Abstract
The genome of the hepatitis B virus (HBV) contains a sequence, designated X, capable of encoding a protein of 154 amino acids. To determine whether the putative protein synthesized from this region is antigenic, we examined the sera of HBV-infected patients for the ability to react with a hybrid protein that contained 133 amino acids encoded by the X region and portions of the bacterial ompF and beta-galactosidase genes. Some HBV-positive sera tested contained antibodies that specifically recognized the hybrid protein. All sera were from patients diagnosed as suffering from chronic active hepatitis. We conclude that the X region of HBV encodes a protein and that this protein is antigenic in some patients.
Full text
PDF
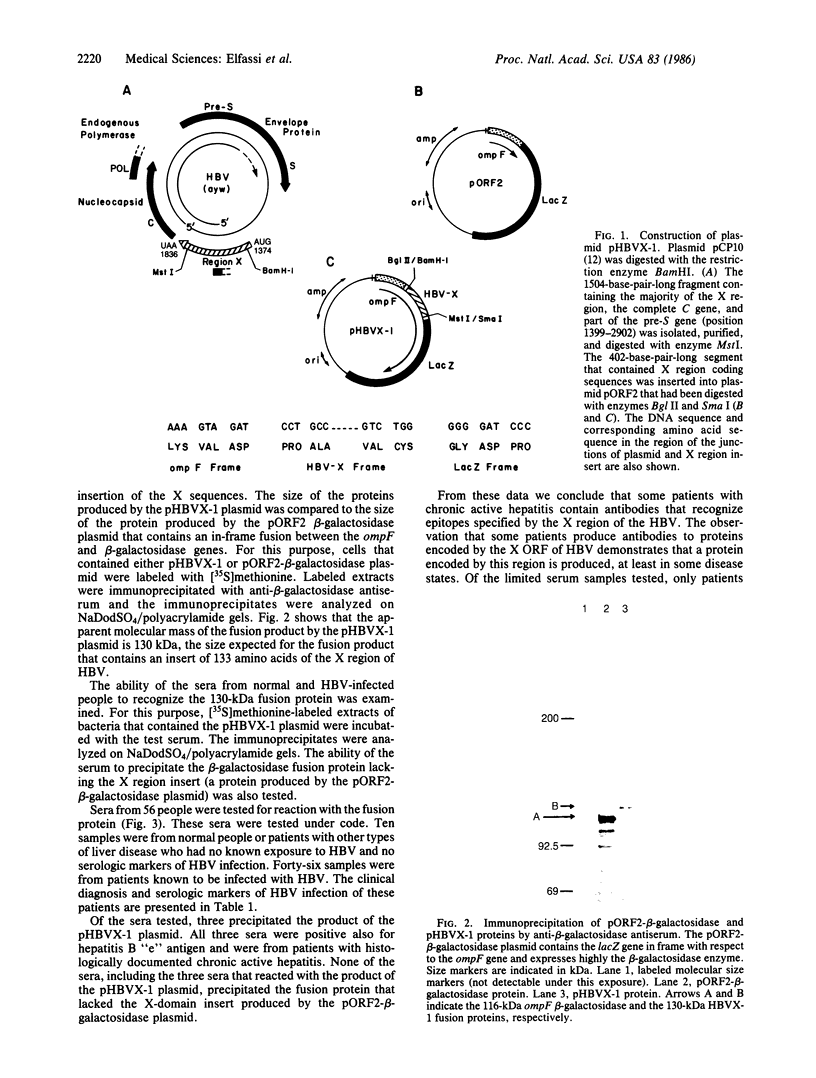
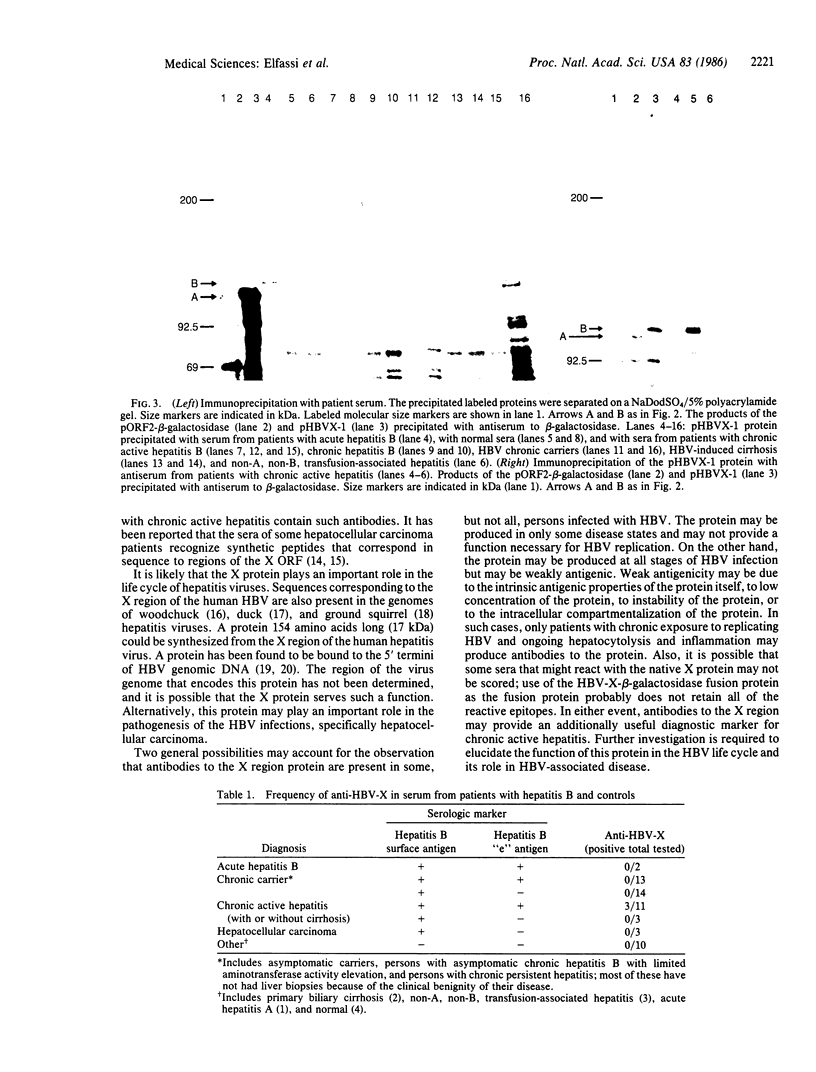

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Brechot C., Pourcel C., Louise A., Rain B., Tiollais P. Presence of integrated hepatitis B virus DNA sequences in cellular DNA of human hepatocellular carcinoma. Nature. 1980 Jul 31;286(5772):533–535. doi: 10.1038/286533a0. [DOI] [PubMed] [Google Scholar]
- Dubois M. F., Pourcel C., Rousset S., Chany C., Tiollais P. Excretion of hepatitis B surface antigen particles from mouse cells transformed with cloned viral DNA. Proc Natl Acad Sci U S A. 1980 Aug;77(8):4549–4553. doi: 10.1073/pnas.77.8.4549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edman J. C., Gray P., Valenzuela P., Rall L. B., Rutter W. J. Integration of hepatitis B virus sequences and their expression in a human hepatoma cell. Nature. 1980 Jul 31;286(5772):535–538. doi: 10.1038/286535a0. [DOI] [PubMed] [Google Scholar]
- Galibert F., Chen T. N., Mandart E. Nucleotide sequence of a cloned woodchuck hepatitis virus genome: comparison with the hepatitis B virus sequence. J Virol. 1982 Jan;41(1):51–65. doi: 10.1128/jvi.41.1.51-65.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galibert F., Mandart E., Fitoussi F., Tiollais P., Charnay P. Nucleotide sequence of the hepatitis B virus genome (subtype ayw) cloned in E. coli. Nature. 1979 Oct 25;281(5733):646–650. doi: 10.1038/281646a0. [DOI] [PubMed] [Google Scholar]
- Gerlich W. H., Robinson W. S. Hepatitis B virus contains protein attached to the 5' terminus of its complete DNA strand. Cell. 1980 Oct;21(3):801–809. doi: 10.1016/0092-8674(80)90443-2. [DOI] [PubMed] [Google Scholar]
- Joseph C. A., Joseph C. R., Beasley C. Effects on free recall and recognition of transforming visually presented words and pictures into trace or imagery form. J Gen Psychol. 1982 Jan;106(1ST):21–26. doi: 10.1080/00221309.1982.9710969. [DOI] [PubMed] [Google Scholar]
- Kay A., Mandart E., Trepo C., Galibert F. The HBV HBX gene expressed in E. coli is recognised by sera from hepatitis patients. EMBO J. 1985 May;4(5):1287–1292. doi: 10.1002/j.1460-2075.1985.tb03774.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mandart E., Kay A., Galibert F. Nucleotide sequence of a cloned duck hepatitis B virus genome: comparison with woodchuck and human hepatitis B virus sequences. J Virol. 1984 Mar;49(3):782–792. doi: 10.1128/jvi.49.3.782-792.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Molnar-Kimber K. L., Summers J., Taylor J. M., Mason W. S. Protein covalently bound to minus-strand DNA intermediates of duck hepatitis B virus. J Virol. 1983 Jan;45(1):165–172. doi: 10.1128/jvi.45.1.165-172.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moriarty A. M., Alexander H., Lerner R. A., Thornton G. B. Antibodies to peptides detect new hepatitis B antigen: serological correlation with hepatocellular carcinoma. Science. 1985 Jan 25;227(4685):429–433. doi: 10.1126/science.2981434. [DOI] [PubMed] [Google Scholar]
- Robinson W. S., Clayton D. A., Greenman R. L. DNA of a human hepatitis B virus candidate. J Virol. 1974 Aug;14(2):384–391. doi: 10.1128/jvi.14.2.384-391.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seeger C., Ganem D., Varmus H. E. Nucleotide sequence of an infectious molecularly cloned genome of ground squirrel hepatitis virus. J Virol. 1984 Aug;51(2):367–375. doi: 10.1128/jvi.51.2.367-375.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shafritz D. A., Kew M. C. Identification of integrated hepatitis B virus DNA sequences in human hepatocellular carcinomas. Hepatology. 1981 Jan-Feb;1(1):1–8. doi: 10.1002/hep.1840010102. [DOI] [PubMed] [Google Scholar]
- Szmuness W. Hepatocellular carcinoma and the hepatitis B virus: evidence for a causal association. Prog Med Virol. 1978;24:40–69. [PubMed] [Google Scholar]
- Tiollais P., Charnay P., Vyas G. N. Biology of hepatitis B virus. Science. 1981 Jul 24;213(4506):406–411. doi: 10.1126/science.6264599. [DOI] [PubMed] [Google Scholar]
- Weinstock G. M., ap Rhys C., Berman M. L., Hampar B., Jackson D., Silhavy T. J., Weisemann J., Zweig M. Open reading frame expression vectors: a general method for antigen production in Escherichia coli using protein fusions to beta-galactosidase. Proc Natl Acad Sci U S A. 1983 Jul;80(14):4432–4436. doi: 10.1073/pnas.80.14.4432. [DOI] [PMC free article] [PubMed] [Google Scholar]