Abstract
Low antituberculosis (TB) drug levels are common, but their clinical significance remains unclear, and methods of measurement are resource intensive. Subjects initiating treatment for sputum smear-positive pulmonary TB were enrolled from Kibong'oto National TB Hospital, Tanzania, and levels of isoniazid, rifampin, ethambutol, and pyrazinamide were measured at the time of typical peak plasma concentration (C2 h). To evaluate the significance of the effect of observed drug levels on Mycobacterium tuberculosis growth, a plasma TB drug activity (TDA) assay was developed using the Bactec MGIT system. Time to detection of plasma-cocultured M. tuberculosis versus time to detection of control growth was defined as a TDA ratio. TDA assays were later performed using the subject's own M. tuberculosis isolate and C2 h plasma from the Tanzanian cohort and compared to drug levels and clinical outcomes. Sixteen subjects with a mean age of 37.8 years ± 10.7 were enrolled. Fourteen (88%) had C2 h rifampin levels and 11 (69%) had isoniazid levels below 90% of the lower limit of the expected range. Plasma spiked with various concentrations of antituberculosis medications found TDA assay results to be unaffected by ethambutol or pyrazinamide. Yet with a range of isoniazid and rifampin concentrations, TDA exhibited a statistically significant correlation with drug level and drug MIC, and a TDA of ∼1.0 indicated the presence of multidrug-resistant TB. In Tanzania, low (≤2.0) TDA was significantly associated with both lower isoniazid and rifampin C2 h levels, and very low (≤1.5) TDA corresponded to a trend toward lack of cure. Study of TDA compared to additional clinical outcomes and as a therapeutic management tool is warranted.
INTRODUCTION
Tuberculosis (TB) is the leading cause of death from a curable infectious disease worldwide, and resource-limited settings bear a disproportionate burden of TB prevalence and poor treatment outcomes (30). Even in TB patients receiving appropriate multidrug therapy, treatment outcomes can be poor due to immunosuppressive comorbidities, delayed presentation to medical care, and impaired adherence to treatment requirements but can also be secondary to inadequate pharmacotherapy. Peak plasma levels (estimated Cmax) of antituberculosis drugs below the expected range occur commonly in patients, and yet the exact role of low drug levels in treatment outcome is not fully understood (2, 3, 11, 13–15). Given that the majority of patients with TB reside in resource-limited settings, widespread application of drug level monitoring is impractical and too costly (2, 14). Thus, alternative means of identification of patients possibly at risk of poor treatment outcome due to low drug levels, and strategies to optimize existing drug regimens, are of critical research importance (6, 21, 22).
We therefore developed an assay that could potentially determine the impact of low drug levels and serve as an accessible clinical tool. The assay uses a patient's plasma or serum collected during TB treatment and the patient's own Mycobacterium tuberculosis isolate and measures time to detection in liquid culture. The principles of the assay are derived from prior study of serum bactericidal dilutions for the management of endocarditis (19). Results are reported as a ratio of the time to detection of plasma-cocultured M. tuberculosis to the time to detection of M. tuberculosis alone. We modeled the assay on the work by Wallis et al. in whole-blood culture studies (25–27) in which time to detection in the Bactec MGIT system (Becton Dickinson, Sparks, MD) is used in replacement of conventional colony counting. Others have found a 1-log decrement in levels of bacilli to be approximately equivalent to a 1.2- to 1.3-fold increase in time to detection (4, 18). We additionally chose the MGIT system for its ease of reproducibility and current WHO-endorsed suitability for scaling up for use in intermediate-volume laboratories in resource-limited areas. Furthermore, we utilized plasma or serum without leukocytes to constrain analysis to drug effects.
MATERIALS AND METHODS
Tanzania.
We first sought to describe the extent of low drug levels in a population of patients starting treatment in a setting of high TB prevalence and at relatively low risk of malabsorption. Subjects initiating TB treatment at Kibong'oto National TB Hospital (KNTH) in Kilimanjaro, Tanzania, were recruited for enrollment. Inclusion criteria specified subjects ≥ 18 years of age who had no prior TB treatment history, were HIV negative, and had newly diagnosed sputum smear-positive pulmonary TB. Per the KNTH protocol, all subjects received fixed-dose combination tablets that included isoniazid (INH) (75 mg), rifampin (RMP) (150 mg), ethambutol (EMB) (275 mg), and pyrazinamide (PZA) (400 mg) based on weight: for patients who weighed <50 kg, 3 tablets were administered; and for patients who weighed ≥50 kg, 4 tablets were administered. Subjects who had any recent history of nausea, vomiting, or diarrhea were excluded. Specimen processing and analysis were performed at Kilimanjaro Christian Medical College (KCMC) in Moshi, Tanzania. Written consent was obtained from all subjects, and protocols were approved by the Institutional Review Boards of Tumaini University at KCMC and the University of Virginia.
Prior to the initiation of TB treatment, sputum was collected for culture in the automated Bactec MGIT 960 system. Standard analyses of drug susceptibilities to INH, RMP, EMB, and streptomycin were performed with a Bactec SIRE kit to detect critical concentrations of INH (0.4 μg/ml), RMP (1.0), EMB (5.0), and streptomycin (1.0). Blood was collected at 14 days following TB treatment initiation and at 2 h after the observed administration of all antituberculosis medications. Subjects were served a meal of porridge approximately 1 h before medication administration per hospital routine. Blood was transported on ice to KCMC, where plasma was separated from the blood draw within 2 h and stored at −80°C for shipment to the Radboud University Nijmegen Medical Centre in the Netherlands, where high-performance liquid chromatography (HPLC) measurements for INH, RMP, EMB, and PZA were performed by validated methods. For INH, measurement of the acetyl-INH level was also performed to determine the acetylator phenotype. A plasma acetyl-INH/INH ratio > 1.0 was categorized as representing a “fast” metabolizer of INH and a ratio ≤ 1.0 as representing a “slow” metabolizer (22). An intermediate INH acetylator phenotype was not determined, as, when the assay had been performed previously, it had required blood draws at additional time points following dose administration. Drug levels were compared to established C2 h reference ranges and categorized as “low” if below 90% of the expected lower limit (12, 15). Subjects were reevaluated at 2 months and 6 months for sputum smear conversion, change in weight, subjective improvement based on assessment by a local TB clinician blinded to study results, and mortality. TB drug activity (TDA) testing was later performed at KCMC with the patient's own M. tuberculosis isolate and plasma.
TDA assay development.
Concentrations within and below the expected C2 h range for INH and RMP (Sigma-Aldrich, St. Louis, MO) were spiked into plasma of a healthy, tuberculin skin test-negative volunteer. Expected C2 h concentrations of EMB (MP Biomedicals, Solon, OH) and PZA (BD Diagnostic System, Sparks, MD) were also added for further comparisons. INH, EMB, and PZA were dissolved in sterile distilled water and RMP in dimethyl sulfoxide.
The M. tuberculosis isolates used in the study were H37Rv (ATCC 27294), two recent clinical isolates susceptible to INH, RMP, EMB and PZA, and two recent clinical isolates resistant to INH and RMP (referred to here as multidrug-resistant TB [MDR-TB] isolates). For susceptibility testing, a 1.0 McFarland suspension was made, and 10−2 and 10−4 dilutions were inoculated onto Middlebrook 7H10 agar with and without drug and incubated at 35°C. Conventional testing was carried out according to the 1% proportions method using established critical concentrations for INH, RMP, and EMB (31). Serial dilutions of INH, RMP, and EMB were used to establish the MIC, defined as the lowest concentration of drug that inhibited more than 99% of the bacterial population after 21 days from inoculation. PZA susceptibility testing was performed in PZA-specific Bactec MGIT media.
TDA assays were performed by adding a 500-μl suspension of a 10−1 dilution of 0.5 McFarland for each M. tuberculosis isolate to a 2-ml screw-top tube; the suspension was centrifuged at 12,000 rpm for 10 min at room temperature, the supernatant was removed, and 300 μl of PBS was added and followed by 300 μl of plasma. Tubes were incubated for 72 h at 37°C and then centrifuged at 12,000 rpm for 5 min, and the supernatant was removed. A 1-ml volume of sterile distilled water was then added with repeat vortexing, prior to a final centrifugation at 12,000 rpm for 10 min. The supernatant was discarded, 500 μl of Middlebrook 7H9–10% oleic acid-albumin-dextrose-catalase (OADC) was added, and the mixture was subjected to vortexing, transferred to a prefilled 7-ml MGIT tube, and incubated in the MGIT 320 machine until time to detection. For control tubes, identical 500-μl inocula were added for each of the isolates and incubated until the time to detection was determined. TDA was reported as the ratio of the time to detection of plasma-cocultured TB in hours to the time to detection of control. All experiments were approved by the Institutional Biosafety Committee of the University of Virginia.
Statistics.
For all analyses, means were compared using a t test or medians by a Mann-Whitney test for nonparametric data. All M. tuberculosis TDA cultures experiments were performed in duplicate or triplicate, except in Tanzania, where they were performed once. The correlation between TDA ratios and Cmax/MIC ratios for INH or RMP was calculated using the Pearson coefficient. TDA ratios were compared to clinical characteristics and outcomes in the Tanzanian cohort by χ2 or Fisher's exact testing when appropriate. All P values were from two-tailed tests.
RESULTS
Tanzania: plasma drug levels.
A total of 16 subjects with newly diagnosed smear-positive pulmonary TB were evaluated. All were inpatients and received directly observed medication administration. The mean patient age was 37.8 years ± 10.7, and 13 (81%) of the patients were male. The mean weight at the time of diagnosis was 48.8 kg ± 7.9. Eight (50%) of subjects were below 50 kg in weight and hence were prescribed 3 fixed-dose combination tablets. Drug susceptibility testing revealed 15 of the isolates from the subjects to be susceptible to INH, RMP, EMB, and streptomycin. One subject's isolate was resistant to EMB only.
HPLC testing demonstrated the majority of patient plasma samples to have very low levels of INH, RMP, and EMB (Table 1). Fourteen (88%) had low C2 h levels of RMP (expected C2 h range, 8 to 24 μg/ml), with a mean of 2.5 ± 2.9 μg/ml. Two patients had trace RMP levels below the limit of quantification. Eleven (69%) patients had low C2 h levels of INH (expected C2 h range, 3 to 5 μg/ml), with a mean of 1.85 μg/ml ± 1.3. Acetyl-INH testing revealed a fast acetylator phenotype in 6 (38%) patients. Of the patients with low C2 h levels of INH, 3 (27%) were fast acetylators compared to 1 (20%) of those with levels in the expected range (P = not significant [NS]). An additional 12 (75%) patients had low C2 h levels of EMB (expected C2 h range, 2 to 6 μg/ml), with a mean of 1.25 ± 0.8 μg/ml. Drug levels were not explained by the concentrations of medication administered. For instance, of the 8 patients with the highest-concentration RMP dose (≥11.0 mg/kg), 5 (63%) had RMP C2 h levels < 1 μg/ml.
Table 1.
C2 h levels of antituberculosis drugs and doses administered to Tanzanian subjects as fixed-dose tablet combinations at 14 days of treatment for pulmonary TBa
| Subject no. | Wt (kg) | INH concn (mg/kg) | C2 h INH | INH acetylator category | RMP concn (mg/kg) | C2 h RMP | EMB concn (mg/kg) | C2 h EMB | PZA concn (mg/kg) | C2 h PZA |
|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 52 | 5.8 | 2.5 | Slow | 11.5 | 7.9 | 21.1 | 2.9 | 30.7 | 33 |
| 2 | 41 | 5.5 | 1.3 | Slow | 11.0 | 0.19 | 20.1 | 0.70 | 29.3 | 17 |
| 3 | 45 | 5.0 | 2.8 | Fast | 10.0 | 7.8 | 18.3 | 2.5 | 26.7 | 38 |
| 4 | 66 | 4.5 | 0.45 | Fast | 9.1 | 0.17 | 16.6 | 0.53 | 24.2 | 17 |
| 5 | 49 | 4.6 | 3.6 | Slow | 9.2 | 6.1 | 16.8 | 2.1 | 24.5 | 32 |
| 6 | 41 | 5.5 | 1.2 | Slow | 11.0 | 0.17 | 20.1 | 0.66 | 29.3 | 20 |
| 7 | 40 | 5.6 | 0.5 | Fast | 11.3 | 0.48 | 20.6 | 0.76 | 30.0 | 20 |
| 8 | 51 | 5.8 | 0.25 | Slow | 11.8 | tr | 21.6 | 0.36 | 31.3 | 7.7 |
| 9 | 60 | 5.0 | 1.6 | Slow | 10.0 | 0.97 | 18.3 | 1.6 | 26.7 | 24 |
| 10 | 51 | 5.8 | 1.2 | Slow | 11.8 | 0.79 | 21.6 | 0.99 | 31.3 | 26 |
| 11 | 52 | 5.7 | 3.6 | Slow | 11.5 | 3.8 | 21.2 | 1.2 | 30.8 | 30 |
| 12 | 51 | 5.8 | 4.2 | Slow | 11.8 | 4.0 | 21.6 | 1.0 | 31.3 | 34 |
| 13 | 58 | 5.2 | 0.23 | Fast | 10.3 | tr | 19.0 | 0.55 | 27.6 | 8 |
| 14 | 42 | 5.4 | 1.9 | Slow | 10.7 | 0.75 | 19.6 | 1.0 | 28.6 | 19 |
| 15 | 37 | 6.1 | 3.2 | Slow | 12.1 | 6.2 | 22.3 | 2.5 | 32.4 | 36 |
| 16 | 45 | 5.0 | 1.1 | Slow | 10.0 | 0.23 | 18.3 | 0.71 | 26.7 | 17 |
The fixed-dose tablets administered included isoniazid (INH) (75 mg), rifampin (RMP) (150 mg), ethambutol (EMB) (275 mg), and pyrazinamide (PZA) (400 mg); for patients weighing <50 kg, 3 tablets were given, and for those weighing ≥50 kg, 4 tablets were given. Values shown in bold are within 90% of the expected C2 h range (reported in micrograms per milliliter). Expected C2 h range for INH, 3 to 5 μg/ml; for RMP, 8 to 24 μg/ml; for EMB, 2 to 6 μg/ml; for PZA, 20 to 50 μg/ml. INH acetylator phenotype values represent ratios of plasma levels of acetyl-isoniazid to those of isoniazid. Values greater than 1.0 were categorized as representing fast acetylation; values less than or equal to 1.0 were categorized as representing slow acetylation.
Development of the TDA assay.
We postulated that the TDA measurements would predominantly reflect the activity of INH and RMP, since pyrazinamide (PZA) is inactive at the pH of standard MGIT media and EMB is largely bacteriostatic (23). To examine this supposition, we compared the mean TDA for plasma without drug to the mean TDA for plasma spiked with EMB at 5 μg/ml (expected C2 h range, 2 to 6 μg/ml) and plasma spiked with PZA at 30 μg/ml (expected C2 h range, 20 to 50 μg/ml) (see Fig. S1 in the supplemental material). For the drug-susceptible clinical isolates (isolates 1 and 2) and H37Rv, the EMB MICs were 2.5 μg/ml for each. The mean TDA values were 0.99 ± 0.05 for plasma across all isolates, 1.16 ± 0.1 with the addition of EMB, and 0.9 ± 0.16 with the addition of PZA (P = NS).
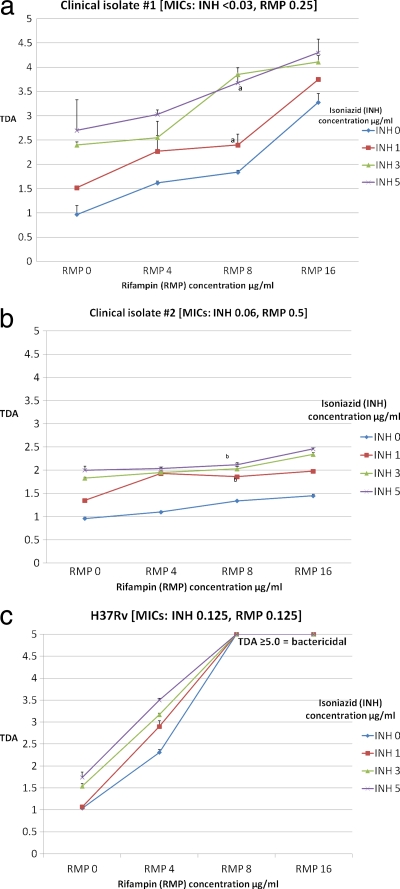
Given the finding of minimal to no activity of EMB and PZA, we sought to examine the effects of INH and RMP. Concentrations of INH (0 to 5 μg/ml) and RMP (0 to 16 μg/ml) starting from below the expected C2 h range to the highest concentration in the expected range were studied in checkerboard format (Fig. 1 ). A dose-response relationship was observed in which higher plasma concentrations of drug resulted in a measurable increase in TDA. Keeping a single drug concentration constant at the lower range of normal but increasing the level of the alternative drug produced a significantly increased TDA. However, the response attributable to INH or RMP was more pronounced in the isolates for which the MICs of those agents were lower (Fig. 1). For example, the mean change in TDA upon increasing the INH concentration from 0 to 5.0 μg/ml across fixed RMP concentrations was 1.48 ± 0.36 for isolate 1 (INH MIC, <0.03 μg/ml; RMP MIC, 0.25 μg/ml) but was only 0.95 ± 0.1 for isolate 2, which had a higher INH MIC (INH MIC, 0.06 μg/ml, RMP MIC, 0.5 μg/ml) (P = 0.03). The effect of altering the RMP concentration was more pronounced; the mean change in TDA resulting from increasing the RMP concentration from 0 to 16.0 μg/ml across fixed INH concentrations was 1.95 ± 0.35 for isolate 1 but was only 0.53 ± 0.15 for isolate 2, for which the RMP MIC was higher (P < 0.001). Indeed, combinations that included RMP at 8.0 μg/ml or greater were completely sterilizing for the H37Rv laboratory isolate that had the lowest RMP MIC (Fig. 1c). As predicted, there was no significant change in TDA for isolates 1 and 2 when EMB at 5 μg/ml was added to combinations of INH at 3 μg/ml plus RMP at 8 μg/ml or INH at 5 μg/ml plus RMP at 16 μg/ml (data not shown).
Fig. 1.
Comparison of anti-TB drug activities (TDA) of isoniazid and rifampin concentrations tested with the M. tuberculosis clinical isolates and laboratory strain H37Rv presented in checkerboard format. TDA assays were performed using M. tuberculosis isolates and volunteer plasma alone or plasma spiked with various concentrations of isoniazid (INH) or rifampin (RMP), including concentrations below the expected C2 h range (INH, 1 μg/ml; RMP, 4 μg/ml), in the low-normal range (INH, 3 μg/ml; RMP, 8 μg/ml), and in the high-normal range (INH, 5 μg/ml; RMP, 16 μg/ml). The expected Cmax range for INH was 3 to 5 μg/ml and for RMP was 8 to 24 μg/ml. TDA was reported as a ratio of time to positivity of plasma-cocultured M. tuberculosis versus time to detection of M. tuberculosis alone, where a TDA ratio of 1.0 indicates stasis, a TDA ratio of >1.0 indicates killing, and a TDA ratio of <1.0 indicates growth. All isolates were susceptible to INH and RMP according to results determined by the 1% proportions method. For the combination of INH at 1 μg/ml with RMP at 8 μg/ml and INH at 5 μg/ml with RMP at 8 μg/ml, the mean TDA levels were 2.55 ± 0.06 and 4.11 ± 0.18 for isolate 1 (P = 0.008) (panel a) and 1.86 ± 0.02 and 2.12 ± 0.06 for isolate 2 (P = 0.03) (panel b). For H37Rv, RMP concentrations of ≥ 8.0 μg/ml were sterilizing.
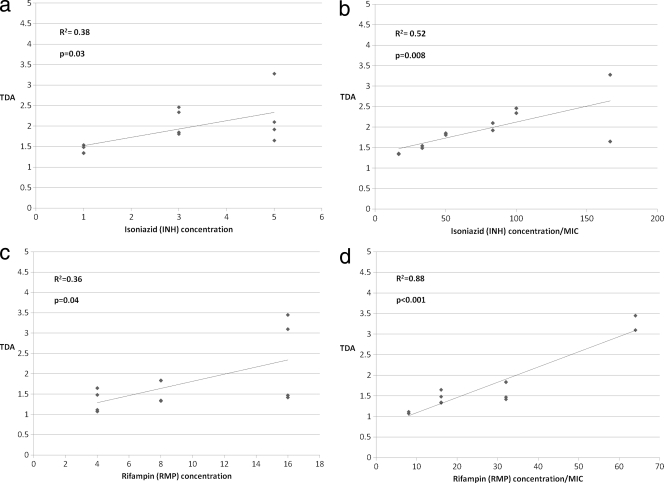
We then examined the extent to which TDA correlated with INH or RMP drug levels. Since both INH and RMP affect TDA, for this analysis we examined these drugs in isolation. While a statistically significant correlation between TDA and drug level was observed for both INH and RMP (Fig. 2a and c), the correlation was improved when TDA was analyzed against INH level/MIC and RMP level/MIC, respectively, and was highest for the latter (Fig. 2b and d). Thus, the TDA assay yielded a metric of both drug level and MIC, particularly for RMP level/MIC.
Fig. 2.
Comparison of TB drug activity (TDA) to isoniazid and rifampin drug concentrations and drug concentrations/MIC. TDA assays were performed using plasma spiked with isoniazid (INH) or rifampin (RMP) in isolation for clinical isolates. TDA was compared to INH concentration (a), INH concentration/MIC (b), RMP concentration (c), and RMP concentration/MIC (d). Correlation determinations were performed using the Pearson coefficient.
Tanzania: TDA assay and clinical outcomes.
We then sought to examine TDA for the 16 subjects from Tanzania on 4-drug therapy. TDA assays were performed onsite in Tanzania where MIC testing was not available. The mean time to detection for control tubes among all isolates was 146.4 ± 40.8 h, and the mean TDA ratio for all isolates was 1.9 ± 0.5 (range, 1.1 to 3.2). No patient's plasma was completely sterilizing, and low values were common. For instance, 5 (31%) subjects had a TDA of ≤1.5, which was clearly at the lowest end of our in vitro results.
In subjects with low (≤2.0) TDA, statistically lower mean concentrations of INH, RMP, and EMB were found compared to those with TDA > 2.0 (Table 2). Furthermore, in analyzing subjects with the lowest (≤1.5) TDA, 3 (60%) exhibited smear-negative results at 2 months compared with 10 (91%) subjects with TDA > 1.5 (P = 0.17); 2 (40%) were cured (sputum smear negative at 5 months and 6 months of medication completed), and 10 (91%) were cured (P = 0.06). Mean weight gain at 6 months was 5.6 ± 4.4 kgs versus 7.4 ± 4.5 in the two groups, respectively (P = 0.1), and mortality was 0% for the members of the cohort.
Table 2.
TB drug activity (TDA) values and C2 h drug levels at 14 days of TB treatment for Tanzanian patientsa
| Drug | Mean drug C2 h ± SD (μg/ml) |
P value | |
|---|---|---|---|
| TDA ≤ 2.0 (n = 9) | TDA > 2.0 (n = 7) | ||
| Isoniazid | 1.31 ± 1.2 | 2.56 ± 1.2 | 0.05 |
| Rifampin | 0.77 ± 1.3 | 4.65 ± 3.2 | 0.005 |
| Ethambutol | 0.83 ± 0.37 | 1.68 ± 0.93 | 0.03 |
| Pyrazinamide | 20.3 ± 7.3 | 28.0 ± 10.7 | 0.11 |
The plasma samples used for C2 h drug level and TDA measurements were from same blood draw. Comparisons of C2 h levels for isoniazid and rifampin were performed by t test.
TDA performance in subjects with MDR-TB.
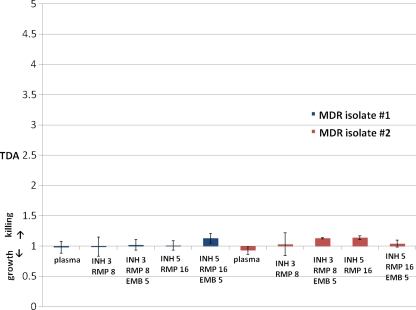
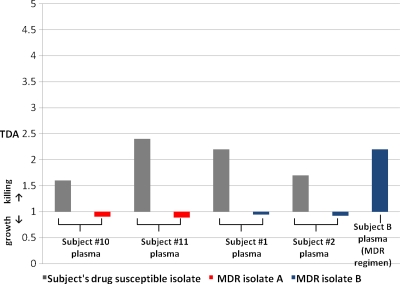
Given that the TDA assay yielded information on drug concentration/MIC, we predicted it would indicate MDR-TB, for which MICs are extremely high. In testing a range concentrations of INH and RMP in plasma with two MDR-TB isolates, TDA values were near 1.0 and were never observed above 1.19, even when EMB was added to EMB-susceptible MDR-TB isolate 1 (Fig. 3). In Tanzania, two locally identified strains of MDR-TB were used for testing plasma from four of the previously enrolled patients on a drug-susceptible regimen (Fig. 4). Each strain was susceptible to EMB. Plasma from two patients (patients 10 and 11) was tested using MDR-TB isolate A, and plasma from two other patients (patients 1 and 2) was tested using MDR-TB isolate B. These plasma samples exhibited a mean TDA of 0.92 ± 0.03 with the MDR-TB isolates versus 2.0 ± 0.38 with their own susceptible isolates (P < 0.001). The individual who produced MDR-TB isolate B was able to be subsequently enrolled while receiving ethambutol, pyrazinamide, amikacin, levofloxacin, and cycloserine, and his TDA on this regimen was 2.2 (Fig. 4).
Fig. 3.
TB drug activity (TDA) with plasma alone compared to concentrations of isoniazid, rifampin, and ethambutol for multidrug-resistant (MDR) M. tuberculosis isolates. TB drug activity (TDA) ratios were determined for volunteer plasma without drug versus plasma spiked with a various concentrations of isoniazid (INH), rifampin (RMP), and ethambutol (EMB) within the expected C2 h range. The drug MICs for MDR isolate 1 were as follows: INH, 32 μg/ml (100% resistant as determined by the proportions method); RMP, 32 μg/ml (100% resistant); EMB, 5 μg/ml (<1% susceptible). The drug MICs for MDR isolate 2 were as follows: INH, 16 μg/ml (63% resistant); RMP, 16 μg/ml (77% resistant); EMB, 10 μg/ml (27% resistant).
Fig. 4.
Use of TB drug activity (TDA) determinations in MDR-TB treatment. Plasma from subjects 1, 2, 10, and 11 under treatment for drug-susceptible TB with a regimen of isoniazid, rifampin, ethambutol, and pyrazinamide administration was tested using the TDA assay and recent MDR-TB isolates from Kibong'oto National TB Hospital in Tanzania. In addition to the initial 16 subjects with drug-susceptible TB, subject B produced MDR-TB isolate B and had a TDA assay performed while on an MDR-TB regimen of ethambutol, pyrazinamide, amikacin, levofloxacin, and cycloserine.
DISCUSSION
The major result of this work was the development of an assay that can provide individualized measurements of INH and RMP activity in TB patients during treatment. Importantly, low circulating drug levels of INH and RMP confer a cost of less killing that is quantifiable in vitro, particularly for isolates with higher MICs still considered susceptible by conventional testing. Therefore, TDA may provide an adjunctive tool to optimize TB therapy for certain patients.
The fact that TDA represents a metric whose determinations are largely constrained to INH and RMP activities in patients on a typical 4-drug regimen is in our view fortunate, since these are the medications most important in therapeutic outcome, most often continued throughout the entirety of the treatment course, and most likely to be dose adjusted. Indeed, in our experience, INH and RMP doses can be increased for low levels without toxicity and to within the expected range with a single adjustment, which may be of clinical benefit in patients with slow response to TB therapy (11). A growing body of evidence suggests that RMP in particular may be at the lower end of the dose-response curve, and reports are emerging that higher doses of RMP may be able to inhibit strains with low-level resistance (RMP MIC, ∼1.0 to 2.0 μg/ml) (5, 9, 20, 24). Determinations of TDA provide some substantiation for dose adjustment through enhanced killing and increases in Cmax/MIC, especially for strains that are highly INH or RMP susceptible.
Interestingly, TDA was integrally dependent upon the INH and RMP MICs for the M. tuberculosis isolate. While not surprising for conventionally “resistant” isolates, this result was not altogether obvious for isolates deemed “susceptible,” as low drug levels remain above the MIC. Thus, this work suggests a possible role for MIC testing of susceptible isolates, particularly when dose adjustment is being considered. As regimens based on higher dosess of RMP are being studied in clinical trials (24), MIC testing may aid interpretations of results.
Overall, the TDA values observed in this Tanzanian cohort were surprisingly low. Values reached as low as 1.1, which in vitro was akin to a subject having MDR-TB. The low TDA was presumably due to subjects having very low C2 h INH and RMP levels and is particularly worrisome, considering that potential subjects who had HIV or gastrointestinal symptoms that would have predisposed the patient to malabsorption were excluded (15, 28, 29). Few studies have examined drug levels in similar African settings, but in a cohort from Botswana that included HIV-infected patients, a similar proportion (88%) had low Cmax levels of RMP (3). Unfortunately, determining which subjects have low INH or RMP levels is not readily accomplished: in our study, a fast acetylator phenotype did not account for the majority of those with low INH levels, and there was no association with initial dosing concentrations for other medications based on standard fixed-dose combinations. These findings reinforce the need for implementation of individualized patient management tools such as TDA.
Additionally, we envision a role for TDA in the management of MDR-TB that necessitates further study. As there have been no randomized trials to guide the treatment of MDR-TB with currently available drugs (7) and as the optimal treatment duration is unknown (8), whether patients with favorable clinical improvement can be treated with a shorter course of drug administration has yet to be determined (1, 17). For a patient with known MDR-TB and limited therapeutic options, TDA may offer an indication of therapeutic activity. For instance, since MDR patient B had a respectable TDA level of 2.2 on the 5-drug regimen and we understand EMB and PZA to have little effect, TDA may represent the activity of other second-line drugs, such as the fluoroquinolones and aminoglycosides, with extracellular and concentration-dependent killing.
There were several limitations to this work. The Tanzanian cohort was not designed to discern a relationship between TDA and later clinical outcomes such as relapse and acquired drug resistance. While a trend toward a lack of cure at 6 months was found in subjects with the lowest TDA values, we speculate that repeated measurements might be more informative than a single measurement. Despite recent in vitro models and animal studies that suggest that the area under the concentration-time curve over 24 h in the steady state divided by the MIC (AUC/MIC) ratio may be the best pharmacodynamic index to explain RMP and INH activity, Cmax and AUC do correlate well (10). We also acknowledge that the use of C2 h plasma may not identify patients with delayed absorption and therefore that drug activity in some subjects may have been underestimated (15, 16). Food intake may have further delayed the Cmax for RMP and may explain why some subjects with very low C2 h drug levels were still able to give sputum smear-negative results at 2 months and improve symptomatically.
Furthermore, since TDA is particularly affected by certain drugs (INH and RMP), TDA determinations may underestimate the overall activity resulting from a treatment regimen and accordingly may not reveal intracellular killing levels as effectively as whole-blood culture (26). That said, we feel that erring on the side of underestimation is safer than the alternative. Additionally, TDA levels are dependent upon circulating drug levels, which may not represent the concentrations achieved at the predominant site of infection (32). In the absence of data on MIC values, it may be difficult to determine the predominant effect of either INH or RMP within the assay for any given patient. Yet among the members of the current cohort, no subject with a TDA value of <2.0 had an RMP level within the expected range, posing the clinical issue of whether all of the members of this at-risk subset should have their RMP doses increased. Finally, as in any study of M. tuberculosis culture, the populations of bacilli isolated may not have been representative of the entirety of the populations within the host, and thus TDA may select for the measurement of killing of subpopulations only in the rapid-growth phase.
Despite these caveats, there are precious few therapeutic management tools to bring to bear on treatment issues for individual TB patients; this is particularly so for those with poor treatment response. In this context, TDA assays are accessible to laboratories capable of liquid culture experiments and may offer a useful adjunct to standard testing.
Supplementary Material
ACKNOWLEDGMENTS
This work was supported by a National Institutes of Health grant (R01 AI093358 to E.R.H.), a National Institutes of Health/Fogarty training grant (D43 TW008270 to E.R.H. and G.S.K.), and the Virginia Department of Health.
We are grateful to Robert Wallis for initial guidance in assay development.
Footnotes
Supplemental material for this article may be found at http://aac.asm.org/.
Published ahead of print on 3 October 2011.
REFERENCES
- 1. Caminero J. A. 2006. Treatment of multidrug-resistant tuberculosis: evidence and controversies. Int. J. Tuberc. Lung Dis. 10:829–837 [PubMed] [Google Scholar]
- 2. Chang K. C., et al. 2008. Peak plasma rifampicin level in tuberculosis patients with slow culture conversion. Eur. J. Clin. Microbiol. Infect. Dis. 27:467–472 [DOI] [PubMed] [Google Scholar]
- 3. Chideya S., et al. 2009. Isoniazid, rifampin, ethambutol and pyrazinamide pharmacokinetics and treatment outcomes among predominately HIV-infected cohort of adults with tuberculosis from Botswana. Clin. Infect. Dis. 48:1685–1694 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Diacon A. H., et al. 2010. Time to detection of the growth of Mycobacterium tuberculosis in MGIT 960 for determining the early bactericidal activity of antituberculosis agents. Eur. J. Clin. Microbiol. Infect. Dis. 29:1561–1565 [DOI] [PubMed] [Google Scholar]
- 5. Diacon A. H., et al. 2007. Early bactericidal activity of high-dose rifampin in patients with pulmonary tuberculosis evidenced by positive sputum smears. Antimicrob. Agents Chemother. 51:2994–2996 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Dooley K. E., Chaisson R. E. 2009. Tuberculosis and diabetes mellitus: convergence of two epidemics. Lancet Infect. Dis. 9:737–746 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Espinal M., Farmer P. 2009. The Cambridge Declaration: towards clinical trials for drug-resistant tuberculosis. Int. J. Tuberc. Lung Dis. 13:1–2 [PubMed] [Google Scholar]
- 8. Gandhi N. R., et al. 2010. Multidrug-resistant and extensively drug-resistant tuberculosis: a threat to global control of tuberculosis. Lancet 375:1830–1843 [DOI] [PubMed] [Google Scholar]
- 9. Goutelle S., et al. 2009. Population modeling and Monte Carlo simulation study of the pharmacokinetics and antituberculosis pharmacodynamics of rifampin in lungs. Antimicrob. Agents Chemother. 53:2974–2981 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Hall R. G., Leff R. D., Gumbo T. 2009. Treatment of active pulmonary tuberculosis in adults: current standards and recent advances. Insights from the Society of Infectious Diseases Pharmacists. Pharmacotherapy 29:1468–1481 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Heysell S. K., Moore J. L., Keller S. J., Houpt E. R. 2010. Therapeutic drug monitoring among slow responders to tuberculosis therapy in a state control program, Virginia, USA. Emerg. Infect. Dis. 16:1546–1553 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Holland D. P., et al. 2009. Therapeutic drug monitoring of antimycobacterial drugs in patients with both tuberculosis and advanced human immunodeficiency virus infection. Pharmacotherapy 29:503–510 [DOI] [PubMed] [Google Scholar]
- 13. Mehta J. B., et al. 2001. Utility of rifampin blood levels in the treatment and follow-up of active pulmonary tuberculosis in patients who were slow to respond to routine directly observed therapy. Chest 120:1520–1524 [DOI] [PubMed] [Google Scholar]
- 14. Narita M., et al. 2001. Tuberculosis recurrence: multivariate analysis of serum levels of tuberculosis drugs, human immunodeficiency virus status, and other risk factors. Clin. Infect. Dis. 32:515–517 [DOI] [PubMed] [Google Scholar]
- 15. Peloquin C. A. 2002. Therapeutic drug monitoring in the treatment of tuberculosis. Drugs 62:2169–2183 [DOI] [PubMed] [Google Scholar]
- 16. Peloquin C. A., Namdar R., Singleton M. D., Nix D. E. 1999. Pharmacokinetics of rifampin under fasting conditions, with food, and with antacids. Chest 115:12–18 [DOI] [PubMed] [Google Scholar]
- 17. Pérez-Guzmán C., Vargas M. H., Martinez-Rossier L. A., Torres-Cruz A., Villarreal-Velarde H. 2002. Results of a 12-month regimen for drug-resistant pulmonary tuberculosis. Int. J. Tuberc. Lung Dis. 6:1102–1109 [PubMed] [Google Scholar]
- 18. Pheiffer C., et al. 2008. Time to detection of Mycobacterium tuberculosis in BACTEC systems as a viable alternative to colony counting. Int. J. Tuberc. Lung Dis. 12:792–798 [PubMed] [Google Scholar]
- 19. Reller L. B., Stratton C. W. 1977. Serum dilution test for bactericidal activity. II. Standardization and correlation with antimicrobial assays and susceptibility tests. J. Infect. Dis. 136:196–204 [DOI] [PubMed] [Google Scholar]
- 20. Ruslami R., et al. 2006. Evaluation of high- versus standard-dose rifampin in Indonesian patients with pulmonary tuberculosis. Antimicrob. Agents Chemother. 50:822–833 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Rylance J., Pai M., Lienhardt C., Garner P. 2010. Priorities for tuberculosis research: a systematic review. Lancet Infect. Dis. 10:886–892 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Seifart H. I., Parkin D. P., Botha F. J., Donald P. R., van der Walt B. J. 2001. Population screening for isoniazid acetylator phenotype. Pharmacoepidemiol. Drug Saf. 10:127–134 [DOI] [PubMed] [Google Scholar]
- 23. Srivastava S., et al. 2010. Efflux-pump-derived multiple drug resistance to ethambutol monotherapy in Mycobacterium tuberculosis and the pharmacokinetics and pharmacodynamics of ethambutol. J. Infect. Dis. 201:1225–1231 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. van Ingen J., et al. 2011. Why do we use 600 mg of rifampicin in tuberculosis treatment? Clin. Infect. Dis. 52:e194–e199 [DOI] [PubMed] [Google Scholar]
- 25. Wallis R. S., et al. 2001. A whole blood bactericidal assay for tuberculosis. J. Infect. Dis. 183:1300–1303 [DOI] [PubMed] [Google Scholar]
- 26. Wallis R. S., et al. 2003. Whole blood bactericidal activity during treatment of pulmonary tuberculosis. J. Infect. Dis. 187:270–278 [DOI] [PubMed] [Google Scholar]
- 27. Wallis R. S., Vinhas S., Janulionis E. 2009. Strain specificity of antimycobacterial immunity in whole blood culture after cure of tuberculosis. Tuberculosis 89:221–224 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Weiner M., et al. 2003. Low isoniazid concentrations and outcome of tuberculosis treatment with once-weekly isoniazid and rifapentine. Am. J. Respir. Crit. Care Med. 167:1341–1347 [DOI] [PubMed] [Google Scholar]
- 29. Weiner M., et al. 2005. Association between acquired rifamycin resistance and the pharmacokinetics of rifabutin and isoniazid among patients with HIV and tuberculosis. Clin. Infect. Dis. 40:1481–1491 [DOI] [PubMed] [Google Scholar]
- 30. World Health Organization 2009. (21 July 2010, accession date.) Global tuberculosis control: a short update to the 2009 report. http://www.who.int/tb/publications/global_report/2009/update/en/index.html World Health Organization, Geneva, Switzerland [Google Scholar]
- 31. World Health Organization 2008. Policy guidance on drug-susceptibility testing (DST) of second-line antituberculosis drugs. World Health Organization, Geneva, Switzerland: [PubMed] [Google Scholar]
- 32. Ziglam H. M., Baldwin D. R., Daniels I., Andrew J. M., Finch R. G. 2002. Rifampicin concentrations in bronchial mucosa, epithelial lining fluid, alveolar macrophages and serum following a single 600 mg oral dose in patients undergoing fibre-optic bronchoscopy. J. Antimicrob. Chemother. 50:1011–1015 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.