Abstract
Human monocytic ehrlichiosis, an influenza-like illness accompanied by signs of hepatitis, is caused by infection of monocytes/macrophages with a lipopolysaccharide-deficient bacterium, Ehrlichia chaffeensis. The E. chaffeensis strain Wakulla induces diffuse hepatitis with neutrophil infiltration in mice with severe combined immunodeficiency, which is accompanied by strong CXCL2 (mouse functional homolog of interleukin-8 [IL-8]) and tumor necrosis factor alpha (TNF-α) expression in the liver. In this study, we found that expression of IL-1β, CXCL2, and TNF-α was induced by strain Wakulla in mouse bone marrow-derived macrophages; this expression was dependent on MyD88, but not on TRIF, TLR2/4, IL-1R1/IL-18R1, or endosome acidification. When the human leukemia cell line THP-1 was exposed to E. chaffeensis, significant upregulation of IL-8, IL-1β, and TNF-α mRNA and extracellular regulated kinase 2 (ERK2) activation were detected. U0126 (inhibitor of mitogen-activated protein kinase/extracellular signal-regulated kinase kinase 1/2 [MEK1/2] upstream of ERK), manumycin A (Ras inhibitor), BAY43-9006 (Raf-1 inhibitor), and NS-50 (inhibitor of NF-κB nuclear translocation) inhibited the cytokine gene expression. A luciferase reporter assay using HEK293 cells, which lack Toll-like receptors (TLRs), showed activation of both the IL-8 promoter and NF-κB by E. chaffeensis. Activation of the IL-8 promoter in transfected HEK293 cells was inhibited by manumycin A, BAY43-9006, U0126, and transfection with a dominant-negative Ras mutant. These results indicate that the E. chaffeensis Wakulla strain can induce inflammatory responses through MyD88-dependent NF-κB and ERK pathways, without the involvement of TRIF and TLRs.
INTRODUCTION
Human monocytic ehrlichiosis (HME), discovered in 1986 (27), is one of the most prevalent life-threatening tick-borne zoonoses in North America (31). HME is an acute febrile illness characterized by headache, malaise, nausea, myalgia and/or arthralgia and is frequently accompanied by leukopenia, thrombocytopenia, anemia, and elevation of hepatic transaminase levels (38). HME patients may develop a fulminant toxic or septic shock-like syndrome, particularly individuals with HIV infection or who are otherwise immunocompromised (39). The small numbers of bacteria detected in the blood and tissues of patients suggest that the clinical disease is mediated largely by proinflammatory cytokines (41).
HME is caused by Ehrlichia chaffeensis, a monocyte-tropic obligatory intracellular bacterium (9). Previously, we demonstrated that the Wakulla strain is more virulent than the type strain Arkansas in mice with severe combined immunodeficiency (SCID mice) (33). The Wakulla strain of E. chaffeensis causes a fatal illness in SCID mice; the mice develop fulminant hepatitis and show upregulation of tumor necrosis factor alpha (TNF-α) and interleukin-1β (IL-1β), several chemokines, including CXCL2 (Mip2, a mouse homolog of human IL-8), and chemokine receptors in the inflammatory liver (32). The Arkansas strain of E. chaffeensis induces expression of IL-1β, IL-8, and IL-10 mRNA and proteins in the human monocytic leukemia cell line THP-1 at 2 and 24 h postexposure, respectively (23). Transcriptome analysis also determined induction of IL-1β, IL-8, and TNF-α in E. chaffeensis Arkansas-infected THP-1 cells (56). These studies demonstrate that E. chaffeensis can induce inflammatory cytokines and chemokines upon interaction with mammalian host cells.
It is well known that pathogen-associated molecular patterns (PAMPs) such as lipopolysaccharide (LPS), flagella, and peptidoglycan are able to induce cytokines/chemokines by innate immune cells (14, 37, 45). Although E. chaffeensis is a Gram-negative bacterium, these PAMPs are not encoded in the E. chaffeensis genome (10, 25). This suggests that the cytokine and chemokine induction by E. chaffeensis is dependent on other types of PAMPs or the signaling pathway. For example, ehrlichial ankyrin repeat-containing protein p200 binds to the promoter region of 456 host genes, including TNF-α, and it was suggested that this leads to transcriptional activation of TNF-α (58). PAMPs are recognized by the pattern-recognition receptors (PRRs) such as Toll-like receptors (TLRs), retinoic acid-inducible gene I-like receptors, and nucleotide-binding oligomerization domain-like receptors (20). Other than a single report describing a prolonged infection by E. chaffeensis of C3H/HeJ mice deficient in TLR4 function (46), the role of PRRs in E. chaffeensis pathogenesis and immunity is unknown.
To investigate the E. chaffeensis cytokine induction pathways, in the present study we determined cytokine induction in bone marrow-derived macrophages (BMDMs) from various mouse strains deficient in TLRs or adaptor molecules as well as in THP-1 cells in response to E. chaffeensis Wakulla. To further analyze the signaling for IL-8 induction, we developed a luciferase reporter assay system using HEK293 cells that can be infected with E. chaffeensis. We present here potentially new cytokine induction pathways activated by E. chaffeensis Wakulla.
MATERIALS AND METHODS
Ehrlichia, antibodies, and reagents.
Arkansas and Wakulla strains of E. chaffeensis were propagated in DH82 cells as previously described (33). Antibodies used were rabbit anti-extracellular regulated kinase (anti-ERK) antibody, mouse anti-phosphorylated ERK monoclonal antibody (both from Cell Signaling, Danvers, MA), and mouse anti-tubulin monoclonal antibody (Santa Cruz, Santa Cruz, CA). Reagents used were manumycin A, BAY43-9006, U0126, Go 6983, and bisindolylmaleimide I (all from Calbiochem, San Diego, CA), SN-50 (Enzo Life Sciences, Farmingdale, NY), chloroquine, and bafilomycin A1 (Sigma, St. Louis, MO).
BMDMs.
MyD88−/− and TRIF−/− mice, originally developed by S. Akira (Osaka University) (1, 50), were crossbred to generate MyD88−/−, TRIF−/−, and MyD88−/− TRIF−/− mice. Wild-type, TLR2−/−, TLR4−/−, IL-1R1−/−, and IL-18R1−/− C57BL/6 mice were purchased from Jackson Laboratory (Bar Harbor, ME). All animal experiments were performed under the animal protocol approved by the Institutional Animal Care and Use Committee (IACUC) at The Ohio State University. The mice were euthanized with CO2 gas, and the femur and tibia of the hind limbs were dissected to prepare bone marrow cells. Cells were cultured in RPMI medium with 10% fetal bovine serum, 2 mM l-glutamine (GIBCO-Invitrogen, Carlsbad, CA), 10% conditioned medium of L929 cells, and 1% antibiotic mixture (100 U/ml penicillin, 100 μg/ml streptomycin, 0.25 μg/ml amphotericin B; GIBCO-Invitrogen) for 5 to 7 days. Adherent BMDMs were harvested and washed and then seeded in 24-well plates.
IL-8 promoter-luciferase construct.
To construct an IL-8 promoter reporter plasmid, a DNA fragment containing the human IL-8 promoter from −243 to +46 bp (where +1 is the transcriptional start site) (51) was amplified by PCR (Table 1) using genomic DNA purified from THP-1 cells as a template. The PCR product was digested with XhoI and HindIII and inserted upstream of the luciferase gene between the XhoI and HindIII sites of pGL4.17 (Promega, Madison, WI) to obtain pK666.
Table 1.
Primer pairs used for genomic PCR or RT-PCR
| Gene | Primer sequence (5′→3′) |
|---|---|
| Human IL-8 promoter | CATCTCGAGTTCACCAAATTGTGGAGCTT |
| CATAAGCTTGAAGCTTGTGTGCTCTGCTG | |
| E. chaffeensis 16S rRNA | AGCAATGCCTCCTGCACCACCAAC |
| CCACATCACCCCTCTACCTC | |
| Human GAPDH | AGCAATGCCTCCTGCACCACCAAC |
| CCGGAGGGGCCATCCACAGTCT | |
| Human TNF-α | CCCCAGGGACCTCTCTCTAA |
| TGAGGTACAGGCCCTCTGAT | |
| Human IL-1β | ACAGATGAAGTGCTCCTTCCA |
| GTCGGAGATTCGTAGCTGGAT | |
| Human IL-8 | CTGCGCCAACACAGAAATTA |
| ATTGCATCTGGCAACCCTAC | |
| Mouse GAPDH | GGCATTGCTCTCAATGACAA |
| TGTGAGGGAGATGCTCAGTG | |
| Mouse TNF-α | CATCTTCTCAAAATTCGAGTGACAA |
| TGGGAGTAGACAAGGTACAACCC | |
| Mouse IL-1β | GGGCCTCAAAGGAAAGAATC |
| TACCAGTTGGGGAACTCTGC | |
| Mouse CXCL2 | CTCTCAAGGGCGGTCAAAAAGTT |
| TCAGACAGCGAGGCACATCAGGTA |
Stimulation of THP-1 cells and mouse BMDMs.
THP-1 cells were incubated with E. chaffeensis freshly isolated from infected DH82 cells as previously described (23). Briefly, heavily infected DH82 cells (6 ×107) were sonicated and centrifuged at 1,000 × g for 5 min to remove unbroken cells and nuclei. The supernatant was centrifuged at 10,000 × g for 10 min to harvest host cell-free bacteria. As a control, an extract of uninfected DH82 cells was prepared under the same conditions. Freshly prepared bacteria or corresponding uninfected DH82 cell extract was suspended in 400 μl phosphate-buffered saline (PBS; 137 mM NaCl, 2.68 mM KCl, 1.47 mM KH2PO4, 8.06 mM Na2HPO4), and 50 μl of this suspension was added to THP-1 cells (1 × 106/well) in 6-well plates, and 25 μl was added to mouse BMDMs (5 × 105/well) in 24-well plates. This corresponds to approximately 20 inclusion-forming units (IFU)/cell. IFU were determined by immunofluorescence labeling of THP-1 cells infected for 24 h at 37°C. Cells were stained with dog anti-E. chaffeensis serum and rhodamine-conjugated anti-dog IgG, and positively stained inclusions were scored in 100 cells. For cytokine assay, after incubation at 37°C for 2 h, cells were harvested and total RNA was extracted with an RNeasy minikit (Qiagen, Valencia, CA). When required, inhibitors were added to the cell culture 45 or 60 min prior to the addition of bacteria and remained present until the cells were harvested.
Stimulation of HEK293 cells and luciferase assay.
For IL-8 and NF-κB reporter assays, HEK293 cells (ATCC, Manassas, VA) cultured in a 96-well plate at 105 cells/well were transfected using FuGene HD (Roche, Basel, Switzerland) with two plasmids: pGL4.73 (simian virus 40 [SV40]-Renilla luciferase; Promega) and either pK666 (IL-8 promoter luciferase) or pGL4.32 (NF-κB response element and minimal promoter luciferase; Promega). To examine the involvement of Ras, HEK293 cells were triply transfected with pGL4.73, pK666 or pGL4.32, and pCMV-Ras or pCMV-RasN17H encoding human wild-type Ras or dominant-negative Ras, respectively (Clontech, Mountain View, CA). Host cell-free E. chaffeensis was added at 24 h posttransfection and incubated for 2 h at 37°C. The dual-luciferase assay was performed using a dual-luciferase reporter assay system (Promega). Firefly luciferase activity of each sample was normalized to the internal control, Renilla luciferase activity (42). When required, inhibitors were added 45 min prior to the addition of E. chaffeensis. RNA was extracted from cells for quantitative reverse transcription-PCR (RT-PCR).
Quantitative RT-PCR.
cDNAs were prepared from 1 μg total RNA using SuperScript III reverse transcriptase (Invitrogen) or a Maxima first-strand cDNA synthesis kit (Fermentas, Glen Burnie, MD) with an oligo(dT) primer. The amount of cDNA for each of the selected genes was quantified by real-time PCR using gene-specific primers (Table 1). Expression of each gene was normalized against GAPDH levels.
Western blot analysis.
Cells were harvested and suspended in PBS supplemented with protease and phosphatase inhibitor cocktail (Calbiochem) followed by sonication and centrifugation to prepare clear lysate. After heat denaturation, proteins in the cell lysate were resolved by 12.5% SDS-PAGE. Proteins in the gel were transferred to a nitrocellulose membrane and incubated with a 1:1,000 dilution of each antibody at 4°C. After treatment with the second antibody, the membrane was developed by using ECL Western blotting substrate (Thermo Fisher Scientific, Waltham, MA).
Statistical analysis.
One-way analysis of variance (ANOVA) with the Tukey honestly significant difference (HSD) test or Student's t test was performed to determine the significance of differences between groups. A P value of <0.05 was considered significant.
RESULTS
Induction of proinflammatory cytokines is MyD88 dependent.
TLR signaling occurs through two distinct pathways based on Toll/IL-1R homology (TIR) domain-containing adapter molecules: MyD88- and TRIF (TICAM-1)-dependent molecules. MyD88 is essential for the downstream signaling of TLRs, except for TLR3. TLR3 recruits TRIF, and TLR4 triggers both MyD88- and TRIF-dependent signaling (45).
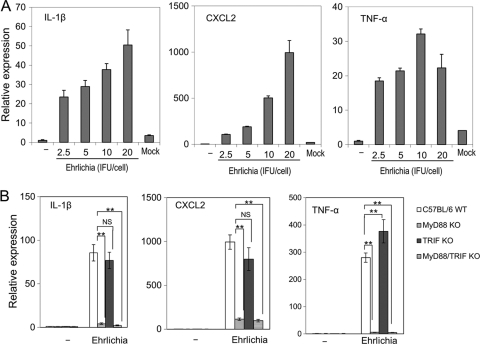
The E. chaffeensis Wakulla strain induces neutrophil infiltration and profound expression of proinflammatory cytokines, including TNF-α and IL-1β, and several chemokines, such as CXCL2 in the liver of infected SCID mice (32). To analyze the signaling pathways that are involved in cytokine/chemokine expression by E. chaffeensis Wakulla, we used BMDMs from C57BL/6 mice. E. chaffeensis Wakulla strongly induced the expression of IL-1β, CXCL2, and TNF-α mRNA in a dose-dependent manner, although TNF-α induction reached a plateau at a lower bacterial titer than did IL-8 and IL-1β (Fig. 1 A). DH82 lysate, which may be present in host cell-free preparations of E. chaffeensis, did not activate these cytokines (Fig. 1A, Mock). To determine the involvement of TLR signaling in the induction of cytokines/chemokines, we used BMDMs from MyD88−/−, TRIF−/−, and MyD88−/− TRIF−/− double-knockout mice. Induction of IL-1β, CXCL2, and TNF-α by Wakulla was reduced in BMDMs from MyD88−/− and MyD88−/− TRIF−/− double-knockout mice, whereas no reduction was detected for TRIF−/− mouse-derived BMDMs (Fig. 1B). These results show that induction of IL-1β, CXCL2, and TNF-α by Wakulla in mouse BMDMs is MyD88 dependent but TRIF independent. This result also rules out involvement of TLR3 in IL-1β, CXCL2, and TNF-α induction by Wakulla.
Fig. 1.
Proinflammatory cytokine induction in mouse BMDMs by E. chaffeensis is MyD88 dependent but TRIF independent. (A) Dose response of IL-1β, CXCL2, and TNF-α induction in mouse BMDMs to E. chaffeensis Wakulla was determined by quantitative RT-PCR. Bacteria were added at different IFU/ml to each well, and the plates were incubated at 37°C for 2 h. The transcript amount of each gene is represented as expression relative to untreated BMDMs. All data are normalized to mouse GAPDH mRNA. Mock, extract of uninfected DH82 cells corresponding to 20 IFU of Ehrlichia/cell. (B) IL-1β, CXCL2, and TNF-α induction in BMDMs from MyD88, TRIF, or MyD88/TRIF double-knockout mice (KO). Bacteria derived from 5 × 105 infected cells were added/well and incubated at 37°C for 2 h. C57BL/6 WT, wild-type control. −, PBS. Data shown are means and standard deviations from triplicate assays. **, significantly different from each other (ANOVA, P < 0.01). NS, not significantly different (ANOVA, P > 0.05). Data are representative of results of at least three independent experiments.
Induction of proinflammatory cytokines by E. chaffeensis Wakulla does not require TLR2/4, endosome acidification, or IL-1R/IL-18R.
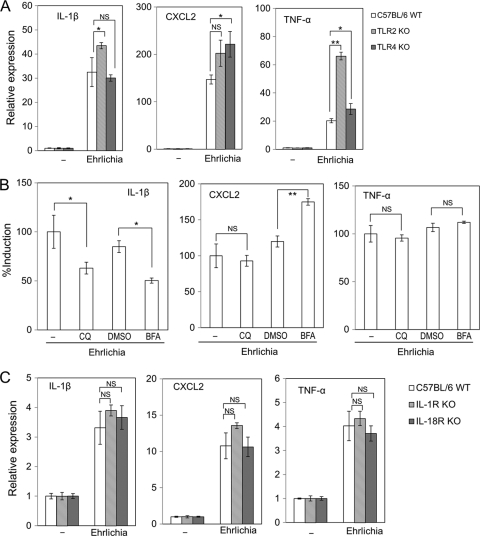
We next examined whether MyD88-recruiting TLRs (37, 45) are involved in the cytokine/chemokine signaling elicited by E. chaffeensis infection. TLR2 and TLR4 recognize peptidoglycan/lipoprotein and LPS, respectively, which induce MyD88-dependent signaling. Induction of IL-1β, CXCL2, and TNF-α by E. chaffeensis Wakulla was not impaired in BMDMs from TLR2−/− or TLR4−/− mice compared to that in BMDMs from wild-type mice (Fig. 2 A), indicating that neither TLR2 nor TLR4 mediates the cytokine induction by E. chaffeensis Wakulla. Instead, we observed enhanced expression of IL-1β and TNF-α in TLR2−/− BMDMs, and of CXCL2 in TLR4−/− BMDMs, suggesting the presence of TLR2−/−- or TLR4−/−-dependent immunosuppressive components in E. chaffeensis Wakulla.
Fig. 2.
Induction of proinflammatory cytokines by E. chaffeensis is independent of TLR2/4, acidification, and IL-1R1/18R1. (A) IL-1β, CXCL2, and TNF-α induction in BMDMs from TLR2 or TLR4 knockout (KO) mice in response to E. chaffeensis Wakulla. (B) Effects of acidification inhibitors on CXCL2 induction in mouse BMDMs. Inhibitors were added to the cell culture at 37°C 60 min prior to infection at the final concentration of 10 μM chloroquine (CQ) or 50 nM bafilomycin A1 (BFA). −, PBS control for CQ; DMSO, dimethyl sulfoxide (solvent control for BFA). (C) IL-1β, CXCL2, and TNF-α induction in BMDMs from IL-1R1 or IL-18R1 knockout mice. C57BL/6 WT, wild-type control. −, PBS control. Bacteria were added to each well at 20 IFU/cell and incubated at 37°C for 2 h. Data shown are means and standard deviations from triplicate assays. *, significantly different from control (ANOVA, P < 0.05); **, significantly different from all others (ANOVA, P < 0.01). NS, not significantly different (ANOVA, P > 0.05). Data are representative of results of at least three independent experiments.
The endosomal TLRs TLR3, TLR7, and TLR9 recognize viral double-stranded RNA, viral single-stranded RNA, and nonmethylated DNA with CpG motifs, respectively (37, 45). As acidification of endosomes is required for endosomal TLR-dependent signaling (8, 28, 54), we investigated the effects of the acidification inhibitors chloroquine (weak base) and bafilomycin A1 (inhibitor of vacuolar-type H+-ATPase). Pretreatment of BMDMs with either inhibitor did not abrogate IL-1β, CXCL2, or TNF-α induction in mouse BMDMs (Fig. 2B). These results indicate that induction of IL-1β, CXCL2, and TNF-α by Wakulla occurs largely independently of endosome acidification, and thus of TLR3, TLR7, and TLR9 as well. The observed partial reduction of IL-1β induction by both chloroquine and bafilomycin A1 and the enhanced expression of CXCL2 in the presence of bafilomycin A1 suggest that Wakulla induces some acidification-dependent signaling pathways.
Secretion of IL-1β or IL-18 is regulated by the processing enzyme caspase-1 (11). Following secretion of IL-1β or IL-18, IL-1β and IL-18 receptors (IL-1R and IL-18R) become involved in positive-feedback regulation of proinflammatory cytokine induction by recruiting MyD88 (37). There are two kinds of IL-1 receptors, type I (IL-1R1) and type II (IL-1R2). Because IL-1R2 lacks a TIR domain which binds to MyD88 (37), IL-1R2 is not functional in MyD88 recruitment (3). There are two kinds of IL-18 receptors, IL-18R1 (or IL-18Rα) and IL-18R2 (or IL-18Rβ). IL-18R1 and IL-18R2 form a heterodimer to function (3). To determine whether IL-1R and/or IL-18R pathways are required for induction of proinflammatory cytokines, we performed a cytokine induction assay using IL-1R1−/− or IL-18R1−/− mice. Induction of IL-1β, CXCL2, and TNF-α was not impaired in BMDMs from these knockout mice (Fig. 2C). Taken together, these data suggest that proinflammatory cytokine/chemokine induction in mouse BMDMs by E. chaffeensis Wakulla does not require TLR2, -3, -4, -7, or -9 or IL-1R1/IL-18R1.
E. chaffeensis Wakulla induces a strong cytokine response by THP-1 cells.
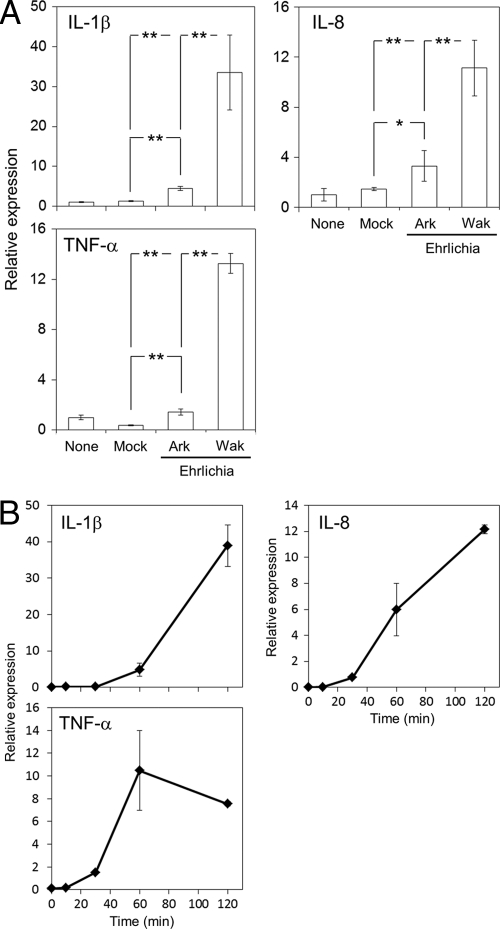
We next explored signaling pathways associated with induction of inflammatory cytokines/chemokines using the human macrophage cell line THP-1. Although genetic manipulation, such as transfection and gene knockout, is not practical for this cell line, it is a useful model for studying cytokine/chemokine induction in the context of E. chaffeensis pathogenesis of human cells. In concordance with previous observations of cytokine/chemokine induction in SCID mouse liver (32), significantly higher levels of IL-1β, IL-8, and TNF-α were induced in human THP-1 cells in response to strain Wakulla than to the same number of cells of strain Arkansas (Fig. 3 A). DH82 lysate did not activate these cytokines (Fig. 3A, Mock). Expression of IL-8 and TNF-α was elevated within 30 min of exposure to E. chaffeensis Wakulla, whereas IL-1β induction occurred with a slight delay (Fig. 3B), suggesting that induction of IL-1β may involve additional steps. In addition, TNF-α peaked at 60 min, while IL-8 and IL-1β continued to be activated at 2 h (Fig. 3B).
Fig. 3.
E. chaffeensis Wakulla induces a stronger cytokine response than Arkansas by THP-1 cells. (A) Activation of cytokine genes by E. chaffeensis Wakulla (Wak) and Arkansas (Ark) strains. THP-1 cells were exposed for 2 h at 37°C to the bacteria at 20 IFU/cell or to extract of corresponding uninfected cells. The amount of two strains of bacteria was normalized according to the copy number of bacterial genomic DNA determined with real-time PCR of E. chaffeensis 16S rRNA genes. The transcript amount of each gene is represented as expression relative to that of untreated THP-1 cells. All data are normalized to human GAPDH mRNA. **, significantly different from all others (ANOVA, P < 0.01). *, significantly different from mock control (ANOVA, P < 0.05). (B) Time course of cytokine gene activation in THP-1 cells stimulated with E. chaffeensis Wakulla at 20 IFU/cell. Data shown are means and standard deviations from triplicate assays. The y axis indicates the transcript amount at each time point relative to that at time zero. Data are representative of results of at least three independent experiments.
Induction of proinflammatory cytokines in THP-1 cells by E. chaffeensis is dependent on ERK and NF-κB pathways.
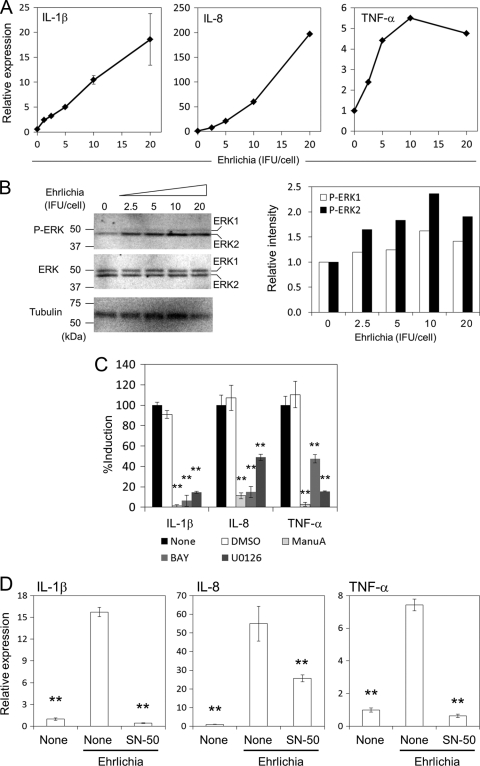
The ability of E. chaffeensis Arkansas to limit MAPK activity has been suggested as an important virulence mechanism (26). Therefore, we examined whether ERK1/2 (p44/p42) is activated by strain Wakulla and whether this is dose dependent. IL-1β, IL-8, and TNF-α were all found to be induced by E. chaffeensis Wakulla in a dose-dependent manner, although TNF-α induction reached a plateau at a lower titer of E. chaffeensis than did IL-8 and IL-1β (Fig. 4 A), similar to the effect in mouse BMDMs (Fig. 1A). At 2 h postinoculation (h p.i.), phosphorylation of ERK2, but not ERK1, was upregulated in an E. chaffeensis dose-dependent manner (Fig. 4B and C).
Fig. 4.
Induction of proinflammatory cytokines in THP-1 cells by E. chaffeensis is dependent on ERK and NF-κB pathways. (A) IL-1β, IL-8, and TNF-α induction in THP-1 cells by E. chaffeensis Wakulla was dose dependent as determined by real-time RT-PCR. Bacteria were added at different IFU/cell and incubated at 37°C for 2 h. (B) Phosphorylation of ERK in THP-1 cells treated with E. chaffeensis Wakulla was dose dependent. The titer of bacteria is the same as in panel A. Densities of phosphorylated ERK1 and ERK2 relative to those at 0 h as determined by densitometric analysis are shown on the right. (C) Effects of Ras, Raf, and MEK inhibitors on cytokine induction in THP-1 cells by E. chaffeensis Wakulla at 20 IFU/well. All inhibitors were added to the cell culture 45 min prior to infection and were present during infection. Final concentrations were 10 μM for manumycin A (ManuA, Ras inhibitor), 10 nM for U0126 (MEK inhibitor), 250 nM for BAY43-9006 (BAY, Raf-1 inhibitor). DMSO (1%) was added as a negative control. Data shown are means and standard deviations from triplicate assays. **, significantly different from either the nontreated or DMSO-treated control (ANOVA, P < 0.01). (D) THP-1 cells were incubated with or without E. chaffeensis Wakulla at 20 IFU/cell for 2 h in the presence or absence of 100 μg/ml SN-50, a cell-permeable NF-κB inhibitory peptide. Expression of cytokine genes was determined by quantitative RT-PCR. Data shown are means and standard deviations from triplicate assays. **, significantly different from Ehrlichia, nontreated control (ANOVA, P < 0.01). Data are representative of results of at least three independent experiments.
The best studied canonical ERK1/2 activation pathway is the Ras GTP-binding protein activating the nonreceptor tyrosine kinase Raf-1, which then activates mitogen-activated protein kinase/extra cellular signal-regulated kinase kinase (MEK) by tyrosine phosphorylation (19). To assess whether the cytokine induction involves the Ras-Raf-MEK-ERK signaling cascade, the effects of specific inhibitors of the pathway were examined. As shown in Fig. 4C, manumycin A, BAY43-9006, and U0126, inhibitors of Ras, Raf-1, and MEK1/2, respectively (4, 35, 48), significantly inhibited the induction of IL-1β, IL-8, and TNF-α by strain Wakulla.
NF-κB is a well-known signaling mediator that activates cytokine genes in macrophages exposed to LPS (14). NF-κB is also activated in THP-1 cells in response to E. chaffeensis Arkansas (24). An inhibitor of NF-κB nuclear translocation, SN-50, significantly inhibited induction of IL-1β, IL-8, and TNF-α in THP-1 cells (Fig. 4D), suggesting that NF-κB is also required to activate cytokine genes in response to E. chaffeensis.
Cytokine induction in HEK293 cells by E. chaffeensis Wakulla is mediated by ERK and NF-κB pathways.
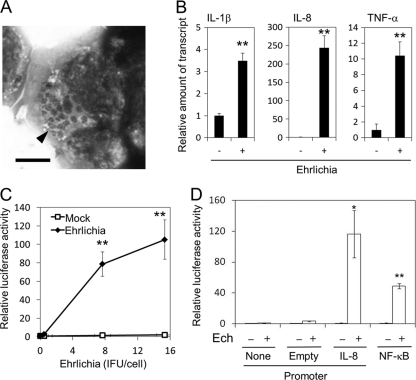
To establish a simple reporter assay system to examine signaling pathways for cytokine induction, we used the easily transfectable cell line HEK293, derived from human embryonic kidney cells. We found that HEK293 cells can be readily infected with E. chaffeensis (Fig. 5 A) and therefore must have proteins required to support the growth of this bacterium. Furthermore, HEK293 cells do not express any TLRs (HEK293 microarray data set, Gene Expression Omnibus [GEO] data set number GDS686) (55), allowing us to analyze TLR-independent signaling pathways associated with E. chaffeensis-induced cytokine expression. Indeed, E. chaffeensis Wakulla can activate IL-1β, IL-8, and TNF-α genes in HEK293 cells (Fig. 5B). Dose-dependent activation of the IL-8 promoter by E. chaffeensis Wakulla was also confirmed in HEK293 cells transfected with pK666 harboring a fusion of the human IL-8 promoter and a luciferase reporter gene, whereas DH82 lysate (mock treated) did not activate the IL-8 promoter (Fig. 5C and D). Thus, Wakulla can induce IL-1β, IL-8, and TNF-α in a TLR-independent manner.
Fig. 5.
E. chaffeensis induces cytokines and activates IL-8 and NF-κB promoters in HEK293 cells. (A) HEK293 cells infected with E. chaffeensis. Host-free E. chaffeensis was inoculated and incubated with HEK293 cells for 6 days. The cells were stained with Diff-Quik. An arrowhead indicates multiple morulae of E. chaffeensis in the cytoplasm of a HEK293 cell. Bar = 5 μm. (B) IL-1β, IL-8, and TNF-α induction in HEK293 cells 2 h after exposure to E. chaffeensis Wakulla. Bacteria were added at 40 IFU/cell and incubated at 37°C for 2 h. Quantitative RT-PCR. (C) Dose-dependent activation of the IL-8 promoter. Luciferase reporter activity of pK666-transfected HEK293 cells was determined after incubation with bacteria at 0 to 15 IFU/cell or corresponding uninfected DH82 cell extract control (Mock) at 37°C for 2 h. **, significantly different from control (Student's t test, P < 0.01). (D) Luciferase activity of HEK293 cells transfected with pGL4.17 (Empty, vector harboring promoterless luciferase), pK666 (IL-8, IL-8-luciferase), pGL4.32 (NF-κB, NF-κB responsive element-luciferase) or none 2 h after incubation with E. chaffeensis Wakulla (Ech +) at 15 IFU/cell or corresponding uninfected DH82 cells (Ech −) at 37°C. Data shown are means and standard deviations from triplicate assays. * and **, significantly different from no or empty plasmid control (Student's t test; P < 0.05 and P < 0.01), respectively. Data are representative of results of at least three independent experiments.
Induction of IL-1β, IL-8, and TNF-α in THP-1 cells by E. chaffeensis was dependent on ERK and NF-κB pathways (Fig. 4). IL-1β, IL-8, and TNF-α promoters contain the NF-κB element (12, 40, 49). Therefore, we examined whether NF-κB was activated in HEK293 cells by E. chaffeensis Wakulla. As shown in Fig. 5D, luciferase expression under the control of the NF-κB responsive element was observed, indicating NF-κB activation.
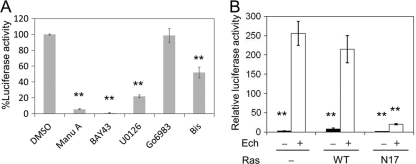
Ras is typically activated by growth hormones through receptor tyrosine kinases and Grb2/SOS, but may also receive other signals (7). Specific inhibitors of the Ras-Raf-MEK-ERK pathway were examined for their effect on IL-8 induction in HEK293 cells by E. chaffeensis Wakulla. As shown in Fig. 6 A, manumycin A, BAY43-9006, and U0126 significantly inhibited IL-8 induction in the luciferase reporter assay. Dominant-negative Ras suppressed IL-8 activation by E. chaffeensis Wakulla, whereas wild-type Ras did not affect the activation (Fig. 6B). Protein kinase C ζ (PKCζ)-dependent, but Ras- or Raf-independent, activation of ERK has been demonstrated in LPS-exposed (34) and in Mycobacterium tuberculosis-infected (52) macrophages. Hence, we examined the involvement of PKC in induction of IL-8 by E. chaffeensis Wakulla. Inhibitors of PKCζ (bisindolylmaleimide I and Go6983) had no or partial inhibitory effects (Fig. 6A), implying that PKCζ activation is not the major pathway. Taken together, these results suggest that the ERK pathway is involved in IL-8 induction by E. chaffeensis Wakulla. These results agree with the results for the THP-1 cells shown in Fig. 4, indicating that similar signal pathways are involved in activating proinflammatory cytokines and chemokines in THP-1- and TLR-deficient HEK293 cells.
Fig. 6.
IL-8 induction in HEK293 cells by E. chaffeensis is mediated by ERK. (A) Effects of inhibitors on IL-8 promoter activation in HEK293 cells in response to E. chaffeensis Wakulla added at 40 IFU/cell and incubated at 37°C for 2 h. All inhibitors were added to the cell culture 45 min prior to infection and were present during infection for 2 h. Final concentrations were 10 μM for manumycin A (Ras inhibitor), 20 μM for U0126 (MEK inhibitor), 5 μM for BAY43-9006 (Raf-1 inhibitor), and 10 μM for Go 6983 and bisindolylmaleimide I (Bis) as PKC inhibitors. DMSO (1%) was used as a negative control. Data shown are means and standard deviations from triplicate assays. **, significantly different from DMSO control (ANOVA, P < 0.01). (B) Effect of dominant-negative Ras. Wild-type Ras (WT)- or dominant-negative Ras (N17)-transfected and untransfected HEK293 cells were incubated with or without E. chaffeensis Wakulla (Ech + or Ech −, respectively) at 40 IFU/well for 2 h. Data shown are means and standard deviations from triplicate samples. **, significantly different from Ehrlichia-infected cells without transfection (−) (ANOVA, P < 0.01). Data are representative of results of at least three independent experiments.
DISCUSSION
In this study, we showed that E. chaffeensis induces cytokines and a chemokine in BMDMs through MyD88, but independently of TRIF. MyD88 is also a key signaling adaptor molecule for IL-1R and IL-18R, but we found that neither IL-1R nor IL-18R is required for induction of cytokines and a chemokine in response to E. chaffeensis. Recently Koh et al. reported MyD88-dependent clearance of Ehrlichia muris in mice (18). E. muris induced IL-12 p40 in mouse bone marrow-derived dendritic cells (DCs), but not in macrophages, and this is also MyD88 dependent (18). In the same study, caspase-1-deficient DCs did not show any differences in IL-12 p40 induction and E. muris infection compared to wild-type DCs. Taken together, MyD88, but not TRIF or the IL-1R/IL-18R family, plays a critical role in Ehrlichia sp. recognition by innate immune cells. This is in stark contrast to gamma interferon (IFN-γ) secretion by natural killer T (NKT) cells in response to E. muris infection, which is a CD1d-dependent but MyD88-independent process, and during which an endogenous glycolipid may be an E. muris PAMP for NKT cell stimulation (29).
In agreement with previous observations of cytokine/chemokine induction in SCID mice (32), significantly higher levels of proinflammatory cytokines were induced in THP-1 cells in response to strain Wakulla than to the same number of cells of strain Arkansas. Thus, strain Wakulla can provide a convenient in vitro model to study proinflammatory cytokine induction by E. chaffeensis. TLR2 and TLR4 did not mediate cytokine and chemokine induction by E. chaffeensis Wakulla. Because TLR1 and TLR6 function as a heterodimer with TLR2 (45), the result also may imply lack of TLR1 and TLR6 involvement in induction of IL-1β, CXCL2, and TNF-α by Wakulla. Although TLR5, TLR11, and TLR13 also recruit MyD88 for their downstream signaling (20, 43), TLR5 is so far not known to recognize any PAMPs other than flagella, which the E. chaffeensis genome does not encode (10). Furthermore, although bacterial ligands of TLR11, TLR12, and TLR13 have not been identified, none of these receptors is encoded in the human genome (45). Therefore, we can exclude involvement of TLR11, -12, and -13 because E. chaffeensis is nevertheless able to induce cytokines in human cells. TLR8 is a human counterpart of mouse TLR7, and the mouse tlr10 gene is disrupted by insertion of an endogenous retrovirus (37, 45). DNase or RNase treatment of freeze-thawed E. chaffeensis Wakulla did not reduce cytokine induction (data not shown). Bacterial DNA needs to be processed in the acidic endosome to be recognized by TLR9 (2). It is possible that TLR9 is not present in E. chaffeensis inclusions or that E. chaffeensis DNA cannot be processed properly, since bacterial inclusion does not fuse with lysosomes. Therefore, it appears that none of these TLRs (TLR1, -2, -3, -4, -5, -6, -7, -9, -11, -12, or -13) mediates proinflammatory cytokine induction by E. chaffeensis. This is corroborated by the fact that HEK cells, which do not express TLRs, could be induced to express proinflammatory cytokines and a chemokine in response to E. chaffeensis stimulation. Combined with the report that IL-12 p40 secretion by mouse bone marrow-derived DCs in response to E. muris occurs independently of TLR1, -2, -3, -4, -5, -6, -7, -9, and -11 (18), these observations support an emerging concept of TLR-independent activation of innate immune cells by Ehrlichia spp.
There are several recent reports about TLR-independent cytokine induction by bacteria or bacterial components. For example, Burkholderia pseudomallei can induce IL-8 via NF-κB and MAPK pathways, aided by the Bsa type III secretion system (T3SS), without involving TLRs (16). VP1680, a T3SS effector protein of Vibrio parahaemolyticus, is responsible for IL-8 induction in Caco-2 cells, which is mediated by activation of both the ERK signaling pathway and NF-κB (44). Phosphoglycolipids from thermophilic bacteria such as Meiothermus taiwanensis induce IL-1 in human monocytes through a mechanism involving PKC-α, MEK1/2, and JNK, but independently of TLRs (53). In all of these cases, cytokine induction is independent of MyD88 or MyD88 dependency is unknown. E. chaffeensis recombinant TRP120 (2 tandem repeats) was reported to induce strong chemokine responses (IL-8, MCP-1, MIP-1β) (57). While cytokine induction was not reported with another tandem repeat protein, p47 of E. chaffeensis, it can interact with a number of host cytoplasmic signaling proteins (47). It is possible that p47 or other E. chaffeensis proteins may directly activate the ERK signaling pathway and NF-κB in a MyD88-dependent manner. While p47 sequences are more than 99% identical between Wakulla and Arkansas (33), proteins of variable amino acid sequences or expression amounts between Wakulla and Arkansas strains (33) may be responsible for strain-variable cytokine induction. We previously reported that the E. chaffeensis Arkansas strain induces IL-1β, IL-8, and IL-10 in THP-1 cells (23). Because viable E. chaffeensis organisms are not required for induction of IL-1β, IL-8, or IL-10 (23, 57), and freeze-thawed Wakulla induces strong proinflammatory cytokine induction (data not shown), bacterial infection does not seem to be required for this early response.
NF-κB is thought to be a key transcription factor in regulating inflammatory cytokine expression, including IL-8 (22). Indeed, both our previous study with the less-virulent E. chaffeensis Arkansas strain (23) and the present study using the virulent Wakulla strain showed a critical role for NF-κB in induction of cytokines and chemokines by E. chaffeensis. In addition, the present study revealed that the ERK pathway is also required for induction of these cytokines. It has been reported that LPS induces cytokines/chemokines (IL-1β, TNF-α, IL-8, etc.) through the TLR4-dependent MAPK pathway and activation of NF-κB (14, 17). M. tuberculosis induces TNF-α through TLR2 and PKCζ-mediated ERK activation in human monocytes/macrophages (52). Chlamydia trachomatis, an obligatory intracellular Gram-negative bacterium, induces IL-8 in infected epithelial cells through the ERK pathway (6). C. trachomatis LPS is associated with cytokine induction through TLR4/CD14- and NF-κB-dependent signaling (15). In contrast to those reports, the present study showed that the IL-8 gene and NF-κB were activated in HEK293 cells by E. chaffeensis Wakulla without transfection of any TLR genes, despite other reports that HEK293 cells require transfection with one or two specific TLR genes, such as the TLR2/6, TLR4, and TLR8 genes, to activate NF-κB or cytokine genes (21, 30, 36). Taken together, our results indicate that E. chaffeensis does not require any TLRs to activate ERK and NF-κB. Elucidation of E. chaffeensis PRRs awaits further investigation.
Ras is a GTPase that is inactive when GDP-bound and becomes activated when a nucleotide exchange factor (GEF) stimulates GDP dissociation allowing rapid replacement by the more abundant GTP. To date, 5 families of GEF molecules have been identified: SOS, RasGRF, RasGRP, CNRasGEF, and PLCε (7). Among them, the SOS family is ubiquitously expressed to signal downstream of receptor tyrosine kinases. In addition, THP-1 cells seem to have RasGRP2 and PLCε, according to results of a transcriptome analysis (GEO data set number GSE4110) (13). HEK293 cells appear to express RasGRF1, RasGRP1, and RasGRP2 (GEO data set number GDS686) (55). Human monocytes seem to have RasGRF2, RasGRP1, RasGRP2, RasGRP4, and PLCε (GEO data set number GSE6054). These findings imply that SOS and/or RasGRP2 may be commonly involved in the Ras-Raf-MEK-ERK pathway to induce cytokines by E. chaffeensis in these cells.
C. trachomatis induces IL-8 expression at a markedly later time (15 h p.i.), which is dependent on bacterial intracellular growth and protein synthesis. IL-8 induction occurs only within inclusion-containing cells and is independent of exogenous factors in the supernatant, indicating a requirement for chlamydial products within the host cell (5). In contrast, because E. chaffeensis induces cytokines within 2 h p.i., this indicates that preformed bacterial factors can induce inflammation independent of infection.
We cannot rule out involvement of additional signaling pathways in E. chaffeensis cytokine/chemokine induction. However, taken altogether, the present study shows that LPS- and peptidoglycan-deficient E. chaffeensis cells induce monocyte inflammatory responses through MyD88, ERK, and NF-κB, but not via TLRs, suggesting involvement of previously unrecognized PAMPs and cognate PRRs.
ACKNOWLEDGMENTS
This work was funded by grant R01AI47885 from the National Institutes of Health.
We thank Prosper Boyaka for providing a TLR4−/− mouse and Abhay Satoskar and Patrick Reville for instruction in the bone marrow macrophage preparation procedure.
Footnotes
Published ahead of print on 19 September 2011.
REFERENCES
- 1. Adachi O., et al. 1998. Targeted disruption of the MyD88 gene results in loss of IL-1- and IL-18-mediated function. Immunity 9:143–150 [DOI] [PubMed] [Google Scholar]
- 2. Akira S., Uematsu S., Takeuchi O. 2006. Pathogen recognition and innate immunity. Cell 124:783–801 [DOI] [PubMed] [Google Scholar]
- 3. Arend W. P., Palmer G., Gabay C. 2008. IL-1, IL-18, and IL-33 families of cytokines. Immunol. Rev. 223:20–38 [DOI] [PubMed] [Google Scholar]
- 4. Bain J., et al. 2007. The selectivity of protein kinase inhibitors: a further update. Biochem. J. 408:297–315 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Buchholz K. R., Stephens R. S. 2006. Activation of the host cell proinflammatory interleukin-8 response by Chlamydia trachomatis. Cell. Microbiol. 8:1768–1779 [DOI] [PubMed] [Google Scholar]
- 6. Buchholz K. R., Stephens R. S. 2007. The extracellular signal-regulated kinase/mitogen-activated protein kinase pathway induces the inflammatory factor interleukin-8 following Chlamydia trachomatis infection. Infect. Immun. 75:5924–5929 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Buday L., Downward J. 2008. Many faces of Ras activation. Biochim. Biophys. Acta 1786:178–187 [DOI] [PubMed] [Google Scholar]
- 8. Crozat K., Beutler B. 2004. TLR7: a new sensor of viral infection. Proc. Natl. Acad. Sci. U. S. A. 101:6835–6836 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Dawson J. E., et al. 1991. Isolation and characterization of an Ehrlichia sp. from a patient diagnosed with human ehrlichiosis. J. Clin. Microbiol. 29:2741–2745 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Dunning Hotopp J. C., et al. 2006. Comparative genomics of emerging human ehrlichiosis agents. PLoS Genet. 2:e21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Fantuzzi G., Puren A. J., Harding M. W., Livingston D. J., Dinarello C. A. 1998. Interleukin-18 regulation of interferon gamma production and cell proliferation as shown in interleukin-1beta-converting enzyme (caspase-1)-deficient mice. Blood 91:2118–2125 [PubMed] [Google Scholar]
- 12. Foxwell B., et al. 1998. Efficient adenoviral infection with IkappaB alpha reveals that macrophage tumor necrosis factor alpha production in rheumatoid arthritis is NF-kappaB dependent. Proc. Natl. Acad. Sci. U. S. A. 95:8211–8215 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Goldin-Lang P., et al. 2007. Effect of ionizing radiation on cellular procoagulability and co-ordinated gene alterations. Haematologica 92:1091–1098 [DOI] [PubMed] [Google Scholar]
- 14. Guha M., Mackman N. 2001. LPS induction of gene expression in human monocytes. Cell. Signal. 13:85–94 [DOI] [PubMed] [Google Scholar]
- 15. Heine H., Muller-Loennies S., Brade L., Lindner B., Brade H. 2003. Endotoxic activity and chemical structure of lipopolysaccharides from Chlamydia trachomatis serotypes E and L2 and Chlamydophila psittaci 6BC. Eur. J. Biochem. 270:440–450 [DOI] [PubMed] [Google Scholar]
- 16. Hii C. S., et al. 2008. Interleukin-8 induction by Burkholderia pseudomallei can occur without Toll-like receptor signaling but requires a functional type III secretion system. J. Infect. Dis. 197:1537–1547 [DOI] [PubMed] [Google Scholar]
- 17. Hoffmann E., Dittrich-Breiholz O., Holtmann H., Kracht M. 2002. Multiple control of interleukin-8 gene expression. J. Leukoc. Biol. 72:847–855 [PubMed] [Google Scholar]
- 18. Koh Y. S., Koo J. E., Biswas A., Kobayashi K. S. 2010. MyD88-dependent signaling contributes to host defense against ehrlichial infection. PLoS One 5:e11758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Kolch W. 2000. Meaningful relationships: the regulation of the Ras/Raf/MEK/ERK pathway by protein interactions. Biochem. J. 351:289–305 [PMC free article] [PubMed] [Google Scholar]
- 20. Kumar H., Kawai T., Akira S. 2009. Toll-like receptors and innate immunity. Biochem. Biophys. Res. Commun. 388:621–625 [DOI] [PubMed] [Google Scholar]
- 21. Lan T., et al. 2007. Stabilized immune modulatory RNA compounds as agonists of Toll-like receptors 7 and 8. Proc. Natl. Acad. Sci. U. S. A. 104:13750–13755 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Lawrence T. 2009. The nuclear factor NF-kappaB pathway in inflammation. Cold Spring Harb. Perspect. Biol. 1:a001651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Lee E. H., Rikihisa Y. 1996. Absence of tumor necrosis factor alpha, interleukin-6 (IL-6), and granulocyte-macrophage colony-stimulating factor expression but presence of IL-1beta, IL-8, and IL-10 expression in human monocytes exposed to viable or killed Ehrlichia chaffeensis. Infect. Immun. 64:4211–4219 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Lee E. H., Rikihisa Y. 1997. Anti-Ehrlichia chaffeensis antibody complexed with E. chaffeensis induces potent proinflammatory cytokine mRNA expression in human monocytes through sustained reduction of IkappaB-alpha and activation of NF-kappaB. Infect. Immun. 65:2890–2897 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Lin M., Rikihisa Y. 2003. Ehrlichia chaffeensis and Anaplasma phagocytophilum lack genes for lipid A biosynthesis and incorporate cholesterol for their survival. Infect. Immun. 71:5324–5331 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Lin M., Rikihisa Y. 2004. Ehrlichia chaffeensis downregulates surface Toll-like receptors 2/4, CD14 and transcription factors PU.1 and inhibits lipopolysaccharide activation of NF-kB, ERK 1/2 and p38 MAPK in host monocytes. Cell. Microbiol. 6:175–186 [DOI] [PubMed] [Google Scholar]
- 27. Maeda K., et al. 1987. Human infection with Ehrlichia canis, a leukocytic rickettsia. N. Engl. J. Med. 316:853–856 [DOI] [PubMed] [Google Scholar]
- 28. Matsumoto M., et al. 2003. Subcellular localization of Toll-like receptor 3 in human dendritic cells. J. Immunol. 171:3154–3162 [DOI] [PubMed] [Google Scholar]
- 29. Mattner J., et al. 2005. Exogenous and endogenous glycolipid antigens activate NKT cells during microbial infections. Nature 434:525–529 [DOI] [PubMed] [Google Scholar]
- 30. McGowin C. L., Ma L., Martin D. H., Pyles R. B. 2009. Mycoplasma genitalium-encoded MG309 activates NF-kappaB via Toll-like receptors 2 and 6 to elicit proinflammatory cytokine secretion from human genital epithelial cells. Infect. Immun. 77:1175–1181 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. McQuiston J. H., Paddock C. D., Holman R. C., Childs J. E. 1999. The human ehrlichioses in the United States. Emerg. Infect. Dis. 5:635–642 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Miura K., Rikihisa Y. 2009. Liver transcriptome profiles associated with strain-specific Ehrlichia chaffeensis-induced hepatitis in SCID mice. Infect. Immun. 77:245–254 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Miura K., Rikihisa Y. 2007. Virulence potential of Ehrlichia chaffeensis strains of distinct genome sequences. Infect. Immun. 75:3604–3613 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Monick M. M., Carter A. B., Flaherty D. M., Peterson M. W., Hunninghake G. W. 2000. Protein kinase C zeta plays a central role in activation of the p42/44 mitogen-activated protein kinase by endotoxin in alveolar macrophages. J. Immunol. 165:4632–4639 [DOI] [PubMed] [Google Scholar]
- 35. Nagase T., et al. 1997. Manumycin and gliotoxin derivative KT7595 block Ras farnesylation and cell growth but do not disturb lamin farnesylation and localization in human tumour cells. Br. J. Cancer. 76:1001–1010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Nahori M. A., et al. 2005. Differential TLR recognition of leptospiral lipid A and lipopolysaccharide in murine and human cells. J. Immunol. 175:6022–6031 [DOI] [PubMed] [Google Scholar]
- 37. O'Neill L. A., Bowie A. G. 2007. The family of five: TIR-domain-containing adaptors in Toll-like receptor signalling. Nat. Rev. Immunol. 7:353–364 [DOI] [PubMed] [Google Scholar]
- 38. Paddock C. D., Childs J. E. 2003. Ehrlichia chaffeensis: a prototypical emerging pathogen. Clin. Microbiol. Rev. 16:37–64 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Paddock C. D., et al. 2001. Infections with Ehrlichia chaffeensis and Ehrlichia ewingii in persons coinfected with human immunodeficiency virus. Clin. Infect. Dis. 33:1586–1594 [DOI] [PubMed] [Google Scholar]
- 40. Rajaiya J., Sadeghi N., Chodosh J. 2009. Specific NFkappaB subunit activation and kinetics of cytokine induction in adenoviral keratitis. Mol. Vis. 15:2879–2889 [PMC free article] [PubMed] [Google Scholar]
- 41. Sehdev A. E., Dumler J. S. 2003. Hepatic pathology in human monocytic ehrlichiosis. Ehrlichia chaffeensis infection. Am. J. Clin. Pathol. 119:859–865 [DOI] [PubMed] [Google Scholar]
- 42. Sherf B. A., Navarro S. L., Hannah R. R., Wood K. V. 1996. Dual-luciferase reporter assay: an advanced co-reporter technology integrating firefly and Renilla luciferase assays. Promega Notes 57:2–8 [Google Scholar]
- 43. Shi Z., et al. 2011. A novel Toll-like receptor that recognizes vesicular stomatitis virus. J. Biol. Chem. 286:4517–4524 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Shimohata T., et al. 2011. Vibrio parahaemolyticus infection induces modulation of IL-8 secretion through dual pathway via VP1680 in Caco-2 cells. J. Infect. Dis. 203:537–544 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Takeuchi O., Akira S. 2010. Pattern recognition receptors and inflammation. Cell 140:805–820 [DOI] [PubMed] [Google Scholar]
- 46. Telford S. R., Dawson J. E. 1996. Persistent infection of C3h/HeJ mice by Ehrlichia chaffeensis. Vet. Microbiol. 52:103–112 [DOI] [PubMed] [Google Scholar]
- 47. Wakeel A., Kuriakose J. A., McBride J. W. 2009. An Ehrlichia chaffeensis tandem repeat protein interacts with multiple host targets involved in cell signaling, transcriptional regulation, and vesicle trafficking. Infect. Immun. 77:1734–1745 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Wilhelm S. M., et al. 2004. BAY 43-9006 exhibits broad spectrum oral antitumor activity and targets the RAF/MEK/ERK pathway and receptor tyrosine kinases involved in tumor progression and angiogenesis. Cancer Res. 64:7099–7109 [DOI] [PubMed] [Google Scholar]
- 49. Wu C. H., Yeh C. T., Shih P. H., Yen G. C. 2010. Dietary phenolic acids attenuate multiple stages of protein glycation and high-glucose-stimulated proinflammatory IL-1beta activation by interfering with chromatin remodeling and transcription in monocytes. Mol. Nutr. Food Res. 54(Suppl. 2):S127–S140 [DOI] [PubMed] [Google Scholar]
- 50. Yamamoto M., et al. 2003. Role of adaptor TRIF in the MyD88-independent toll-like receptor signaling pathway. Science 301:640–643 [DOI] [PubMed] [Google Scholar]
- 51. Yamaoka Y., et al. 2004. Role of interferon-stimulated responsive element-like element in interleukin-8 promoter in Helicobacter pylori infection. Gastroenterology 126:1030–1043 [DOI] [PubMed] [Google Scholar]
- 52. Yang C. S., et al. 2007. Protein kinase C zeta plays an essential role for Mycobacterium tuberculosis-induced extracellular signal-regulated kinase 1/2 activation in monocytes/macrophages via Toll-like receptor 2. Cell. Microbiol. 9:382–396 [DOI] [PubMed] [Google Scholar]
- 53. Yang F. L., et al. 2008. TLR-independent induction of human monocyte IL-1 by phosphoglycolipids from thermophilic bacteria. Glycoconj. J. 25:427–439 [DOI] [PubMed] [Google Scholar]
- 54. Yasuda K., Ogawa Y., Yamane I., Nishikawa M., Takakura Y. 2005. Macrophage activation by a DNA/cationic liposome complex requires endosomal acidification and TLR9-dependent and -independent pathways. J. Leukoc. Biol. 77:71–79 [DOI] [PubMed] [Google Scholar]
- 55. Zagranichnaya T. K., Wu X., Danos A. M., Villereal M. L. 2005. Gene expression profiles in HEK-293 cells with low or high store-operated calcium entry: can regulatory as well as regulated genes be identified? Physiol. Genomics 21:14–33 [DOI] [PubMed] [Google Scholar]
- 56. Zhang J. Z., Sinha M., Luxon B. A., Yu X. J. 2004. Survival strategy of obligately intracellular Ehrlichia chaffeensis: novel modulation of immune response and host cell cycles. Infect. Immun. 72:498–507 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Zhang X., McBride J. W. 2009. Early Ehrlichia chaffeensis-monocyte interaction induces response dominated by inflammatory CC family chemokines, abstr. 87, p. 77 23rd Meet. Am. Soc. Rickettsiol., Hilton Head Island, SC. [Google Scholar]
- 58. Zhu B., et al. 2009. Nuclear translocated Ehrlichia chaffeensis ankyrin protein interacts with a specific adenine-rich motif of host promoter and intronic Alu elements. Infect. Immun. 77:4243–4255 [DOI] [PMC free article] [PubMed] [Google Scholar]