Abstract
After the first report in May 2008, the National Reference Center for Susceptibility Testing confirmed 113 cases of infection or colonization by KPC-producing members of the family Enterobacteriaceae in Poland by the end of 2009. The vast majority of patients were found in 18 hospitals; three patients were diagnosed at outpatient clinics. Most of the institutions were in the Warsaw area, including three hospitals with the highest numbers of cases. When available, the data on previous hospitalizations often indicated that these hospitals were the probable acquisition sites; one patient arrived from New York. The group of 119 unique isolates consisted of Klebsiella pneumoniae (n = 114), followed by Klebsiella oxytoca (n = 3), and Escherichia coli (n = 2). The K. pneumoniae isolates were dominated by the clone sequence type 258 (ST258) (n = 111); others were ST11 and ST23. The ST258 group was heterogeneous, with 28 pulsed-field gel electrophoresis (PFGE) subtypes, ∼25 plasmid profiles, and nine β-lactamase patterns differing by KPC variants (KPC-2 mainly), and SHV-12, CTX-M-3, and TEM-1-like enzymes. Plasmids carrying blaKPC genes varied in size (∼48 to 250 kb), structure, and conjugation potential. Transferable IncFIIK plasmids of ∼110 to 160 kb, probably pKpQIL or its derivatives, were observed in all K. pneumoniae clones and in K. oxytoca. Also prevalent were nontypeable pETKp50-like plasmids of ∼50 kb, found in K. pneumoniae ST258 and E. coli isolates (ST93 and ST224). Two K. pneumoniae-E. coli pairs from single patients might represent the in vivo transfer of such plasmids. The striking diversity of KPC producers at the early stage of dissemination could result from several introductions of these bacteria into the country, their multidirectional evolution during clonal spread, and transfer of the plasmids.
INTRODUCTION
Resistance to carbapenems in members of the family Enterobacteriaceae has become a matter of the highest concern in recent years (6, 37). It has been associated largely with various carbapenem-hydrolyzing β-lactamases, including KPC-like enzymes, which hydrolyze all β-lactams that are in use (28, 32). Of several variants identified since the first report (43), KPC-2 and KPC-3 have been the most frequent types, and Klebsiella pneumoniae has been the main producer species (28, 32). blaKPC genes are located on plasmids with different replicon types (e.g., IncFII, IncL/M, IncN, IncR, and ColE1), size (∼12 to ∼180 kb), conjugation ability, and resistance repertoire (1, 10, 16, 18, 20, 24, 25, 27). The direct context of blaKPC is formed by Tn4401-like transposons of some structural diversity (10, 22, 23, 27, 29). KPC producers vary in their level of resistance to β-lactams which may increase owing to modifications in blaKPC promoters, blaKPC duplications, acquisition of other β-lactamase genes, or porin alterations (10, 16, 22, 27, 43). The presence of non-β-lactam resistance mechanisms and the fact that bacteria commonly possess these mechanisms means that KPC producers are often susceptible to few drugs, e.g., gentamicin, amikacin, and/or colistin (32).
Since the late 1990s, KPC-producing organisms have spread in the United States (35), while since 2005, their expansion has been observed in Israel (37, 38) and worldwide (32). Although KPCs have been found in numerous K. pneumoniae clones and in other species (1, 9, 10, 18, 23, 25, 38), this spread has been strongly associated with K. pneumoniae sequence type 258 (ST258) and related clones, like ST11 (1, 3, 23, 25, 34, 36). Identified in many countries and continents, this hyperepidemic clonal complex carries either blaKPC-2 or blaKPC-3 on different plasmids, such as the ∼110-kb IncFIIK pKpQIL, identified and fully sequenced in Israel (25).
The aim of this study was to analyze the dissemination of KPC producers in Poland, following the first report in May 2008 (3). Isolates identified in 2008-2009 were subjected to typing and plasmid and β-lactamase analyses.
MATERIALS AND METHODS
Clinical isolates.
A total of 119 KPC-producing Klebsiella pneumoniae (n = 114), Klebsiella oxytoca (n = 3), and Escherichia coli (n = 2) isolates were collected in 2008 and 2009 (n = 33 and 86, respectively) by the National Reference Center for Susceptibility Testing (NRCST) as a part of its surveillance activity (Table 1). Most of these isolates (n = 115) were from patients in 18 hospitals in Poland, including 12 hospitals in the Warsaw area (Fig. 1) that were tertiary care teaching, tertiary care specialist, secondary care regional, or primary care local institutions. One isolate was cultured from a nursing home resident upon her admission to hospital 11 (h11) (in Warsaw suburbs), and three others were from outpatients (two in Warsaw). The isolates originated from 113 patients; in some cases, the isolates from one patient gave different species (two pairs of K. pneumoniae and E. coli) or typing results, which were included in the study. Ten hospitals sent 1 or 2 organisms each, while others had multiple KPC isolations, including h3, h4, and h5 with 19, 24, and 23 isolates, respectively. Many isolates were from infections, namely, urinary tract (urine samples; n = 61), invasive (blood or peritoneal fluid samples; n = 22), respiratory tract (bronchoalveolar lavage samples; n = 8), or skin and soft tissue (n = 6) infections. The other isolates represented probable cases of colonization of gastrointestinal (stool samples or rectal swabs; n = 17) or respiratory (sputum samples or nasopharyngeal swabs; n = 5) tracts. Species were identified with the Vitek 2 system (bioMérieux, Marcy l'Etoile, France).
Table 1.
KPC-producing isolates and their most important characteristicsa
| Species | Medical center (n)b |
Total no. of isolates | PFGE subtype | MLSTc | KPC typed | ESBL (n)b,d | Other acquired β-lactamases (n)b,d | Size (kb) of blaKPC plasmid(s) |
blaKPC plasmid(s) in R+c,e |
Tn4401 | Size and type of ESBL plasmid(s) in R+c,e,g | ||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| In the Warsaw area | In other areas | Size (kb)f | PstI fingerprinting restriction pattern | Replicon typeg | |||||||||||
| K. pneumoniae | h1 (4), h4 (1) | 5 | A1 | ST258 | 2 | SHV-12 | TEM-1 | ∼110 | ∼110 | c | FIIK and FIB | a | ∼40 kb, NT | ||
| h4 (2) | 2 | A2 | ND | 2 | SHV-12 | TEM-1* | ∼160 and ∼250 | − | ND | ND | a | ∼40 kb, NT | |||
| h2 (2), h3 (7), h4 (1), h7 (1), h11 (6), h12 (1) | h13 (2) and a3 (1) | 21 | A3 | ST258 | 2 | SHV-12 (13) and SHV-12 and CTX-M-3 (7) | TEM-1* | ∼110 | ND | ND | ND | a | ND | ||
| h4 (14), h5 (11), h9 (2), h10 (2), h11 (1), a1 (1), a2 (1) | h14 (1) and h15 (6) | 39 | A4 | ST258 | 2 | CTX-M-3 (6) | TEM-1* (7) | ∼50 | ∼50 | b | NT | a | ∼90 kb, L/M | ||
| h4 (1) | 1 | A5 | ND | 2* | SHV-12* | TEM-1* | ∼110 and ∼230 | ND | ND | ND | a | ND | |||
| h3 (4), h4 (1), h6 (1) | 6 | A6 | ND | 2* | SHV-12 | TEM-1* | ∼110 | ND | ND | ND | a | ND | |||
| h3 (2) | 2 | A7 | ND | 2 | SHV-12* | TEM-1* | ∼48 and ∼110 | ∼110 | c | FIIK and FIB | a | ND | |||
| h11 (1) | 1 | A8 | ND | 2* | SHV-12* and CTX-M-3 | TEM-1* | ∼110 | ND | ND | ND | a | ND | |||
| h3 (1) | 1 | A9 | ND | 2* | SHV-12* | TEM-1* | ∼110 | ND | ND | ND | a | ND | |||
| h3 (1) | 1 | A10 | ND | 2 | SHV-12 | TEM-1* | ∼130 | ∼130 | c | FIIK and FIB | a | ∼40 kb, NT | |||
| h3 (1) | 1 | A11 | ST258 | 2 | CTX-M-3 | TEM-1* | ∼120 | ∼120 | c | FIIK and FIB | a | ∼90 kb, L/M | |||
| h3 (1) | 1 | A12 | ND | 2 | SHV-12 | TEM-1* | ∼125 | ∼125 | c | FIIK and FIB | a | ∼40 kb, NT | |||
| h2 (1) | 1 | A13 | ST258 | 3 | CTX-M-3 | TEM-1* | ∼48 and ∼70 | ∼48 | a | R | b | ∼90 kb, L/M | |||
| h3 (1) | 1 | A14 | ND | 2* | SHV-12* | TEM-1* | ∼110 | ND | ND | ND | ND | ||||
| h3 (1) | 1 | A15 | ST258 | 2 | SHV-12 | − | ∼200 | − | ND | ND | a | ∼40 kb, NT | |||
| h1 (2) | 2 | A16 | ST258 | 2 | SHV-12 | TEM-1* | ∼110 and ∼220 | ∼110 | c | FIIK and FIB | a | ∼40 kb, NT | |||
| − | h18 (1) | 1 | A17 | ST258 | 3 | − | TEM-1* | ∼110 | ∼110 | c | FIIK and FIB | a | − | ||
| h2 (1), h6 (1) | h16 (1) | 3 | A18 | ST258 | 2* | SHV-12* and CTX-M-3 | TEM-1* | ∼110 | ND | ND | ND | a | ∼90 kb, L/M | ||
| h1 (1), h7 (1), h12 (1) | 3 | A19 | ST258 | 2 | SHV-12 | TEM-1* | ∼110 and ∼230 | ∼110 | c | FIIK and FIB | a | ∼40 kb, NT | |||
| h1 (1), h2 (2), h11 (1) | 4 | A20 | ND | 2* | − | TEM-1* | ∼110 | ND | ND | ND | a | − | |||
| h4 (1) and h8 (1) | 2 | A21 | ST258 | 2* | SHV-12* | − | ∼110 | ND | ND | ND | a | ND | |||
| h6 (1) | 1 | A22 | ST258 | 2* | SHV-12* | TEM-1* | ∼160 | ND | ND | ND | a | ND | |||
| h7 (1) | 1 | A23 | ST258 | 2 | SHV-12 and CTX-M-3 | TEM-1* | ∼160 | ∼160 | d | FIIK and FIB | a | ∼40 kb, NT; ∼90 kb, L/M | |||
| h5 (5) | 5 | A24 | ND | 2* | − | − | ∼50 | ∼50 | b | NT | a | − | |||
| h4 (1) | 1 | A25 | ND | 2 | SHV-12 | TEM-1* | ∼250 | − | ND | ND | a | ∼40 kb, NT | |||
| h4 (1) | 1 | A26 | ND | 2 | SHV-12 | − | ∼160 | ∼160 | d | FIIK and FIB | a | ∼40 kb, NT | |||
| h5 (2) | 2 | A27 | ST258 | 2* | − | − | ∼50 | ND | ND | ND | a | − | |||
| h4 (1) | 1 | A28 | ND | 2 | SHV-12 | TEM-1* | ∼160 | ∼160 | d | FIIK and FIB | a | ∼40 kb, NT | |||
| − | h17 (1) | 1 | B | ST23 | 2* | − | TEM-1* | ∼110 | ND | ND | ND | a | − | ||
| h6 (2) | 2 | C | ST11 | 2 | SHV-12* and CTX-M-3 | TEM-1* | ∼110 | ∼110 | c | FIIK and FIB | a | ∼90 kb, L/M | |||
| K. oxytoca | h5 (3) | 3 | a | ND | 2 | − | TEM-1* | ∼110 | ∼110 | c | FIIK and FIB | a | − | ||
| E. coli | h5 (1) | 1 | α | ST224 | 2 | − | TEM-1* | ∼50 | ∼50 | b | NT | a | − | ||
| h5 (1) | 1 | β | ST93 | 2 | − | TEM-1* | ∼50 | ∼50 | b | NT | a | − | |||
The isolates are ordered in the table according to the species and PFGE data.
n is the number of isolates from a particular medical center or number of isolates producing a particular type of β-lactamase.
ND, not determined.
The isolates for which sequencing was not performed are indicated by asterisks; the identification was based on the PCR and RsaI digestion data (blaKPC genes) or the isoelectric focusing, PCR, and sequencing data obtained for other isolates (blaSHV and blaTEM genes).
R+, E. coli A15 transconjugant or E. coli TOP10 transformant.
−, neither transconjugant nor transformant was obtained for these isolates.
NT, nontypeable by PBRT (7).
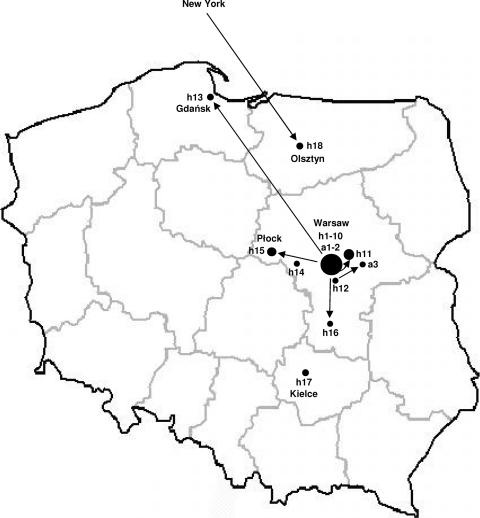
Fig. 1.
Map of Poland with the geographic locations of the medical centers in which KPC-positive isolates were identified. h1 to 18, hospitals; a1 to 3, outpatient clinics. The arrows show the movement of patients with KPC-producing organisms between locales.
KPC detection and genetic context of blaKPC genes.
The detection of KPC was assessed by the disk test with phenylboronic acid (12) and confirmed by PCR (30). blaKPC amplicons were cut with RsaI (Fermentas, Vilnius, Lithuania) to distinguish between blaKPC-2- and blaKPC-3-like genes (26) and directly sequenced for selected isolates. The location of blaKPC within Tn4401-like elements was analyzed by PCR mapping as previously proposed (29).
Identification of other β-lactamases.
β-Lactamase profiling was performed by isoelectric focusing (5). Genes coding for CTX-M-1- and SHV-like extended-spectrum β-lactamases (ESBLs) (blaCTX-M and blaSHV, respectively), and TEM-type enzymes (blaTEM) were amplified and sequenced as described previously (2, 13).
Typing.
Pulsed-field gel electrophoresis (PFGE) was performed as reported previously (39), using the XbaI enzyme (Fermentas); DNA patterns were analyzed visually by using the criteria of Tenover et al. (40). K. pneumoniae and E. coli isolates were subjected to multilocus sequence typing (MLST) as described previously (11, 42); the Klebsiella pneumoniae MLST database (www.pasteur.fr/recherche/genopole/PF8/mlst/Kpneumoniae.html) and the Escherichia coli MLST Database (http://mlst.ucc.ie/mlst/dbs/Ecoli) were used for assigning sequence types (STs).
Plasmid analysis.
Plasmid profiling was carried out by PFGE of total DNA from isolates that had been cut with nuclease S1 (New England BioLabs, Beverly, MA) (36). After electrophoresis, DNA was blotted onto Hybond-N+ (Amersham Pharmacia Biotech, Little Chalfont, United Kingdom) and hybridized with a blaKPC PCR probe, using the ECL Random-Prime Labeling and Detection system (Amersham Pharmacia Biotech). Mating was done with E. coli A15 Rifr (5); transconjugants were selected with 100 μg/ml rifampin (Polfa Tarchomin, Warsaw, Poland) and 0.5 μg/ml imipenem (Merck, Sharp & Dohme Research, Rahway, NJ) or 2 μg/ml cefotaxime (Polfa). Plasmid DNA from the isolates, purified with the Qiagen Plasmid Midi kit (Qiagen, Hilden, Germany), was electroporated into E. coli TOP10 cells (Invitrogen, Carlsbad, CA), and selected with 0.5 μg/ml imipenem. Plasmids from the E. coli recombinants were characterized by PstI fingerprinting (2) and by PCR-based replicon typing (PBRT) (7); the IncR-type replicon was detected as described previously (27) and sequenced. Plasmids of the pKpQIL type (25) were studied by PCR mapping covering seven regions, with primers designed on the basis of the pKpQIL sequence (GenBank accession no. GU595196) (Table 2).
Table 2.
Pairs of primers used in the PCR mapping assay analyzing the pKpQIL-like plasmids
| Primer | Sequence (5′ → 3′) | Annealing positionsa | Product size (bp) | pKpQIL fragment analyzeda | Resultb |
|---|---|---|---|---|---|
| RepAF | GCTGAGCGTAAATCTCAC | 4439–4456 | 159 | IncFIIK replicon region (FIIKrepA gene) | + |
| RepAR | CTCAACCTCACGCTTCAG | 4580–4597 | |||
| RepAF | GCTGAGCGTAAATCTCAC | 4439–4456 | 1,497 | Junction between the FIIK replicon region and the pNYC-derived region | + |
| ORF8R | CAGACGTGATTTTCTGACCAC | 5915–5935 | |||
| ORF11R | GCATGTTTGAACTGGTCAG | 8347–8365 | 682 | Junction between Tn4401 and its 5′ flank within the pNYC region | + |
| tnpRF | CGACGTCGATGTATTTGCATG | 9008–9028 | |||
| IS26LF | GTCGGTGGTGATAAAC | 25556–25571 | 2,847 | IS26 insertion within 1331 the pKPN4-derived Tn | +/− |
| TEM-F | TTACTGTCATGCCATCC | 28386–28402 | |||
| OXA-9F | CTGCTGCATATGTTGGTG | 26542–26559 | 1,861 | Junction between blaOXA-9 and blaTEM-1 within the pKPN4 Tn1331 | +/− |
| TEM-F | TTACTGTCATGCCATCC | 28386–28402 | |||
| rep2R | TCTGCTGGTTATTGGGTGAG | 53337–53356 | 312 | FIB-like repA gene within the pKPN4 region | + |
| rep2F | GTCTTCGCAGCACAACTATC | 53629–53648 | |||
| TraXF | CGCAATGTTCTATGCTGTG | 112918–112936 | 1,576 | Junction between the pKPN4 region (traX gene) and the FIIK replicon region | +/− |
| ORF2R | CAGAATAACTGCTGCTCAG | 838–856 |
Susceptibility testing.
The MICs of antimicrobial agents were evaluated with Etest strips (AB bioMérieux, Solna, Sweden) or M.I.C. Evaluator strips (Oxoid, Basingstoke, United Kingdom). The results were interpreted according to the CLSI guidelines (8); for tigecycline and colistin, the criteria of EUCAST were applied (www.eucast.org).
RESULTS
Epidemiology in Warsaw area.
The first KPC-positive K. pneumoniae isolates in Poland were identified by the NRCST in May-June 2008; the patient was hospitalized on two different occasions in two Warsaw centers (h1 and h2) (3). Soon after, the next non-carbapenem-susceptible enteric isolates were sent by Polish hospitals for reference diagnostic analysis; by the end of 2008, 33 KPC-producing isolates from 31 patients in five Warsaw centers were confirmed. Hospitals h1 and h2 had two cases and one case, respectively, but the other hospitals recorded clusters of cases (h3, h4, and h5 with 19, 6, and 5 patients, respectively), comprising patients in different wards infected or colonized mainly with K. pneumoniae (three K. oxytoca cases in h5). In 2009, the five centers continued to identify KPC-positive K. pneumoniae cases, with h3, h4, and h5 being the most affected (h4, 18 patients; h5, 15 patients; h3 stopped confirming cases at the NRCST). From two patients in h5, both K. pneumoniae and E. coli isolates were recovered. Five more Warsaw centers (h6 to h10) and two in the suburbs (h11 and h12) recorded KPC cases. Finally, two patients were diagnosed in Warsaw outpatient clinics (a1 and a2). The data on the patients' movement in the area often could not be obtained; in other cases, the information was fragmentary or indicated several hospitals, both the affected ones and those that did not report KPC-producing isolates. In h11, which started screening all patients at admission in early 2009, KPC-producing isolates were identified in a patient previously treated in h4, a nursing home patient, and three dialysis outpatients. The latter patients had complicated histories of earlier hospitalizations, and where they acquired KPC-producing K. pneumoniae is unclear.
Epidemiology outside Warsaw.
In May 2009, the first KPC-producing K. pneumoniae isolate was identified in a hospital in Gdańsk, Poland (h13), and by the end of 2009, five other centers (h14 to h18) and one outpatient clinic (a3) recorded such organisms. These were mainly single or a few cases, except for a hospital in Płock (h15) where an outbreak with six patients occurred. The first patients in h13 and h16 arrived from Warsaw h3, the outbreak in h15 commenced with a patient from h5, while the patient from a3 had stayed in h12. The patient in Olsztyn, Poland (h18) had been hospitalized in New York. Only two cases (h14 and h17) were not linked to previous care elsewhere.
Typing.
All but three K. pneumoniae isolates belonged to a single PFGE type, type A, split into 28 subtypes, A1 to A28 (Table 1). Similarity between two patterns varied from ∼72% to 94% (Dice coefficient calculated manually). Subtypes A4 and A3 were highly prevalent, grouping 39 and 21 isolates from nine and eight sites, respectively. Of the hospitals in Warsaw that were the most affected, h5 had a relatively homogeneous population of K. pneumoniae KPC (subtypes A4 and A24 mostly), while others were notably diverse. The isolate from Olsztyn, Poland, and New York was of unique subtype (A17). MLST classified 16 selected type A isolates with the ST258 clone. The three remaining K. pneumoniae isolates were split into two other PFGE types and STs. The single ST23 strain was the only organism from a hospital in Kielce, Poland (h17). Two isolates from a Warsaw hospital (h6) represented ST11. The three K. oxytoca isolates from h5 produced identical PFGE patterns. In contrast, the two E. coli isolates from the same site differed from each other and were classified as clones ST93 and ST224.
β-Lactamase content.
Two unique K. pneumoniae ST258 isolates carried blaKPC-3 (subtype A13 from Warsaw h2 and subtype A17 from Olsztyn/New York); all the others likely had blaKPC-2, confirmed by sequencing for 19 isolates (Table 1). Twenty-two K. pneumoniae isolates also expressed β-lactamases encoded by blaCTX-M-1-like genes; the genes were found to be blaCTX-M-3 in 11 isolates. Fifty-nine K. pneumoniae isolate produced enzymes correlating with amplicons of blaSHV-5/12-like genes, identified as blaSHV-12 in 13 isolates. CTX-M-3 and SHV-12 were coexpressed in 14 isolates. Seventy-six isolates had β-lactamases corresponding to blaTEM-1-like genes. The β-lactamase profiles correlated with the PFGE types/subtypes; only the K. pneumoniae ST258 subtypes A4 and A3 varied regarding the presence of CTX-M-3, SHV-12, and/or TEM-1.
Plasmids with blaKPC genes.
Plasmid DNA of the isolates revealed high variation with around 30 S1 profiles (data not shown). The profiles consisted of 1 to 4 molecules varying in size from ∼40 kb to ∼350 kb and in general correlated with PFGE types/subtypes and β-lactamase patterns. All S1 profiles were hybridized with the blaKPC probe, revealing a number of molecules split into five size groups, of ∼48 to 50 kb, ∼70 kb, ∼110 to 130 kb, ∼160 kb and ∼200 to 250 kb (Table 1). In six profiles, two plasmids carried blaKPC. The ∼110- to 130-kb molecules were observed alone or with other sized molecules in 62 isolates of ∼15 S1 profiles from K. pneumoniae ST11, ST23, and ST258 of 17 subtypes (including the A17 KPC-3 isolate from Olsztyn/New York), and K. oxytoca isolates. The second common plasmid was of ∼50 kb, identified in 46 K. pneumoniae ST258 isolates of three subtypes and two S1 profiles, and E. coli isolates.
Twenty-four isolates with all blaKPC plasmids, including K. oxytoca and both E. coli and K. pneumoniae pairs from the same patient, were used in mating, which worked for 13 isolates. Then, DNA from the nonmating isolates was subjected to electroporation, which yielded seven transformants (Table 1). Plasmids from all 20 E. coli recombinants had four types of PstI restriction patterns, namely, type a for the plasmid of ∼48 kb, type b for plasmids of ∼50 kb, type c for plasmids of ∼110 to 130 kb, and type d for plasmids of ∼160 kb (patterns shared at least 80% or 90% of bands of >1.5 kb within a type). The fingerprints of type c matched well with the fingerprint of the pKpQIL plasmid with blaKPC-3 from Israel (25), which was generated in silico. The type b plasmids of ∼50 kb in the E. coli and K. pneumoniae isolates from the same patient had very similar fingerprints. Plasmids that did not transfer were those of ∼70 kb and ∼200 to 250 kb.
The PCR assay checking for several pKpQIL regions (Table 2) confirmed that plasmids of ∼110 to 130 kb represented several variants of that structure (25); moreover, the ∼160-kb plasmids also behaved in a similar way. The IncFIIK-type repA gene (25, 41), amplifiable also with PBRT primers for FIIAS (7), was always observed, so was the additional replicon FIB. The position of FIIK repA was stable regarding the downstream region with Tn4401 but not with respect to the upstream tra operon (25). The plasmids of ∼50 kb and ∼48 kb were nontypeable by PBRT (7); however, the latter plasmid carried the IncR-type repB gene (27).
Plasmids with blaCTX-M-3 and blaSHV-12 genes.
Nineteen isolates with CTX-M-3 (n = 6) or SHV-12 (n = 13) were used in conjugation assays (Table 1); only two of the SHV-12 producers did not mate. The ∼90-kb plasmids from the CTX-M-3 transconjugants had PstI fingerprints and L/M-type replicons like the pCTX-M3-type molecules (15). The ∼40-kb plasmids from SHV-12 strains showed identical fingerprints and were nontypeable by PBRT (3).
Genetic context of blaKPC genes.
All but one of a wide group of isolates tested carried the Tn4401a element (Table 1), with a 100-bp deletion between ISKpn7 and blaKPC (29). Only the K. pneumoniae ST258 A13 KPC-3 isolate from Warsaw h2 had the Tn4401b variant.
Susceptibility testing.
Thirty-seven representative isolates were subjected to susceptibility testing (see Table S1 in the supplemental material). All were resistant to penicillins, penicillin-inhibitor combinations, ceftazidime, and aztreonam. Considering the confluent growth around antibiotic strips, the MICs of carbapenems varied (MICs, 0.5 to >32 μg/ml); however, multiple in-ellipse colonies were usually observed (32). Except for K. pneumoniae ST23, K. oxytoca, and E. coli ST93, all isolates were resistant to ciprofloxacin. Amikacin and gentamicin were often active; however, K. pneumoniae ST11 and some ST258 isolates were resistant to these two drugs. All the isolates tested were susceptible to colistin; 18 isolates were intermediate to tigecycline.
DISCUSSION
This study shows the early stage of KPC-producing K. pneumoniae dissemination in Poland in 2008-2009. Although microbiology laboratories are not obliged to send pathogens on the alert or watch list to the NRCST, years of experience and good reciprocal contacts allow us to assume that the isolates reflected the situation well. It is impossible to know whether the KPC-producing K. pneumoniae history in Poland commenced with the first report of K. pneumoniae ST258 in hospitals h1 and h2 in May-June 2008 (3). This organism belonged to PFGE subtype A1 and had plasmids of ∼40 kb (blaSHV-12), ∼110 kb (blaKPC-2 and blaTEM-1), and ∼200 kb. The following 30 K. pneumoniae ST258 isolates, collected by the end of 2008, exhibited 13 PFGE subtypes, six β-lactamase patterns, and all blaKPC plasmid types (∼10 S1 profiles), and only three isolates (two from h1) were like the original ones. It is likely that there were several occurrences of K. pneumoniae ST258 in Warsaw hospitals, possibly also before May 2008. The notable rate of asymptomatic carriage and the diagnostic problems and lack of awareness at the time could have caused KPC-producing organisms to be overlooked.
In 2008, the NRCST started promulgating practical knowledge of KPC-producing bacteria and subsequently published national guidelines for the laboratory detection and infection control (www.korld.edu.pl). The increased awareness but also the progressive spread resulted in the higher number of isolations in 2009 (86 isolates; 82 patients). A remarkable role has been played by Warsaw hospitals h3, h4, and h5, which have become sites where KPC-producing bacteria are endemic and export KPC strains to other places. It is hard to understand the situation in hospitals with several separate KPC cases (e.g., h1, h2, or h11) that could be due to new entries and/or “leaks” in infection control systems. Several institutions seem to have counteracted the KPC spread well as a result of strict precaution procedures implemented just after identification of the index case.
The analysis showed serious difficulties in tracking the dissemination of KPC-producing K. pneumoniae. Although most of the isolates (97.4%) belonged to the pandemic clone ST258, they revealed high diversity in PFGE subtypes, S1 profiles, blaKPC-carrying plasmids, and β-lactamase patterns. All these differences confused the matter; only in some cases were clear situations of ongoing spread (e.g., subtype A3 in h3 or A4 in h4 and h5) or interhospital transfer (A4 in h5 and h15) observed. In general, it was difficult to sort out whether an ST258 organism was introduced independently into a hospital or the country (for example, the A17 organism was introduced into Olsztyn, Poland, from New York) or evolved from another one at the site by changes in chromosomal and/or plasmid DNA. The ST258 intrinsic diversity has been observed in other studies; however, isolates with around ∼80% similarity in PFGE and significant differences in plasmids were collected over rather large areas with longer KPC history so far (14, 23). To our knowledge, the heterogeneity level described here has been extreme for the time scale and geographic scale of this study.
Plasmids with the blaKPC genes largely contributed to this diversity by size, structure, and transfer ability. The major group were the conjugative pKpQIL-type molecules (IncFIIK) originally identified in ST258 in Israel, dominating KPC-3-producing K. pneumoniae in that country (24, 25). By size one may suspect that such plasmids with blaKPC-2 or blaKPC-3 have been present in ST258 in other countries (36); moreover, plasmids identified by PBRT as with replicon FIIAS (10) might also belong to this group (primers for FIIAS amplify the pKpQIL FIIK repA). In Poland, the pKpQIL-like plasmids were observed in all the K. pneumoniae clones and K. oxytoca and showed variety in size and structure. Except for the isolate with blaKPC-3 from Olsztyn/New York, all the other isolates had blaKPC-2. This study revealed high plasticity of the pKpQIL-like plasmids and further proved their crucial role in the KPC spread, with no strict correlation with K. pneumoniae ST258 as reported (31).
Other prevalent plasmids were those of ∼50 kb, observed first in another study on five K. pneumoniae isolates from h4 and designated pETKp50 (44). Compared to pKpQIL, the pETKp50-like molecules were present in a more uniform group of isolates. Of six isolates tested, only two yielded transconjugants, and they were actually a pair of E. coli and K. pneumoniae from one patient. This result showed some conjugative potential for pETKp50 and might illustrate yet another case of in vivo transfer of a blaKPC plasmid (4, 17). Although ∼50-kb plasmids were found in other countries, it is difficult to compare these because of their nontypeability or lack of replicon data (10, 24). IncR plasmids with blaKPC-2 have been identified in the United States (27), but again the data reported do not allow us to compare these plasmids with the ∼48-kb blaKPC-3 plasmid from the unique A13 isolate. The presence of several blaKPC plasmid types was observed on a country or global scale (1, 10, 18, 24); however, to our knowledge, the molecules of ∼200 to ∼250 kb have been the largest ones identified so far. Again, it is hard to reveal how far this variety resulted from plasmid rearrangements during spread in Poland or from independent introductions of KPC-producing organisms. Most probably, the pKpQIL-like molecules illustrated both possibilities, and the presence of two blaKPC plasmids in some isolates was indicative of Tn4401 transposition between these plasmids.
KPC producers also varied by other acquired β-lactamases and plasmids. Similar to other countries (14, 21, 33, 36), many K. pneumoniae isolates (ST11 and ST258) produced SHV-12, encoded by genes carried on nontypeable plasmids of ∼40 kb. Specific to Poland was the presence of CTX-M-3, which was also observed in the earlier report (44). CTX-M-3, encoded by highly conjugative IncL/M plasmids of the pCTX-M3 family (15), has been the most widespread ESBL in the country (2, 13). Indeed, our results showed that pCTX-M3-like plasmids have already penetrated into KPC-producing K. pneumoniae ST11 and ST258 populations in several hospitals.
This has been one of the few reports showing a comprehensive view of the spread of KPC-producing K. pneumoniae on a country-wide scale (23, 24), with striking complexity considering its early stage. This study confirmed and expanded previous data on the two crucial factors of the “success” of KPC-producing K. pneumoniae, namely, the K. pneumoniae ST258 clone and the pKpQIL-like plasmids. The multidimensional heterogeneity of ST258, in the context of its global spread, causes difficulties in outbreak investigations and tracking KPC-producing organisms, indicating the need of more precise typing, as well as the superior role of detailed clinical-epidemiological data. In Europe, Poland is one of the countries most affected by KPC producers (19); the increased awareness shown by many hospital infection control teams offers hope that Poland will not become the next region where these alarming organisms are endemic.
Supplementary Material
ACKNOWLEDGMENTS
We are very thankful to Alessandra Carattoli and Izabela Kern-Zdanowicz for helpful consultations.
This work was partially financed by the grant Narodowy Program Ochrony Antybiotyków (NPOA) from the Polish Ministry of Health and grants SPUB MIKROBANK and 934/6. PR UE/2009/7 from the Polish Ministry of Science and Higher Education.
Footnotes
Supplemental material for this article may be found at http://aac.asm.org/.
Published ahead of print on 19 September 2011.
REFERENCES
- 1. Andrade L. N., et al. 2011. Dissemination of blaKPC-2 by the spread of Klebsiella pneumoniae clonal complex 258 clones (ST258, ST11, ST437) and plasmids (IncFII, IncN, IncL/M) among Enterobacteriaceae species in Brazil. Antimicrob. Agents Chemother. 55: 3579–3583 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Baraniak A., Fiett J., Sulikowska A., Hryniewicz W., Gniadkowski M. 2002. A countrywide spread of CTX-M-3 extended-spectrum β-lactamase (ESBL)-producing microorganisms of the family Enterobacteriaceae in Poland. Antimicrob. Agents Chemother. 46: 151–159 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Baraniak A., et al. 2009. The emergence of Klebsiella pneumoniae ST258 with KPC-2 in Poland. Antimicrob. Agents Chemother. 53: 4565–4567 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Barbier F., et al. 2010. Genesis of a KPC-producing Klebsiella pneumoniae after in vivo transfer from an imported Greek strain. Euro Surveill. 15: pii=19457. [DOI] [PubMed] [Google Scholar]
- 5. Bauernfeind A., Grimm H., Schweighart S. 1990. A new plasmidic cefotaximase in a clinical isolate of Escherichia coli. Infection 18: 294–298 [DOI] [PubMed] [Google Scholar]
- 6. Bush K. 2010. Alarming β-lactamase-mediated resistance in multidrug-resistant Enterobacteriaceae. Curr. Opin. Microbiol. 13: 558–564 [DOI] [PubMed] [Google Scholar]
- 7. Carattoli A., et al. 2005. Identification of plasmids by PCR-based replicon typing. J. Microbiol. Methods 63: 219–228 [DOI] [PubMed] [Google Scholar]
- 8. Clinical Laboratory Standards Institute 2011. Performance standards for antimicrobial susceptibility testing; twenty-first informational supplement. CLSI M100-S21 Clinical and Laboratory Standards Institute, Wayne, PA [Google Scholar]
- 9. Curiao T., et al. 2010. Emergence of blaKPC-3-Tn4401a associated with a pKPN3/4-like plasmid within ST384 and ST388 Klebsiella pneumoniae clones in Spain. J. Antimicrob. Chemother. 65: 1608–1614 [DOI] [PubMed] [Google Scholar]
- 10. Cuzon G., et al. 2010. Worldwide diversity of Klebsiella pneumoniae that produces β-lactamase blaKPC-2 gene. Emerg. Infect. Dis. 16: 1349–1356 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Diancourt L., Passet V., Verhoef J., Grimont P. A., Brisse S. 2005. Multilocus sequence typing of Klebsiella pneumoniae nosocomial isolates. J. Clin. Microbiol. 43: 4178–4182 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Doi Y., et al. 2008. Simple disk-based method for detection of Klebsiella pneumoniae carbapenemase-type β-lactamase by use of a boronic acid compound. J. Clin. Microbiol. 46: 4083–4086 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Empel J., et al. 2008. Molecular survey of β-lactamases conferring resistance to newer β-lactams in Enterobacteriaceae isolates from Polish hospitals. Antimicrob. Agents Chemother. 52: 2449–2454 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Endimiani A., et al. 2009. Characterization of blaKPC-containing Klebsiella pneumoniae isolates detected in different institutions in the Eastern USA. J. Antimicrob. Chemother. 63: 427–437 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Gołębiewski M., et al. 2007. Complete nucleotide sequence of the pCTX-M3 plasmid and its involvement in spread of the extended-spectrum β-lactamase (ESBL) gene blaCTX-M-3. Antimicrob. Agents Chemother. 51: 3789–3795 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Gootz T. D., et al. 2009. Genetic organization of transposase regions surrounding blaKPC carbapenemase genes on plasmids from Klebsiella strains isolated in a New York City hospital. Antimicrob. Agents Chemother. 53: 1998–2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Goren M. G., et al. 2010. Transfer of carbapenem-resistant plasmid from Klebsiella pneumoniae ST258 to Escherichia coli in patient. Emerg. Infect. Dis. 16: 1014–1017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Goren M. G., Navon-Venezia S., Chmelnitsky I., Carmeli Y. 2010. Carbapenem-resistant KPC-2-producing Escherichia coli in a Tel-Aviv Medical Center, 2005 to 2008. Antimicrob. Agents Chemother. 54: 2687–2691 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Grundmann H., et al. 2010. Carbapenem-non-susceptible Enterobacteriaceae in Europe: conclusions from a meeting of national experts. Euro Surveill. 15: pii=19711. [DOI] [PubMed] [Google Scholar]
- 20. Jiang Y., et al. 2010. Complete nucleotide sequence of Klebsiella pneumoniae multidrug resistance plasmid pKP048, carrying blaKPC-2, blaDHA-1, qnrB4, and armA. Antimicrob. Agents Chemother. 54: 3967–3969 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Kassis-Chikhani N., et al. 2010. Outbreak of Klebsiella pneumoniae producing KPC-2 and SHV-12 in a French hospital. J. Antimicrob. Chemother. 65: 1539–1540 [DOI] [PubMed] [Google Scholar]
- 22. Kitchel B., et al. 2010. Genetic factors associated with elevated carbapenem resistance in KPC-producing Klebsiella pneumoniae. Antimicrob. Agents Chemother. 54: 4201–4207 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Kitchel B., et al. 2009. Molecular epidemiology of KPC-producing Klebsiella pneumoniae isolates in the United States: clonal expansion of multilocus sequence type 258. Antimicrob. Agents Chemother. 53: 3365–3370 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Leavitt A., et al. 2010. Molecular epidemiology, sequence types, and plasmid analyses of KPC-producing Klebsiella pneumoniae strains in Israel. Antimicrob. Agents Chemother. 54: 3002–3006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Leavitt A., Chmelnitsky I., Carmeli Y., Navon-Venezia S. 2010. Complete nucleotide sequence of KPC-3-encoding plasmid pKpQIL in the epidemic Klebsiella pneumoniae sequence type 258. Antimicrob. Agents Chemother. 54: 4493–4496 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Lopez J. A., et al. 2011. Intercontinental spread from Israel to Colombia of a KPC-3-producing Klebsiella pneumoniae strain. Clin. Microbiol. Infect. 17: 52–56 [DOI] [PubMed] [Google Scholar]
- 27. Mataseje L. F., et al. 2011. Plasmid comparison and molecular analysis of Klebsiella pneumoniae harbouring blaKPC from New York City and Toronto. J. Antimicrob. Chemother. 66: 1273–1277 [DOI] [PubMed] [Google Scholar]
- 28. Munoz-Price L. S., Quinn J. P. 2009. The spread of Klebsiella pneumoniae carbapenemases: a tale of strains, plasmids and transposons. Clin. Infect. Dis. 49: 1739–1741 [DOI] [PubMed] [Google Scholar]
- 29. Naas T., et al. 2008. Genetic structures at the origin of acquisition of the β-lactamase blaKPC gene. Antimicrob. Agents Chemother. 52: 1257–1263 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Navon-Venezia S., et al. 2006. Plasmid-mediated imipenem-hydrolyzing enzyme KPC-2 among multiple carbapenem-resistant Escherichia coli clones in Israel. Antimicrob. Agents Chemother. 50: 3089–3101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Navon-Venezia S., et al. 2009. First report on a hyperepidemic clone of KPC-3-producing Klebsiella pneumoniae in Israel genetically related to a strain causing outbreaks in the United States. Antimicrob. Agents Chemother. 53: 818–820 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Nordmann P., Cuzon G., Naas T. 2009. The real threat of Klebsiella pneumoniae carbapenemase-producing bacteria. Lancet Infect. Dis. 9: 228–236 [DOI] [PubMed] [Google Scholar]
- 33. Pournaras S., et al. 2009. Clonal spread of KPC-2 carbapenemase-producing Klebsiella pneumoniae strains in Greece. J. Antimicrob. Chemother. 64: 348–352 [DOI] [PubMed] [Google Scholar]
- 34. Qi Y., et al. 2011. ST11, the dominant clone of KPC-producing Klebsiella pneumoniae in China. J. Antimicrob. Chemother. 66: 307–312 [DOI] [PubMed] [Google Scholar]
- 35. Queenan A. M., Bush K. 2007. Carbapenemases: the versatile β-lactamases. Clin. Microbiol. Rev. 20: 440–458 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Samuelsen Ø., et al. 2009. Emergence of clonally related Klebsiella pneumoniae isolates of sequence type 258 producing plasmid-mediated KPC carbapenemase in Norway and Sweden. J. Antimicrob. Chemother. 63: 654–658 [DOI] [PubMed] [Google Scholar]
- 37. Schwaber M., Carmeli Y. 2008. Carbapenem-resistant Enterobacteriaceae: a potential threat. JAMA 300: 2911–2913 [DOI] [PubMed] [Google Scholar]
- 38. Schwaber M. J., et al. 2011. Containment of a country-wide outbreak of carbapenem-resistant Klebsiella pneumoniae in Israeli hospitals via a nationally implemented intervention. Clin. Infect. Dis. 52: 848–855 [DOI] [PubMed] [Google Scholar]
- 39. Struelens M. J., et al. 1993. Pseudomonas aeruginosa and Enterobacteriaceae bacteremia after biliary endoscopy: an outbreak investigation using DNA macrorestriction analysis. Am. J. Med. 95: 489–498 [DOI] [PubMed] [Google Scholar]
- 40. Tenover F. C., et al. 1995. Interpreting chromosomal DNA restriction patterns produced by pulsed-field gel electrophoresis: criteria for bacterial strain typing. J. Clin. Microbiol. 33: 2233–2239 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Villa L., García-Fernández A., Fortini D., Carattoli A. 2010. Replicon sequence typing of IncF plasmids carrying virulence and resistance determinants. J. Antimicrob. Chemother. 65: 2518–2529 [DOI] [PubMed] [Google Scholar]
- 42. Wirth T., et al. 2006. Sex and virulence in Escherichia coli: an evolutionary perspective. Mol. Microbiol. 60: 1136–1151 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Yigit H., et al. 2001. Novel carbapenem-hydrolyzing β-lactamase, KPC-1, from a carbapenem-resistant strain of Klebsiella pneumoniae. Antimicrob. Agents Chemother. 45: 1151–1161 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Zacharczuk K., et al. 2011. Emergence of Klebsiella pneumoniae coproducing KPC-2 and 16S rRNA methylase ArmA in Poland. Antimicrob. Agents Chemother. 55: 443–446 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.