Abstract
During germination of barley grains, the cell walls of the starchy endosperm are degraded by (1→3,1→4)-β-glucanases (EC 3.2.1.73) secreted from the aleurone and scutellar tissues. The complete sequence of the aleurone (1→3,1→4)-β-glucanase isoenzyme II comprises 306 amino acids and was determined by sequencing nine tryptic peptides (110 residues) and aligning them with the amino acid sequence deduced from a cDNA clone encoding the 291 NH2-terminal residues. Although no amino acid sequence homology with a bacterial (1→3)(1→4)-β-glucanase is apparent, close to 50% homology is found with two large regions of a (1→3)-β-glucanase from tobacco pith tissue. The gene for barley (1→3,1→4)-β-glucanase isoenzyme II shares with that for the α-amylase isoenzyme 1 a strongly preferred use of codons with G and C in the wobble position (94% and 90%, respectively). Both enzymes are secreted from the aleurone cells during germination. Such one-sided codon usage is not characteristic for the gene encoding the (1→3)-β-glucanase of tobacco pith tissue or the hor2-4 gene encoding the B1 hordein storage protein in the endosperm.
Keywords: cell-wall degradation, cDNA, codon usage, tissue specificity
Full text
PDF

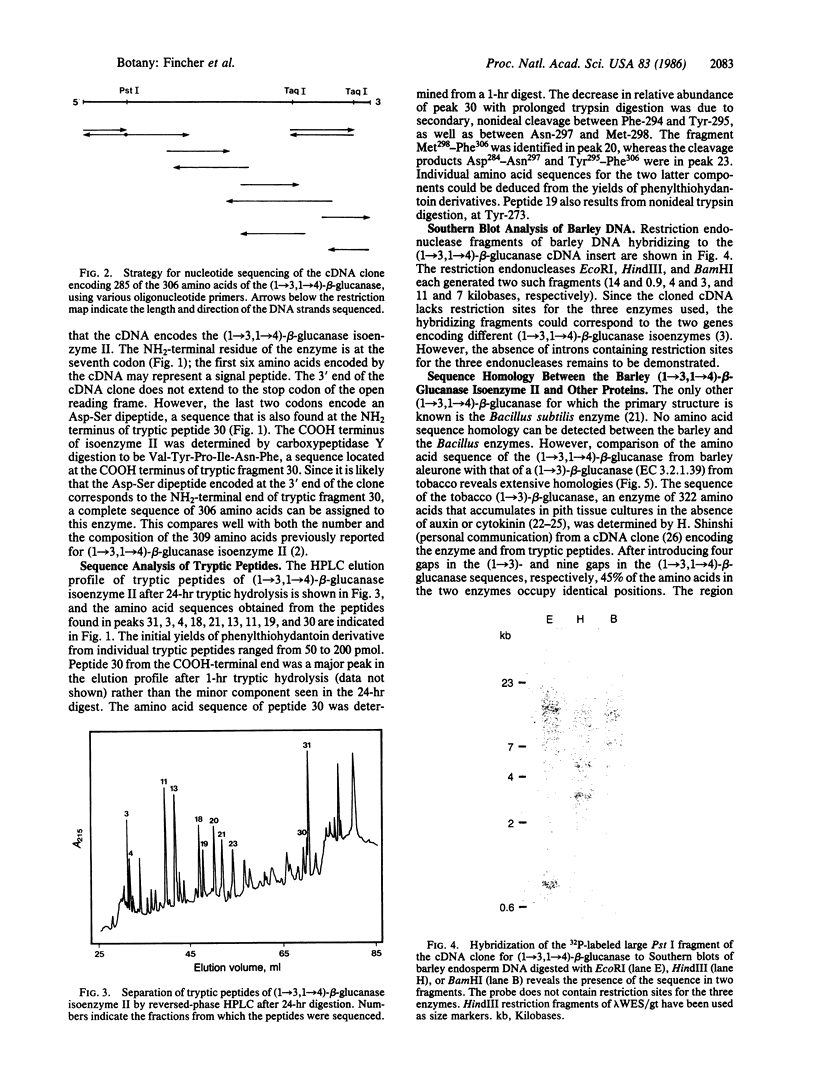


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Birnboim H. C., Doly J. A rapid alkaline extraction procedure for screening recombinant plasmid DNA. Nucleic Acids Res. 1979 Nov 24;7(6):1513–1523. doi: 10.1093/nar/7.6.1513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Denhardt D. T. A membrane-filter technique for the detection of complementary DNA. Biochem Biophys Res Commun. 1966 Jun 13;23(5):641–646. doi: 10.1016/0006-291x(66)90447-5. [DOI] [PubMed] [Google Scholar]
- Gubler U., Hoffman B. J. A simple and very efficient method for generating cDNA libraries. Gene. 1983 Nov;25(2-3):263–269. doi: 10.1016/0378-1119(83)90230-5. [DOI] [PubMed] [Google Scholar]
- Hanahan D. Studies on transformation of Escherichia coli with plasmids. J Mol Biol. 1983 Jun 5;166(4):557–580. doi: 10.1016/s0022-2836(83)80284-8. [DOI] [PubMed] [Google Scholar]
- Hu N. T., Peifer M. A., Heidecker G., Messing J., Rubenstein I. Primary structure of a genomic zein sequence of maize. EMBO J. 1982;1(11):1337–1342. doi: 10.1002/j.1460-2075.1982.tb01319.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meinkoth J., Wahl G. Hybridization of nucleic acids immobilized on solid supports. Anal Biochem. 1984 May 1;138(2):267–284. doi: 10.1016/0003-2697(84)90808-x. [DOI] [PubMed] [Google Scholar]
- Mills K. K., Bauer W. D. Rhizobium attachment to clover roots. J Cell Sci Suppl. 1985;2:333–345. doi: 10.1242/jcs.1985.supplement_2.18. [DOI] [PubMed] [Google Scholar]
- Mohnen D., Shinshi H., Felix G., Meins F. Hormonal regulation of beta1,3-glucanase messenger RNA levels in cultured tobacco tissues. EMBO J. 1985 Jul;4(7):1631–1635. doi: 10.1002/j.1460-2075.1985.tb03830.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mundy J., Brandt A., Fincher G. B. Messenger RNAs from the Scutellum and Aleurone of Germinating Barley Encode (1-->3,1-->4)-beta-d-Glucanase, alpha-Amylase and Carboxypeptidase. Plant Physiol. 1985 Nov;79(3):867–871. doi: 10.1104/pp.79.3.867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murphy N., McConnell D. J., Cantwell B. A. The DNA sequence of the gene and genetic control sites for the excreted B. subtilis enzyme beta-glucanase. Nucleic Acids Res. 1984 Jul 11;12(13):5355–5367. doi: 10.1093/nar/12.13.5355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pedersen K., Devereux J., Wilson D. R., Sheldon E., Larkins B. A. Cloning and sequence analysis reveal structural variation among related zein genes in maize. Cell. 1982 Jul;29(3):1015–1026. doi: 10.1016/0092-8674(82)90465-2. [DOI] [PubMed] [Google Scholar]
- Rigby P. W., Dieckmann M., Rhodes C., Berg P. Labeling deoxyribonucleic acid to high specific activity in vitro by nick translation with DNA polymerase I. J Mol Biol. 1977 Jun 15;113(1):237–251. doi: 10.1016/0022-2836(77)90052-3. [DOI] [PubMed] [Google Scholar]
- Rogers J. C., Milliman C. Isolation and sequence analysis of a barley alpha-amylase cDNA clone. J Biol Chem. 1983 Jul 10;258(13):8169–8174. [PubMed] [Google Scholar]
- Sanger F., Nicklen S., Coulson A. R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Southern E. M. Detection of specific sequences among DNA fragments separated by gel electrophoresis. J Mol Biol. 1975 Nov 5;98(3):503–517. doi: 10.1016/s0022-2836(75)80083-0. [DOI] [PubMed] [Google Scholar]
- Suggs S. V., Wallace R. B., Hirose T., Kawashima E. H., Itakura K. Use of synthetic oligonucleotides as hybridization probes: isolation of cloned cDNA sequences for human beta 2-microglobulin. Proc Natl Acad Sci U S A. 1981 Nov;78(11):6613–6617. doi: 10.1073/pnas.78.11.6613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vieira J., Messing J. The pUC plasmids, an M13mp7-derived system for insertion mutagenesis and sequencing with synthetic universal primers. Gene. 1982 Oct;19(3):259–268. doi: 10.1016/0378-1119(82)90015-4. [DOI] [PubMed] [Google Scholar]
- Werr W., Frommer W. B., Maas C., Starlinger P. Structure of the sucrose synthase gene on chromosome 9 of Zea mays L. EMBO J. 1985 Jun;4(6):1373–1380. doi: 10.1002/j.1460-2075.1985.tb03789.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woodward J. R., Fincher G. B. Purification and chemical properties of two 1,3;1,4-beta-glucan endohydrolases from germinating barley. Eur J Biochem. 1982 Jan;121(3):663–669. doi: 10.1111/j.1432-1033.1982.tb05837.x. [DOI] [PubMed] [Google Scholar]
- Zimmerman C. L., Appella E., Pisano J. J. Rapid analysis of amino acid phenylthiohydantoins by high-performance liquid chromatography. Anal Biochem. 1977 Feb;77(2):569–573. doi: 10.1016/0003-2697(77)90276-7. [DOI] [PubMed] [Google Scholar]



