Abstract
Type III secretion systems (T3SSs) secrete needle components, pore-forming translocators, and the translocated effectors. In part, effector recognition by a T3SS involves their N-terminal amino acids and their 5′ mRNA. To investigate whether similar molecular constraints influence translocator secretion, we scrutinized this region within YopD from Yersinia pseudotuberculosis. Mutations in the 5′ end of yopD that resulted in specific disruption of the mRNA sequence did not affect YopD secretion. On the other hand, a few mutations affecting the protein sequence reduced secretion. Translational reporter fusions identified the first five codons as a minimal N-terminal secretion signal and also indicated that the YopD N terminus might be important for yopD translation control. Hybrid proteins in which the N terminus of YopD was exchanged with the equivalent region of the YopE effector or the YopB translocator were also constructed. While the in vitro secretion profile was unaltered, these modified bacteria were all compromised with respect to T3SS activity in the presence of immune cells. Thus, the YopD N terminus does harbor a secretion signal that may also incorporate mechanisms of yopD translation control. This signal tolerates a high degree of variation while still maintaining secretion competence suggestive of inherent structural peculiarities that make it distinct from secretion signals of other T3SS substrates.
INTRODUCTION
A wide variety of Gram-negative bacteria utilize type III secretion systems (T3SSs) to interact with diverse hosts such as humans, animals, plants, fish, and insects (58, 76). Inherent in this host interaction strategy is a multicomponent protein assembly spanning the bacterial envelope that is coupled to an extracellular protruding needle-like appendage. When in contact with eukaryotic cells, this injection device has the capacity to translocate an extensive array of protein cargo from the bacterial cytoplasm and/or the bacterial surface directly into the target cell interior (3, 63). Internalized bacterial proteins dismantle the inner processes of the host cell, creating a more hospitable environment for bacterial survival and colonization. Laboratory-grown bacteria can also use their T3SSs to secrete proteins into the extracellular milieu (31).
In general, three types of protein substrate are secreted by a T3SS: components of the external needle, the translocated effectors, and the translocator proteins (58, 76). The latter proteins are essential for the translocation process and form at the needle tip, a pore-like translocon in the eukaryotic cell plasma membrane (53). These pores may therefore complete an uninterrupted type III secretion (T3S) channel that links the bacterial interior to that of the eukaryotic cell. Although direct experimental evidence is lacking, it is possible that effectors pass through this translocon conduit to localize inside the eukaryotic cell.
Multiple T3S signals for effector substrates are evident. Most effectors require low-molecular-weight chaperones for their stability and/or efficient secretion (26). Some of these chaperones are known to interact with the T3S ATPase energizer at the cytoplasmic base of the T3SS (2, 32). A chaperone-independent secretion signal also exists at the extreme N terminus, represented by a complex combination of the mRNA with the protein sequence (16, 46, 69). While no sequence consensus is visually obvious, there is some evidence of an amphipathic property (47), and various computational approaches based on sophisticated machine-learning methodology can predict T3S substrates on the basis of a conserved secretion signal (6, 48, 64, 84). Nevertheless, the molecular contribution these extensively mapped chaperone-independent signals make to substrate secretion is not yet understood. However, it must be universally recognized, considering that T3SSs are promiscuous, often allowing the secretion of nonnative substrates.
N-terminal secretion signals of the translocator proteins are considerably less defined. Perhaps this putative secretion signal is unique, allowing the T3SS to distinguish translocator cargo from effector cargo (67). A secretion signal of SipB from Salmonella enterica serovar Typhimurium lies between residues 3 and 8 of the N terminus (41). Polar residues in the extreme N terminus contribute to the secretion of IpaC by Shigella flexneri (35). Moreover, secretion of LcrV by Yersinia requires information located between residues 2 and 4 and residues 11 and 13 (12). At the least, these data indicate the existence of an N-terminal chaperone-independent signal for the translocators that is reminiscent of the well-studied effector N-terminal secretion signal. Furthermore, the respective signals are interchangeable without apparent loss of biological function (54).
This study was designed to extend our knowledge of the translocator N terminus by investigating what role this domain plays in the activity of the YopD translocator from Y. pseudotuberculosis. This 306-amino-acid protein possesses multiple functions critical for the Yersinia Ysc-Yop T3S. In the Yersinia cytoplasm, YopD stability depends on an interaction with its customized T3S chaperone, LcrH (20, 27, 79). YopD-LcrH complexes cooperate with the LcrQ regulatory element to bind the 5′ untranslated regions (UTRs) of yop mRNA and impose posttranscriptional silencing of Yop synthesis by blocking translation and/or promoting degradation of mRNA (4, 13). Upon secretion, YopD forms pores in the infected cell plasma membrane through which the effectors might gain access to the host cell interior (34, 55, 57, 72). This extracellular function depends on self-assembly and additional interactions with LcrV and YopB (17). Thus, a ΔyopD null mutant is deregulated for Yop synthesis and, although Yop secretion functions normally, Yop delivery into cells is completely abolished (29, 36, 62, 81). Despite this knowledge of YopD function, information about the chaperone-dependent and -independent signals actually needed for YopD secretion is still lacking. To amend this, a series of N-terminal substitution and deletion mutations, as well as translational reporter fusions, were used to investigate the N-terminal signal for YopD secretion. Our data suggest that as few as five N-terminal residues are sufficient for T3S of YopD. Of importance among these are the isoleucines at positions 3 and 5 or their corresponding codons ATA and ATC. Only a few of the many mutations actually impinged on YopD secretion, suggesting that the molecular framework of the YopD N-terminal secretion signal is extremely robust and capable of tolerating a remarkable degree of physiochemical alteration. Effector translocation was seldom compromised, also suggesting that the N terminus is not required for the extracellular function of YopD. Interestingly, YopD synthesis was diminished in some key variants, indicating a possible role for some aspect of the 5′ end of yopD in translation control. Moreover, domain-swapping experiments involving the N-terminal secretion signals of YopD and the YopE effector molecule compromised T3SS activity. This may indicate that the N-terminal secretion signal has evolved specifically for and functions best solely for their substrate, ensuring timely delivery and/or function of Yops during intimate bacterium-host cell contact.
MATERIALS AND METHODS
Bacterial strains and growth conditions.
Strains used in this study are listed in Table 1. Routine bacterial culturing of Escherichia coli and Y. pseudotuberculosis was performed at 37°C and 26°C, respectively, typically in Luria Bertani (LB) broth. For determinations of protein expression and secretion from Yersinia, strains were grown in brain heart infusion (BHI) broth, in the absence (BHI supplemented with 5 mM EGTA, 20 mM MgCl2 [T3S-permissive medium]) or presence (2.5 mM CaCl2 [T3S-nonpermissive medium]) of calcium. Under both sets of conditions, bacteria were grown in the presence of 0.025% (vol/vol) Triton X-100. This treatment detached Yops prone to associate to the bacterial surface (3), thereby ensuring that our T3S analysis would include all Yops secreted beyond the bacterial envelope. When appropriate, antibiotics at the following concentrations were used to select for plasmid maintenance during culturing: carbenicillin (Cb) at 100 μg/ml, chloramphenicol (Cm) at 25 μg/ml, and kanamycin (Km) at 50 μg/ml. The plasmids are listed in Table S1 in the supplemental material.
Table 1.
Bacterial strains used in this study
| Strain | Genotype or phenotypea | Source or reference |
|---|---|---|
| Escherichia coli | ||
| DH5 | F−recA1 endA1 hsdR17 supE44 thi-1 gyrA96 relA1 | Vicky Shingler |
| S17-1λpir | recA thi pro hsdR(r− m+) Smr [RP4:2-Tc:Mu:Ku:Tn7] Tpr | 65 |
| TOP10 | F−mcrA Δ(mrr-hsdRMS-mcrBC) φ80lacZΔM15 ΔlacX74 recA1 araD139 (Δara-leu)7697 galU galK rpsL (Smr) endA1 nupG | Invitrogen |
| Yersinia pseudotuberculosis | ||
| YPIII/pIB102 | yadA::Tn5, Kmr (parent) | Hans Wolf-Watz |
| YPIII/pIB619 | pIB102, yopB and yopD in-frame deletion, Kmr | 20 |
| YPIII/pIB619-75 | pIB619, yscU in-frame deletion of codons 25 to 329, Kmr | This study |
| YPIII/pIB75 | pIB102, yscU in-frame deletion, Kmr | 45 |
| YPIII/pIB75-26 | pIB75, lcrQ in-frame deletion, Spr Kmr | This study |
| YPIII/pIB522 | pIB102, yopE in-frame deletion, Kmr | 62 |
| YPIII/pIB522-625 | pIB522, yopD in-frame deletion of codons 4 to 20, Kmr | 57 |
| YPIII/pIB625 | pIB102, yopD in-frame deletion of codons 4 to 20, Kmr | 57 |
| YPIII/pIB577 | pIB102, yopE harboring the YopD secretion signal between codons 2 and 15, Kmr | This study |
| YPIII/pIB578 | pIB102, yopE harboring the YopH secretion signal between codons 2 and 15, Kmr | This study |
| YPIII/pIB62501 | pIB625, yopD harboring the YopE secretion signal between codons 2 and 15, Kmr | This study |
| YPIII/pIB62501-577 | pIB62501, yopE harboring the YopD secretion signal between codons 2 and 15, Kmr | This study |
| YPIII/pIB62502 | pIB625, yopD with a +1 frameshift mutation after codon 1, Kmr | This study |
| YPIII/pIB62503 | pIB625, yopD with a −1 frameshift mutation after codon 3, Kmr | This study |
| YPIII/pIB62304 | pIB625, yopD with several wobble-base mutations between codons 2 and 16 altering only the mRNA sequence, Kmr | This study |
| YPIII/pIB62505 | pIB625, yopD in-frame deletion of codons 2 and 3, Kmr | This study |
| YPIII/pIB62506 | pIB625, yopD in-frame deletion of codons 4 and 5, Kmr | This study |
| YPIII/pIB62507 | pIB625, yopD in-frame deletion of codons 6 and 7, Kmr | This study |
| YPIII/pIB62508 | pIB625, yopD in-frame deletion of codons 8 and 9, Kmr | This study |
| YPIII/pIB62509 | pIB625, yopD in-frame deletion of codons 10 and 11, Kmr | This study |
| YPIII/pIB62510 | pIB625, yopD in-frame deletion of codons 12 and 13, Kmr | This study |
| YPIII/pIB62511 | pIB625, yopD in-frame deletion of codons 14 and 15, Kmr | This study |
| YPIII/pIB62512 | pIB625, yopD in-frame deletion of codons 16 and 17, Kmr | This study |
| YPIII/pIB62513 | pIB625, yopD in-frame deletion of codons 18 and 19, Kmr | This study |
| YPIII/pIB62514 | pIB625, yopD with a synthetic hypothetical 7-codon high-secretion signal, Kmr | This study |
| YPIII/pIB62537 | pIB625, yopD with a synthetic hypothetical duplicated 7-codon high-secretion signal, Kmr | This study |
| YPIII/pIB62515 | pIB625, yopD with a synthetic hypothetical 7-codon low-secretion signal, Kmr | This study |
| YPIII/pIB62538 | pIB625, yopD with a synthetic hypothetical duplicated 7-codon low-secretion signal, Kmr | This study |
| YPIII/pIB62524 | pIB625, yopD in-frame deletion of codons 5 to 19, Kmr | This study |
| YPIII/pIB62525 | pIB625, yopD in-frame deletion of codons 6 to 19, Kmr | This study |
| YPIII/pIB62526 | pIB625, yopD in-frame deletion of codons 7 to 19, Kmr | This study |
| YPIII/pIB62527 | pIB625, yopD in-frame deletion of codons 8 to 19, Kmr | This study |
| YPIII/pIB62528 | pIB625, yopD in-frame deletion of codons 9 to 19, Kmr | This study |
| YPIII/pIB62529 | pIB625, yopD in-frame deletion of codons 10 to 19, Kmr | This study |
| YPIII/pIB62530 | pIB625, yopD in-frame deletion of codons 11 to 19, Kmr | This study |
| YPIII/pIB62531 | pIB625, yopD in-frame deletion of codons 12 to 19, Kmr | This study |
| YPIII/pIB62532 | pIB625, yopD in-frame deletion of codons 13 to 19, Kmr | This study |
| YPIII/pIB62533 | pIB625, yopD in-frame deletion of codons 14 to 19, Kmr | This study |
| YPIII/pIB62534 | pIB625, yopD in-frame deletion of codons 15 to 19, Kmr | This study |
| YPIII/pIB62535 | pIB625, yopD in-frame deletion of codons 16 to 19, Kmr | This study |
| YPIII/pIB62536 | pIB625, yopD in-frame deletion of codons 17 to 19, Kmr | This study |
| YPIII/pIB62549 | pIB625, yopD in-frame deletion of codons 2 to 4, Kmr | This study |
| YPIII/pIB62550 | pIB625, yopD in-frame deletion of codons 2 to 5, Kmr | This study |
| YPIII/pIB62544 | pIB625, yopD in-frame deletion of codons 3 to 4, Kmr | This study |
| YPIII/pIB62555 | pIB625, yopD in-frame deletion of codons 3 to 5, Kmr | This study |
| YPIII/pIB62561 | pIB625, yopD in-frame deletion of codons 3 to 6, Kmr | This study |
| YPIII/pIB62556 | pIB625, yopD in-frame deletion of codons 3 to 7, Kmr | This study |
| YPIII/pIB62562 | pIB625, yopD in-frame deletion of codons 5 and 6, Kmr | This study |
| YPIII/pIB62551 | pIB625, yopD in-frame deletion of codons 5 to 7, Kmr | This study |
| YPIII/pIB62549 | pIB625, yopD in-frame deletion of codons 5 to 8, Kmr | This study |
| YPIII/pIB62563 | pIB625, yopD in-frame deletion of codons 5 to 9, Kmr | This study |
| YPIII/pIB62545 | pIB625, yopD in-frame deletion of codons 5 to 10, Kmr | This study |
| YPIII/pIB62560 | pIB625, yopD in-frame deletion of codons 6 to 10, Kmr | This study |
| YPIII/pIB62557 | pIB625, yopD in-frame deletion of codons 7 to 10, Kmr | This study |
| YPIII/pIB62565 | pIB625, yopD in-frame deletion of codons 8 to 10, Kmr | This study |
| YPIII/pIB62552 | pIB625, yopD in-frame deletion of codons 9 and 10, Kmr | This study |
| YPIII/pIB62546 | pIB625, yopD encoding the substitution of T2G, Kmr | This study |
| YPIII/pIB62547 | pIB62503, yopD with a −1 frameshift mutation after codon 3 and a substitution of T2G, Kmr | This study |
| YPIII/pIB62539 | pIB625, yopD encoding the substitution of T2N, Kmr | This study |
| YPIII/pIB62540 | pIB62503, yopD with a −1 frameshift mutation after codon 3 and a substitution of T2N, Kmr | This study |
| YPIII/pIB62554 | pIB625, yopD encoding the substitution of T2K, Kmr | This study |
| YPIII/pIB62558 | pIB62503, yopD with a −1 frameshift mutation after codon 3 and a substitution of T2K, Kmr | This study |
| YPIII/pIB62541 | pIB625, yopD encoding the substitution of I3G, Kmr | This study |
| YPIII/pIB62553 | pIB62503, yopD with a −1 frameshift mutation after codon 3 and a substitution of I3G, Kmr | This study |
| YPIII/pIB62548 | pIB625, yopD encoding the substitution of I3N, Kmr | This study |
| YPIII/pIB62569 | pIB62503, yopD with a −1 frameshift mutation after codon 3 and a substitution of I3N, Kmr | This study |
| YPIII/pIB62542 | pIB625, yopD encoding the substitution of I3K, Kmr | This study |
| YPIII/pIB62543 | pIB62503, yopD with a −1 frameshift mutation after codon 3 and a substitution of I3K, Kmr | This study |
| YPIII/pIB62559 | pIB625, yopD encoding the substitutions of I3N and I5N, Kmr | This study |
| YPIII/pIB62564 | pIB62503, yopD with a −1 frameshift mutation after codon 3 and the substitutions of I3N and I4N, Kmr | This study |
| YPIII/pIB62577 | pIB625, yopD harboring the YopB secretion signal between codons 2 and 15, Kmr | This study |
Tp, trimethoprim; Sp, spectinomycin; Sm, streptomycin.
Mutant construction.
N-terminal YopD variants were created by the overlap PCR method using the various primer pairs listed in Table S2 in the supplemental material. PCR fragments were cloned directly into pCR4-TOPO (Invitrogen), and each mutation was confirmed by sequence analysis (Eurofins MWG Operon, Ebersberg, Germany). Confirmed DNA fragments were then added to the pDM4 suicide mutagenesis vector (52) following XhoI-XbaI restriction. E. coli S17-1λpir strains harboring the different mutagenesis constructs were used as the donor strains in conjugations with Y. pseudotuberculosis. Appropriate allelic exchange events were monitored by measurement of Cm sensitivity and sucrose resistance. All mutants were confirmed by a combination of PCR and sequence analysis.
Exchange of the YopD or YopE N-terminal region with the equivalent sequence from other Yop translocator or effector substrates was also performed by overlap PCR with the primer combinations listed in Table S2 in the supplemental material. Unless otherwise stated, the region exchanged encompassed residues 2 to 15. Significantly, each chimeric variant was again introduced in cis on the Y. pseudotuberculosis virulence plasmid to ensure that expression occurred in the context of native regulatory elements.
mRNA structural predictions.
Sequences 5′ of various yopD alleles, including 45 nucleotides (nt) upstream and 57 nt downstream of the AUG start codon, were predicted using RNA Mfold version 3.2 software (87). Structures were defined with default settings.
Transcriptional analysis by semiquantitative RT-PCR.
The isolation of total RNA from Yersinia, the reverse transcription (RT) of the mRNA into cDNA, and its use as a template for subsequent PCR amplification with the gene-specific primers listed in Table S2 in the supplemental material were performed as described in detail elsewhere (14).
Analysis of Yop synthesis and type III secretion.
Yop synthesis and secretion by Y. pseudotuberculosis was analyzed after log-phase growth in permissive (without Ca2+) and nonpermissive (with Ca2+) fresh BHI media for 1 h at 26°C and, following the addition of 0.025% (vol/vol) Triton X-100, for a further 3 h at 37°C. Measurements of optical density at 600 nm (OD600) were used to standardize each culture. Samples of the suspensions were then collected to represent the total protein fraction. Bacteria were then collected by a 2-min centrifugation, after which samples of the cleared bacterial supernatant (representing the secreted Yop fraction) were taken. All samples were added to 4× sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) loading buffer (200 mM Tris-HCl [pH 6.8], 8% SDS, 0.4% bromophenol blue, 40% glycerol, 20% β-mercaptoethanol), denatured, and then fractionated by 12% sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) and Western blot analysis using rabbit α-YopD, α-YopB, α-LcrV, and α-YopE polyclonal antisera (Agrisera, Vännäs, Sweden) in combination with α-rabbit antiserum conjugated with horseradish peroxidase (GE Healthcare, Buckinghamshire, United Kingdom). Homemade chemiluminescent solutions were used to detect individual protein bands. Quantification by Western blotting mirrored previously published methods (8) and was performed using Quantity One software, version 4.52 (Bio-Rad).
Intracytoplasmic YopD stability assay.
To assess the stability of premade YopD built up in the bacterial cytoplasm, we employed the intrabacterial stability assay of Feldman and colleagues (24) and either chloramphenicol or tetracycline as the de novo protein synthesis inhibitor. Note that this steady-state experiment was designed to measure the steady-state stability of accumulated YopD or YopD-Bla variants and not the efficiency of de novo translation.
Low-calcium growth measurements.
Determination of the Yersinia low-calcium-response growth phenotypes resulting from growth under high- and low-Ca2+ conditions at 37°C were performed by measuring absorbance at 600 nm during growth in liquid Thoroughly Modified Higuchi's (TMH) medium (minus Ca2+) or TMH medium supplemented with 2.5 mM CaCl2 (plus Ca2+) (17). Parental Yersinia (YPIII/pIB102) bacteria are defined as calcium dependent (CD), since they are unable to grow in the absence of Ca2+ at 37°C, while Yersinia bacteria lacking the lcrQ allele, such as the ΔyscU ΔlcrQ mutant (YPIII/pIB75-26), are termed temperature sensitive (TS), reflecting an inability to grow at 37°C.
Cytotoxicity assay.
Cultivation and infection of HeLa cells for cytotoxicity assays were performed using coverslips and our standard methods (29, 62). At numerous intervals postinfection, the culture medium was replaced by 2% paraformaldehyde fixation solution and the resulting mixture was then mounted on glass slides. The extent of morphological change was visualized by phase-contrast microscopy using a Nikon Eclipse 90i microscope. Cytotoxicity resulting from infection with parental Y. pseudotuberculosis (YPIII/pIB102) defined the upper limit of morphological change, while the lower limit was defined by the results seen with a ΔyopD deletion mutant, YPIII/pIB625 (YopDΔ4-20) (57).
Bacterial viability in the presence of eukaryotic cells.
Essentially, the method of Bartra and coworkers (7) was used to establish bacterial viability in the presence of murine macrophage-like J774 cells. In essence, bacteria lacking a fully functional T3SS are more readily phagocytosed and are therefore more susceptible to the antimicrobial effects of J774 cells. Reductions in viability were determined by performing CFU counts for relevant bacterial strains in infected eukaryotic cell lysates.
Construction and analysis of YopD translationally fused to β-Lac lacking signal.
A 5-prime-truncated bla gene was amplified using pAJR104 for the template DNA and the primer pair listed in Table S1 in the supplemental material. The Kpn-EcoRI DNA fragment was cloned into pMMB208, thereby placing the bla reporter under IPTG (isopropyl-β-d-thiogalactopyranoside)-inducible control. Translational fusions of various lengths linking the 5-prime region of yopD, including the predicted Shine-Dalgarno (SD) sequence, to truncated bla were then generated in this background. This was achieved by BamHI-KpnI cloning using two methods. Larger (>75-bp) DNA fragments were first amplified by PCR using the appropriate primer pairs listed in Table S2 in the supplemental material and lysed YPIII/pIB102 as a source of template DNA. Smaller (<45-bp) DNA fragments were formed by the annealing of two complementary oligonucleotides prior to DNA ligation to the vector (see Table S2 in the supplemental material). Analysis of recombinant β-lactamase (β-Lac) synthesis and secretion followed the procedure described for Yop synthesis and secretion. After Western blotting of fractionated protein, fusion proteins were detected with a primary rabbit polyclonal anti-β-Lac antibody (Millipore AB, Solna, Sweden) followed by incubation with α-rabbit antiserum conjugated with horseradish peroxidase (GE Healthcare). Relative Western blot signal intensities were quantified using an established protocol (8) and Quantity One software, version 4.52 (Bio-Rad).
Statistics.
Values are expressed as means ± standard errors (SE) of the results of multiple independent experiments. A two-tailed nonparametric Mann-Whitney U test was used to analyze the differences in (i) percent Yop secretion efficiency compared to native Yop secretion data, (ii) Yop-Bla secretion efficiency compared to YopD20-Bla data, and (iii) relative viability determined by the ratio (in CFU per milliliter) of the results seen with mutant bacteria (producing the chimeras) to those seen with bacteria producing YopDhigh(x2), which was phenotypically indistinguishable from parental bacteria. Differences with a probability value of P < 0.05 were considered significant.
RESULTS
YopD secretion is affected by an N-terminal frameshift mutation.
A T3S signal sequence is located in the N terminus of various effector proteins (16, 46, 69). It probably comprises a combination of the 5-prime mRNA sequence and the amino acid sequence. A similar combination of signals may also exist for the translocator proteins, but this has not been conclusively demonstrated (12, 35, 41, 54). YopD, a translocator protein functioning as a component of the Ysc-Yop T3SS of Yersinia, possesses an N-terminal domain necessary for efficient secretion (57). To dissect how this region contributes to YopD secretion, a series of in-cis mutations were generated in the yopD allele. This enabled production under normal regulatory control of YopD variants with alterations to the first 15 residues of their N termini. The first mutant (YopDFrame+1) harbors a +1 frameshift in which an A nucleotide was inserted immediately after the start codon of YopD and then compensated for by the removal of a T nucleotide at position 46 to restore the reading frame after codon 15 (Table 2). The second mutant (YopDFrame−1) was a −1 frameshift. Since a deletion of the first nucleotide after the start codon would result in a premature stop codon, the ninth nucleotide (an A) was removed and a T was inserted at position 46 to again restore the reading frame after codon 15 (Table 2). These mutants were designed to assess whether the secretion signal is protein based; both constructs carry altered amino acid sequences, but the mRNA sequences closely resemble that of native YopD (YopDwild type). Another mutant (YopDScramble) was constructed by mutating all possible nucleotides in the wobble position of each codon in the YopD N terminus while still maintaining a wild-type amino acid sequence. This resulted in a YopD variant with a scrambled 5′ mRNA coding sequence designed to gauge the contribution of mRNA to YopD secretion. In fact, 16 nt substitutions of a possible 45 were generated (Table 2). Importantly, none of the mutations affected the stability of accumulated cytoplasmic-located YopD, since all variants were as resistant to intrabacterial proteases as YopDwild type (Fig. 1).
Table 2.
Comparison of the nucleotide and amino acid sequence changes in the YopD and YopE variants used in this studya
Shading in light gray indicates the YopDwt nucleotide triplets and their corresponding amino acid sequences. Dark gray shading indicates YopEwt sequence. Amino acid sequences boxed in a broken line are identical to those of wild-type YopD, but the nucleotide sequence is altered (YopDScramble). Those regions left unmarked possess different amino acid sequences, whereas the nucleotide sequence is essentially the same (YopDFrame+1 and YopDFrame−1). The sequence shaded in light blue is artificial and in another study (47) was seen to either promote (high) or abolish (low) YopE secretion. Boxes crossed with a dark blue diagonal line highlight highly hydrophobic residues. Bold font indicates site-directed substitution mutations. The single asterisk (row 8, rightmost column) indicates that synthesis of this variant was impaired.
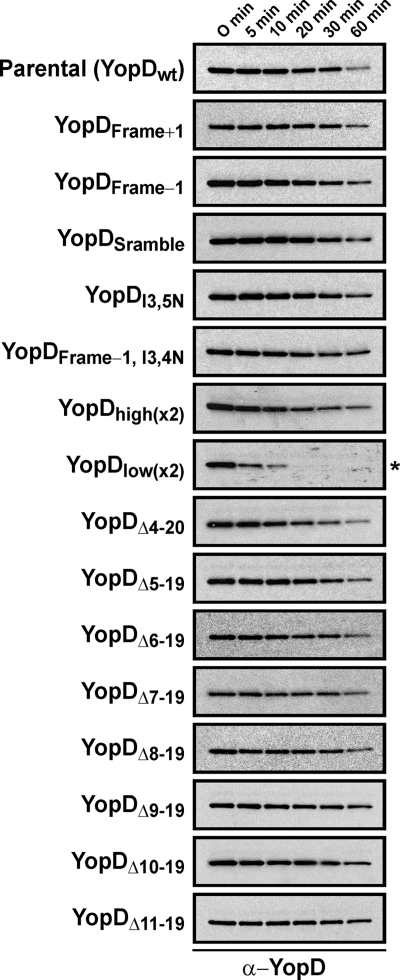
Fig. 1.
Intrabacterial stability of preformed pools of various YopD mutant proteins. Bacteria were first cultured for 1 h in noninducing (i.e., including 2.5 mM CaCl2) BHI broth at 37°C. The protein synthesis inhibitor chloramphenicol (50 μg/ml) was added at time point 0 min. Samples were then collected at subsequent time points. Protein levels associated with pelleted bacteria were detected by Western blotting using polyclonal anti-YopD antiserum. Panels: Parental (YopDwt), YPIII/pIB102; YopDFrame+1, YPIII/pIB62502; YopDFrame−1, YPIII/pIB62503; YopDScramble, YPIII/pIB62504; YopDI3,5N, YPIII/pIB62559; YopDFrame−1, I3,4N, YPIII/pIB62564; YopDhigh(x2), YPIII/pIB62537; YopDlow(x2), YPIII/pIB62538; YopDΔ4-20, YPIII/pIB625; YopDΔ5-19, YPIII/pIB62524; YopDΔ6-19, YPIII/pIB62525; YopDΔ7-19, YPIII/pIB62526; YopDΔ8-19, YPIII/pIB62527; YopDΔ9-19, YPIII/pIB62528; YopDΔ10-19, YPIII/pIB62529; YopDΔ11-19, YPIII/pIB62530. The asterisk (*) highlights YopDlow(x2) as the only visibly unstable variant.
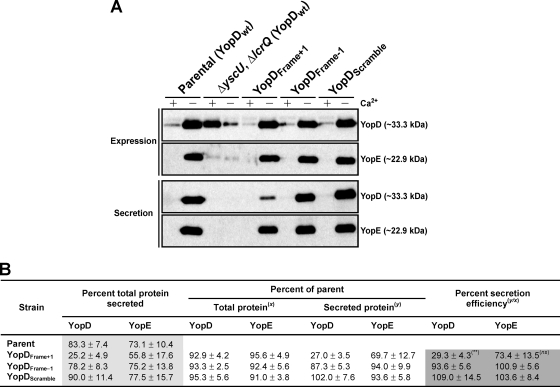
Yop synthesis and secretion were next examined by growing bacteria in either Yop-inducing (minus Ca2+) or noninducing (plus Ca2+) BHI broth. The extent of mutated YopD associated with bacteria did not deviate from that observed for parental bacteria (Fig. 2A, upper panel). This was confirmed by quantifying total YopDFrame+1 (92.9%), YopDFrame−1 (93.3%), and YopDScramble (95.3%) synthesis relative to native YopD synthesis (Fig. 2B). The results indicated that the well-established Ca2+-dependent regulation of Yop synthesis is not affected by these alterations to the YopD N terminus. In contrast, a marked reduction of secretion of YopDFrame+1, but not YopDFrame−1 or YopDScramble, was evident in the supernatant fraction of these bacteria after growth in Yop-inducing media (Fig. 2A, lower panel). In fact, this equated to only 25.2% of the synthesized YopDFrame+1 that was actually secreted (Fig. 2B, light gray box). Moreover, the efficiency of YopDFrame+1 secretion was significantly reduced to 29.3% of native YopD secretion efficiency (Fig. 2B, dark gray box [P = 0.0079, indicated by double asterisks]). The poor YopDFrame+1 secretion efficiency was not caused by a general secretion defect, because the slight effect on the secretion of other Yops such as YopE (73.4% secretion efficiency) was not statistically significant (Fig. 2B, dark gray box [P = 0.1143]). Finally, secretion was dependent on the Ysc-Yop T3SS, because mutant bacteria lacking the yscU and lcrQ alleles did not secrete any Yops (Fig. 2A and data not shown). We therefore conclude that YopDFrame+1 possesses an intrinsic T3S defect.
Fig. 2.
A YopD frameshift mutant altering the amino acid sequence of the N-terminal secretion signal specifically affects secretion. (A) Overnight cultures were subcultured into BHI broth either containing (+) or lacking (−) calcium and grown at 26°C for 1 h and then at 37°C for 3 h. Protein samples were fractionated by 12% SDS-PAGE and then transferred onto a membrane support for immune detection. Expression fractions (upper panels) represent total protein associated with bacteria and also released into the culture supernatant. Secretion fractions (lower panels) signify protein freely released into the culture supernatant. Lanes: Parental (YopDwt), YPIII/pIB102; ΔyscU, ΔlcrQ (YopDwt), YPIII/pIB75-26; YopDFrame+1, YPIII/pIB62502; YopDFrame−1, YPIII/pIB62503; YopDScramble, YPIII/pIB62504. Molecular mass values shown in parentheses were deduced from primary amino acid sequences. (B) At least three independent experiments were used to quantify relative YopD and YopE synthesis and secretion values ± standard errors of the means using Quantity One software, version 4.52 (Bio-Rad). Percent total secretion (lighter gray) values reflect the ratio (expressed as a percentage) of secreted protein to the amount synthesized in each respective strain. Percent secretion efficiency values reflect the extent of Yop secretion occurring in mutant bacteria relative to what occurs in parental bacteria (darker gray). It is calculated from the ratio of secreted protein seen with the parent (y) to the total protein seen with the parent (x). The median secretion efficiency of YopDFrame+1 was significantly lower (**, P = 0.079, two-tailed nonparametric Mann-Whitney U test; P < 0.05) than that of native YopD; it was also lower than the secretion efficiencies of both YopDFrame−1 and YopDScramble. In contrast, YopE secretion efficiency in the same strains was not statistically different from that of the parent bacteria (ns, not significant; P = 0.1143).
These data primarily suggest an involvement of the amino acid sequence in ensuring efficient YopD secretion. However, they do not necessarily rule out a role for mRNA. We were therefore curious to model the predicted mRNA secondary structure of the yopD alleles, focusing on sequences encompassing the AUG start codon and 45 nt upstream and 57 nt downstream. This modeling revealed very similar mRNA structures for YopDwild type (see Fig. S1A in the supplemental material), YopDFrame+1 (see Fig. S1B in the supplemental material), and YopDFrame−1 (see Fig. S1C in the supplemental material), whereas the YopDScramble mRNA structure was considerably different (see Fig. S1D in the supplemental material). Given that YopDFrame+1 was the only poorly secreted variant, it is therefore hard to envision how these mRNA structures could constitute a secretion signal per se.
N-terminal isoleucines contribute to YopD secretion.
In light of the defect in YopDFrame+1 secretion, we were curious why the secretion of YopDFrame−1 was unaffected. We tested the possibility that the remaining wild-type polar threonine and isoleucine residues at positions 2 and 3, respectively (Table 2), were adequate to promote secretion of this variant. However, individual substitution mutations replacing those two residues with amino acids (glycine, asparagine, and lysine) with various physical properties in both the YopDwild-type and YopDFrame−1 backgrounds had no effect on Yop synthesis or secretion (see Fig. S2 in the supplemental material). Nevertheless, an additional isoleucine residue is also located at position 4 in YopDFrame−1 and position 5 in native YopD (Table 2). Since a few studies have suggested that isoleucine is a vital aspect of T3S targeting for some Yops (59, 60), we generated two double mutants, exchanging both isoleucines for asparagine and giving rise to YopDI3,5N and YopDFrame−1, I3,4N. Intracellular pools of both mutants were stable (Fig. 1) and permitted generous synthesis of all Yops during bacterial growth under inducing conditions (Fig. 3A, upper panel, and data not shown), although the I3,5N mutation did alter the migration of YopD on SDS-PAGE. More interesting, however, was that this YopDI3,5N variant secreted just 51.4% of synthesized protein, while the YopDFrame−1, I3,4N variant secreted 75.6% (Fig. 3A, lower panel, and Fig. 3B, light gray box). Compared to the native YopD results, this represented a significant reduction in YopDI3,4N secretion efficiency of 55.5% (Fig. 3B, dark gray box [P = 0.0079, indicated by double asterisks]). However, the calculated YopDFrame−1, I3,4N secretion efficiency of 79.8% was not considered to be statistically different (P = 0.1508). Crucially, this secretion defect was not observed for any other Yop, including YopE (Fig. 3A, lower panel, Fig. 3B, and data not shown). In silico mRNA secondary-structure predictions cannot easily reconcile these differences, because the generated models of YopDI3,5N (see Fig. S1E in the supplemental material), YopDFrame−1, I3,4N (see Fig. S1F in the supplemental material), and YopDwild type (see Fig. S1A in the supplemental material) mRNA all appear appreciably different from each other. Hence, these data do not explain why YopDFrame−1 was still efficiently secreted. Nevertheless, they do highlight a combined contribution of the N-terminal residues Ile-3 and Ile-5 to the secretion of native YopD.
Fig. 3.
N-terminal isoleucine residues contribute to secretion of native YopD. (A) Overnight cultures were subcultured into BHI broth either containing (+) or lacking (−) calcium and grown at 26°C for 1 h and then at 37°C for 3 h. Protein samples were fractionated by 12% SDS-PAGE and then transferred onto a membrane support for immune detection. Expression fractions (upper panels) represent total protein associated with bacteria and also released into the culture supernatant. Secretion fractions (lower panels) signify protein freely released into the culture supernatant. Lanes: Parental (YopDwt), YPIII/pIB102; ΔyscU, ΔlcrQ (YopDwt), YPIII/pIB75-26; YopDFrame−1, YPIII/pIB62503; YopDI3,5N, YPIII/pIB62559; YopDFrame−1, I3,4N, YPIII/pIB62564. Molecular mass values shown in parentheses were deduced from primary amino acid sequences. (B) The quantification of YopD and YopE secretion efficiency ± standard error of the mean was calculated from a minimum of three independent experiments using Quantity One software, version 4.52 (Bio-Rad). See the legend to Fig. 2 for explanations of “Percent total secretion” (lighter gray) and “Percent secretion efficiency” (darker gray). Compared to native YopD, the median secretion efficiency of YopDI3,5N was significantly lower than that of YopDFrame−1, I3,4N (*, P = 0.0317, two-tailed nonparametric Mann-Whitney U test; P < 0.05) or YopDFrame−1 (**, P = 0.079). Conversely, the observed secretion deficiency of YopDFrame−1, I3,4N was not considered to be statistically different from that of YopDFrame−1 or native YopD (ns, not significant; P = 0.1508).
YopD secretion is supported by an artificial amphipathic N-terminal signal sequence.
A recent molecular analysis of T3S signals was performed by replacing amino acids 2 to 8 of the secreted protein YopE with all combinations of synthetic serine and isoleucine sequences (47). This revealed that amphipathic N-terminal sequences containing four or five serine or isoleucine residues are more likely to target YopE for secretion than stretches of hydrophobic or hydrophilic sequences. We therefore wondered whether amphipathicity is also a feature of the N-terminal YopD secretion signal. This was investigated by appending artificial sequences to the YopD N terminus that induced efficient YopE secretion (NH3-Ile-Ile-Ser-Ser-Ile-Ser-Ser-CO2) (“high”) or abolished YopE secretion (NH3-Ile-Ile-Ile-Ile-Ser-Ile-Ile-CO2) (“low”) (47). These sequences were first used in single-copy form to replace amino acids 2 to 8 of YopD (YopDhigh and YopDlow) (Table 2). Once again, to avoid any copy-number effects, the constructs were placed in cis on the virulence plasmid. In contrast to the results of the previously cited YopE study (47), no difference in synthesis or secretion could be observed for either YopDhigh or YopDlow (Fig. 4). Thus, T3S of YopD is supported by an artificial secretion signal appended to the N terminus. Moreover, while the two studies were performed differently—YopE was produced in trans, while YopD was produced in cis—the fact that YopDlow was still secreted could also imply that native secretion signals of both middle (translocator) and late (effector) substrates differ with respect to the degree of amphipathicity required for their respective levels of secretion.
Fig. 4.
Amphipathicity of the N terminus is not an obvious mediator of YopD secretion. Overnight cultures were subcultured into BHI broth either containing (+) or lacking (−) calcium and grown at 26°C for 1 h and then at 37°C for 3 h. Protein samples were fractionated by 12% SDS-PAGE and then transferred onto a membrane support for immune detection. Expression fractions (upper panels) represent total protein associated with bacteria and also released into the culture supernatant. Secretion fractions (lower panels) signify protein freely released into the culture supernatant. Lanes: Parental (YopDwt), YPIII/pIB102; ΔyscU, ΔlcrQ (YopDwt), YPIII/pIB75-26; YopDhigh, YPIII/pIB62514; YopDlow YPIII/pIB62515; YopDhigh(x2), YPIII/pIB62537; YopDlow(x2), YPIII/pIB62538. Molecular mass values shown in parentheses were deduced from primary amino acid sequences.
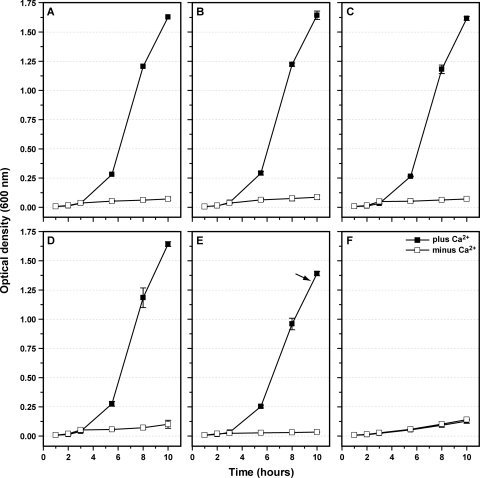
We attempted to address that aspect further by creating another two additional constructs, YopDhigh(x2) and YopDlow(x2). In those variants, the respective “high” and “low” synthetic sequences were duplicated, thereby replacing codons 2 to 15 of native YopD (Table 2). We hypothesized that these two constructs would represent the maximal extremes in possible amphipathic tendencies at the YopD N terminus. YopDhigh(x2) synthesis and secretion (Fig. 4) and the stability of premade pools accumulated in the cytoplasm (Fig. 1) still occurred in a manner reminiscent of YopDwild type results. In contrast, production of YopDlow(x2) was, surprisingly, very low under our assay conditions (Fig. 4). To investigate the basis for this phenotype, we first isolated mRNA and reverse transcribed it into cDNA. Using this cDNA as the template in a PCR with yopD gene-specific primers, no differences with respect to Ca2+-dependent transcription from the various mutated yopD alleles were observed (Fig. 5). In fact, this yopD transcription pattern mirrored the Ca2+-dependent expression of the yopE gene encoding a late Ysc-Yop T3SS effector substrate (Fig. 5). We also examined the regulatory phenotype of the YopDlow(x2) mutant by the use of a dual approach that incorporated a low-calcium-response growth assay in parallel with a more thorough analysis of type III substrate synthesis and secretion. When grown at 37°C, parental bacteria typically displayed a strict CD growth phenotype (Fig. 6A), which was mirrored by the phenotype of strains producing variants YopDhigh (Fig. 6B), YopDlow (Fig. 6C), and YopDhigh(x2) (Fig. 6D). The ΔyscU lcrQ double mutant exhibited a characteristic TS growth phenotype irrespective of the Ca2+ concentration (Fig. 6F). However, bacteria producing YopDlow(x2) were intermediate with respect to their CD growth (Fig. 6E), a phenotype we have previously referred to as representing CD-like growth (17). Consistent with this subtle alteration in low calcium responsiveness, mutant bacteria producing YopDlow(x2) also showed less calcium-dependent control of Yop synthesis. Production of YopB and LcrV was more abundant under conditions that were nonpermissive for T3S and also resulted in secretion of LcrV (Fig. 4). Collectively, these are all key indicators of compromised regulatory control of yop in Yersinia (17, 28, 57, 66, 81), suggesting that YopDlow(x2)-producing bacteria are subtly deregulated with respect to Yop synthesis. Curiously, this modest regulatory defect was not evident at the transcriptional level; yop transcription was restricted to T3S-permissive growth conditions (Fig. 5). This prompted us to compare the secondary structures of 5′ mRNAs derived from yopD alleles producing YopDhigh(x2) and YopDlow(x2). Interestingly, the AUG start codon of mRNA encoding YopDlow(x2) is potentially buried in an extended stem-loop structure (compare Fig. S1H in the supplemental material with Fig. S1G in the supplemental material), which could conceivably alter translational control. Even if the rate of translation is unperturbed, low YopDlow(x2) amounts could be partly explained by an observed decrease in intracytoplasmic stability. Oddly, of all the YopD mutants constructed, the accumulated premade pools of YopDlow(x2) were notably the most sensitive to endogenous protease digestion (Fig. 1). Why this particular genetic exchange results in a YopD variant with elevated steady-state instability is not yet understood. Collectively, though, these unexpected observations curtailed any further investigation into the role of N-terminal sequence amphipathicity in YopD secretion. However, YopDlow(x2) might prove a useful tool for investigations of unknown mechanisms of translational control of YopD.
Fig. 5.
RT-PCR of mRNA isolated from Y. pseudotuberculosis. RNA was isolated from log-phase bacterial cultures grown at 37°C in BHI medium with (+) and without (−) Ca2+. Samples were subjected to RT-PCR using primers specific for rpoA (used as a loading control) and the T3SS genes yopD and yopE. Lanes: Parental (YopDwt), YPIII/pIB102; YopDΔ4-20, YPIII/pIB625; YopDhigh, YPIII/pIB62514; YopDlow, YPIII/pIB62515; YopDhigh(x2), YPIII/pIB62537; YopDlow(x2), YPIII/pIB62538. Images were acquired using a Fluor-S MultiImager (Bio-Rad). The images were then inverted using Quantity One quantitation software, version 4.52 (Bio-Rad). Numbers in parentheses indicate the approximate size of the amplified DNA fragment in base pairs.
Fig. 6.
Low-calcium-response growth phenotypes of Y. pseudotuberculosis producing various YopD variants. Bacteria were grown at 37°C in nonsupplemented TMH medium (minus Ca2+) or in TMH medium supplemented with 2.5 mM CaCl2 (plus Ca2+). Three different growth phenotypes were detected: calcium-dependent (CD) growth (A to D); CD-like (i.e., moderately calcium-dependent) growth (E); and TS growth (i.e., bacteria were sensitive to elevated temperature regardless of the presence of calcium) (F). Panels: A, parental (YPIII/pIB102); B, YopDhigh (YPIII/pIB62514); C, YopDlow (YPIII/pIB62515); D, YopDhigh(x2) (YPIII/pIB62537); E, YopDlow(x2) (YPIII/pIB62538); F, ΔyscU, ΔlcrQ (YPIII/pIB75-26). The arrow highlights the subtle growth restriction of Y. pseudotuberculosis producing YopDlow(x2).
YopD secretion is affected by N-terminal deletions.
To further dissect the YopD N terminus, we created a series of 13 progressively smaller in cis deletion mutations between codons 4 and 20. These mutated alleles encoded the variants YopDΔ5-19, YopDΔ6-19, YopDΔ7-19, YopDΔ8-19, YopDΔ9-19, YopDΔ10-19, YopDΔ11-19, YopDΔ12-19, YopDΔ13-19, YopDΔ14-19, YopDΔ15-19, YopDΔ16-19, and YopDΔ17-19. As a control, we used Yersinia producing YopDΔ4-20, which has been described previously (57). All were examined for their ability to be produced and secreted by Y. pseudotuberculosis. As expected, secretion of YopDΔ4-20 was virtually undetectable under our assay conditions (57), a phenotype now also shared by a new mutant producing YopDΔ5-19 (Fig. 7A, lower panel). When quantified, the levels of YopDΔ4-20 and YopDΔ5-19 secretion accounted for only 10.2% and 3.2% of the total protein produced, respectively (Fig. 7B, light gray box). Moreover, deletion mutants YopDΔ6-19 (26.8%), YopDΔ7-19 (49%), YopDΔ8-19 (37.8%), YopDΔ9-19 (27.9%), and YopDΔ10-19 (54.5%) also displayed reduced secretion levels. However, mutants with progressively smaller deletions (such as YopDΔ11-19, YopDΔ12-19, YopDΔ13-19, YopDΔ14-19, YopDΔ15-19, YopDΔ16-19,and YopDΔ17-19) as well as native YopD all maintained secretion competency (Fig. 7A, lower panel). For example, when expressed quantitatively, the results represented secretion levels on the order of 81.4% for YopDΔ11-19 and 89.9% for native YopD (Fig. 7B, light gray box). As expected, secretion was totally absent from a control Yersinia strain (ΔyscU ΔlcrQ) lacking a functional T3SS (Fig. 7A, lower panel). However, it was evident following quantification that the larger deletions all tended to negatively impact the amounts of Yop accumulated (Fig. 7B). This was observed for both YopD and YopE, with accumulated intracellular pools reaching only between 36.3% and 70.4% of the levels seen with the parent bacteria. At least for the YopD variants, we ruled out instability as a factor for this clear reduction in steady-state levels, for native YopD and the deletion variants were equally resistant to endogenous proteases in vivo (Fig. 1). As reduced levels of intracellular pools of protein would compromise secretion, we compensated for this effect by specifically quantifying the secretion efficiency of YopD and YopE from mutant bacteria compared to the efficiency seen with the parent bacteria. Notably, the secretion efficiency of all YopD larger-deletion variants—within a range of 3.4% (for YopDΔ5-19) to 60.5% (YopDΔ10-19)—was still only a fraction of that of native YopD (Fig. 7B, dark gray box). This contrasted with the higher secretion efficiencies of the smaller-deletion variants such as YopDΔ11-19 (90.1%) (Fig. 7B, dark gray box, and data not shown). Critically, the efficiency of YopE secretion by all these mutant bacteria was also equivalent to that seen with the parental bacteria (Fig. 7B, dark gray box). Thus, we can conclude that the YopDΔ4-20, YopDΔ5-19, YopDΔ6-19, YopDΔ7-19, YopDΔ8-19, YopDΔ9-19, and YopDΔ10-19 variants all possess a bona fide secretion defect, in addition to and despite having lower intracellular pools of accumulated protein.
Fig. 7.
Expression and secretion of YopD containing various in-frame deletions of the N-terminal secretion signal. (A) Overnight cultures were subcultured into BHI broth either containing (+) or lacking (−) calcium and grown at 26°C for 1 h and then at 37°C for 3 h. Protein samples were fractionated by 12% SDS-PAGE and then transferred onto a membrane support for immune detection. Expression fractions (upper panels) represent total protein associated with bacteria and also released into the culture supernatant. Secretion fractions (lower panels) signify protein freely released into the culture supernatant. Lanes: Parental (YopDwt), YPIII/pIB102; ΔyscU, ΔlcrQ (YopDwt), YPIII/pIB75-26; YopDΔ4-20, YPIII/pIB625; YopDΔ5-19, YPIII/pIB62524; YopDΔ6-19, YPIII/pIB62525; YopDΔ7-19, YPIII/pIB62526; YopDΔ8-19, YPIII/pIB62527; YopDΔ9-19, YPIII/pIB62528; YopDΔ10-19, YPIII/pIB62529; YopDΔ11-19, YPIII/pIB62530; YopDΔ12-19, YPIII/pIB62531; YopDΔ13-19, YPIII/pIB62532; YopDΔ14-19, YPIII/pIB62533; YopDΔ15-19, YPIII/pIB62534; YopDΔ16-19, YPIII/pIB62535; YopDΔ17-19, YPIII/pIB62536. Molecular mass values shown in parentheses were deduced from primary amino acid sequences. (B) For a selection of mutants, YopD and YopE synthesis and secretion efficiency were quantified from the results of at least three independent experiments using Quantity One software, version 4.52 (Bio-Rad). Definitions of terms are provided in the legend to Fig. 2.
To scrutinize whether any particular codon or group of codons in this region is critically important for YopD secretion, extra sets of in cis yopD deletion mutations were created. We divided the analysis into three categories, resulting in various combinations of in-frame deletions targeting (i) residues 2 to 7 (YopDΔ2-3, YopDΔ2-4, YopDΔ2-5, YopDΔ3-4, YopDΔ3-5, YopDΔ3-6, YopDΔ3-7, and YopDΔ4-5) (see Fig. S3 in the supplemental material), (ii) residues 5 to 10 (YopDΔ5-6, YopDΔ5-7, YopDΔ5-8, YopDΔ5-9, YopDΔ5-10, YopDΔ6-10, YopDΔ7-10, YopDΔ9-10, and YopDΔ9-10) (see Fig. S4 in the supplemental material), and (iii) all sequential residues through position 19 (YopDΔ2-3, YopDΔ4-5, YopDΔ6-7, YopDΔ8-9, YopDΔ10-11, YopDΔ12-13, YopDΔ14-15, YopDΔ16-17, and YopDΔ18-19) (see Fig. S5 in the supplemental material). However, in no case did our analysis reveal any defect in YopD synthesis or secretion by Y. pseudotuberculosis. Hence, a systematic analysis of smaller deletions of the N-terminal region failed to pinpoint any one codon, or group of codons, responsible for the YopD secretion defect observed with the larger deletions described for Fig. 7. It is also interesting that, unlike our observations for YopDI3,5N, none of the mutants with a smaller deletion lacking codon 3 and 5 were altered in YopD secretion (see Fig. 3). Currently, we have no definitive explanation for this disparity. Collectively, the vagaries of these results do suggest that no particular N-terminal amino acid(s) is essential for YopD secretion so long as compensatory residues maintain the necessary physical characteristics of this N-terminal region.
Reduced YopD secretion impairs Yop translocation into eukaryotic cells.
We now had multiple YopD variants with modifications of the N terminus; only a few were defective in secretion. This presented an opportunity to ascertain whether sequences within the YopD N-terminal secretor domain play any role in the ability of Yersinia to intoxicate target eukaryotic cells with Yop effector toxins. Thus, we performed a HeLa cell cytotoxicity assay that measures the effect of the presence of intracellularly localized YopE, a GTPase-activating protein of the Rho family, on the morphology of infected target cells (62). As a control, we included a strain producing YopDΔ4-20 that is known from an earlier study to be incapable of Yop translocation (57). We detected a similar impairment of the cytotoxicity response of eukaryotic cells only when the cells were infected by Yersinia producing the poorly secreted YopD variants; bacteria producing YopDlow(x2), YopDΔ5-19, and YopDΔ6-19 were noncytotoxic, while the onset of cytotoxicity of the mutant producing YopDFrame+1 was quite delayed (Fig. 8). All other YopD variants, including those with smaller N-terminal deletions or even those with a dramatically altered N-terminal coding sequence such as YopDFrame−1, YopDhigh, YopDlow, and YopDhigh(x2), still maintained the capacity to efficiently intoxicate nonimmune epithelial cells with the YopE cytotoxin (Fig. 8; see also Fig. S6 in the supplemental material). Our interpretation of these data is that the YopD N terminus encompassing residues 2 to 20 appears to play no role in the translocation process per se other than to ensure that YopD is secreted.
Fig. 8.
Defects in YopD secretion impair Yop intoxication of infected eukaryotic cells. Strains were allowed to infect a monolayer of growing HeLa cells. At 20, 40, 60, and 120 min postinfection, samples were subjected to fixation and the effect of the bacteria on the HeLa cells was recorded by phase-contrast microscopy. Translocation of the YopE cytotoxin, a GTPase-activating protein, causes a distinct change in cell shape, from oblong to rounded (cytotoxicity), of affected HeLa cells (see panels A, D, E, and G). HeLa cells not intoxicated with YopE show normal uninfected cell morphology (see panels B, F, and H). Some YopD variants reduced the efficiency of YopE translocation, which delayed the onset of cytotoxicity (see panels C and I). Panels: A, YopDwt (parent) (YPIII/pIB102); B, YopDΔ4-20 (YPIII/pIB625); C, YopDFrame+1 (YPIII/pIB62502); D, YopDFrame−1 (YPIII/pIB62503); E, YopDI3,5N (YPIII/pIB62559); F, YopDhigh(x2) (YPIII/pIB62537); G, YopDlow(x2) (YPIII/pIB62538); H, YopDE-Nterm (YPIII/pIB62501-577); I, YopDΔ5-19, (YPIII/pIB62524); J, YopDΔ6-19 (YPIII/pIB62525).
The YopD N terminus may assist with orchestrating secretion.
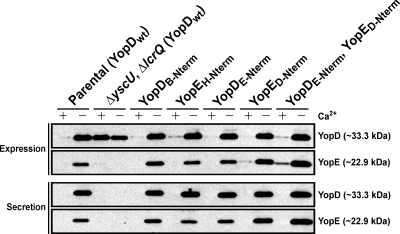
We have previously shown that even minimal quantities of secreted native YopD are adequate for efficient Yop delivery into cells (19). Why, then, are Y. pseudotuberculosis strains that secrete low but reproducibly detectable levels of YopD variants that are altered in their N termini such poor translocators of Yop effectors (see Fig. 8)? We hypothesized that this chaperone-independent N-terminal secretion signal may contribute to temporal secretion control such that the timing of secretion of these modified YopD variants might be compromised. Since translocators are believed to function by forming pores in the eukaryotic cell plasma membrane through which effectors could possibly pass to gain access to the eukaryotic cell interior (53), failure to secrete them before the effectors could reduce translocation efficiency. We therefore examined whether the N terminus of secreted Yop substrates plays a part in orchestrating secretion. Chimeras were generated in which residues 2 to 15 of YopD were exchanged for the equivalent residues from YopE (YopDE-Nterm) and vice versa (YopED-Nterm). Each chimeric allele was introduced in cis onto the Ysc-Yop-encoding virulence plasmid to generate Y. pseudotuberculosis capable of producing either or both of YopDE-Nterm and YopED-Nterm. No matter what the strain background, these chimeras were easily detected in association with bacteria and freely secreted into culture media (Fig. 9). As expected, a thorough quantification confirmed that these three chimeras were secreted with a level of efficiency equivalent to that of the native protein (data not shown). Thus, under in vitro growth conditions of Ca2+ depletion in which T3S is considered to be at maximal activity, the YopD N-terminal secretion signal can be replaced with the equivalent region from an effector substrate without consequence. Moreover, the YopD N terminus was also able to support secretion of the YopE substrate. At face value, this result supports current dogma asserting that conserved physical and/or chemical features of the N terminus, rather than a consensus sequence of amino acids, determine a signal for T3S for all substrates.
Fig. 9.
Expression and secretion of YopD and YopE chimeras with a reciprocally exchanged N-terminal secretion signal. Overnight cultures were subcultured into BHI broth either containing (+) or lacking (−) calcium and grown at 26°C for 1 h and then at 37°C for 3 h. Protein samples were fractionated by 12% SDS-PAGE and then transferred onto a membrane support for immune detection. Expression fractions (upper panels) represent total protein associated with bacteria and also released into the culture supernatant. Secretion fractions (lower panels) signify protein freely released into the culture supernatant. Lanes: Parental (YopDwt), YPIII/pIB102; ΔyscU, ΔlcrQ (YopDwt), YPIII/pIB75-26; YopDB-Nterm, YPIII/pIB62577; YopEH-Nterm, YPIII/pIB578; YopDE-Nterm, YPIII/pIB62501; YopED-Nterm, YPIII/pIB577; YopDE-Nterm, YopED-Nterm, YPIII/pIB62501-577. Molecular mass values shown in parentheses were deduced from primary amino acid sequences.
However, in vitro induction of T3S is an all-or-nothing phenomenon that might not be synchronous, given the various stages of T3SS assembly in a growing culture. Such a lack of synchronicity would negate any efforts to illustrate hierarchal secretion. Therefore, we turned to testing the temporal secretion of our chimeras in a bacterial viability assay associated with infections of macrophage-like J774-1 cell monolayers. This immune cell-based assay is far more stringent than the traditional epithelial cell-based YopE-dependent cytotoxic assay. In the latter assay, whether or not Yersinia can translocate YopE into HeLa cells has no consequence on bacterial survival. In contrast, the immune cell-based assay effectively distinguishes T3SSs with respect to functional status, because T3S-defective Yersinia bacteria, or those devoid of one or more effectors, are phagocytosed and destroyed by the antibacterial effects of an intracellular macrophage environment (7). In other words, such bacteria would be identifiable by reduced extracellular proliferation in experiments performed with eukaryotic cells. Significantly, in such a cell-based assay, only those T3SSs on the surface of bacteria that are in direct contact with eukaryotic cells are presumed to be functionally competent—thereby conferring synchronous effector translocation (63). Bacteria were incubated with cell monolayers for a sufficient time to attain target cell contact. Bacteria in suspension were removed, and the proliferation of bacteria at the cell surface and after internalization was monitored over time by performing counts of total viable numbers. Data were expressed as the ratio of mutant bacteria to parental bacteria. The latter remain predominately extracellular and therefore ably proliferate during 6 h of incubation. Thus, a ratio below 1.0 implies that the mutant bacteria are less viable than the parent bacteria, whereas a ratio equivalent to 1.0 means that the mutant bacteria are as viable as the parent bacteria. As a positive control, we used bacteria producing YopDhigh(x2) with a dramatically altered N-terminal sequence that in no way affects the function of YopD. As expected, a determination of a ratio of ∼1.0 at every time interval indicated that this mutant was as viable as the parental bacteria (Fig. 10). This confirms our previous findings (see Fig. 8) that the YopD N-terminal sequence is not directly involved in the translocation process per se. For negative-control experiments, we infected cells with bacteria lacking yopD or yopE or both. Progressively fewer bacteria were recovered as the experiment proceeded, such that, at its conclusion (at 6 h postinfection), around 5-fold fewer of these mutant bacteria were recovered compared to the parent bacteria (Fig. 10). Although not quite to the same extent, bacteria producing the YopDE-Nterm and/or YopED-Nterm chimeras were also significantly less viable after 4 h and 6 h postinfection than Yersinia bacteria producing native YopD or YopDhigh(x2) (Mann-Whitney U test; P < 0.05) (Fig. 10). Hence, these chimeric bacterial strains were impaired in their ability to efficiently resist phagocytosis by the macrophage-like cells and were therefore subsequently exposed to various intracellular antibacterial killing strategies of the infected immune cells. Thus, these data might favor the idea of the existence of translocator- and effector-type N-terminal secretion signals that assist establishment of appropriate temporal Yop secretion that enables Yersinia bacteria to orchestrate Yop effector translocation to prevent bacterial uptake and avoid exposure to the antibacterial effects of an intracellular macrophage environment.
Fig. 10.
Swapping Yop substrate N-terminal secretion signals compromises T3SS activity during contact with eukaryotic cells. (A) Yersinia bacteria were used to infect monolayers of macrophage J774-1 cells. Those bacteria with a compromised T3SS were more rapidly phagocytosed and killed by the antibacterial activities of the cell. Bacterial viability was tested at the time of inoculation and at 2 h, 4 h, and 6 h postinfection. The data represent numbers of CFU per milliliter expressed as the ratio of mutant to parent bacteria. Each symbol represents one independent experiment; each error bar represents ± the standard error of the mean, which in turn is indicated as a short horizontal line. Bacteria producing either or both of YopDE-Nterm and YopED-Nterm were always less viable, suggesting a compromised order of YopD and YopE secretion. Bacteria either lacking YopE or failing to secrete YopD were even less viable. Strains: YopDΔ4-20, YPIII/pIB625; ΔyopE, YPIII/pIB522; YopDΔ4-20, ΔyopE, YPIII/pIB522-625; YopDhigh(x2), YPIII/pIB62537; YopDB-Nterm, YPIII/pIB62577; YopEH-Nterm, YPIII/pIB578; YopDE-Nterm, YPIII/pIB62501; YopED-Nterm, YPIII/pIB577; YopDE-Nterm, YopED-Nterm, YPIII/pIB62501-577. The asterisks (*, **, or ***) indicate that the chimeric variants were statistically less viable (two-tailed parametric Mann-Whitney U test, P < 0.05) than parent bacteria or bacteria producing YopDhigh(x2) after 4 h and 6 h of incubation in the presence of J774-1 cell monolayers. On the other hand, the viability of bacteria producing YopEH-Nterm was comparable to that of the control bacteria. ns, not statistically significant.
It is also possible that secretion signals have evolved specifically for and function best for only their cognate substrate. Thus, under stringent in vivo conditions, any chimeric T3S substrate harboring a heterologous N-terminal secretion signal might by default be defective in delivery and/or function. To examine this possibility, we constructed hybrid proteins with secretion signals from proteins of the same T3S substrate class. In particular, the N-terminal region of the YopB translocator was incorporated into YopD (YopDB-Nterm) and the secretion signal of the YopH effector into YopE (YopEH-Nterm). These two chimeras also were secreted with efficiency similar to that of the native protein (Fig. 9 and data not shown). We next performed a viability assay with bacteria producing these chimeric hybrids. Intriguingly, the viability of bacteria producing the YopEH-Nterm chimera over the entire 6-h infection period remained comparable to that of Yersinia bacteria producing native YopD or YopDhigh(x2) (Fig. 10). In contrast, Yersinia bacteria producing YopDB-Nterm survived poorly in our viability assay (Fig. 10). Hence, in the presence of host immune cells, YopEH-Nterm, with an alternative secretion signal from the same substrate class, maintained proper secretion and function. Conversely, YopDB-Nterm, with one other translocator secretion signal, did not. Thus, it appears that Yop effector N termini can be interchanged without any obvious detrimental effect so long as the swapped secretion signal sequence is derived from the same T3S substrate class (effector) and not from a different class (translocator). On the other hand, YopD secretion and/or function is poorly preserved regardless of whether the exchanged sequence originated from an alternative translocator or from an effector. The native YopD N terminus is therefore particularly critical for YopD function when the effectiveness of the Ysc-Yop T3SS is crucial (i.e., in the presence of “enemy” immune cells). In general terms, therefore, our data support those of an earlier study (67) that suggested that translocators and effectors do have distinct chaperone-independent N-terminal secretion signals to assist in orchestrating temporal secretion.
Interestingly, when we used the standard HeLa cell cytotoxicity assay as a measure of YopE translocation, no differences in the translocation rates of native YopE or YopED-Nterm produced by Yersinia possessing either native YopD or YopDE-Nterm could be detected (Fig. 8; see also Fig. S6 in the supplemental material). As has already been suggested (12, 17, 19), the YopE-based cytotoxicity assay may not permit detection of subtle defects in Ysc-Yop T3SS functionality. Indeed, even some mutant bacteria capable of only modest YopD secretion (YopDI3,5N, YopDΔ7-19, YopDΔ8-19, YopDΔ9-19, and YopDΔ10-19) were still fully cytotoxic to HeLa cell monolayers.
The first 15 N-terminal residues act as an efficient T3S signal.
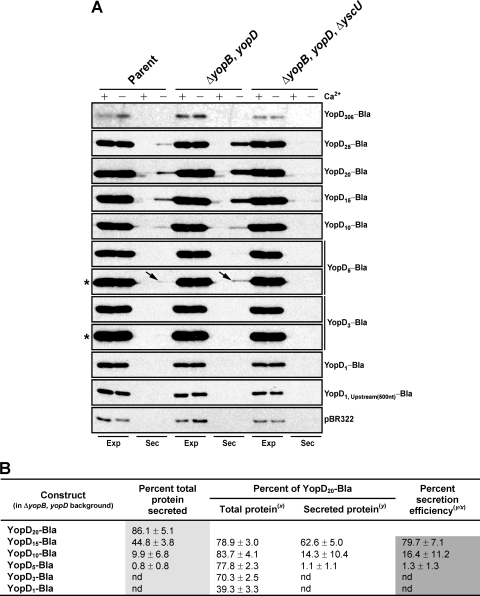
Thus far, our analysis has indicated that the extreme YopD N terminus contributes to secretion efficiency and may possibly influence temporal secretion control, although those effects apparently do not directly extend to effector translocation per se. To complete our study, we were curious as to whether those N-terminal residues could also function as an independent secretion signal to promote the T3S of β-lactamase, which is a reporter lacking a secretion signal. A series of translational fusions between the 5′ end of yopD (including the native SD sequence) and a bla allele lacking a secretion signal and promoter were generated. Plasmids were maintained in trans, and expression of each fusion was controlled by an IPTG-inducible promoter. For a control experiment, we also generated a fusion in which the full-length yopD open reading frame was appended to promoterless bla. However, this generated a poorly expressed (Fig. 11A) and unstable (see Fig. S7 in the supplemental material) product that prevented meaningful comparisons to the shorter N-terminal fusions. Nevertheless, sequences of yopD encoding the first 25, 20, 15, and 10 amino acids were all sufficient to produce generous levels of β-lactamase, a portion of which was secreted. Secretion by Yersinia lacking the native yopB and yopD alleles and also (to a lesser extent) by parental bacteria was readily observed (Fig. 11A). This secretion was dependent on the presence of a functional T3SS, because an isogenic yopB yopD mutant also lacking YscU, an integral T3SS component, failed to secrete these fusions. The finding of inhibited secretion by parental bacteria producing endogenous YopB and YopD is not surprising, because one would expect native substrates to be preferentially secreted—with the preferential secretion presumably mediated via the action of the cognate T3S chaperone LcrH—and this would then cause transient blockage of the T3SS channel. Critically, the secretion efficiencies of YopD10-Bla (16.4%) and YopD15-Bla (79.7%) were less than that seen with YopD20-Bla (Fig. 11B, dark gray box). Additionally, the YopD5-Bla fusion was also secreted. However, secretion of the YopD5-Bla fusion occurred at an efficiency of 1.3%, which was only a fraction of the secretion efficiency of YopD20-Bla (Fig. 11B, dark gray box), and was seen only in the translocator mutant background and often required overexposure of the immunoblot image (Fig. 11A). On the other hand, the smallest yopD fusions of 3 and 1 amino acids did not visibly promote secretion of the reporter; neither did a fusion containing the start codon together with 500 nt of upstream sequence (Fig. 11A). Hence, an efficient secretion signal of YopD consists of probably more than 10 but fewer than 16 N-terminal residues, whereas the first 5 amino acids may constitute the absolutely minimal YopD secretion signal. Moreover, steady-state levels of accumulated YopD1-Bla fusion, at only 39.3% of the level seen with synthesized YopD20-Bla, were dramatically diminished (Fig. 11B). This reduction in accumulation could not be explained by a simple increase in protein turnover, for no differences among these smaller fusions with respect to stability were observed (see Fig. S7 in the supplemental material). This might suggest the finding that the extreme YopD N-terminal sequence also contains an element(s) necessary for translation control. However, we acknowledge that there may other explanations for the low abundance of YopD1-Bla in these steady-state experiments.
Fig. 11.
Formation of the T3S of a β-lactamase reporter by appending the YopD N terminus. (A) Derivatives of pMMB208 contained yopD::bla translational fusions placed under the control of an IPTG-inducible promoter. The constructs were maintained in parental (YPIII/pIB102), ΔyopB yopD (YPIII/pIB619), and ΔyopB yopD ΔyscU (YPIII/pIB619-75) bacteria. Overnight cultures of these bacteria were subcultured into BHI broth either containing (+) or lacking (−) calcium and grown at 26°C for 1 h and then at 37°C for 3 h. Expression fractions (Exp) representing total protein associated with bacteria and also released into the culture supernatant and secretion fractions (Sec) representing protein freely released into the culture supernatant were fractionated by 12% SDS-PAGE and then transferred onto a membrane support for immune detection using rabbit polyclonal antisera recognizing β-lactamase. Panels: YopD306-Bla, pAA113; YopD25-Bla, pAA020 and pAA020; YopD20-Bla, pAA022; YopD15-Bla, pAA025; YopD10-Bla, pAA026; YopD5-Bla, pAA027; YopD3-Bla, pAA069; YopD1-Bla, pAA028 and pBR322 (containing native β-lactamase). The asterisks highlight overexposed panels. The overexposure enabled visualization of YopD5::Bla fusion secretion (indicated by arrows) but not visualization of secretion of the YopD3-Bla fusion; codons 1 to 5 may therefore represent the minimal N-terminal YopD secretion signal. The synthesis and secretion of selected fusions were quantified according to the legend to Fig. 2. Limited by an acutely unstable full-length YopD306-Bla fusion, relative synthesis and secretion of the smaller truncated fusions were compared with synthesis and secretion of YopD20-Bla.
DISCUSSION
We investigated how the N-terminal secretion signal influences the activity of the YopD translocator from enteropathogenic Y. pseudotuberculosis. While we could not definitively rule out the possibility that an mRNA-based secretion signal is involved, the results of experiments performed with frame-shifted mutants and the use of mRNA structural predictions indicate that the N-terminal secretion signal of YopD is more likely to be protein based. In addition, codons for isoleucine at positions 3 and 5 were found to be required for full YopD secretion. These two codons were a part of the absolute minimal secretion signal encompassing the first five residues of YopD. This signal enabled the reporter protein β-lactamase lacking signal to be secreted via a T3S-dependent mechanism, although secretion levels increased appreciably when a larger YopD N-terminal sequence was appended. These translational fusion studies also indicated that this extreme N-terminal sequence might contribute to the control of translation—an underappreciated feature of T3S control. Moreover, swapping the N-terminal secretion signal of YopD with the equivalent region derived from the translocated YopE effector or the YopB translocator affected T3S function during Yersinia-immune cell contact, despite exhibiting a normal Yop secretion profile in vitro. Collectively, these data identify the N-terminal sequence of YopD not only as a genuine secretion signal but also as a possible mediator of translation control. Logically, these could be critical features necessary to coordinate the multiple activities of YopD both in the bacterial cytoplasm and at the zone of contact between bacteria and the host cell.
By serendipity, we observed an apparent correlation between the length of the yopD 5′ coding sequence and the production level. This was most evident when examining the yopD-bla translation fusions; the presence of only the yopD-derived AUG start codon dramatically reduced the level of β-lactamase synthesis obtained. Efforts to further investigate this interesting phenotype were beyond the scope of this study. While we are still to rule out trivial differences related to transcription levels or mRNA stability, at least we know that the low YopD1-Bla yield was not due to an increase in protein turnover. Thus, it is tempting to speculate that a feature within the 5′ region of the yopD mRNA transcript might contribute to control of YopD translation. Several mechanisms of translation control are described in the literature and can involve both cis- and trans-acting factors, all of which are dependent upon the sequence and structure of the mRNA transcript (30, 42). If, as suspected, features of the 5′ end of the yopD mRNA transcript are important for translation control, a coupling between translation and secretion, an event likely to help prioritize YopD for secretion, is a theoretical possibility. Interestingly, translation and secretion coupling has already been proposed for substrates of the flagellum-dependent T3SS (40).
The YopDFrame+1 variant with an altered N-terminal protein sequence and only minor changes to the mRNA sequence was poorly secreted. One might therefore consider the efficient secretion of the alternative frameshifted YopDFrame−1 variant to represent a contradiction. This frameshift mutation was placed after codon 4 to avoid introducing a premature stop codon. However, site-directed mutagenesis confirmed that retention of these native codons (encoding Thr2 and Ile3) was not the reason for efficient secretion. We believe that a more likely scenario is that the physical and/or chemical characteristics of N-terminal residues in YopDFrame−1 fortuitously combine to enable its continued secretion. Of significance is the recent prediction that the overall amphipathicity of the N-terminal secretion signal, with an enrichment of serine, threonine, and proline and, possibly, even short stretches of hydrophobic residues, is a characteristic of a working T3S signal (6, 48, 64, 84). Interestingly, YopDFrame−1 still harbors an N terminus interspersed with hydrophobic residues and a relatively high proportion (5 of 15) of serine, threonine, and/or proline residues, a pattern also observed for other secretion-competent YopD N-terminal sequences (see Table 2). In contrast, it is evident that the nonsecreted YopDFrame+1 secretion signal contains very few hydrophobic residues and also essentially lacks (i.e., possesses only 1 of 15) serine, threonine, and proline residues. We therefore assume that these differences account for the secretion disparities between the two frameshifted mutants. This could also explain the slight reduction in YopDI3,5N secretion compared to YopDFrame−1, I3,4N secretion; whereas the proportions of serine, threonine, and proline residues are consistent between the two, the former protein has a more restricted distribution of hydrophobic amino acids (see Table 2). It is also worth keeping in mind that N-terminal-mediated substrate secretion can be influenced by the sequence composition further downstream; secretion of the T3S of YopR (also termed YscH) by Yersinia requires a distinct mRNA motif nearer the C terminus that cooperates with the typical N-terminal amino acid signal (9). The presence of such an internal secondary mRNA sequence in YopD has not been investigated.
Due in part to inherent limitations of conventional in vitro assays, evidence of a secretion hierarchy among Yersinia T3S translocator and effector substrates is essentially absent. Ysc-Yop T3SS assembly in a growing laboratory culture would be asynchronous, disguising any evidence of ordered secretion between early, middle, and late substrates. Orchestrated secretion is also masked in vitro by the low calcium response, an “all-or-none” phenomenon that reveals massive amounts of synthesized and secreted Yops when Ca2+ is specifically depleted (80). In contrast, bacteria in close association with target host cells are thought to possess functional T3SSs only at the contact zone (63). Therefore, hierarchal secretion is more likely to be observed during bacterium-host cell contact, the only setting where subsets of T3SSs are turned on simultaneously. In fact, real-time monitoring of this process has been reported for other bacteria (21, 51, 78, 82), but our laboratory is not yet equipped to perform such sophisticated experimentation. However, we did observe that, during infections of J774.1 macrophage-like cells, bacteria secreting YopD and YopE chimeras in which their respective N-terminal secretion signal domains were reciprocally exchanged were significantly more susceptible to host cell antibacterial killing than were parental Yersinia bacteria. These chimeras therefore sufficiently impair the T3S process to ensure that bacteria are less able to use their T3SSs to promote survival when under siege from host cell innate immune defense mechanisms. This could imply that swapping the respective N-terminal secretion signals compromised temporal delivery of YopD and YopE.
If this hierarchal secretion model is correct, why do the chimera-producing strains all behave like the parental strain in the HeLa cell cytotoxicity assay? To begin with, the cytotoxicity assay is not capable of detecting subtle defects in Ysc-Yop T3SS functionality (12, 17, 19). In addition, Yersinia-induced cytotoxicity and antiphagocytosis are phenotypically unlinked; highly attenuated yopE point mutants are still cytotoxic with respect to HeLa cell monolayers, whereas bacteria defective in antiphagocytosis are always avirulent (1, 75, 77). Accordingly, for use as an indirect measure of Yersinia-mediated antiphagocytosis, we believe the viability assay to be a superior tool to ascertain T3SS function. In view of this, it might also be expected that bacteria coproducing YopDE-Nterm and YopED-Nterm would show a more drastic reduction in viability (the timing being completely wrong, as YopE would be secreted before YopD) in comparison with bacteria producing one or the other chimera (YopD and YopE would be secreted at the same time). However, one caveat regarding the viability readout is that it is not solely a measure of translocated YopE function. For example, Yersinia antiphagocytosis also requires immediate delivery of the YopH phosphatase to the host cell interior (3, 5). It is likely that the influence of intracellular YopH activity would mask some of the subtle variances in viability of the three different YopD-YopE chimeric strains. It is also notable that T3S chaperones contribute to cognate substrate secretion (26). Since both YopDE-Nterm and YopED-Nterm still maintain their cognate binding domains for the LcrH and SycE chaperones, respectively (27, 83), a reasonable assumption would be that the native T3S chaperone-piloting function is retained. Thus, this could also curb some of the effects of N-terminal domain swapping within the cognate substrates. With some justification, therefore, we believe that our data are a reliable initial indicator that the N-terminal domain might be involved in orchestrating T3S, although definitive proof is still lacking.
So how might the chaperone-independent and chaperone-dependent secretion signals prioritize substrate secretion? Recent data indicate the existence of a large multiprotein cytoplasmic complex that could function as a T3S substrate-sorting platform (44). Within this platform exists a conserved ATPase present in all T3SSs that is responsible for energizing substrate secretion (68, 70). Ample biochemical evidence indicates that effectors can interact with their cognate ATPases either directly or indirectly via their T3S chaperones (2, 10, 15, 33, 50, 68, 70, 73, 74). However, there is still no published report demonstrating binding between the ATPase and translocator-chaperone complexes. Moreover, tangible proof of the specificity underpinning the putative recognition mechanisms is thwarted by a scarcity of structural information on T3S ATPases, either alone or in association with T3S chaperone and/or substrate complexes (2, 37, 38, 86). It is therefore prudent to highlight alternative modes for substrate recognition and the creation of hierarchal secretion. In particular, translocator class T3S chaperones can selectively engage a membrane-associated T3SS component that exists in both flagellar T3SSs (termed FliJ) (23) and nonflagellar T3SSs (e.g., InvI in Salmonella and YscO in Yersinia) (22). These protein complexes are thought to rapidly recycle empty translocator chaperones to collect new substrates before the appearance of the late effector substrates to advance their secretion. Members of the InvE protein family present in a variety of T3SS-containing bacteria may also physically discriminate between middle and late substrate classes to prioritize translocator secretion (11, 18, 43, 49, 56, 85). In Yersinia, YopN and TyeA share homology with the N- and C-terminal regions of the InvE protein family, respectively. However, their loss does not specifically decrease YopB and YopD translocator secretion (25, 71), even though a YopD complex with TyeA has been previously reported (39). Finally, the integral inner membrane protein YscU—a core component of all T3SSs—might also distinguish between middle and late T3S substrates on the basis of differential recognition of the substrate N terminus (61, 67), although this is not true for all homologues (8). We are currently endeavoring to unravel the relative contributions of all the parameters that may enable Yersinia to prioritize translocator secretion before secretion of the Yop effector arsenal begins.
At least for the Ysc-Yop T3SS, our data indicate that the N terminus may contain structural information specific for the translocator and effector substrate classes. This finding is not without precedent; in flagellar T3SSs, the molecular basis of early and late flagellum substrate sorting is believed to involve such a structural demarcation (70). The fact that a YopE chimera containing the secretion signal of the YopH effector N terminus did not compromise Yersinia T3S function supports this view. Yop effector substrates might contain a generic N-terminal sequence with shared characteristics that assist with ordering their secretion after the translocators. However, the situation appears far more complex for the multipotent YopD protein, since the YopD chimeric function at the zone of bacterium-host cell contact was disrupted regardless of whether the exchanged N-terminal sequence was of translocator (YopB) or effector (YopE) origin. Whether this is a universal phenomenon characteristic of all T3SSs remains unclear. The reciprocal exchange of N-terminal T3S secretion signals of enteropathogenic Escherichia coli (EPEC) translocator and effector proteins does not seem to interfere with function (54). While this observation might be at odds with our study results, it is prudent to highlight that the EPEC study relied solely on in trans experimentation; all our chimeric constructs are stably placed in cis in the genome as monocopy alleles. There can be no doubting that this key difference would impact phenotypic output, making meaningful interpretation more difficult.
Given our evidence for the requirement of specific characteristics within individual N-terminal secretion signals for translocator and effector functions, it is both impressive and curious that the artificial “highx2” sequence functions normally for YopD (this study) and for YopE (47). It would be rewarding to learn what features of this synthetic sequence universally permit substrate secretion and function. However, these can really be appreciated only once the specific characteristics of each individual N-terminal secretion signal are unequivocally defined. We have used extensive molecular methods to reveal that YopD harbors an N-terminal secretion signal with peculiarities that make it distinct from other signals, but it has still proven impossible to pinpoint what the peculiarities are. Thus, it is now necessary to undertake complementing biochemical and biophysical approaches with the intention of contributing crucial advances in our knowledge of the T3S substrate secretion signal.
Supplementary Material
ACKNOWLEDGMENTS
This work, performed within the framework of the Umeå Centre for Microbial Research (UCMR) Linnaeus Program (LP), was supported by grants from the Carl Tryggers Foundation for Scientific Research (M.S.F.), Swedish Research Council (Å.F., M.S.F.), Foundation for Medical Research at Umeå University (M.S.F.), Swedish Medical Association (M.S.F.), and J. C. Kempe Memorial Fund (A.A.A.A.).
We express gratitude to Hans Wolf-Watz for the gift of the parental Yersinia strain and various anti-Yop and anti-LcrV antibodies as well as to Andrew Roe for providing the plasmid pAJR104.
Footnotes
Supplemental material for this article may be found at http://jb.asm.org/.
Published ahead of print on 30 September 2011.
REFERENCES
- 1. Aili M., Isaksson E. L., Hallberg B., Wolf-Watz H., Rosqvist R. 2006. Functional analysis of the YopE GTPase-activating protein (GAP) activity of Yersinia pseudotuberculosis. Cell Microbiol. 8:1020–1033 [DOI] [PubMed] [Google Scholar]
- 2. Akeda Y., Galan J. E. 2005. Chaperone release and unfolding of substrates in type III secretion. Nature 437:911–915 [DOI] [PubMed] [Google Scholar]
- 3. Akopyan K., et al. 2011. Translocation of surface-localized effectors in type III secretion. Proc. Natl. Acad. Sci. U. S. A. 108:1639–1644 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Anderson D. M., Ramamurthi K. S., Tam C., Schneewind O. 2002. YopD and LcrH regulate expression of Yersinia enterocolitica YopQ by a posttranscriptional mechanism and bind to yopQ RNA. J. Bacteriol. 184:1287–1295 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Andersson K., Magnusson K. E., Majeed M., Stendahl O., Fallman M. 1999. Yersinia pseudotuberculosis-induced calcium signaling in neutrophils is blocked by the virulence effector YopH. Infect. Immun. 67:2567–2574 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Arnold R., et al. 2009. Sequence-based prediction of type III secreted proteins. PLoS Pathog. 5:e1000376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Bartra S., Cherepanov P., Forsberg A., Schesser K. 2001. The Yersinia YopE and YopH type III effector proteins enhance bacterial proliferation following contact with eukaryotic cells. BMC Microbiol. 1:22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Björnfot A. C., Lavander M., Forsberg A., Wolf-Watz H. 2009. Autoproteolysis of YscU of Yersinia pseudotuberculosis is important for regulation of expression and secretion of Yop proteins. J. Bacteriol. 191:4259–4267 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Blaylock B., Sorg J. A., Schneewind O. 2008. Yersinia enterocolitica type III secretion of YopR requires a structure in its mRNA. Mol. Microbiol. 70:1210–1222 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Boonyom R., Karavolos M. H., Bulmer D. M., Khan C. M. 2010. Salmonella pathogenicity island 1 (SPI-1) type III secretion of SopD involves N- and C-terminal signals and direct binding to the InvC ATPase. Microbiology 156:1805–1814 [DOI] [PubMed] [Google Scholar]
- 11. Botteaux A., Sory M. P., Biskri L., Parsot C., Allaoui A. 2009. MxiC is secreted by and controls the substrate specificity of the Shigella flexneri type III secretion apparatus. Mol. Microbiol. 71:449–460 [DOI] [PubMed] [Google Scholar]
- 12. Bröms J. E., Francis M. S., Forsberg A. 2007. Diminished LcrV secretion attenuates Yersinia pseudotuberculosis virulence. J. Bacteriol. 189:8417–8429 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Cambronne E. D., Schneewind O. 2002. Yersinia enterocolitica type III secretion: yscM1 and yscM2 regulate yop gene expression by a posttranscriptional mechanism that targets the 5′ untranslated region of yop mRNA. J. Bacteriol. 184:5880–5893 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Carlsson K. E., Liu J., Edqvist P. J., Francis M. S. 2007. Extracytoplasmic-stress-responsive pathways modulate type III secretion in Yersinia pseudotuberculosis. Infect. Immun. 75:3913–3924 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Cooper C. A., et al. 2010. Structural and biochemical characterization of SrcA, a multi-cargo type III secretion chaperone in Salmonella required for pathogenic association with a host. PLoS Pathog. 6:e1000751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Cornelis G. R. 2003. How Yops find their way out of Yersinia. Mol. Microbiol. 50:1091–1094 [DOI] [PubMed] [Google Scholar]
- 17. Costa T. R., et al. 2010. YopD self-assembly and binding to LcrV facilitate type III secretion activity by Yersinia pseudotuberculosis. J. Biol. Chem. 285:25269–25284 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Deng W., et al. 2005. Regulation of type III secretion hierarchy of translocators and effectors in attaching and effacing bacterial pathogens. Infect. Immun. 73:2135–2146 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Edqvist P. J., Aili M., Liu J., Francis M. S. 2007. Minimal YopB and YopD translocator secretion by Yersinia is sufficient for Yop-effector delivery into target cells. Microbes Infect. 9:224–233 [DOI] [PubMed] [Google Scholar]
- 20. Edqvist P. J., et al. 2006. Tetratricopeptide repeats in the type-III-secretion chaperone, LcrH: their role in substrate binding and secretion. Mol. Microbiol. 59:31–44 [DOI] [PubMed] [Google Scholar]
- 21. Enninga J., Mounier J., Sansonetti P., Tran Van Nhieu G. 2005. Secretion of type III effectors into host cells in real time. Nat. Methods 2:959–965 [DOI] [PubMed] [Google Scholar]
- 22. Evans L. D., Hughes C. 2009. Selective binding of virulence type III export chaperones by FliJ escort orthologues InvI and YscO. FEMS Microbiol. Lett. 293:292–297 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Evans L. D., Stafford G. P., Ahmed S., Fraser G. M., Hughes C. 2006. An escort mechanism for cycling of export chaperones during flagellum assembly. Proc. Natl. Acad. Sci. U. S. A. 103:17474–17479 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Feldman M. F., Muller S., Wuest E., Cornelis G. R. 2002. SycE allows secretion of YopE-DHFR hybrids by the Yersinia enterocolitica type III Ysc system. Mol. Microbiol. 46:1183–1197 [DOI] [PubMed] [Google Scholar]
- 25. Forsberg Å., Viitanen A. M., Skurnik M., Wolf-Watz H. 1991. The surface-located YopN protein is involved in calcium signal transduction in Yersinia pseudotuberculosis. Mol. Microbiol. 5:977–986 [DOI] [PubMed] [Google Scholar]
- 26. Francis M. S. 2010. Type III secretion chaperones: a molecular toolkit for all occasions, p. 79–147 In Durante P., Colucci L. (ed.), Handbook of molecular chaperones: roles, structures and mechanisms. Nova Science Publishers, Inc., Hauppauge, NY [Google Scholar]
- 27. Francis M. S., Aili M., Wiklund M. L., Wolf-Watz H. 2000. A study of the YopD-LcrH interaction from Yersinia pseudotuberculosis reveals a role for hydrophobic residues within the amphipathic domain of YopD. Mol. Microbiol. 38:85–102 [DOI] [PubMed] [Google Scholar]
- 28. Francis M. S., Lloyd S. A., Wolf-Watz H. 2001. The type III secretion chaperone LcrH co-operates with YopD to establish a negative, regulatory loop for control of Yop synthesis in Yersinia pseudotuberculosis. Mol. Microbiol. 42:1075–1093 [DOI] [PubMed] [Google Scholar]
- 29. Francis M. S., Wolf-Watz H. 1998. YopD of Yersinia pseudotuberculosis is translocated into the cytosol of HeLa epithelial cells: evidence of a structural domain necessary for translocation. Mol. Microbiol. 29:799–813 [DOI] [PubMed] [Google Scholar]
- 30. Fredrick K., Ibba M. 2010. How the sequence of a gene can tune its translation. Cell 141:227–229 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Galán J. E. 2009. Common themes in the design and function of bacterial effectors. Cell Host Microbe 5:571–579 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Galán J. E. 2008. Energizing type III secretion machines: what is the fuel? Nat. Struct. Mol. Biol. 15:127–128 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Gauthier A., Finlay B. B. 2003. Translocated intimin receptor and its chaperone interact with ATPase of the type III secretion apparatus of enteropathogenic Escherichia coli. J. Bacteriol. 185:6747–6755 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Goure J., Broz P., Attree O., Cornelis G. R., Attree I. 2005. Protective anti-V antibodies inhibit Pseudomonas and Yersinia translocon assembly within host membranes. J. Infect. Dis. 192:218–225 [DOI] [PubMed] [Google Scholar]
- 35. Harrington A. T., et al. 2003. Structural characterization of the N terminus of IpaC from Shigella flexneri. Infect. Immun. 71:1255–1264 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Hartland E. L., Green S. P., Phillips W. A., Robins-Browne R. M. 1994. Essential role of YopD in inhibition of the respiratory burst of macrophages by Yersinia enterocolitica. Infect. Immun. 62:4445–4453 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Imada K., Minamino T., Kinoshita M., Furukawa Y., Namba K. 2010. Structural insight into the regulatory mechanisms of interactions of the flagellar type III chaperone FliT with its binding partners. Proc. Natl. Acad. Sci. U. S. A. 107:8812–8817 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Imada K., Minamino T., Tahara A., Namba K. 2007. Structural similarity between the flagellar type III ATPase FliI and F1-ATPase subunits. Proc. Natl. Acad. Sci. U. S. A. 104:485–490 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Iriarte M., et al. 1998. TyeA, a protein involved in control of Yop release and in translocation of Yersinia Yop effectors. EMBO J. 17:1907–1918 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Karlinsey J. E., Lonner J., Brown K. L., Hughes K. T. 2000. Translation/secretion coupling by type III secretion systems. Cell 102:487–497 [DOI] [PubMed] [Google Scholar]
- 41. Kim B. H., Kim H. G., Kim J. S., Jang J. I., Park Y. K. 2007. Analysis of functional domains present in the N-terminus of the SipB protein. Microbiology 153:2998–3008 [DOI] [PubMed] [Google Scholar]
- 42. Kozak M. 2005. Regulation of translation via mRNA structure in prokaryotes and eukaryotes. Gene 361:13–37 [DOI] [PubMed] [Google Scholar]
- 43. Kubori T., Galán J. E. 2002. Salmonella type III secretion-associated protein InvE controls translocation of effector proteins into host cells. J. Bacteriol. 184:4699–4708 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Lara-Tejero M., Kato J., Wagner S., Liu X., Galan J. E. 2011. A sorting platform determines the order of protein secretion in bacterial type III systems. Science 331:1188–1189 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Lavander M., et al. 2002. Proteolytic cleavage of the FlhB homologue YscU of Yersinia pseudotuberculosis is essential for bacterial survival but not for type III secretion. J. Bacteriol. 184:4500–4509 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Lloyd S. A., Forsberg Å., Wolf-Watz H., Francis M. S. 2001. Targeting exported substrates to the Yersinia TTSS: different functions for different signals? Trends Microbiol. 9:367–371 [DOI] [PubMed] [Google Scholar]
- 47. Lloyd S. A., Sjöström M., Andersson S., Wolf-Watz H. 2002. Molecular characterization of type III secretion signals via analysis of synthetic N-terminal amino acid sequences. Mol. Microbiol. 43:51–59 [DOI] [PubMed] [Google Scholar]
- 48. Löwer M., Schneider G. 2009. Prediction of type III secretion signals in genomes of gram-negative bacteria. PLoS One 4:e5917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Martinez-Argudo I., Blocker A. J. 2010. The Shigella T3SS needle transmits a signal for MxiC release, which controls secretion of effectors. Mol. Microbiol. 78:1365–1378 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Miki T., Shibagaki Y., Danbara H., Okada N. 2009. Functional characterization of SsaE, a novel chaperone protein of the type III secretion system encoded by Salmonella pathogenicity island 2. J. Bacteriol. 191:6843–6854 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Mills E., Baruch K., Charpentier X., Kobi S., Rosenshine I. 2008. Real-time analysis of effector translocation by the type III secretion system of enteropathogenic Escherichia coli. Cell Host Microbe 3:104–113 [DOI] [PubMed] [Google Scholar]
- 52. Milton D. L., O'Toole R., Horstedt P., Wolf-Watz H. 1996. Flagellin A is essential for the virulence of Vibrio anguillarum. J. Bacteriol. 178:1310–1319 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Mueller C. A., Broz P., Cornelis G. R. 2008. The type III secretion system tip complex and translocon. Mol. Microbiol. 68:1085–1095 [DOI] [PubMed] [Google Scholar]
- 54. Munera D., Crepin V. F., Marches O., Frankel G. 2010. N-terminal type III secretion signal of enteropathogenic Escherichia coli translocator proteins. J. Bacteriol. 192:3534–3539 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Neyt C., Cornelis G. R. 1999. Insertion of a Yop translocation pore into the macrophage plasma membrane by Yersinia enterocolitica: requirement for translocators YopB and YopD, but not LcrG. Mol. Microbiol. 33:971–981 [DOI] [PubMed] [Google Scholar]
- 56. O'Connell C. B., et al. 2004. SepL, a protein required for enteropathogenic Escherichia coli type III translocation, interacts with secretion component SepD. Mol. Microbiol. 52:1613–1625 [DOI] [PubMed] [Google Scholar]
- 57. Olsson J., et al. 2004. The YopD translocator of Yersinia pseudotuberculosis is a multifunctional protein comprised of discrete domains. J. Bacteriol. 186:4110–4123 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Pallen M. J., Beatson S. A., Bailey C. M. 2005. Bioinformatics, genomics and evolution of non-flagellar type-III secretion systems: a Darwinian perpective. FEMS Microbiol. Rev. 29:201–229 [DOI] [PubMed] [Google Scholar]
- 59. Ramamurthi K. S., Schneewind O. 2005. A synonymous mutation in Yersinia enterocolitica yopE affects the function of the YopE type III secretion signal. J. Bacteriol. 187:707–715 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Ramamurthi K. S., Schneewind O. 2003. Yersinia yopQ mRNA encodes a bipartite type III secretion signal in the first 15 codons. Mol. Microbiol. 50:1189–1198 [DOI] [PubMed] [Google Scholar]
- 61. Riordan K. E., Schneewind O. 2008. YscU cleavage and the assembly of Yersinia type III secretion machine complexes. Mol. Microbiol. 68:1485–1501 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Rosqvist R., Forsberg Å., Wolf-Watz H. 1991. Intracellular targeting of the Yersinia YopE cytotoxin in mammalian cells induces actin microfilament disruption. Infect. Immun. 59:4562–4569 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Rosqvist R., Magnusson K. E., Wolf-Watz H. 1994. Target cell contact triggers expression and polarized transfer of Yersinia YopE cytotoxin into mammalian cells. EMBO J. 13:964–972 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Samudrala R., Heffron F., McDermott J. E. 2009. Accurate prediction of secreted substrates and identification of a conserved putative secretion signal for type III secretion systems. PLoS Pathog. 5:e1000375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Simon R., Priefer U., Pühler A. 1983. A broad host range mobilisation system for in vivo genetic engineering: transposon mutagenesis in Gram negative bacteria. Nat. Biotechnol 1:784–791 [Google Scholar]
- 66. Skrzypek E., Straley S. C. 1995. Differential effects of deletions in lcrV on secretion of V antigen, regulation of the low-Ca2+ response, and virulence of Yersinia pestis. J. Bacteriol. 177:2530–2542 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Sorg I., et al. 2007. YscU recognizes translocators as export substrates of the Yersinia injectisome. EMBO J. 26:3015–3024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Sorg J. A., Blaylock B., Schneewind O. 2006. Secretion signal recognition by YscN, the Yersinia type III secretion ATPase. Proc. Natl. Acad. Sci. U. S. A. 103:16490–16495 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Sorg J. A., Miller N. C., Schneewind O. 2005. Substrate recognition of type III secretion machines—testing the RNA signal hypothesis. Cell Microbiol. 7:1217–1225 [DOI] [PubMed] [Google Scholar]
- 70. Stafford G. P., et al. 2007. Sorting of early and late flagellar subunits after docking at the membrane ATPase of the type III export pathway. J. Mol. Biol. 374:877–882 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Sundberg L., Forsberg A. 2003. TyeA of Yersinia pseudotuberculosis is involved in regulation of Yop expression and is required for polarized translocation of Yop effectors. Cell Microbiol. 5:187–202 [DOI] [PubMed] [Google Scholar]
- 72. Tardy F., et al. 1999. Yersinia enterocolitica type III secretion-translocation system: channel formation by secreted Yops. EMBO J. 18:6793–6799 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Thomas J., Stafford G. P., Hughes C. 2004. Docking of cytosolic chaperone-substrate complexes at the membrane ATPase during flagellar type III protein export. Proc. Natl. Acad. Sci. U. S. A. 101:3945–3950 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Thomas N. A., et al. 2005. CesT is a multi-effector chaperone and recruitment factor required for the efficient type III secretion of both LEE- and non-LEE-encoded effectors of enteropathogenic Escherichia coli. Mol. Microbiol. 57:1762–1779 [DOI] [PubMed] [Google Scholar]
- 75. Thorslund S. E., et al. 2011. The RACK1 signaling scaffold protein selectively interacts with Yersinia pseudotuberculosis virulence function. PLoS One 6:e16784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Troisfontaines P., Cornelis G. R. 2005. Type III secretion: more systems than you think. Physiology (Bethesda) 20:326–339 [DOI] [PubMed] [Google Scholar]
- 77. Trülzsch K., Sporleder T., Igwe E. I., Rüssmann H., Heesemann J. 2004. Contribution of the major secreted Yops of Yersinia enterocolitica O:8 to pathogenicity in the mouse infection model. Infect. Immun. 72:5227–5234 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Van Engelenburg S. B., Palmer A. E. 2008. Quantification of real-time Salmonella effector type III secretion kinetics reveals differential secretion rates for SopE2 and SptP. Chem. Biol. 15:619–628 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Wattiau P., Bernier B., Deslee P., Michiels T., Cornelis G. R. 1994. Individual chaperones required for Yop secretion by Yersinia. Proc. Natl. Acad. Sci. U. S. A. 91:10493–10497 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Wiley D. J., Rosqvist R., Schesser K. 2007. Induction of the Yersinia type 3 secretion system as an all-or-none phenomenon. J. Mol. Biol. 373:27–37 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Williams A. W., Straley S. C. 1998. YopD of Yersinia pestis plays a role in negative regulation of the low-calcium response in addition to its role in translocation of Yops. J. Bacteriol. 180:350–358 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Winnen B., et al. 2008. Hierarchical effector protein transport by the Salmonella Typhimurium SPI-1 type III secretion system. PLoS One 3:e2178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Woestyn S., Sory M. P., Boland A., Lequenne O., Cornelis G. R. 1996. The cytosolic SycE and SycH chaperones of Yersinia protect the region of YopE and YopH involved in translocation across eukaryotic cell membranes. Mol. Microbiol. 20:1261–1271 [DOI] [PubMed] [Google Scholar]
- 84. Yang Y., Zhao J., Morgan R. L., Ma W., Jiang T. 2010. Computational prediction of type III secreted proteins from gram-negative bacteria. BMC Bioinformatics 11(Suppl. 1):S47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Yu X. J., Liu M., Holden D. W. 2004. SsaM and SpiC interact and regulate secretion of Salmonella pathogenicity island 2 type III secretion system effectors and translocators. Mol. Microbiol. 54:604–619 [DOI] [PubMed] [Google Scholar]
- 86. Zarivach R., Vuckovic M., Deng W., Finlay B. B., Strynadka N. C. 2007. Structural analysis of a prototypical ATPase from the type III secretion system. Nat. Struct. Mol. Biol. 14:131–137 [DOI] [PubMed] [Google Scholar]
- 87. Zuker M. 2003. Mfold web server for nucleic acid folding and hybridization prediction. Nucleic Acids Res. 31:3406–3415 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.