Abstract
Acinetobacter baumannii isolates T23, W35, and H1 were isolated from three patients who had been injured in the Haiti earthquake in January 2010. Those isolates, corresponding to two distinct clones, were identified as extended-spectrum β-lactamase (ESBL) producers and found to be blaCTX-M-15-positive. That ESBL gene was associated with ISEcp1, involved in its acquisition by a one-ended transposition mechanism. In all isolates, the ISEcp1-blaCTX-M-15 compound transposon was apparently chromosomally located.
TEXT
A growing number of β-lactamases conferring resistance to broad-spectrum cephalosporins have been identified in Acinetobacter spp. Extended-spectrum β-lactamase (ESBL) genes identified in those species are mostly of VEB or PER types but TEM, SHV, GES, and CTX-M derivatives have also been reported (1, 5, 10, 14). In particular, it is noteworthy that blaCTX-M genes that are so widespread in Enterobacteriaceae have been identified so rarely in Acinetobacter spp., with one CTX-M-2-producing Acinetobacter baumannii isolate in Japan (11) and a few CTX-M-15-producing A. baumannii isolates in India (19).
Acinetobacter baumannii isolates T23, H1, and W35 were obtained, respectively, from wound specimens (n = 2) and a rectal swab specimen (n = 1) from three victims of the earthquake that struck southern Haiti in January 2010 (8). Isolates H1 and W35 had been recovered in Haiti at the field hospital, whereas isolate T23 was obtained in Miami, FL, within 24 h of admission at the Jackson Hospital. Identification of those isolates at the species level was performed by using the API32GN system (bioMérieux, Marcy l'Etoile, France) and was confirmed by 16S rRNA gene sequencing as described previously (6). Susceptibility testing was performed at 37°C on Mueller-Hinton-containing plates by disk diffusion assay (Sanofi-Diagnostic Pasteur, Marnes-la-Coquette, France) and using the Etest technique (bioMérieux, Marcy l'Etoile, France), and results were interpreted in accordance with the guidelines of the CLSI (2). The three A. baumannii isolates were resistant to penicillins, broad-spectrum cephalosporins, and aztreonam but remained susceptible to carbapenems (2). Addition of clavulanic acid or tazobactam significantly decreased the MICs of amoxicillin, ticarcillin, and piperacillin, as well as ceftazidime and cefotaxime (Table 1). Synergies between ceftazidime and clavulanic acid suggested the production of an ESBL. In addition, two isolates (T23 and W35) showed resistance to fluoroquinolones, whereas isolate H1 remained susceptible. All isolates showed susceptibility to colistin (Table 1). A. baumannii W35 and T23 were susceptible to amikacin, and A. baumannii H1 was of intermediate susceptibility to amikacin (Table 1). Whole-cell DNA of A. baumannii isolates was extracted as described previously (13). Those DNAs were used as templates under standard PCR conditions (18) with a series of primers designed for the detection of class A β-lactamase genes (blaTEM, blaSHV, blaPER-1, blaVEB-1, blaGES-1, and blaCTX-M) (3, 16). Genes encoding CTX-M-type ESBL were detected in all three A. baumannii isolates, and sequencing identified blaCTX-M-15.
Table 1.
MICs of β-lactams for A. baumannii T23, W35, and H1 and E. coli TOP10(pT23), TOP10(pW35), TOP10(pH1), and the TOP10 reference straina
| β-Lactam(s)b | MIC (μg/ml) for: |
||||||
|---|---|---|---|---|---|---|---|
|
A. baumannii |
E. coli |
||||||
| T23 | W35 | H1 | TOP10(pT23) | TOP10(pW35) | TOP10(pH1) | TOP10 | |
| Amoxicillin | >256 | >256 | >256 | >256 | >256 | >256 | 2 |
| Amoxicillin + CLA | 16 | 16 | 16 | 8 | 8 | 8 | 2 |
| Ticarcillin | >256 | >256 | >256 | >256 | >256 | >256 | 2 |
| Ticarcillin + CLA | 32 | 32 | 32 | 32 | 32 | 32 | 2 |
| Piperacillin | >256 | >256 | >256 | >256 | >256 | >256 | 1 |
| Piperacillin + TZB | 2 | 2 | 2 | 2 | 2 | 2 | 1 |
| Cephalothin | >256 | >256 | >256 | >256 | >256 | >256 | 4 |
| Cefuroxime | >256 | >256 | >256 | >256 | >256 | >256 | 2 |
| Cefoxitin | 64 | 64 | 64 | 2 | 2 | 2 | 2 |
| Cefotaxime | >256 | >256 | >256 | 256 | 256 | 256 | 0.06 |
| Cefotaxime + CLA | 16 | 16 | 16 | 0.5 | 0.5 | 0.25 | 0.06 |
| Ceftazidime | 64 | 64 | 64 | 128 | 128 | 128 | 0.12 |
| Ceftazidime + CLA | 2 | 2 | 2 | 0.25 | 0.25 | 0.12 | 0.06 |
| Cefepime | >256 | >256 | >256 | 32 | 32 | 32 | 0.06 |
| Aztreonam | >256 | >256 | >256 | >256 | >256 | >256 | 0.03 |
| Meropenem | 0.5 | 0.5 | 0.5 | 0.03 | 0.03 | 0.03 | 0.03 |
| Imipenem | 0.25 | 0.25 | 0.25 | 0.25 | 0.25 | 0.25 | 0.12 |
| Colistin | 1 | 1 | 1 | 0.5 | 0.5 | 0.5 | 0.5 |
| Tigecycline | 0.5 | 0.5 | 0.25 | 0.25 | 0.25 | 0.25 | 0.25 |
| Amikacin | 4 | 4 | 16 | 1 | 1 | 1 | 1 |
| Rifampin | 2 | 2 | 2 | NDc | ND | ND | ND |
E. coli TOP10(pT23), TOP10(pW35), and TOP10(pH1), respectively, correspond to the E. coli TOP10 recombinant strains harboring plasmids containing inserts obtained from the A. baumannii T23, W35, and H1 isolates.
CLA, clavulanic acid (4 μg/ml); TZB, tazobactam (4 μg/ml).
ND, not determined.
Genotypic comparison was performed by multilocus sequence typing (MLST), sequence-type multiplex PCR, and pulsed-field gel electrophoresis (PFGE) according to the manufacturer's recommendations (Bio-Rad, Marnes-la-Coquette, France). PFGE analysis, performed as described previously (9), showed that two out of the three isolates (T23 and W35) were clonally related (and designated clone A), although isolate H1 corresponded to a distinct clone (clone B) (data not shown). The occurrence of the same clone in two different patients could result from a nosocomial transmission originally at the field hospital.
Further analysis with sequence-type multiplex PCR as described by Turton et al. (20) showed that clone A belonged to international clone II, whereas clone B did not correspond to any of the well-defined international clones I to III, amplicons being obtained with group I-specific ompA and blaOXA-51-like primers but with group II-specific csuE primers. The blaOXA-51-like gene identified in isolates T23 and W35 corresponded to blaOXA-180, whereas that identified in isolate H1 encoded a newly described point mutant derivative of OXA-64.
MLST analysis performed for each clone as described by Diancourt et al. (4) showed that clones A and B belonged to two new sequence types (STs) named ST117 (12-37-2-2-9-2-14) and ST118 (35-2-11-2-9-1-2), respectively (http://www.pasteur.fr/cgi-bin/genopole/PF8/mlstdbnet.pl?file=acin_isolates.xml). Those two novel STs are distantly related to all of the others known (no more than 4 out of the 7 alleles in common).
Transfer of the blaCTX-M-15 gene from the three A. baumannii isolates by conjugation or electroporation, as described previously (9), using rifampin-resistant A. baumannii BM45.47 as the recipient failed, suggesting a chromosomal location for the blaCTX-M-15 gene.
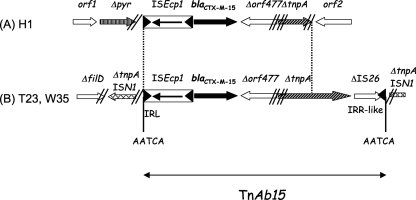
In order to further investigate the genetic support and environment of the blaCTX-M-15 gene in those isolates, shotgun cloning using HindIII-restricted genomic DNA and HindIII-restricted pACYC184 plasmid was performed as described previously (12). Recombinant plasmids were selected onto Trypticase soy agar (TSA) plates containing cefotaxime (10 μg/ml) and chloramphenicol (30 μg/ml). The resulting recombinant strains, Escherichia coli TOP10(pW35), TOP10(pT23), and TOP10(pH1), displayed an ESBL phenotype with high-level resistance to broad-spectrum cephalosporins and aztreonam, remaining susceptible to cefoxitin and carbapenems (Table 1). Sequencing of the insert of these three recombinant plasmids revealed that the entire ISEcp1 element was always present immediately upstream of the blaCTX-M-15 gene. The right boundary of ISEcp1 was located 48 bp upstream of the start codon of the blaCTX-M-15 gene in the three isolates, as previously described (7). The 701 bp located immediately downstream of the blaCTX-M-15 gene were identical in all three isolates, the first 345 bp corresponding to a truncated part of Orf477 previously described downstream of the chromosome-borne blaCTX-M-3 gene in the Kluyvera ascorbata progenitor (17). The last 356 bp corresponded to a Tn3 family transposase, which was truncated in A. baumannii H1 (Fig. 1). These observations further suggest that structures identified in A. baumannii derive from those identified in the progenitor. In A. baumannii T23 and W35, the whole sequence of the Tn3 family transposase was identified and was followed by a truncated part of IS26, as already found in the blaCTX-M-3-carrying plasmid pEK204 (22). The 14 bp located at the 3′ extremity of IS26 corresponded to an inverted repeat 2 (IRR2) sequence that could be used as a right inverted repeat (IRR) by ISEcp1, as demonstrated previously (15). This sequence shared 8 out of the 14 bp with the original IRR1 of ISEcp1. The identification of a 5-bp target site duplication (AATCA) at the 5′ extremity of ISEcp1 and at the right-hand extremity of the IS26 fragment was the signature of a transposition event and led us to identify a new compound transposon named TnAb15, comprising ISEcp1, blaCTX-M-15, Δorf477, the Tn3 family transposase, and a truncated IS26 (Fig. 1). This transposon was inserted into a gene encoding a transposase sharing 100% amino acid identity with that of ISN1 previously identified in A. baumannii AB0057 (accession no. NC_011586.1) (Fig. 1). An filD gene encoding a pilus assembly protein, sharing 100% amino acid identity with that identified in A. baumannii AYE (21), was identified upstream of the ISN1 transposase, further supporting chromosomal integration of the ISEcp1-blaCTX-M-15 transposon. In A. baumannii H1, transposon TnAb15 had been truncated at its right extremity, and no target site duplication was identified, suggesting some deletions/rearrangements (Fig. 1). However, this acquired genetic structure was inserted into a gene encoding a pyrimidine utilization transporter sharing significant similarity with that identified among A. baumannii genomes, again supporting a chromosomal location.
Fig. 1.
Schematic map of blaCTX-M-15-positive structures identified in A. baumannii isolates. (A) The structure identified in A. baumannii H1; (B) the structures from A. baumannii isolates T23 and W35. The genes and their corresponding transcriptional orientations are indicated by horizontal arrows. The AATCA target site duplications identified on both extremities of transposon TnAb15 are indicated. Sequences between vertical dashes are strictly identical. Δpyr corresponds to a truncated gene encoding a putative pyrimidine utilization transporter.
We report here three CTX-M-15-producing A. baumannii isolates. In all isolates, the blaCTX-M-15 gene was linked to ISEcp1, which might be responsible for its acquisition. ISEcp1 and blaCTX-M-15 were part of a transposon that integrated into the chromosome of A. baumannii through a likely transposition process. This corresponds to the second description of CTX-M-15-producing A. baumannii isolates after that reported in India (19). Despite the high prevalence of blaCTX-M-15 in Enterobacteriaceae, that gene is still rarely identified in A. baumannii. This is probably due to the fact that blaCTX-M-15-carrying plasmids, often of the IncF or IncH1 types, are of narrow host range and cannot replicate in A. baumannii. However, since ISEcp1 is a very efficient genetic tool in terms of gene mobilization, it is possible that integration of the ISEcp1-blaCTX-M-15 element into the chromosome of A. baumannii may have occurred.
The emergence and spread of ESBL-producing A. baumannii strains are worrisome, as this combination of events will enhance extensive use of carbapenems, thus increasing the risk of emergence of carbapenem-resistant isolates. In addition, it must be emphasized that detection of ESBL production by phenotypic methods is quite difficult in A. baumannii because of the frequent accumulation of enzymatic and nonenzymatic resistance mechanisms such as impermeability and efflux. Therefore, it could facilitate a “silent” spread of those particular isolates.
Nucleotide sequence accession number.
The nucleotide sequences data reported in this work have been deposited in the GenBank nucleotide database under accession no. JN788266 and JN788267.
Acknowledgments
This work was partially funded by a grant from the INSERM (U914) and by grants from the European Community (TROCAR, HEALTH-F3-2008-223031 and TEMPOtest-QC, HEALTH-2009-241742). It was also partially funded by an IISP Research Grant from Merck & Co., Inc.
Footnotes
Published ahead of print on 19 September 2011.
REFERENCES
- 1. Bonnin R. A., et al. 2011. PER-7, an extended-spectrum β-lactamase with increased activity toward broad-spectrum cephalosporins in Acinetobacter baumannii. Antimicrob. Agents Chemother. 55: 2424–2427 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Clinical and Laboratory Standards Institute 2011. Performance standards for antimicrobial susceptibility testing: 21st informational supplement. CLSI M100-S21. Clinical and Laboratory Standards Institute, Wayne, PA [Google Scholar]
- 3. Cuzon G., et al. 2010. Worldwide diversity of Klebsiella pneumoniae that produce β-lactamase blaKPC-2 gene. Emerg. Infect. Dis. 16: 1349–1356 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Diancourt L., Passet V., Nemec A., Dijkshoorn L., Brisse S. 2010. The population structure of Acinetobacter baumannii: expanding multiresistant clones from an ancestral susceptible genetic pool. PLoS One 5: e10034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Endimiani A., et al. 2007. Spread in an Italian hospital of a clonal Acinetobacter baumannii strain producing the TEM-92 extended-spectrum β-lactamase. Antimicrob. Agents Chemother. 51: 2211–2214 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Ibrahim A., Gerner-Smidt P., Liesack W. 1997. Phylogenetic relationship of the twenty-one DNA groups of the genus Acinetobacter as revealed by 16S ribosomal DNA sequence analysis. Int. J. Syst. Bacteriol. 47: 837–841 [DOI] [PubMed] [Google Scholar]
- 7. Lartigue M.-F., Poirel L., Nordmann P. 2004. Diversity of genetic environment of genes. FEMS Microbiol. Lett. 234: 201–207 [DOI] [PubMed] [Google Scholar]
- 8. Lichtenberger P., et al. 2010. Infection control in field hospitals after a natural disaster: lessons learned after the 2010 earthquake in Haiti. Infect. Control Hosp. Epidemiol. 31: 951–957 [DOI] [PubMed] [Google Scholar]
- 9. Mugnier P., Poirel L., Pitout M., Nordmann P. 2008. Carbapenem-resistant and OXA-23-producing Acinetobacter baumannii isolates in the United Arab Emirates. Clin. Microbiol. Infect. 14: 879–882 [DOI] [PubMed] [Google Scholar]
- 10. Naas T., Namdari F., Réglier-Poupet H., Poyart C., Nordmann P. 2007. Panresistant extended-spectrum β-lactamase SHV-5-producing Acinetobacter baumannii from New York City. J. Antimicrob. Chemother. 60: 1174–1176 [DOI] [PubMed] [Google Scholar]
- 11. Nagano N., Nagano Y., Cordevant C., Shibata N., Arakawa Y. 2004. Nosocomial transmission of CTX-M-2 β-lactamase-producing Acinetobacter baumannii in a neurosurgery ward. J. Clin. Microbiol. 42: 3978–3984 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Nordmann P., et al. 1993. Characterization of a novel extended-spectrum β-lactamase from Pseudomonas aeruginosa. Antimicrob. Agents Chemother. 37: 962–969 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Philippon L. N., Naas T., Bouthors A. T., Barakett V., Nordmann P. 1997. OXA-18, a class D clavulanic-acid inhibited extended-spectrum β-lactamase from Pseudomonas aeruginosa. Antimicrob. Agents Chemother. 41: 2188–2195 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Poirel L., Bonnin R. A., Nordmann P. Genetic basis of antibiotic resistance in pathogenic Acinetobacter species. IUBMB Life, in press. [DOI] [PubMed] [Google Scholar]
- 15. Poirel L., Lartigue M.-F., Decousser J.-W., Nordmann P. 2005. ISEcp1B-mediated transposition of blaCTX-M in Escherichia coli. Antimicrob. Agents Chemother. 49: 447–450 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Poirel L., Le Thomas I., Naas T., Karim A., Nordmann P. 2000. Biochemical sequence analyses of GES-1, a novel class A extended-spectrum-β-lactamase, and the class 1 integron In52 from Klebsiella pneumoniae. Antimicrob. Agents Chemother. 44: 622–632 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Rodriguez M. M., et al. 2004. Chromosome-encoded CTX-M-3 from Kluyvera ascorbata: a possible origin of plasmid-borne CTX-M-1-derived cefotaximases. Antimicrob. Agents Chemother. 48: 4895–4897 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Sambrook J., Fritsch E. F., Maniatis T. 1989. Molecular cloning: a laboratory manual, 2nd ed. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, NY [Google Scholar]
- 19. Shakil S., Khan A. U. 2010. Detection of CTX-M-15-producing and carbapenem-resistant Acinetobacter baumannii strains from urine from an Indian hospital. J. Chemother. 22: 324–327 [DOI] [PubMed] [Google Scholar]
- 20. Turton J. F., Gabriel S. N., Valderrey C., Kaufmann M. E., Pitt T. L. 2007. Use of sequence-based typing and multiplex PCR to identify clonal lineages of outbreak strains of Acinetobacter baumannii. Clin. Microbiol. Infect. 13: 807–815 [DOI] [PubMed] [Google Scholar]
- 21. Vallenet D., et al. 2008. Comparative analysis of Acinetobacter: three genomes for three lifestyles. PLoS One 3: e1805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Woodford N., et al. 2009. Complete nucleotide sequences of plasmids pEK204, pEK499, and pEK516, encoding CTX-M enzymes in three major Escherichia coli lineages from the United Kingdom, all belonging to the international O25:H4-ST131 clone. Antimicrob. Agents Chemother. 53: 4472–4482 [DOI] [PMC free article] [PubMed] [Google Scholar]