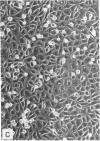
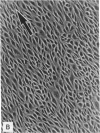
Abstract
The effects of hemodynamic forces upon vascular endothelial cell turnover were studied by exposing contact-inhibited confluent cell monolayers to shear stresses of varying amplitude in either laminar or turbulent flow. Laminar shear stresses (range, 8-15 dynes/cm2; 24 hr) induced cell alignment in the direction of flow without initiating the cell cycle. In contrast, turbulent shear stresses as low as 1.5 dynes/cm2 for as short a period as 3 hr stimulated substantial endothelial DNA synthesis in the absence of cell alignment, discernible cell retraction, or cell loss. The results of these in vitro experiments suggest that in atherosclerotic lesion-prone regions of the vascular system, unsteady blood flow characteristics, rather than the magnitude of wall shear stress per se, may be the major determinant of hemodynamically induced endothelial cell turnover.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bussolari S. R., Dewey C. F., Jr, Gimbrone M. A., Jr Apparatus for subjecting living cells to fluid shear stress. Rev Sci Instrum. 1982 Dec;53(12):1851–1854. doi: 10.1063/1.1136909. [DOI] [PubMed] [Google Scholar]
- Caro C. G., Fitz-Gerald J. M., Schroter R. C. Atheroma and arterial wall shear. Observation, correlation and proposal of a shear dependent mass transfer mechanism for atherogenesis. Proc R Soc Lond B Biol Sci. 1971 Feb 16;177(1046):109–159. doi: 10.1098/rspb.1971.0019. [DOI] [PubMed] [Google Scholar]
- Cornhill J. F., Roach M. R. A quantitative study of the localization of atherosclerotic lesions in the rabbit aorta. Atherosclerosis. 1976 May-Jun;23(3):489–501. doi: 10.1016/0021-9150(76)90009-5. [DOI] [PubMed] [Google Scholar]
- Davies P. F., Dewey C. F., Jr, Bussolari S. R., Gordon E. J., Gimbrone M. A., Jr Influence of hemodynamic forces on vascular endothelial function. In vitro studies of shear stress and pinocytosis in bovine aortic cells. J Clin Invest. 1984 Apr;73(4):1121–1129. doi: 10.1172/JCI111298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davies P. F., Reidy M. A., Goode T. B., Bowyer D. E. Scanning electron microscopy in the evaluation of endothelial integrity of the fatty lesion in atherosclerosis. Atherosclerosis. 1976 Oct;25(1):125–130. doi: 10.1016/0021-9150(76)90054-x. [DOI] [PubMed] [Google Scholar]
- Davies P. F., Ross R. Mediation of pinocytosis in cultured arterial smooth muscle and endothelial cells by platelet-derived growth factor. J Cell Biol. 1978 Dec;79(3):663–671. doi: 10.1083/jcb.79.3.663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeForrest J. M., Hollis T. M. Relationship between low intensity shear stress, aortic histamine formation, and aortic albumin uptake. Exp Mol Pathol. 1980 Jun;32(3):217–225. doi: 10.1016/0014-4800(80)90056-8. [DOI] [PubMed] [Google Scholar]
- Friedman M. H., Hutchins G. M., Bargeron C. B., Deters O. J., Mark F. F. Correlation between intimal thickness and fluid shear in human arteries. Atherosclerosis. 1981 Jun;39(3):425–436. doi: 10.1016/0021-9150(81)90027-7. [DOI] [PubMed] [Google Scholar]
- Fry D. L. Acute vascular endothelial changes associated with increased blood velocity gradients. Circ Res. 1968 Feb;22(2):165–197. doi: 10.1161/01.res.22.2.165. [DOI] [PubMed] [Google Scholar]
- Langille B. L., Adamson S. L. Relationship between blood flow direction and endothelial cell orientation at arterial branch sites in rabbits and mice. Circ Res. 1981 Apr;48(4):481–488. doi: 10.1161/01.res.48.4.481. [DOI] [PubMed] [Google Scholar]
- Lutz R. J., Cannon J. N., Bischoff K. B., Dedrick R. L., Stiles R. K., Fry D. L. Wall shear stress distribution in a model canine artery during steady flow. Circ Res. 1977 Sep;41(3):391–399. doi: 10.1161/01.res.41.3.391. [DOI] [PubMed] [Google Scholar]
- Motomiya M., Karino T. Flow patterns in the human carotid artery bifurcation. Stroke. 1984 Jan-Feb;15(1):50–56. doi: 10.1161/01.str.15.1.50. [DOI] [PubMed] [Google Scholar]
- Schwartz S. M., Benditt E. P. Cell replication in the aortic endothelium: a new method for study of the problem. Lab Invest. 1973 Jun;28(6):699–707. [PubMed] [Google Scholar]
- Selden S. C., 3rd, Rabinovitch P. S., Schwartz S. M. Effects of cytoskeletal disrupting agents on replication of bovine endothelium. J Cell Physiol. 1981 Aug;108(2):195–211. doi: 10.1002/jcp.1041080210. [DOI] [PubMed] [Google Scholar]
- Wright H. P. Mitosis patterns in aortic endothelium. Atherosclerosis. 1972 Jan-Feb;15(1):93–100. doi: 10.1016/0021-9150(72)90042-1. [DOI] [PubMed] [Google Scholar]
- Zarins C. K., Giddens D. P., Bharadvaj B. K., Sottiurai V. S., Mabon R. F., Glagov S. Carotid bifurcation atherosclerosis. Quantitative correlation of plaque localization with flow velocity profiles and wall shear stress. Circ Res. 1983 Oct;53(4):502–514. doi: 10.1161/01.res.53.4.502. [DOI] [PubMed] [Google Scholar]