Abstract
Eukaryotic transcriptional coactivators are multi-subunit complexes that both modify chromatin and recognize histone modifications. Until recently, structural information on these large complexes has been limited to isolated enzymatic domains or chromatin-binding motifs. This review summarizes recent structural studies of the SAGA coactivator complex that have greatly advanced our understanding of the interplay between its different subunits. The structure of the four-protein SAGA deubiquitinating module has provided a first glimpse of the larger organization of a coactivator complex, and illustrates how interdependent subunits interact with each other to form an active and functional enzyme complex. In addition, structures of the histone binding domains of ATXN7 and Sgf29 shed light on the interactions with chromatin that help recruit the SAGA complex.
INTRODUCTION
Eukaryotic cells rely on a diverse array of transcriptional coactivator complexes to regulate the expression of inducible genes [1]. These coactivators, which are typically large multi-protein complexes and contain enzymatic subunits that modify chromatin, as well as domains that recognize specific histone modifications and help recruit the transcription pre-initiation complex [2,3]. The SAGA (Spt-Ada-Gcn5-Acetyltransferase) complex is 1.8 MDa transcriptional coactivator that activates around 10% of yeast genes in response to environmental stresses [4-6], and has served as a paradigm for understanding eukaryotic gene activation. SAGA performs multiple functions including acetylating core histones, recruiting the pre-initiation complex, and deubiquitinating monoubiquitinated histone H2B (H2B-Ub) [7]. Removal of monoubiquitin from Lys123 H2B is required for downstream events including recruitment of the Ctk1 kinase, which phosphorylates the C-terminal domain (CTD) of RNA polymerase II [8]. In addition to its role in activating transcription, SAGA also promotes transcription elongation [9] and export of the nascent mRNA through the nuclear pore complex [10]. Studies have shown that the activities first described for the yeast complex are largely conserved in Drosophila and human SAGA [11,12].
The 21 proteins that make up the SAGA complex are broadly conserved from yeast to man [5] and contain a variety of domains whose functions are known, including the catalytic core of the GCN5 histone acetyltransferase (HAT) [13], the catalytic domain of the deubiquitinating enzyme (DUB), Ubp8 [14], domains that bind various histone modifications, zinc fingers, and protein-protein interaction domains [5]. Crystal structures of the GCN5 HAT domain [15,16] and bromodomain (which binds acetyl lysine) [17] have been determined, and a variety of other domains have been annotated based on sequence similarities [5]. Many of the SAGA polypeptides, however, contain a few recognizable domains, and there is limited information on how the SAGA subunits are organized within their sub-complexes. Several studies have shown that the activities of the enzymatic subunits of SAGA are altered when they are incorporated into larger sub-complexes [18,19], underscoring the need for structural information on larger complexes with other SAGA subunits. In this review, we describe recent advances in understanding the spatial organization of components of the SAGA complex, and how the interactions among subunits govern both the structure and enzymatic activity of the individual components.
The SAGA complex
The SAGA proteins are organized into four sub-complexes with distinct functions: the deubiquitinating module (DUBm), the histone acetyltransferase (HAT) module, and the SPT and TAF modules, which are implicated in pre-initiation complex assembly and SAGA architecture, respectively [5]. Figure 1 shows a current view of the organization of SAGA subunits based on a recent mass spectrometry study [20]*. The HAT module contains the GCN5 acetyltransferase in complex with Ada2, Ada3 and Sgf29 [20]. The DUBm comprises four proteins: Ubp8, Sgf11, Sus1 and Sgf73 [19,21]. While the full-length Ubp8, Sgf11 and Sus1 proteins are contained within the DUBm, Sgf73 has multiple roles in forming part of the DUBm, tethering the DUBm to the rest of the SAGA complex and recruiting the DUBm to its substrate [19]. Interestingly, the Sus1 protein has a distinct role in mRNA export [22], forming a part of the TREX-2 complex together with Cdc31, Sac3 and Thp1 [10].
Figure 1. SAGA is a modular complex.
The 21 yeast SAGA proteins are arranged into four distinct modules: The HAT module (green) consists of Ada3, Ada2, Sgf29 and the histone acetyltransferase (HAT) Gcn5. The DUB module (red) consists of Sgf11, Sgf73, Sus1 and the deubiquitinating enzyme, Ubp8. The SPT module is shown in purple and the TAF module is shown in blue. The spatial positions of the SAGA proteins in this figure are derived from a model based on data from a recent mass spectrometric study [20].
A defining feature of the DUB module is the functional interdependence of the four subunits. Although Ubp8 contains a catalytic domain that is similar in sequence to members of the USP family of deubiquitinating enzymes [23], this DUB module subunit is inactive on its own [19]. Ubp8 only becomes enzymatically active when it forms a complex with Sgf11, Sus1, and the N-terminus of Sgf73. Of the 657 residues of Sgf73, amino acids 1-96 comprise the minimal fragment needed for activity on an artificial ubiquitin-AMC substrate, while a fragment extending to residue 104 exhibits greater activity on H2B-Ub [19]. The corresponding fragment of the human homologue of Sgf73, ATXN7, is the location of a polyglutamine expansion that disrupts SAGA function and is the cause of Spinocerebellar Ataxia type 7 [24].
Structure of the DUB module
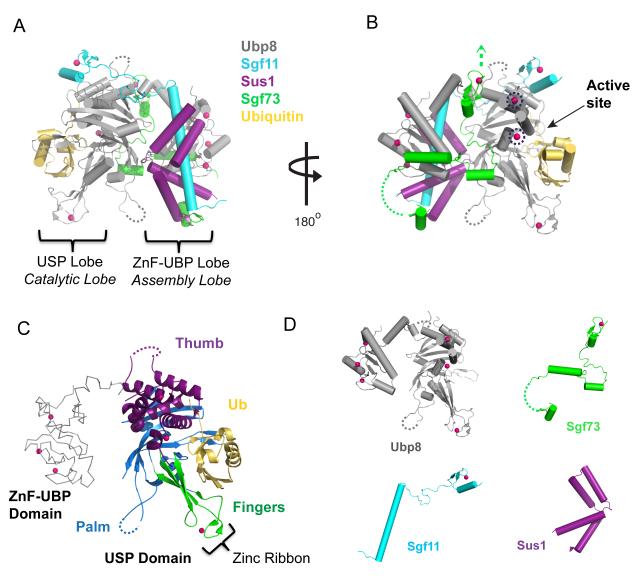
Recent structure determinations of the DUB module ([25]** and [26]**) have provided clues as to how Ubp8 is activated and targeted to its chromatin substrate. Most surprisingly, the structures reveal an unprecedented organization of the four subunits in the complex. The DUB module structures reported contain the full Ubp8, Sgf11 and Sus1 proteins and an Sgf73 N-terminal fragment extending to either residue 96 [26] or 104 [25] (we shall refer to this fragment as Sgf73N-term). The structures show Ubp8, Sgf11, Sus1 and Sgf73N-term to be highly intertwined (Figure 2, A and B), with each protein contacting the other three. Remarkably, Ubp8 is the only protein that contains globular domains, while the other polypeptide chains are fairly extended and, with the exception of a zinc finger in Sgf11 and in Sgf73, lack independently folding domains (Figure 2D). The DUB module is organized into two lobes (Figure 2, A and B), each containing one of the two globular domains of Ubp8: the USP, or catalytic lobe, which contains the C-terminal USP domain and the ZnF-UBP, or assembly lobe, which contains the N-terminal Zinc Finger-Ubiquitin Binding Protein (ZnF-UBP) domain [25]. While some ZnF-UBP domains have been implicated in ubiquitin binding [27,28], this domain of Ubp8 lacks the conserved residues that are needed for ubiquitin binding, instead containing hydrophobic residues that interact with Sgf11.
Figure 2. The structure of the SAGA DUB module.
A) The structure of the DUB module bound to ubiquitin aldehyde. The DUB module proteins Ubp8, Sgf11, Sus1 and Sgf73 (colors indicated in figure, zinc atoms are colored dark pink) form a highly intertwined complex. The four proteins are organized around the two globular domains of Ubp8 forming the ZnF-UBP/assembly lobe, and the USP/catalytic lobe. Ubiquitin aldehyde (yellow) is bound to the ubiquitin-binding pocket with its C-terminal tail extending into the Ubp8 active site. B) View of the DUB module, rotated 180°. Two novel USP zinc-binding sites (blue circles) are conserved in the human and Drosophila homologues of Ubp8. A green arrow points in the direction of SAGA, indicating the region where the DUBm may be anchored to the rest of SAGA, and the Ubp8 active site is indicated. C) The USP domain of Ubp8, indicating the thumb, palm and fingers regions. The globular portion of ubiquitin (yellow) binds to the fingers region. D) While Ubp8 contains globular domains, the other three proteins are mostly non-globular and adopt conformations that depend on their incorporation into the complex.
Running through the core of the ZnF-UBP lobe is the long N-terminal helix of Sgf11 (Figure 2A), which is largely buried by binding of the Ubp8 ZnF-UBP domain on one face and Sus1 on the other. Sus1 wraps around the Sgf11 helix, adopting a hinged helical hairpin fold [29]*. Sus1 also contacts the ZnF-UBP domain of Ubp8, further cementing the binding of these two proteins to Sgf11. Sgf11 spans the two lobes of the DUB module via a long linker region that joins the Sgf11 helix to a C-terminal zinc finger domain, which binds to the Ubp8 catalytic domain adjacent to the active site (Figure 2A). As discussed below, its proximity to the active site suggests that the Sgf11 zinc finger might play a role in activating Ubp8. Between the two lobes lies Sgf73, whose meandering linker and C-terminal zinc finger domain appear to bring together the two globular domains of Ubp8. The Sgf73 zinc finger is critical to the incorporation of this subunit into the DUB module, as a deletion of just a few of the zinc finger residues (the 1-89 fragment, as compared to 1-96) dramatically disrupts binding of Sgf73 to the other DUB module proteins [25]. The remaining N-terminal residues of Sgf73 contain additional alpha-helical segments that interact with all three proteins in the ZnF-UBP (assembly) lobe and cap off the N-terminus of the Sgf11 helix.
The catalytic domain of Ubp8 is similar in overall fold to several other USP-type DUBs whose structures have been determined [30-32]. This fold has been described [30] as containing a thumb, palm and fingers region (Figure 2C), with the latter binding the globular domain of ubiquitin. The distal portion of the fingers domain, which contains a bound zinc atom, is partially disordered in both reported structures of the apo-enzyme [25,26], but is well-ordered in the structure of the DUB module bound to ubiquitin aldehyde [26]. An unusual feature of the catalytic domain is the presence of two zinc-binding sites in the thumb region (Figure 2B), which are found in place of buried hydrophobic core residues that are found in other USP domains. While there is as yet no known functional role for these bound zincs, it is interesting to note that these sites are conserved in the human and Drosophila homologues, USP22 and Nonstop [33,34].
Insights into DUB activation
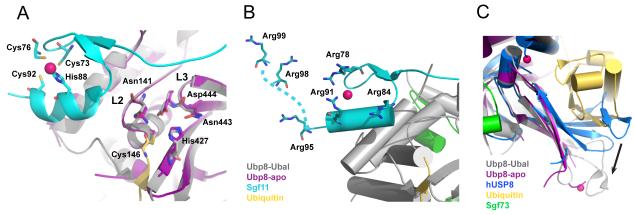
The DUB module structures provide a few clues as to why Ubp8 is only enzymatically active when complexed with Sgf11, Sus1 and Sgf73N-term [19]. Ubp8 is a papain-like cysteine protease with an Asn-His-Cys catalytic triad (Figure 3A). The Sgf11 zinc finger binds immediately adjacent to the Ubp8 active site residues (Figure 3A), placing Sgf11 in a position where it could potentially influence the catalytic activity of Ubp8. There are a number of deubiquitinating enzymes that depend upon interactions with substrates [23] or other protein partners [35] to orient the active site residues in a catalytically competent configuration. However, the catalytic residues Cys146, His427, and Asn443 and the predicted oxyanion hole residues, Asp444 and Asn141, of Ubp8 are properly oriented in both the presence and absence of bound ubiquitin (Figure 3A). Sgf11 contacts loops in Ubp8 that contain four of the five active site residues, raising the possibility that these contacts may maintain the active site residues in a catalytically competent conformation. Consistent with this, deletion of the Sgf11 zinc finger dramatically reduces the catalytic activity of the DUB module [25].
Figure 3. The activation of Ubp8 and substrate recognition of the DUBm.
A) The Sgf11 zinc finger binds adjacent to the Ubp8 active site and interacts with loops L2 and L3, which contain the predicted oxyanion hole residues N141, D444, and the catalytic residues, N443 and C146. Also shown is H427, the third residue in the catalytic triad. The active site residues adopt the same conformation in the absence (purple DUBm) and presence (grey DUBm) of ubiquitin (colors indicated in figure, zinc atoms are colored dark pink). B) Conserved arginine residues that form a basic patch on the Sgf11 zinc finger are indicated, including Arg98 and Arg99, which are disordered in the structure and have been modeled into the figure (indicated by the dashed line). C) The fingers region of Ubp8 adopts an open conformation in the presence (gray) and absence (magenta) of bound ubiquitin. By contrast, fingers region in the structure of human USP8 [36] occludes the ubiquitin-binding pocket, and must undergo a conformational change in order to bind ubiquitin, as indicated by the black arrow.
A second role that has been proposed for Sgf11 is in recognizing the DUB module substrate, H2B-Ub. The Sgf11 zinc finger contains a cluster of six conserved arginines (Figure 3B) that could potentially mediate interactions with nucleosomal DNA and orient the DUB module properly on its substrate. Mutations in several of these conserved arginines affect SAGA function in vivo [25], but further biochemical and structural studies will be needed to determine the role of Sgf11 in substrate binding .
The role of Sgf73 in bringing together the two lobes of the DUB module is also important for DUB activity, as deletions that disrupt the Sgf73 zinc finger or further truncate the N-terminal fragment inactivate enzymatic activity [25]. How Sgf73 maintains the activity of Ubp8, however, is not clear. One hypothesis [26] is that the interactions between Sgf73 and Ubp8 along the “back” side of Ubp8 could promote ubiquitin binding by maintaining the structure of the fingers region. In the structure of the human USP8 apo-enzyme [36] (no relation to Ubp8, whose human homologue is USP22), the fingers region collapses inward and occludes the ubiquitin-binding pocket (Figure 3C), whereas the corresponding region of Ubp8 does not change conformation in the absence of bound ubiquitin. Whether Sgf73 indeed plays a role in reducing the energetic cost of a conformational change, or instead plays a different role in activating Ubp8, awaits further studies.
Insights into complex formation
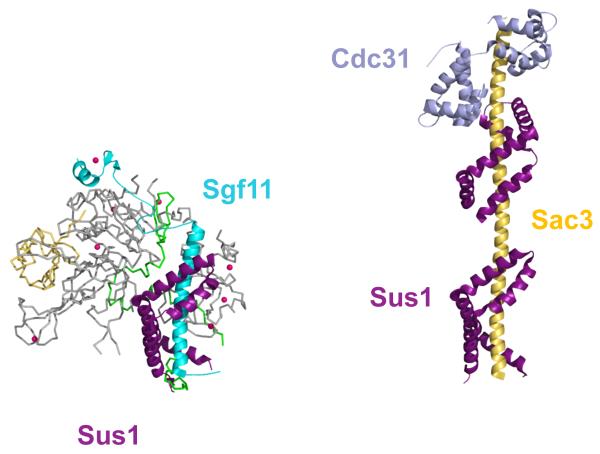
The unusually interdependent arrangement of the DUB module subunits raises the question of how the complex assembles and what distinct structural and functional roles the non-enzymatic subunits play. The non-globular nature of the Sgf11, Sus1 and Sgf73 proteins in the DUB module strongly suggest that their conformations are governed by complex formation. Sgf11 and Sgf73N-term, in particular, contain extended regions lacking any secondary structure that are presumably disordered in the isolated proteins. It is then not surprising, then, that an NMR study of Sgf731-104 found that, with the exception of the zinc finger, the protein is unstructured [37]*. While the long N-terminal helix of Sgf11 would similarly be expected to be unfolded in solution, its complex with Sus1 forms a stable structure, as seen in the crystal structure of an isolated Sgf111-33-Sus1 complex [38]**. This complex is virtually superimposable with the corresponding residues in the DUB module. An exception is the N-terminal helix of Sus1, which adopts variable conformations when in complex with Sgf11 alone but just a single conformation in the three DUB module structures [25,26] that is stabilized by interactions with the other three DUB module subunits.
The architectural role of Sus1 is entirely conserved the structure of TREX-2 complex [29]*, which contains Sus1 in addition to Sac3 and Cdc31 (Figure 4). In that structure, Sus1 binds to the long alpha helix of Sac3, mirroring the complex between Sus1 and the Sgf11 helix. The functional significance of the presence of Sus1 in both the SAGA DUB module and in the mRNA export machinery is unknown. The small size of Sus1 (96 amino acids) and its mutually exclusive interactions with either Sgf11 or Sac3 leaves open the question of how this small protein couples SAGA function with mRNA export.
Figure 4. Sus1 plays a conserved role in the SAGA DUB module and in the TREX-2 complex.
As in the DUB module (left), Sus1 also forms an alpha-helical clamp in the TREX-2 complex (right), which contains two copies of Sus1 and one copy of Cdc31 bound to the extended N-terminal helix of Sac3.
SAGA beyond the DUB module
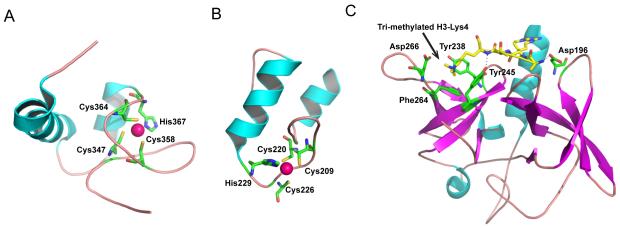
Structures were recently reported for additional SAGA protein domains, further adding to our structural understanding of how they function as components of the SAGA complex. Beyond the N-terminal residues of Sgf73 that are part of the DUB module, this protein contains a conserved ~70 amino acid SCA7 domain, which is implicated in chromatin interactions [39]**. The SCA7 domain derives its name from the human homologue of Sgf73, ATXN7, which is the affected protein in Spinocerebellar Ataxia type 7 [24]. A SCA7 domain is also found in the human homologue of Sgf11, called ATXN7L3, which contains an additional ~150 amino acids not found in the yeast homologue. Recent NMR structures of the SCA7 domain in ATXN7 and ATXN7L3 [39]** reveal a novel zinc-binding fold, with a pair of alpha helices and a zinc atom coordinated by three cysteines and a histidine (Figure 5, A and B). While the overall topology of SCA7 domain is conserved in both proteins, there is a significant difference in the relative angle relating the helices, perhaps as a consequence of the limited sequence identity between the two SCA7 domains. The two helices in ATXN7-SCA7 zinc finger appear to adopt a pseudo-perpendicular orientation, while the helices in the ATXN7L3-SCA7 zinc finger are positioned in an anti-parallel fashion (Figure 5, A and B). The differences in structure and sequence appear to allow ATXN7/Sgf73, but not ATXN7L3, to bind to histone H2A-H2B dimers. Overall, these studies hint at a role for the SCA7 domain of Sgf73/ATXN7 in recruiting the SAGA complex to chromatin, adding another layer of complexity to substrate recognition by the DUB module proteins. The function of the ATXN7L3 SCA7 domain in the human DUB module is still not known, but its presence suggests an additional function that is absent in fungi.
Figure 5. The SCA7 domain of ATNX7 and the Tudor domain of Sgf29.
A) The SCA7 zinc finger of ATXN7 Also shown is the zinc coordinated by a histidine and three cysteines. B) The ATXN7L3 SCA7 zinc finger. Its zinc binding site is similar to that of ATXN7-SCA7. C) Structure of the Tudor domains of Sgf29 bound to a tri-methylated H3K4 peptide. An arrow indicates the tri-methyl group on Lys4, which lies in a hydrophobic pocket formed by Tyr245, Tyr238, and Phe264, and is further stabilized by interactions with Asp266. Tyr245 and Aps196 also form hydrogen bonds with amide groups on the peptide.
Before SAGA is recruited to an activated gene, histone H3-K4 is methylated by the Set1 methyltransferase [40,41], which is required for SAGA recruitment [42]. This activating histone mark is recognized by the Tudor domains of Sgf29, a recently-identified subunit of the SAGA HAT module [20]. Crystal structures of both yeast and human Sgf29 tandem Tudor domains bound to methylated histone peptides [43] explain the specificity of Sgf29 for di- and tri-methylated histone H3K4. The Tudor domains of Sgf29 form a negatively charged pocket that binds the first four residues of the H3-K4 peptide (Figure 5C). Clusters of hydrophobic and acidic residues in the Tudor domains form extensive interactions with the tri-methylated lysine, as well other residues in the peptide, and specifically position the H3-K4 peptide in that pocket. The extensive interactions between Sgf29 and tri-methylated H3-K4 explain how and why Sgf29 specifically recognizes this modification, and not the other methylated lysine residues on histone H3.
Conclusions
After over a decade in which a structural understanding of SAGA function was largely restricted to the GCN5 catalytic domain, structure determinations of the SAGA DUB module and of the histone reading domains of Sgf73 and Sgf29 are a significant advance. The unexpected architecture of the DUB module, with non-globular subunits whose conformations are governed by their integration into the complex, may well turn out to be a more general feature of SAGA structure. It would not be surprising if the remaining three SAGA modules – the HAT, SPT and TAF modules – similarly contained architectural subunits. This suggests a fresh approach to tackling structures of other sub-complexes: rather than identifying further functional domains of interest, a more fruitful approach may be to identify co-folding protein fragments that contribute to a stably folded complex.
Research Highlights.
SAGA is a conserved 21-subunit transcriptional coactivator that is arranged into four modular sub-complexes with distinct functions: histone deubiquitination, acetylation, transcription pre-initiation complex assembly, and coactivator architecture.
Structures of the SAGA deubiquitinating module (DUBm) show the four subunits, Ubp8, Sgf11, Sus1 and Sgf73 to form an unusual intertwined complex.
Sus1 plays a conserved structural role in both the DUB module and the TREX-2 complex, which is involved in mRNA export.
Structures of two SAGA chromatin-binding domains, SCA7 of ATXN7 (the human homologue of Sgf73) and the Tudor domains of Sgf29, provide new information on the interactions that recruit the SAGA complex.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
* of special interest
** of outstanding interest
- 1.Lee TI, Young RA. Transcription of eukaryotic protein-coding genes. Annu Rev Genet. 2000;34:77–137. doi: 10.1146/annurev.genet.34.1.77. [DOI] [PubMed] [Google Scholar]
- 2.Weake VM, Workman JL. Inducible gene expression: diverse regulatory mechanisms. Nat Rev Genet. 2010;11:426–437. doi: 10.1038/nrg2781. [DOI] [PubMed] [Google Scholar]
- 3.Naar AM, Lemon BD, Tjian R. Transcriptional coactivator complexes. Annu Rev Biochem. 2001;70:475–501. doi: 10.1146/annurev.biochem.70.1.475. [DOI] [PubMed] [Google Scholar]
- 4.Baker SP, Grant PA. The SAGA continues: expanding the cellular role of a transcriptional co-activator complex. Oncogene. 2007;26:5329–5340. doi: 10.1038/sj.onc.1210603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Koutelou E, Hirsch CL, Dent SY. Multiple faces of the SAGA complex. Curr Opin Cell Biol. 2010 doi: 10.1016/j.ceb.2010.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Rodriguez-Navarro S. Insights into SAGA function during gene expression. EMBO Rep. 2009;10:843–850. doi: 10.1038/embor.2009.168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Daniel JA, Torok MS, Sun ZW, Schieltz D, Allis CD, Yates JR, 3rd, Grant PA. Deubiquitination of histone H2B by a yeast acetyltransferase complex regulates transcription. J Biol Chem. 2004;279:1867–1871. doi: 10.1074/jbc.C300494200. [DOI] [PubMed] [Google Scholar]
- 8.Wyce A, Xiao T, Whelan KA, Kosman C, Walter W, Eick D, Hughes TR, Krogan NJ, Strahl BD, Berger SL. H2B ubiquitylation acts as a barrier to Ctk1 nucleosomal recruitment prior to removal by Ubp8 within a SAGA-related complex. Mol Cell. 2007;27:275–288. doi: 10.1016/j.molcel.2007.01.035. [DOI] [PubMed] [Google Scholar]
- 9.Govind CK, Zhang F, Qiu H, Hofmeyer K, Hinnebusch AG. Gcn5 promotes acetylation, eviction, and methylation of nucleosomes in transcribed coding regions. Mol Cell. 2007;25:31–42. doi: 10.1016/j.molcel.2006.11.020. [DOI] [PubMed] [Google Scholar]
- 10.Pascual-Garcia P, Govind CK, Queralt E, Cuenca-Bono B, Llopis A, Chavez S, Hinnebusch AG, Rodriguez-Navarro S. Sus1 is recruited to coding regions and functions during transcription elongation in association with SAGA and TREX2. Genes Dev. 2008;22:2811–2822. doi: 10.1101/gad.483308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zhang XY, Varthi M, Sykes SM, Phillips C, Warzecha C, Zhu W, Wyce A, Thorne AW, Berger SL, McMahon SB. The putative cancer stem cell marker USP22 is a subunit of the human SAGA complex required for activated transcription and cell-cycle progression. Mol Cell. 2008;29:102–111. doi: 10.1016/j.molcel.2007.12.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zhao Y, Lang G, Ito S, Bonnet J, Metzger E, Sawatsubashi S, Suzuki E, Le Guezennec X, Stunnenberg HG, Krasnov A, et al. A TFTC/STAGA module mediates histone H2A and H2B deubiquitination, coactivates nuclear receptors, and counteracts heterochromatin silencing. Mol Cell. 2008;29:92–101. doi: 10.1016/j.molcel.2007.12.011. [DOI] [PubMed] [Google Scholar]
- 13.Brownell JE, Zhou J, Ranalli T, Kobayashi R, Edmondson DG, Roth SY, Allis CD. Tetrahymena histone acetyltransferase A: a homolog to yeast Gcn5p linking histone acetylation to gene activation. Cell. 1996;84:843–851. doi: 10.1016/s0092-8674(00)81063-6. [DOI] [PubMed] [Google Scholar]
- 14.Henry KW, Wyce A, Lo WS, Duggan LJ, Emre NC, Kao CF, Pillus L, Shilatifard A, Osley MA, Berger SL. Transcriptional activation via sequential histone H2B ubiquitylation and deubiquitylation, mediated by SAGA-associated Ubp8. Genes Dev. 2003;17:2648–2663. doi: 10.1101/gad.1144003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Rojas JR, Trievel RC, Zhou J, Mo Y, Li X, Berger SL, Allis CD, Marmorstein R. Structure of Tetrahymena GCN5 bound to coenzyme A and a histone H3 peptide. Nature. 1999;401:93–98. doi: 10.1038/43487. [DOI] [PubMed] [Google Scholar]
- 16.Trievel RC, Rojas JR, Sterner DE, Venkataramani RN, Wang L, Zhou J, Allis CD, Berger SL, Marmorstein R. Crystal structure and mechanism of histone acetylation of the yeast GCN5 transcriptional coactivator. Proc Natl Acad Sci U S A. 1999;96:8931–8936. doi: 10.1073/pnas.96.16.8931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Owen DJ, Ornaghi P, Yang JC, Lowe N, Evans PR, Ballario P, Neuhaus D, Filetici P, Travers AA. The structural basis for the recognition of acetylated histone H4 by the bromodomain of histone acetyltransferase gcn5p. EMBO J. 2000;19:6141–6149. doi: 10.1093/emboj/19.22.6141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Balasubramanian R, Pray-Grant MG, Selleck W, Grant PA, Tan S. Role of the Ada2 and Ada3 transcriptional coactivators in histone acetylation. J Biol Chem. 2002;277:7989–7995. doi: 10.1074/jbc.M110849200. [DOI] [PubMed] [Google Scholar]
- 19.Kohler A, Schneider M, Cabal GG, Nehrbass U, Hurt E. Yeast Ataxin-7 links histone deubiquitination with gene gating and mRNA export. Nat Cell Biol. 2008;10:707–715. doi: 10.1038/ncb1733. [DOI] [PubMed] [Google Scholar]
- * 20.Lee KK, Sardiu ME, Swanson SK, Gilmore JM, Torok M, Grant PA, Florens L, Workman JL, Washburn MP. Combinatorial depletion analysis to assemble the network architecture of the SAGA and ADA chromatin remodeling complexes. Mol Syst Biol. 2011;7:503. doi: 10.1038/msb.2011.40. This proteomics study characterized the interrepationship amond the subunits of the the SAGA and ADA coactivator complexes. By using different TAP-tagged proteins as bait and analyzing the associated proteins in wild type and deletion strains lacking one or more subunits, the authors were able to map the four SAGA subcomplexes as well as the ADA complex.
- 21.Lee KK, Swanson SK, Florens L, Washburn MP, Workman JL. Yeast Sgf73/Ataxin-7 serves to anchor the deubiquitination module into both SAGA and Slik(SALSA) HAT complexes. Epigenetics Chromatin. 2009;2:2. doi: 10.1186/1756-8935-2-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Rodriguez-Navarro S, Fischer T, Luo MJ, Antunez O, Brettschneider S, Lechner J, Perez-Ortin JE, Reed R, Hurt E. Sus1, a functional component of the SAGA histone acetylase complex and the nuclear pore-associated mRNA export machinery. Cell. 2004;116:75–86. doi: 10.1016/s0092-8674(03)01025-0. [DOI] [PubMed] [Google Scholar]
- 23.Komander D, Clague MJ, Urbe S. Breaking the chains: structure and function of the deubiquitinases. Nat Rev Mol Cell Biol. 2009;10:550–563. doi: 10.1038/nrm2731. [DOI] [PubMed] [Google Scholar]
- 24.David G, Abbas N, Stevanin G, Durr A, Yvert G, Cancel G, Weber C, Imbert G, Saudou F, Antoniou E, et al. Cloning of the SCA7 gene reveals a highly unstable CAG repeat expansion. Nat Genet. 1997;17:65–70. doi: 10.1038/ng0997-65. [DOI] [PubMed] [Google Scholar]
- ** 25.Kohler A, Zimmerman E, Schneider M, Hurt E, Zheng N. Structural basis for assembly and activation of the heterotetrameric SAGA histone H2B deubiquitinase module. Cell. 2010;141:606–617. doi: 10.1016/j.cell.2010.04.026. This paper reports the structure of the SAGA DUB module, consisting of Ubp8, Sgf73, Sgf11 and Sus1, determined to 2.7 Å resolution. This paper also describes a detailed set of biochemical experiments that identify key components of the DUB module that are essential to full enzymatic activity and complex assembly.
- ** 26.Samara NL, Datta AB, Berndsen CE, Zhang X, Yao T, Cohen RE, Wolberger C. Structural insights into the assembly and function of the SAGA deubiquitinating module. Science. 2010;328:1025–1029. doi: 10.1126/science.1190049. This paper reports the 2.45 Å structure of the apo SAGA DUB module as well as the structure of the DUB module bound to ubiquitin aldehyde, which was determined at 1.9 Å resolution. Comparison of the apo and ubiquitin-bound complexes revealed structural differences due to ubiquitin binding that suggest possible mechanisms of Ubp8 activation by the other DUB module subunits.
- 27.Reyes-Turcu FE, Horton JR, Mullally JE, Heroux A, Cheng X, Wilkinson KD. The ubiquitin binding domain ZnF UBP recognizes the C-terminal diglycine motif of unanchored ubiquitin. Cell. 2006;124:1197–1208. doi: 10.1016/j.cell.2006.02.038. [DOI] [PubMed] [Google Scholar]
- 28.Bonnet J, Romier C, Tora L, Devys D. Zinc-finger UBPs: regulators of deubiquitylation. Trends Biochem Sci. 2008;33:369–375. doi: 10.1016/j.tibs.2008.05.005. [DOI] [PubMed] [Google Scholar]
- * 29.Jani D, Lutz S, Marshall NJ, Fischer T, Kohler A, Ellisdon AM, Hurt E, Stewart M. Sus1, Cdc31, and the Sac3 CID region form a conserved interaction platform that promotes nuclear pore association and mRNA export. Mol Cell. 2009;33:727–737. doi: 10.1016/j.molcel.2009.01.033. The structure of the TREX02 complex, Sus1, Cdc31 and Sac3, shows Sus1 to adopt a conserved helical clamp that binds to the long Sac3 helix. In the SAGA DUB module, Sus1 plays the same structural role in binding to the Sgf11 helix.
- 30.Hu M, Li P, Li M, Li W, Yao T, Wu JW, Gu W, Cohen RE, Shi Y. Crystal structure of a UBP-family deubiquitinating enzyme in isolation and in complex with ubiquitin aldehyde. Cell. 2002;111:1041–1054. doi: 10.1016/s0092-8674(02)01199-6. [DOI] [PubMed] [Google Scholar]
- 31.Hu M, Li P, Song L, Jeffrey PD, Chenova TA, Wilkinson KD, Cohen RE, Shi Y. Structure and mechanisms of the proteasome-associated deubiquitinating enzyme USP14. EMBO J. 2005;24:3747–3756. doi: 10.1038/sj.emboj.7600832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ye Y, Scheel H, Hofmann K, Komander D. Dissection of USP catalytic domains reveals five common insertion points. Mol Biosyst. 2009;5:1797–1808. doi: 10.1039/b907669g. [DOI] [PubMed] [Google Scholar]
- 33.Atanassov BS, Evrard YA, Multani AS, Zhang Z, Tora L, Devys D, Chang S, Dent SY. Gcn5 and SAGA regulate shelterin protein turnover and telomere maintenance. Mol Cell. 2009;35:352–364. doi: 10.1016/j.molcel.2009.06.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Weake VM, Lee KK, Guelman S, Lin CH, Seidel C, Abmayr SM, Workman JL. SAGA-mediated H2B deubiquitination controls the development of neuronal connectivity in the Drosophila visual system. EMBO J. 2008;27:394–405. doi: 10.1038/sj.emboj.7601966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Scheuermann JC, de Ayala Alonso AG, Oktaba K, Ly-Hartig N, McGinty RK, Fraterman S, Wilm M, Muir TW, Muller J. Histone H2A deubiquitinase activity of the Polycomb repressive complex PR-DUB. Nature. 2010;465:243–247. doi: 10.1038/nature08966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Avvakumov GV, Walker JR, Xue S, Finerty PJ, Jr., Mackenzie F, Newman EM, Dhe-Paganon S. Amino-terminal dimerization, NRDP1-rhodanese interaction, and inhibited catalytic domain conformation of the ubiquitin-specific protease 8 (USP8) J Biol Chem. 2006;281:38061–38070. doi: 10.1074/jbc.M606704200. [DOI] [PubMed] [Google Scholar]
- * 37.Lai C, Wu M, Li P, Shi C, Tian C, Zang J. Solution NMR characterization of Sgf73(1-104) indicates that Zn ion is required to stabilize zinc finger motif. Biochem Biophys Res Commun. 2010;397:436–440. doi: 10.1016/j.bbrc.2010.05.118. This NMR study shows that, outside of the zinc finger, this fragment of Sgf73 is unstructured when not incorporated into the DUB module.
- ** 38.Ellisdon AM, Jani D, Koehler A, Hurt E, Stewart M. Structural basis for the interaction between yeast saga complex components SGF11 and SUS1. J Biol Chem. 2009 doi: 10.1074/jbc.M109.070839. This structure of Sus1 bound to an N-terminal fragment of Sgf11 provided the first view of how these proteins associate, and revealed the conserved architectural role of Sus1 in both the TREX-2 complex and SAGA.
- 39.Bonnet J, Wang YH, Spedale G, Atkinson RA, Romier C, Hamiche A, Pijnappel WW, Timmers HT, Tora L, Devys D, et al. The structural plasticity of SCA7 domains defines their differential nucleosome-binding properties. EMBO Rep. 2010;11:612–618. doi: 10.1038/embor.2010.98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Briggs SD, Bryk M, Strahl BD, Cheung WL, Davie JK, Dent SY, Winston F, Allis CD. Histone H3 lysine 4 methylation is mediated by Set1 and required for cell growth and rDNA silencing in Saccharomyces cerevisiae. Genes Dev. 2001;15:3286–3295. doi: 10.1101/gad.940201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Roguev A, Schaft D, Shevchenko A, Pijnappel WW, Wilm M, Aasland R, Stewart AF. The Saccharomyces cerevisiae Set1 complex includes an Ash2 homologue and methylates histone 3 lysine 4. EMBO J. 2001;20:7137–7148. doi: 10.1093/emboj/20.24.7137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Pray-Grant MG, Daniel JA, Schieltz D, Yates JR, 3rd, Grant PA. Chd1 chromodomain links histone H3 methylation with SAGA- and SLIK-dependent acetylation. Nature. 2005;433:434–438. doi: 10.1038/nature03242. [DOI] [PubMed] [Google Scholar]
- 43.Bian C, Xu C, Ruan J, Lee KK, Burke TL, Tempel W, Barsyte D, Li J, Wu M, Zhou BO, et al. Sgf29 binds histone H3K4me2/3 and is required for SAGA complex recruitment and histone H3 acetylation. EMBO J. 2011;30:2829–2842. doi: 10.1038/emboj.2011.193. [DOI] [PMC free article] [PubMed] [Google Scholar]