, 99, 101, 102] which readily cleaves the Swe β-secretase site, with poor efficiency for cleaving the wild-type β-secretase site. BACE1 activity for the wild-type β-secretase substrate is extremely low, illustrated by low kcat/Km values of 40–60 M−1s−1 [102, 105], whereas proteases acting on biological substrates have kcat/Km values that range between ten of thousands to a few millions [85–87, 105]. Cathepsin B, however, has a kcat/Km for cleaving wild-type β-secretase site substrates of 317,000 M−1s−1 [13, 16]. These kinetic properties demonstrate that cathepsin B most effectively cleaves the wild-type β-secretase site of APP for Aβ production.
3.10.2. BACE1 knockout in Swedish APP expressing mice
Since BACE1 cleaves the Swe mutant APP, investigators have utilized transgenic mice expressing human Swe mutant APP as an AD model for studies of BACE1 gene knockout. Indeed, a study with such Swe mutant APP mice [103] showed that knockout of BACE1 reduced brain Aβ and CTFβ, which provides support for involvement of BACE1 as β-secretase for the Swedish mutant APP. These BACE1 studies, though, do not rule out a role for cathepsin B in production of Aβ from wild-type APP.
3.10.3. Beta-secretase activity in BACE1 knockout mice
Studies of BACE1 knockout mice apparently found that brain homogenates lack brain β-secretase activity [104]. However, because the β-secretase assays contained E64, a potent inhibitor of cathepsin B, the assay could detect the cysteine protease activity of cathepsin B. Therefore, the assays of that study do not rule out cathepsin B as representing β-secretase activity.
3.10.4. Aβ production and secretion in neurons of BACE1 knockout mice
A study found lower Aβ levels in media from cultured brain neurons obtained from BACE1 knockout mice which implicated participation of BACE1 [104]. But the Aβ in “conditioned medium” indicates constitutive secretion of Aβ because the neurons were not stimulated to secrete. The study did not measure regulated secretion of Aβ and, therefore, the data do not rule out participation of cathepsin B. The same analysis applies to another study [100], which also found reduced Aβ in conditioned medium secreted from neurons from BACE1 knockout mice, indicating a role for BACE1 in the constitutive secretory pathway. The study did not address regulated secretion and, therefore, does not rule out cathepsin B participation in Aβ production in the regulated secretory pathway.
3.10.5. BACE1 and Aβ production from normal compared to high expression levels of APP
Because AD brains possess physiologically normal levels of APP [106], proteases for APP processing should be effective at normal APP levels. Studies of the effects of BACE1 knockout on Aβ production from normal, endogenous levels of mouse APP showed reduction of brain Aβ1–40 and partial reduction of Aβx-40 [107]; heterozygous BACE1 gene deletion (BACE1 −/+) has no effect on brain Aβ. Overexpression of BACE1 in wild-type mice with normal levels of APP results in no change in brain Aβ levels, which led that group to conclude that BACE1 “has minimal effect on the level of endogenous Abeta” and that “other factors” must be involved in modulation of Aβ production in adult and aging brain” [106]. The “other factor” may include cathepsin B in Aβ production.
In transgenic mice expressing super high levels of APP, BACE1 gene knockout resulted in decreased brain Aβ levels [108]. These findings indicate that BACE1 can function with very high, non-physiological levels of APP. It is known that protease enzyme activities are dependent on substrate levels, with more activity at higher substrate levels [109]. The important question is what protease target can be inhibited under conditions of normal APP levels to reduce Aβ? This question is addressed by results showing that inhibitors of cathepsin B reduce brain Aβ in brains of normal wild-type guinea pigs [11, 12]. These data indicate that cathepsin B functions at normal levels of APP for Aβ production.
3.10.6. Different proteases for preferential processing of the wild-type vs. the Swedish mutant β-secretase site of APP for Aβ production: cathepsin B vs. BACE1 for the AD patient population
The data in the field indicate joint roles for cathepsin B and BACE1 as β-secretases for Aβ production of AD. Their notable difference is that these proteases differ in their cleavage preferences. Cathepsin B prefers to cleave the wild-type β-secretase site, whereas BACE1 prefers to cleave the Swedish mutant β-secretase site. Since the majority of AD patients express APP with the wild-type β-secretase site, cathepsin B is important to the AD population. Therefore, development of cathepsin B inhibitors may be useful for novel therapeutic strategies in AD.
4. The secretory vesicle proteome environment for cathepsins L and B biosynthesis of active peptides
4.1. Summary
The functions of cathepsins L and B in secretory vesicles indicate a previously unknown subcellular location for these cathepsins. These cysteine cathepsins, and other cathepsins, have been known to function in lysosomes for protein degradation but recent studies indicate their biological roles in health and disease [110]. The roles of cathepsins L and B for generation of biologically active peptides in secretory vesicles are distinct from their roles in lysosomes. The secretory vesicle environment provides unique conditions for cathepsin functions which include protein systems to maintain conditions of acidic pH, reduction-oxidation regulation, and numerous key functions of the secretory vesicle proteome that are utilized for cathepsins to generate active peptides in health and disease. Therefore, proteomic studies of secretory vesicles have been conducted to reveal the functional protein systems that provide the appropriate environment for cathepsins L and B to generate active peptides.
4.2. Proteomics of Secretory Vesicles for Biosynthesis and Secretion of Active Peptides
Proteomic studies of neuropeptide-containing secretory vesicles can identify the functional protein categories in secretory vesicles utilized for neuropeptide production and secretion. Recent examination of proteins in model bovine chromaffin secretory vesicles revealed multiple functional protein categories that participate in secretory vesicle production of neuropeptides and catecholamines for cell-cell communication (Figure 12) [111, 112]. Protein systems involved in vesicular neuropeptide biosynthesis were examined in proteomic studies of soluble and membrane fractions of dense core secretory vesicles purified from chromaffin cells of the sympathetic nervous system. Proteomic results revealed functional categories of prohormones, proteases, catecholamine neurotransmitter metabolism, protein folding, redox regulation, ATPases, calcium regulation, signaling components, exocytotic mechanisms, and related functions. Interestingly, these secretory vesicles contained an extensive number of GTP nucleotide-binding proteins related to Rab, Rho, and Ras signaling molecules [113, 114], together with SNARE-related proteins and annexins that are involved in trafficking and exocytosis of secretory vesicle components [115, 116]. These vesicles also contain ATPases that regulate proton translocation [117], combined with components for signaling and exocytosis of neuropeptides. It will be of interest to compare the proteomics of neuropeptide secretory vesicles with that of synaptic vesicles that secrete classic small molecule neurotransmitters [118–120].
Figure 12. The secretory vesicle proteome for biosynthesis and secretion of active peptides.
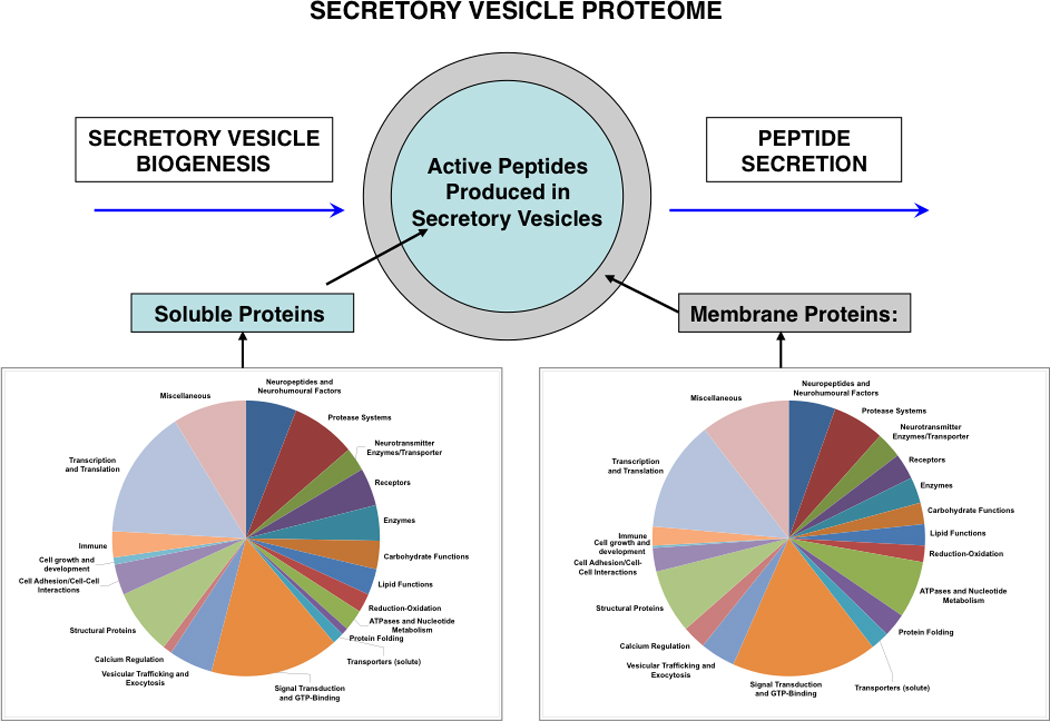
Proteins of the secretory vesicle, known as the neuroproteome, participate in the biosynthesis, storage, and regulated secretion of active peptides, as described in this review article. Thus, the active peptides are generated and secreted by the proteome of regulated secretory vesicles. The secretory vesicle proteome consists of soluble and membrane proteins that participate in secretory vesicle functions for providing neuropeptides for cell-cell communication in the nervous and endocrine systems. Proteomic studies of the soluble and membrane fractions of neuropeptide secretory vesicles isolated from adrenal medullary chromaffin cells of the sympathetic nervous system (bovine) indicate the protein systems participating in production of neuropeptides and β-amyloid for regulated secretion that include neuropeptides and neurohumoural factors, proteases, neurotransmitters enzymes and transporters, receptors, enzymes, carbohydrate functions, lipids, reduction-oxidation, ATPases and nucleotide metabolism, protein folding, signal transduction and GTP-binding proteins, vesicular trafficking and exocytosis, structural proteins, and cell adhesion proteins [111, 112].
Interestingly, the proteomic data revealed that these secretory vesicles contain proteins involved in neurodegeneration including amyloid precursor protein (APP), huntingtin interacting protein, cystatin 7, ataxin 7, and prion protein [112]. These regulated secretory vesicles may be informative for investigation of how such neurodegenerative diseases may involve secretory vesicle mechanisms.
Overall, knowledge of secretory vesicle proteomes provides novel insights into the biosynthesis and secretion of active peptides utilized for cell-cell communication in neuroendocrine systems, and in Alzheimer’s disease and other human diseases.
4.3. Future systems analyses of the secretory vesicle proteome to define protein interaction networks that regulate cysteine cathepsins in the production of active peptides in health and disease
Regulation of brain and neurological disease functions utilizes profiles of active peptides that together function to mediate the complex cell-cell communication network among neuroendocrine systems. Changes in physiological functions are represented by alterations in profiles of active peptides in health and disease. Furthermore, proteomic studies will be useful for biomarker applications for monitoring disease status and the effectiveness of therapeutic agents involving peptide regulation of disease functions. Elucidation of the proteomic systems utilized by the secretory vesicle for producing active neuropeptides for health and for generating toxic peptides such as β-amyloid in Alzheimer’s disease and other neurological diseases, can provide insight into new drug targets for novel disease therapeutics.
5. Conclusions: Novel biological functions of cysteine cathepsins in secretory vesicles for health and disease
This review highlights the paradigm-shifting discoveries of the key biological functions of the cysteine cathepsin L for producing active peptides for neurotransmission and of the key role of cathepsin B for production of Aβ involved in the development of Alzheimer’s disease. It is likely that future research will unveil significant roles of cysteine cathepsins in cellular, physiological, and disease conditions. Such knowledge will provide new target strategies for drug discovery to improve health and disease conditions.
Highlights.
Cathepsin L in secretory vesicles participaes in the biosynthesis of peptide neurotransmitters and hormones
Cathepsin B produces neurotoxic β-amyloid in secretory vesicles and represents a new drug target for Alzheimer’s disease
The secretory vesicle proteome indicates the protein environment that supports cathepsins L and B in the production of active peptides
Cysteine cathepsins possess novel biological functions in secretory vesicles for health and disease
Acknowledgments
The cathepsin L and proteomics research was supported by grants from the National Institutes of Health to V. Hook consisting of R01DA04271, R01NS24553, and R01MH077305 from the NIH. S. Bark was supported by a NIH Mentored Scientist Award (K01DA023065). The authors also appreciate scientific advice by Dr. Shin-Rong Hwang, and technical assistance by Mr. Thomas Toneff, at the Skaggs School of Pharmacy and Pharmaceutical Sciences, Univ. of Calif., San Diego, La Jolla, CA. The authors appreciate collaboration of these studies with Dr. Thomas Reinheckel and Dr. Christoph Peters at the Albert-Ludwigs University, Freiburg, Germany.
The cathepsin B and Alzheimer’s research was supported by the NIA/NIH R21 Grant AG027446 and 1R44AG032784 (to American Life Science Pharmaceuticals (ALSP)), and by an award from the Alzheimer’s Association to V. Hook. V.H. holds equity in ALSP and serves on the Scientific Advisory Board of ALSP. The terms of this agreement have been reviewed by the University of California, San Diego, in accordance with its conflict of interest policies. G.H. holds equity in and is employed by ALSP.
Figures 1–4, 8–11 of this review are reproduced from their original articles (2, 5, 10, 13, 14) with the kind permission from the Proceeding of the National Academy of Sciences USA, Annual Review of Pharmacology and Toxicology, Biological Chemistry, the Journal of Biology Chemistry, and Biochemical and Biophysical Research Communications.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Funkelstein L, Beinfeld M, Minokadeh A, Zadina J, Hook V. Unique biological functions of cathepsin L in secretory vesicles for biosynthesis of neuropeptides. Neuropeptides. 2010;44:457–466. doi: 10.1016/j.npep.2010.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Yasothornsrikul S, Greenbaum D, Medzihradszky KF, Toneff T, Bundey R, Miller R, Schilling B, Petermann I, Dehnert J, Logvinova A, Goldsmith P, Neveu JM, Lane WS, Gibson B, Reinheckel T, Peters C, Bogyo M, Hook VYH. Cathepsin L in secretory vesicles functions as a prohormone-processing enzyme for production of the enkephalin peptide neurotransmitter. Proc. Natl. Acad. Sci. USA. 2003;100:9590–9595. doi: 10.1073/pnas.1531542100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Funkelstein L, Toneff T, Hwang SR, Reinheckel T, Peters C, Hook VYH. Cathepsin L participates in the production of neuropeptide Y in secretory vesicles, demonstrated by protease gene knockout and expression. J. Neurochem. 2008;106:384–391. doi: 10.1111/j.1471-4159.2008.05408.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Funkelstein L, Toneff T, Hwang SR, Beuschlein F, Lichtenauer UD, Reinheckel T, Peters C, Hook VYH. Major role of cathepsin L for producing the peptide hormones ACTH, β-endorphin, and α-MSH, illustrated by protease gene knockout and expression. J. Biol. Chem. 2008;83:35652–35659. doi: 10.1074/jbc.M709010200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hook V, Funkelstein L, Lu WD, Bark S, Wegrzyn J, Hwang SR. Proteases for processing proneuropeptides into peptide neurotransmitters and hormones. Annu. Rev. Pharmacol. Toxicol. 2008;48:393–423. doi: 10.1146/annurev.pharmtox.48.113006.094812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Beinfeld MC, Funkelstein L, Foulon T, Cadel S, Kitagawa K, Toneff T, Reinheckel T, Peters C, Hook VYH. Cathepsin L plays a major role in cholecystokinin production in mouse brain and in pituitary AtT-20 cells: protease gene knockout and inhibitor studies. Peptides. 2009;30:1882–1991. doi: 10.1016/j.peptides.2009.06.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Biswas N, Rodriquez-Flores JL, Courel M, Gayen JR, Vaingankar SM, Mahata M, Torpey JW, Taupenot L, O’Connor DT, Mahata SK. Cathepsin L colocalizes with chromogranin A in chromaffin vesicles to generate active peptides. Endocrinology. 2009;150:3547–3557. doi: 10.1210/en.2008-1613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Minokadeh A, Funklestein L, Toneff T, Hwang SR, Reinheckel T, Peters C, Zadina J, Hook VYH. Cathepsin L participates in dynorphin neuropeptide production in brain cortex, illustrated by protease gene knockout and expression. Mol. Cell. Neurosci. 2010;43:98–107. doi: 10.1016/j.mcn.2009.10.001. [DOI] [PubMed] [Google Scholar]
- 9.Hook VYH, Toneff T, Aaron W, Yasothorsnrikul S, Bundey R, Reisine T. β-Amyloid peptide in regulated secretory vesicles of chromaffin cells: evidence for multiple cysteine proteolytic activities in distinct pathways for β-secretase activity in chromaffin vesicles. J. Neurochem. 2002;81:237–256. doi: 10.1046/j.1471-4159.2002.00794.x. [DOI] [PubMed] [Google Scholar]
- 10.Hook V, Toneff T, Bogyo M, Medzihradszky KF, Neveu J, Lane W, Hook G, Reisine T. Inhibition of cathepsin B reduces β-amyloid production in regulated secretory vesicles of neuronal chromaffin cells: evidence for cathepsin B as a candidate β-secretase of Alzheimer's disease. Biological Chemistry. 2005;386:931–940. doi: 10.1515/BC.2005.108. [DOI] [PubMed] [Google Scholar]
- 11.Hook G, Hook VY, Kindy M. Cysteine protease inhibitors reduce brain beta-amyloid and beta-secrease activity in vivo and are potential Alzheimer’s disease therapeutics. Biol. Chem. 2007;388:979–983. doi: 10.1515/BC.2007.117. [DOI] [PubMed] [Google Scholar]
- 12.Hook V, Kindy M, Hook G. Cysteine protease inhibitors effectively reduce in vivo levels of brain beta-amyloid related to Alzheimer's disease. Biol. Chem. 2007;388:247–252. doi: 10.1515/BC.2007.027. [DOI] [PubMed] [Google Scholar]
- 13.Hook V, Kindy M, Hook G. (2008) Inhibitors of cathepsin B improve memory and reduce beta-amyloid in transgenic Alzheimer’s disease mice expressing the wild type, but not the Swedish mutant, beta-secretase site of the amyloid precursor protein. J. Biol. Chem. 2008;283:7745–7753. doi: 10.1074/jbc.M708362200. [DOI] [PubMed] [Google Scholar]
- 14.Hook V, Kindy M, Reinheckel T, Peters C, Hook G. Genetic cathepsin B deficiency reduces beta-amyloid in transgenic mice expressing human wild-type amyloid precursor protein. Biochem. Biophys. Res. Commun. 2009;386:284–288. doi: 10.1016/j.bbrc.2009.05.131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hook G, Hook V, Kindy M. The cysteine protease inhibitor, E64d, reduces brain amyloid-β and improves memory deficits in Alzheimer's disease animal models by inhibiting cathepsin B, but not BACE1, β-secretase activity. J Alzheimers Dis. 2011 May 25; doi: 10.3233/JAD-2011-110101. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hook V, Hook G, Kindy M. Pharmacogenetic features of cathepsin B inhibitors that improve memory deficit and reduce beta-amyloid related to Alzheimer’s disease. Biol. Chem. 2010;3391:861–872. doi: 10.1515/BC.2010.110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Price DL, Sisodia SS. Mutant genes in familial Alzheimer's disease and transgenic models. Annu. Rev. Neurosci. 1998;21:479–505. doi: 10.1146/annurev.neuro.21.1.479. [DOI] [PubMed] [Google Scholar]
- 18.Selkoe DJ. Alzheimer's disease: genes, proteins, and therapy. Physiol. Rev. 2001;81:741–766. doi: 10.1152/physrev.2001.81.2.741. [DOI] [PubMed] [Google Scholar]
- 19.Gandy S, Martins RN, Buxbaum J. Molecular and cellular basis for anti-amyloid therapy in Alzheimer disease. Alzheimer Dis. Assoc. Disord. 2003;17:259–266. doi: 10.1097/00002093-200310000-00011. [DOI] [PubMed] [Google Scholar]
- 20.Law PY, Wong YH, Loh HH. Molecular mechanisms and regulation of opioid receptor signaling. Annu. Rev. Pharmacol. Toxicol. 2000;40:389–430. doi: 10.1146/annurev.pharmtox.40.1.389. [DOI] [PubMed] [Google Scholar]
- 21.Snyder SH, Pasternak GW. Historical review: opioid receptors. Trends Pharmacol. Sci. 2003;24:198–205. doi: 10.1016/S0165-6147(03)00066-X. [DOI] [PubMed] [Google Scholar]
- 22.Frohman LA. Diseases of the anterior pituitary. In: Felig P, Baxter JD, Frohman LA, editors. Endocrinology and Metabolism. Third Edition. New York: McGraw-Hill, Inc. Health Professions Division; 1995. pp. 293–297. [Google Scholar]
- 23.Steiner RA, Hohmann JG, Holmes A, Wrenn CC, Cadd G, Jureus A, Clifton DK, Luo M, Gutshall M, Ma SY, Mufson EJ, Crawley JN. Galanin transgenic mice display cognitive and neurochemical deficits characteristic of Alzheimer’s disease. Proc. Natl. Acad. Sci. USA. 2001;98:4184–4189. doi: 10.1073/pnas.061445598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Gehlert DR. Role of hypothalamic neuropeptide Y in feeding and obesity. Neuropeptides. 1999;33:329–338. doi: 10.1054/npep.1999.0057. [DOI] [PubMed] [Google Scholar]
- 25.Wieland HA, Hamilton BS, Krist B, Doods HN. The role of NPY in metabolic homeostasis: implications for obesity therapy. Expert Opin. Investig. Drugs. 2000;9:1327–1346. doi: 10.1517/13543784.9.6.1327. [DOI] [PubMed] [Google Scholar]
- 26.Steiner DF. The proprotein convertases. Curr. Opin. Chem. Biol. 1998;2:31–39. doi: 10.1016/s1367-5931(98)80033-1. [DOI] [PubMed] [Google Scholar]
- 27.Seidah NG. A. Prat, Precursor convertases in the secretory pathway, cytosol and extracellular milieu. Essays Biochem. 2002;38:79–94. doi: 10.1042/bse0380079. [DOI] [PubMed] [Google Scholar]
- 28.Cravatt BF, Wright AR, Kozarich JW. Activity-based protein profiling: from enzyme chemistry to proteomic chemistry. Annu. Rev. Biochem. 2008;77:383–414. doi: 10.1146/annurev.biochem.75.101304.124125. [DOI] [PubMed] [Google Scholar]
- 29.Hwang SR, Garza C, Mosier C, Toneff T, Wunderlich E, Goldsmith P, Hook VYH. Cathepsin L expression is directed to secretory vesicles for enkephalin neuropeptide biosynthesis and secretion. J. Biol. Chem. 2007;282:9556–9563. doi: 10.1074/jbc.M605510200. [DOI] [PubMed] [Google Scholar]
- 30.Schiller MR, Mende-Mueller L, Moran K, Meng M, Miller KW, Hook VYH. “Prohormone thiol protease” (PTP) processing of recombinant proenkephalin. Biochemistry. 1995;34:7988–7995. doi: 10.1021/bi00025a004. [DOI] [PubMed] [Google Scholar]
- 31.Azaryan AV, Hook VYH. Unique cleavage specificity of ‘prohormone thiol protease’ related to proenkephalin processing. FEBS Lett. 1994;341:197–202. doi: 10.1016/0014-5793(94)80456-7. [DOI] [PubMed] [Google Scholar]
- 32.Fricker LD. Carboxypeptidase E. Annu. Rev. Physiol. 1988;50:309–321. doi: 10.1146/annurev.ph.50.030188.001521. [DOI] [PubMed] [Google Scholar]
- 33.Yasothornsrikul S, Toneff T, Hwang SR, Hook VYH. Arginine and lysine aminopeptidase activities in chromaffin granules of bovine adrenal medulla: relevance to prohormone processing. J. Neurochem. 1998;70:153–163. doi: 10.1046/j.1471-4159.1998.70010153.x. [DOI] [PubMed] [Google Scholar]
- 34.Hwang SR, O’Neill A, Bark S, Foulon T, Hook VYH. Secretory vesicle aminopeptidase B related to neuropeptide processing: molecular identification and subcellular localization to enkephalin- and NPY-containing chromaffin granules. J. Neurochem. 2007;100:1340–1350. doi: 10.1111/j.1471-4159.2006.04325.x. [DOI] [PubMed] [Google Scholar]
- 35.Walker P, Grouzmann E, Burnier M, Waeber B. The role of neuropeptide Y in cardiovascular regulation. TIPS. 1991;12:111–115. doi: 10.1016/0165-6147(91)90518-w. [DOI] [PubMed] [Google Scholar]
- 36.Miller R, Toneff T, Vishnuvardhan D, Beinfeld M, Hook VYH. Selective roles for the PC2 processing enzyme in the regulation of peptide neurotransmitter levels in brain and peripheral neuroendocrine tissues of PC2 deficient mice. Neuropeptides. 2003;37:140–148. doi: 10.1016/s0143-4179(03)00027-1. [DOI] [PubMed] [Google Scholar]
- 37.Zhang X, Panm H, Peng B, Steiner DF, Pintar JE, Fricker LD. Neuropeptidomic analysis establishes a major role for prohormone convertase-2 in neuropeptide biosynthesis. J. Neurochem. 2010;112 doi: 10.1111/j.1471-4159.2009.06530.x. 168-1179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Miller R, Aaron W, Toneff T, Vishnuvardhan D, Beinfeld M, Hook VYH. Obliteration of α-melanocyte-stimulating hormone derived from POMC in pituitary and brains of PC2-deficient mice. J. Neurochem. 2003;86:556–563. doi: 10.1046/j.1471-4159.2003.01856.x. [DOI] [PubMed] [Google Scholar]
- 39.Akil H, Owens C, Gutstein H, Taylor L, Currran E, Watson S. Endogenous opioids: overview and current issues. Drug Alcohol Depend. 1988;51:127–140. doi: 10.1016/s0376-8716(98)00071-4. [DOI] [PubMed] [Google Scholar]
- 40.Wang Z, Gardell LW, Ossipov MH, Vanderah TW, Brennan MB, Hochgeschwender U, Hruby VJ, Malan TP, Lai J, Porreca F. Pronociceptive actions of dynorphin maintain chronic neuropathic pain. J. Neurosci. 2001;21:1779–1786. doi: 10.1523/JNEUROSCI.21-05-01779.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Shippenberg TS, Zapata Z, Chefer VI. Dynorphin and the pathophysiology of drug addiction. Pharmacol. & Ther. 2007;116:306–321. doi: 10.1016/j.pharmthera.2007.06.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Beinfeld MC, Blum A, Vishnuvardhan D, Fanous S, Marchand JE. Cholecystokinin levels in prohormone convertase 2 knock-out mouse brain regions reveal a complex phenotype of region-specific alterations. J. Biol. Chem. 2005;280:38410–38415. doi: 10.1074/jbc.M500055200. [DOI] [PubMed] [Google Scholar]
- 43.Cain BM, Connolly K, Blum AC, Vishnuvardhan D, Marchand JE, Zhu X, Steiner DF, Beinfeld MC. Genetic inactivation of prohormone convertase (PC1) causes a reduction in cholecystokinin (CCK) levels in the hippocampus, amygdala, pons and medulla in mouse brain that correlates with the degree of colocalization of PC1 and CCK mRNA in these structures in rat brain. J. Neurochem. 2004;89:307–313. doi: 10.1046/j.1471-4159.2003.02295.x. [DOI] [PubMed] [Google Scholar]
- 44.Rehfeld JF, Bundgaard JR, Hannibal J, Zhu X, Norrbom C, Steiner DF, Friis-Hansen L. The cell-specific pattern of cholecystokinin peptides in endocrine cells versus neurons is governed by the expression of prohormone convertases 1/3, 2, and 5/6. Endocrinology. 2008;149:1600–1608. doi: 10.1210/en.2007-0278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Vaingankar SM, Li Y, Biswas N, Gayen J, Choksi S, Rao F, Ziegler MG, Mahata SK, O’Connor DT. Effects of chromogranin A deficiency and excess in vivo: biphasic blood pressure and catecholamine responses. J. Hypertens. 2010;28:817–825. doi: 10.1097/HJH.0b013e328336ed3e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Mahata SK, Mahata M, Fung MM, O’Connor DT. Catestatin: a multifuncional peptide from chromogranin A. Regul. Pept. 2010;165:52–62. doi: 10.1016/j.regpep.2010.09.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Wardman JH, Zhang X, Gagnon S, Castro LM, Zhu X, Steiner DF, Day R, Fricker LD. Analysis of peptides in prohormone convertase 1/3 null mouse brain using quantitative peptidomics. J. Neurochem. 2010;114:215–225. doi: 10.1111/j.1471-4159.2010.06760.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Yokota S, Nishimura Y, Kato K. K. Localization of cathepsin L in rat kidney revealed by immunoenzyme and immunogold techniques. Histochemistry. 1988;90:277–283. doi: 10.1007/BF00495971. [DOI] [PubMed] [Google Scholar]
- 49.Ryvnyak VV, Ryvnyak EI, Tudos RV. Electron histochemical localization of cathepsin L in the liver. Bull. Exp. Biol. Med. 2004;137:90–91. doi: 10.1023/b:bebm.0000024396.17895.77. [DOI] [PubMed] [Google Scholar]
- 50.Ishidoh K, Kominami E. Processing and activation of lysosomal proteinases. Biol. Chem. 2002;383:1827–1831. doi: 10.1515/BC.2002.206. [DOI] [PubMed] [Google Scholar]
- 51.Waguri S, Sato N, Watanabe T, Ishidoh K, Kominami E, Sato K, Uchiyama Y. Cysteine proteinases in GH4C1 cells, a rat pituitary tumor cell line, are secreted by the constitutive and regulated secretory pathways. Eur. J. Cell Biol. 1995;67:308–318. [PubMed] [Google Scholar]
- 52.Collette J, Bocock JP, Ahn K, Chapman RL, Godbold G, Yeyeodu S, Erickson AH. Biosynthesis and alternate targeting of the lysosomal cysteine protease cathepsin L. Int. Rev. Cytol. 2004;241:1–51. doi: 10.1016/S0074-7696(04)41001-8. [DOI] [PubMed] [Google Scholar]
- 53.Hook V, Funkelstein L, Toneff T, Mosier C, Hwang SR. Human pituitary contains dual cathepsin L and prohormone convertase processing pathway components involved in converting POMC into the peptide hormones ACTH, alpha-MSH, and beta-endorphin. Endocrine. 2009;35:429–437. doi: 10.1007/s12020-009-9163-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Duncan EM, Muratore-Schroeder TL, Cook RG, Garcia BA, Shabanowitz J, Hunt DF, Allis CD. Cathepsin L proteolytically processes histone H3 during mouse embryonic stem cell differentiation. Cell. 2008;135:284–294. doi: 10.1016/j.cell.2008.09.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Sullivan S, Tosetto M, Kevans D, Coss A, Wang L, O'Donoghue D, Hyland J, Sheahan K, Mulcahy H, O'Sullivan J. Localization of nuclear cathepsin L and its association with disease progression and poor outcome in colorectal cancer. Int. J. Cancer. 2009;125:54–61. doi: 10.1002/ijc.24275. [DOI] [PubMed] [Google Scholar]
- 56.Mehani S, Gong Q, Panella J, Subbiah S, Peffley DM, Frankfater A. In vivo expression of an alternatively spliced human tumor message that encodes a truncated form of cathepsin B, subcellular distribution of the truncated enzyme in COS cells. J. Biol. Chem. 1998;273:13236–13244. doi: 10.1074/jbc.273.21.13236. [DOI] [PubMed] [Google Scholar]
- 57.Goulet B, Baruch A, Moon NS, Poirier M, Sansregret LL, Erickson AK, Bogyo M, Nepveu A. A cathepsin L isoform that is devoid of a signal peptide localizes o the nucleus in S phase and processes the CDP/Cux transcription factor. Mol. Cell. 2004;14:207–219. doi: 10.1016/s1097-2765(04)00209-6. [DOI] [PubMed] [Google Scholar]
- 58.Obermajer N, Jevnikar Z, Doljak B, Kos J. Role of cysteine cathepsins in marix degradation and cell signaling. Connect Tissue Res. 2008;49:193–196. doi: 10.1080/03008200802143158. [DOI] [PubMed] [Google Scholar]
- 59.Garcia-Touchard A, Henry TD, Sangiorgi G, Spagnoli LG, Mauriello A, Conover C, Schwarz RS. Extracellular proteases in atherosclerosis and restenosis. Atherioscler. Thromb. Vasc. Biol. 2005;25:1119–1127. doi: 10.1161/01.ATV.0000164311.48592.da. [DOI] [PubMed] [Google Scholar]
- 60.Ishidoh K, Kominami E. 1998. Gene regulation and extracellular functions of procathepsin L. Biol. Chem. 1998;379:131–135. doi: 10.1515/bchm.1998.379.2.131. [DOI] [PubMed] [Google Scholar]
- 61.Sisodia SS. Alzheimer's disease: perspectives for the new millennium. J. Clin. Invest. 1999;104(1999):1169–1170. doi: 10.1172/JCI8508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Selkoe DJ, Schenk D. Alzheimer’s disease: molecular understanding predicts amyloid-based therapeutics. Annu. Rev. Pharmacol. Toxicol. 2002;43:545–584. doi: 10.1146/annurev.pharmtox.43.100901.140248. [DOI] [PubMed] [Google Scholar]
- 63.Wolfe MS. Gamma-secretase: structure, function, and modulation for Alzheimer's disease. Curr Top Med Chem. 2008;8:2–8. doi: 10.2174/156802608783334024. [DOI] [PubMed] [Google Scholar]
- 64.Blennow K, de Leon MJ, Zetterberg H. Alzheimer's disease. Lancet. 2006;368:387–403. doi: 10.1016/S0140-6736(06)69113-7. [DOI] [PubMed] [Google Scholar]
- 65.Turner RS. Alzheimer's disease. Semin. Neurol. 2006;26:499–506. doi: 10.1055/s-2006-951622. [DOI] [PubMed] [Google Scholar]
- 66.Masliah E, Rockenstein E. Genetically altered transgenic models of Alzheimer's disease. J. Neural Transm. Suppl. 2000;59(2000):175–183. doi: 10.1007/978-3-7091-6781-6_20. [DOI] [PubMed] [Google Scholar]
- 67.Dodart JC, Mathis C, Bales KR, Paul SM. Does my mouse have Alzheimer's disease? Genes Brain Behav. 2002;1:142–155. doi: 10.1034/j.1601-183x.2002.10302.x. [DOI] [PubMed] [Google Scholar]
- 68.Citron M, Oltersdorf T, Haass C, McConlogue L, Hung AY, Seubert P, Vigo-Pelfrey C, Lieberburg I, Selkoe DJ. Mutation of the β-amyloid precursor protein in familial Alzheimer’s disease increases β-protein production. Nature. 1992;360:672–674. doi: 10.1038/360672a0. [DOI] [PubMed] [Google Scholar]
- 69.Hsiao K, Chapman P, Nilson mS, Eckman C, Harigaya Y, Younkin S, Yang F, Cole G. Correlative memory deficits, Abeta elevation, and amyloid plaques in transgenic mice. Science. 1996;274:99–102. doi: 10.1126/science.274.5284.99. [DOI] [PubMed] [Google Scholar]
- 70.Gumbiner G, Kelly KB. Two distinct intracellular pathways transport secretory and membrane glycoproteins to the surface of pituitary tumor cells. Cell. 1982;28:51–55. doi: 10.1016/0092-8674(82)90374-9. [DOI] [PubMed] [Google Scholar]
- 71.Lodish H, Berk A, Zipursky SL, Matsudaira P, Baltimore D, Darnell J. Molecular Cell Biology. 4th ed. W.H. Freeman: New York; 1999. pp. 691–726. [Google Scholar]
- 72.Brion C, Miller SG, Moore HP. Regulated and constitutive secretion, differential effects of protein synthesis arrest on transport of glycosaminoglycan chains to the two secretory pathways. J. Biol. Chem. 1982;267:1477–1483. [PubMed] [Google Scholar]
- 73.Hook V, Yasothornsrikul S, Greenbaum D, Medzihradszky KF, Troutner K, Toneff T, Bundey R, Reinheckel T, Peters C, Bogyo M. Cathepsin L and Arg/Lys aminopeptidase: a distinct prohormone processing pathway for the biosynthesis of peptide neurotransmitters and hormones. Biol. Chem. 2004;385:473–480. doi: 10.1515/BC.2004.055. [DOI] [PubMed] [Google Scholar]
- 74.Klein DM, Felsenstein KM, Brenneman DE. Cathepsins B and L differentially regulate amyloid precursor protein processing. J Pharmacol Exp Ther. 2009;328:813–821. doi: 10.1124/jpet.108.147082. [DOI] [PubMed] [Google Scholar]
- 75.Vassilacopoulou D, Ripellino JA, Tezapsidis N, Hook VYH, Robakis NK. Full-length and truncated Alzheimer amyloid precursors in chromaffin granules: solubilization of membrane amyloid precursor is mediated by an enzymatic mechanism. J. Neurochem. 1995;64:2140–2146. doi: 10.1046/j.1471-4159.1995.64052140.x. [DOI] [PubMed] [Google Scholar]
- 76.Tezapsidis N, Li HC, Ripellino JA, Efthimiopoulos S, Vassilacopoulou D, Sambamurti K, Toneff T, Yasothornsrikul S, Hook VYH, Robakis NK. Release of nontransmembrane full-length Alzheimer’s amyloid precursor protein from the lumenar surface of chromaffin granule membranes. Biochemistry. 1998;37(1998):1274–1282. doi: 10.1021/bi9714159. [DOI] [PubMed] [Google Scholar]
- 77.Nitsch RM, Slack BE, Wurtman RJ, Growdon JH. Release of Alzheimer’s amyloid precursor derivatives by activation of muscarinic acetylcholine receptors. Science. 1992;258:304–307. doi: 10.1126/science.1411529. [DOI] [PubMed] [Google Scholar]
- 78.Nitsch RJ, Farber SA, Growdon JH, Wurtman RJ. Release of amyloid beta-protein precursor derivatives by electrical depolarization of rat hippocampal slices. Proc. Natl. Acad. Sci. USA. 1993;90:5191–5193. doi: 10.1073/pnas.90.11.5191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Efthimiopoulos S, Vassilacopoulou D, Rippellino JA, Tezapsidis N, Robakis NK. Cholinergic agonists stimulate secretion of soluble full-length amyloid precursor protein in neuroendocrine cells. Proc. Natl. Acad. Sci. USA. 1996;93:8046–8050. doi: 10.1073/pnas.93.15.8046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Jolly-Tornetta C, Gao ZY, Lee VM, Wolf BA. Regulation of amyloid precursor protein secretion by glutamate receptors in human Ntera 2 neurons. J. Biol. Chem. 1998;272:140015–140021. doi: 10.1074/jbc.273.22.14015. [DOI] [PubMed] [Google Scholar]
- 81.Benjannet S, Savaria D, Laslop A, Munzer JS, Chretien M, Marcinkiewicz M, Seidah NG. Alpha1-antitrypsin Portland inhibits processing of precursors mediated by proprotein convertases primarily within the constitutive secretory pathway. J. Biol. Chem. 1997;272:26210–26218. doi: 10.1074/jbc.272.42.26210. [DOI] [PubMed] [Google Scholar]
- 82.Gensberg K, Jan S, Matthews GM. Subtilisin-related serine proteases in the mammalian constitutive secretory pathway. Semin. Cell Dev. Biol. 1998;9:11–17. doi: 10.1006/scdb.1997.0196. [DOI] [PubMed] [Google Scholar]
- 83.Thomas G. Furin at the cutting edge: from protein traffic to embryogenesis and disease. Nat. Rev. Mol. Cell Biol. 2002;3:753–766. doi: 10.1038/nrm934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Ungar A, Phillips JH. Regulation of the adrenal medulla. Physiol. Rev. 1983;63:787–843. doi: 10.1152/physrev.1983.63.3.787. [DOI] [PubMed] [Google Scholar]
- 85.Schechter I, Berger A. On the size of the active site in proteases I. Papain. Biochem Biophys. Res. Commun. 1967;27:157–162. doi: 10.1016/s0006-291x(67)80055-x. [DOI] [PubMed] [Google Scholar]
- 86.Schechter I, Berger A. On the active site of proteases III. Mapping the active site of papain; Specific peptide inhibitors of papain. Biochem. Biophys. Res. Comm. 1968;32:898–902. doi: 10.1016/0006-291x(68)90326-4. [DOI] [PubMed] [Google Scholar]
- 87.Schechter I. Mapping of the active site of proteases in the 1960s and rational design of inhibitors/drugs in the 1990s. Curr Protein Pept Sci. 2005;6:501–512. doi: 10.2174/138920305774933286. [DOI] [PubMed] [Google Scholar]
- 88.Halfon S, Baird TT, Craik CS. Trypsin. In: Barrett AJ, Rawings ND, Woesnner JF, editors. Handbook of Proteolytic Enzymes. San Diego: Elsevier Academic Press; 2004. pp. 1483–1488. [Google Scholar]
- 89.Rawlings ND, Barrett AJ. Introduction: The clans and families of cysteine proteases. In: Barrett AJ, Rawlings ND, Woesnner JF, editors. Handbook of Proteolytic Enzymes. San Diego: Elsevier Academic Press; 2004. pp. 1051–1057. [Google Scholar]
- 90.Nicholson DW, Thornberry NA. Caspases: killer proteases. Trends Biochem. Sci. 1997;22:299–306. doi: 10.1016/s0968-0004(97)01085-2. [DOI] [PubMed] [Google Scholar]
- 91.Bohme L, Hoffmann T, Manhart S, Wolf R, Demuth HU. Isoaspartate-containing amyloid precursor protein-derived peptides alter efficacy and specificity of potential beta-secretases. Biol. Chem. 2008;389:1055–1066. doi: 10.1515/BC.2008.125. [DOI] [PubMed] [Google Scholar]
- 92.Yan R, Bienkowski MJ, Shuck ME, Miao H, Tory MC, Pauley AM, Brashier JR, Stratment NC, Mathews WR, Buhl AE, Carter DB, Tomasselli AG, Parodi LA, Heinrikson RL, Gurney ME. Membrane-anchored aspartyl protease with Alzheimer’s disease beta-secretase activity. Nature. 1999;402:533–537. doi: 10.1038/990107. [DOI] [PubMed] [Google Scholar]
- 93.Sinha S, Anderson JP, Barbour R, Basi GS, Caccavello R, Dsavis D, Don M, Dovey JF, Frigon N, Hong J, Jacobson-Croak K, Jewett N, Keim P, Knops J, Lieberburg I, Power M, Tan H, Tatsuno G, Tung J, Schenk D, Seubert P, Suomensaari SM, Wang S, Walker D, Zhao J, McCologue L, John V. Purification and cloning of amyloid precursor protein β-secretase from human brain. Nature. 1999;402:537–540. doi: 10.1038/990114. [DOI] [PubMed] [Google Scholar]; Greenbaum DC, Arnold WD, Lu F, Hayrapetian L, Baruch A, Krumrine J, Toba S, Chehade K, Brömme D, Kuntz ID, Bogyo M. Small molecule affinity fingerprinting. A tool for enzyme family subclassification, target identification, and inhibitor design. Chem Biol. 2002;9:1085–1094. doi: 10.1016/s1074-5521(02)00238-7. [DOI] [PubMed] [Google Scholar]
- 94.Yamamoto A, Kaji T, Tomoo K, Ishida T, Inoue M, Murata M, Kitamura K. Crystallization and preliminary X-ray study of the cathepsin B complexes with CA074, a selective inhibitor. J. Mol. Biol. 1992;227(1992):942–944. doi: 10.1016/0022-2836(92)90234-b. [DOI] [PubMed] [Google Scholar]
- 95.Towatari T, Nikawa T, Murata M, Yokoo C, Tamai M, Hanada K, Katunuma N. Novel epoxysuccinyl peptides, a selective inhibitor of cathepsin B in vivo. FEBS Lett. 1991;280:311–315. doi: 10.1016/0014-5793(91)80319-x. [DOI] [PubMed] [Google Scholar]
- 96.Buttle DJ, Murata M, Knight CG, Barrett AJ. CA074 methyl ester: a proinhibitor for intracellular cathepsin B. Arch. Biochem. Biophys. 1992;299:377–380. doi: 10.1016/0003-9861(92)90290-d. [DOI] [PubMed] [Google Scholar]
- 97.Montaser M, Lalmanach G, Mach L. CA-074, but not its methyl ester CA-074Me, is a selective inhibitor of cathepsin B within living cells. Biol Chem. 2002;383:1305–1308. doi: 10.1515/BC.2002.147. [DOI] [PubMed] [Google Scholar]
- 98.Mueller-Steiner S, Zhou Y, Arai H, Roberson ED, Sun B, Chen J, Wang X, Yu G, Esposito L, Mucke L, Gan L. Antiamyloidogenic and neuroprotective functions of cathepsin B: implications for Alzheimer's disease. Neuron. 2006;51:703–714. doi: 10.1016/j.neuron.2006.07.027. [DOI] [PubMed] [Google Scholar]
- 99.Vassar R, Bennet BD, Babu-Khan S, Mendiaz EA, Denis P, Teplow DB, Ross S, Amarante P, Loeloff R, Luo Y, Fisher S, Fuller J, Edenson S, Lile J, Jarosinski MA, Biere AL, Curran E, Burgess T, Louis JC, Collins F, Reanor J, Rogers G, Citron M. β-secretase cleavage of Alzheimer’s amyloid precursor protein by the transmembrane aspartic protease BACE. Science. 1999;286:735–741. doi: 10.1126/science.286.5440.735. [DOI] [PubMed] [Google Scholar]
- 100.Cai H, Wang Y, McCarthy D, Wen H, Corchelt DR, Price DL, Wong PC. BACE 1 is the major β-secretase for generation of Aβ by neurons. Nat. Neurosci. 2001;4:233–234. doi: 10.1038/85064. [DOI] [PubMed] [Google Scholar]
- 101.Hussain I, Powell D, Howlett DR, Tew DG, Meek TD, Chapman C, Gloger IS, Murphy KE, Southan CD, Ryan DM, Smith S, Simmons DL, Walsh FS, Dingwall C, Christie G. Identification of a Novel Aspartic Protease (Asp 2) as β-Secretase. Mol. Cell. Neurosci. 1999;14:419–427. doi: 10.1006/mcne.1999.0811. [DOI] [PubMed] [Google Scholar]
- 102.Lin X, Koelsch G, Wu S, Downs D, Dashti A, Tang J. Human aspartic protease memapsin 2 cleaves the beta-secretase site of beta-amyloid precursor protein. Proc. Natl. Acad. Sci. USA. 2000;97:1456–1460. doi: 10.1073/pnas.97.4.1456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Luo Y, Bolon B, Kahn S, Bennet BD, Babu-Khan S, Denis P, Fan W, Kha H, Zhang J, Gong Y, Martin L, Louis JC, Yan Q, Richards WG, Citron M, Vassar R, et al. Mice deficient in BACE 1, the Alzheimer’s β-secretase, have normal phenotype and abolished β-amyloid generation. Nat. Neurosci. 2001;4:231–232. doi: 10.1038/85059. [DOI] [PubMed] [Google Scholar]
- 104.Roberds SL, Anderson J, Basi G, Bienkowski MJ, Branstetter DG, Chen KS, Freedman SB, Frigon NL, Games D, Hu K, Johnson-Wood K, Kappenman E, Kawabe TT, Kola I, Kuehn R, Lee M, Liu W, Motter R, Nichols NF, Power M, Robertson DW, Schenk D, Schoor M, Shopp GM, Shuck ME, Sinha S, Svensoon KA, Tatsuno G, Tintrup H, Wijsman J, Wright S, McConlogue L. BACE knockout mice are healthy despite lacking the primary beta-secretase activity in the brain: implications for Alzheimer’s disease therapeutics. Hum. Mol. Genet. 2001;10:1317–1324. doi: 10.1093/hmg/10.12.1317. [DOI] [PubMed] [Google Scholar]
- 105.Schechter I, Ziv E, Cathepsins S. Cathepsins S, B and L with aminopeptidases display β-secretase activity associated with the pathogenesis of Alzheimer's disease. Biol Chem. 2011;392:555–569. doi: 10.1515/BC.2011.054. [DOI] [PubMed] [Google Scholar]
- 106.Hirata-Fukae C, Sidahmed EH, Gooskens TP, Aisen PS, Dewachter I, Devijver H, Van Leeuven FV, Matsuoka Y. Beta-site amyloid precursor protein-cleaving enzyme-1 (BACE1)-mediated changes of endogenous amyloid beta in wild-type and transgenic mice in vivo. Neursosci. Letters. 2008;435:186–189. doi: 10.1016/j.neulet.2008.02.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Nishitomi K, Sakaguchi G, Horikoshi Y, Gary AJ, Babu-Khan S, Hirata-Fukae C, Becker AG, Hosono M, Sakaguchi I, Minami SS, Nakajima Y, Liu HF, Takeyama C, Kihara T, Ota A, Wong PC, Aisen PS, Kato A, Kinoshita N, Matsuoka Y. BACE1 inhibition reduces endogenous Abeta and alters APP processing in wild-type mice. J. Neurochem. 2006;99:1555–1563. doi: 10.1111/j.1471-4159.2006.04178.x. [DOI] [PubMed] [Google Scholar]
- 108.McConlogue L, Buttini M, Anderson JP, Brigham EF, Chen KS, Freedman SB, Games D, Johnson-Wood K, Lee M, Zeller M, Liu W, Moter R, Sinha S. Partial reduction of BACE1 has dramatic effects on Alzheimer plaque and synaptic pathology in APP transgenic mice. J. Biol. Chem. 2007;282 doi: 10.1074/jbc.M611687200. 26326-2334. [DOI] [PubMed] [Google Scholar]
- 109.Voet D, Voet JG. New York: Biochemistry. John Wiley & Sons; 2004. pp. 480–481. [Google Scholar]
- 110.Reiser J, Adair B, Reinheckel T. Specialized roles for cyseine cathepsins in health and disease. J. Clin. Invest. 2010;20:3421–3431. doi: 10.1172/JCI42918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Wegrzyn J, Lee J, Neveu JM, Lane WS, Hook V. Proteomics of neuroendocrine secretory vesicles reveal distinct functional systems for biosynthesis and exocytosis of peptide hormones and neurotransmitters. J Proteome Res. 2007;6:1652–1665. doi: 10.1021/pr060503p. [DOI] [PubMed] [Google Scholar]
- 112.Wegrzyn JL, Bark SJ, Yap A, Hook V. Proteomics of dense core secretory vesicles reveal distinct protein categories for secretion of neuroeffectors for cell-cell communication. J Proteome Res. 2010;9:5002–5024. doi: 10.1021/pr1003104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Pfeffer S, Aivazian D. Targeting RAB GTPases to distinct membrane compartments. Nat Rev Mol Cell Biol. 2004;5:886–896. doi: 10.1038/nrm1500. [DOI] [PubMed] [Google Scholar]
- 114.Colicelli J. Human RAS superfamily proteins and related GTPases. Sci. STKE. 2004;250:RE13. doi: 10.1126/stke.2502004re13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Ungar D, Hughson FM. SNARE protein structure and function. Annu Rev Cell Dev Biol. 2003;19:493–517. doi: 10.1146/annurev.cellbio.19.110701.155609. [DOI] [PubMed] [Google Scholar]
- 116.Gerke V, Moss SE. Annexins: from structure to function. Physiol Rev. 2002;82:331–371. doi: 10.1152/physrev.00030.2001. [DOI] [PubMed] [Google Scholar]
- 117.Taupenot L, Harper KL, O’Connor DT. Role of H+-ATPase-mediated acidification in sorting and release of the regulated secretory protein chromogranin A: Evidence for a vesiculogenic function. J Biol Chem. 2005;280:3885–3897. doi: 10.1074/jbc.M408197200. [DOI] [PubMed] [Google Scholar]
- 118.Brunner Y, Schvartz D, Couté Y, Sanchez JC. Proteomics of regulated secretory organelles. Mass Spectrom Rev. 2009;28:844–867. doi: 10.1002/mas.20211. [DOI] [PubMed] [Google Scholar]
- 119.Gilchrist A, Au CE, Hiding J, Bell AW, Fernandez-Rodriguez J, Lesimple S, Nagaya H, Roy L, Gosline SJ, Hallett M, Paiement J, Kearney RE, Nilsson T, Bergeron JJ. Quantitative proteomics analysis of the secretory pathway. Cell. 2006;127:1265–1281. doi: 10.1016/j.cell.2006.10.036. [DOI] [PubMed] [Google Scholar]
- 120.Chen X, Walker AK, Strahler JR, Simon ES, Tomanicek-Volk SL, Nelson BB, Hurley MC, Ernst SA, Williams JA, Andrews PC. Organellar proteomics: analysis of pancreatic zymogen granule membranes. Mol Cell Proteomics. 2006;5:306–312. doi: 10.1074/mcp.M500172-MCP200. [DOI] [PubMed] [Google Scholar]


