Abstract
The transcription factor Fur regulates the expression of a number of genes in Vibrio cholerae in response to changes in the level of available iron. Fur usually acts as a repressor, but here we show that Fur positively regulates the expression of ompT, which encodes a major outer membrane porin. OmpT levels increased when the bacteria were grown in medium containing relatively high levels of iron, and this effect required Fur. The level of ompT mRNA also is increased in the presence of iron and Fur. The effect of iron on OmpT levels was independent of the known ompT regulators ToxR and Crp, and it did not require RyhB, which has been shown to be responsible for positive regulation by iron of some V. cholerae genes. Electrophoretic mobility shift assays showed that Fur binds upstream of the ompT transcription start site in a region overlapping known binding sites for ToxR and Crp. These data suggest that Fur and iron positively regulate ompT expression through the direct binding of Fur to the ompT promoter.
INTRODUCTION
Many bacterial genes are regulated in response to the level of intracellular iron. In general, genes encoding iron uptake systems are repressed by iron, while those involved in iron storage or oxidative stress may be positively regulated. The control of iron transport and metabolism allows the bacteria to obtain sufficient iron for essential enzymes and pathways while avoiding the toxicity associated with excess iron (1).
The major iron-responsive regulatory factor in Gram-negative bacteria is the transcription factor Fur (13, 14, 21). When intracellular iron is abundant, Fur forms complexes with iron and binds to sites in the promoters that are termed Fur boxes. Many Fur boxes overlap the −10 or −35 region and prevent the transcription of the downstream genes (11). Although most regulation by iron and Fur is negative, the positive regulation of gene expression by Fur has been observed (21). In some cases this positive regulation is indirect and requires a small RNA, RyhB, which interacts with target mRNAs, leading to their degradation (24). In the presence of iron, Fur represses the expression of ryhB, allowing RyhB target genes, including those encoding superoxide dismutase and aconitase, to be expressed (24, 25). More recently, Fur was shown to directly activate the expression of some genes in Helicobacter pylori (6) and Neisseria meningitidis (7), indicating that Fur can act as both a positive and negative regulator of transcription.
In Vibrio cholerae, the causative agent of cholera, genes encoding iron and heme transport systems and a number of metabolic enzymes are repressed by iron via Fur (31). A smaller number of genes are positively regulated by Fur, including genes within the large integron and Tcp region (31). As in Escherichia coli, some, but not all, of the positive regulation by Fur is due to the repression of ryhB (5, 30). Fur and RyhB are critical to V. cholerae survival in the host and in environments outside the host. fur mutants are attenuated in the infant mouse model (31), while ryhB mutants are defective for biofilm formation (30).
Fur is only one of a number of factors that regulate the expression of V. cholerae genes involved in pathogenesis. Notably, the ToxR regulon is the major regulatory pathway controlling the expression of virulence genes (4, 8, 9). In response to signals that have not been fully elucidated, ToxR, together with TcpP, activates the expression of toxT, and ToxT in turn activates the cholera toxin genes ctxAB (33) and tcpA, encoding the toxin-coregulated pilus (43).
ToxR also controls the expression of two outer membrane porins, OmpU and OmpT, and this effect is independent of ToxT and TcpP (32). ToxR binds directly to the promoters of both ompU and ompT but with opposite effects. Whereas ToxR activates the transcription of ompU, it represses transcription from the ompT promoter. Footprinting studies have shown that ToxR binds a large region within the ompT promoter, spanning the nucleotides −30 to −95 upstream of the ompT transcriptional start site (22). ToxR further inhibits OmpT production by preventing the binding of the cyclic AMP (cAMP) receptor protein (CRP) (23). CRP binds to three discrete regions on the ompT promoter, with a distal site centered at −310 and two proximal sites centered at −85 and −7 (23). If CRP binds the distal upstream site as well as the proximal sites, then CRP acts as a positive regulator. However, if CRP binds the proximal sites without binding the distal site, CRP can act as a negative regulator (23).
Here, we show that OmpT levels are influenced by the level of iron, and that iron activates ompT expression in a Fur-dependent but RyhB-independent manner.
MATERIALS AND METHODS
Strains and growth conditions.
Strains (Table 1) were grown at 37°C with shaking in LB broth or in EZ-rich defined medium (EZ-RDM) (http://www.genome.wisc.edu/resources/protocols/ezmedium.htm), a modification of the medium developed by Neidhardt et al. (35). Minimal defined medium was T medium without added iron (40). Sucrose (0.2%) was added to EZ-RDM and T medium as the carbon source unless otherwise indicated. Iron was added to a final concentration of 5 to 40 μM to induce the expression of ompT. Antibiotics were used at the following concentrations for E. coli: 250 μg/ml carbenicillin, 50 μg/ml kanamycin, 30 μg/ml chloramphenicol, and 25 μg/ml ampicillin. For V. cholerae strains, antibiotics were used at the following concentrations: 125 μg/ml carbenicillin, 50 μg/ml kanamycin, 7.5 μg/ml chloramphenicol, 2.5 μg/ml ampicillin, 10 μg/ml polymyxin B (for El Tor strains), and 75 μg/ml streptomycin (for classical strains).
Table 1.
Strains used in this study
| Strain | Characteristic(s) | Source or reference |
|---|---|---|
| V. cholerae | ||
| N16961 | Wild-type El Tor | R. A. Finkelstein |
| C6706 | Wild-type El Tor | R. A. Finkelstein |
| O139 | Wild-type O139 | R. A. Finkelstein |
| O395 | Wild-type classical | 27 |
| SAC116 | N16961 ΔompT::Km | This study |
| SAC119 | N16961 ΔtoxR::Km | This study |
| ARM711 | N16961 ΔryhB::Km | 30 |
| ARM713 | N16961 Δfur::Cm | This study |
| ARM713/pAMF1 | ARM713 complemented with wild-type fur gene | 31 |
| E. coli | ||
| W3110 | K12 | 15 |
| EWE1008 | W3110 Δfur::kan | This study |
Construction of strains containing chromosomal mutations.
A V. cholerae ompT mutant (SAC116) in which most of the coding sequence was deleted and replaced by a kanamycin resistance gene was generated by allelic exchange. Primer sets OmpT7/OmpT2 and OmpT3/OmpT8 were used to amplify overlapping fragments. All primer sequences are shown in Table 2. The overlap extension product then was amplified using primers OmpT7 and OmpT8. The PCR product was A-tailed and ligated into pGEM-T Easy (Promega, Madison, WI), creating plasmid pGEM-OmpTB. The chloramphenicol resistance cassette from pMTL-cam (47) was introduced as a BamHI fragment into the BamHI-digested pGEM-OmpTB, yielding pGEM-OmpTB-cam. pGEM-OmpTB-cam and the suicide vector pCVD442N (48) were digested with NotI and ligated to create pCVD-OmpTB. pCVD-OmpTB was conjugated from DH5αλpir with the helper strain MM294/pRK2013 (12) into V. cholerae strain N16961. Allelic exchange mutants were isolated by selecting for resistance to 10% (wt/vol) sucrose, indicating the loss of the plasmid and resistance to the antibiotic, as described previously (29). Mutants were verified by PCR. All mutants were confirmed by the DNA sequence analysis of the PCR-amplified DNA region and by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) of whole-cell proteins.
Table 2.
Primers and oligonucleotides used in this study
| Primer name | Sequence |
|---|---|
| OmpT2 | 5′ GCTTCTTGGGATCCAATCCACTGCC 3′ |
| OmpT3 | 5′ GCAGTGGATTGGATCCCAAGAAGCG 3′ |
| OmpT7 | 5′ TAGGGAGATGGTGCGCTTCGTC 3′ |
| OmpT8 | 5′ ACCCTTGTGCTTGCGGTAGTGG 3′ |
| ToxR1 | 5′ TATGACTCCACACGAGTG 3′ |
| ToxR2 | 5′ GGCTACGCCCCGGGGCTTAGGGGATCAAAG 3′ |
| ToxR3 | 5′ CCCTAAGCCCCGGGGCGTAGCCGTCAATATGCC 3′ |
| ToxR4 | 5′ GGCGATCAACATGCCCAAG 3′ |
| ToxRV1 | 5′ TGTAAAGTAAGCTGGTGTAG 3′ |
| ToxRV2 | 5′ CTTAACTTTGCGTAGAAGAG 3′ |
| ToxRV3 | 5′ CCGCATCATCCATAATAAAGAC 3′ |
| ToxRV4 | 5′ CAGTTGCCTCCTTCAATAAATC 3′ |
| fur.745 | 5′ GTTGGCGTTTTTATTCCACCAG 3′ |
| fur.nhe.xho.rev | 5′ GGCTCGAGTATTTAGCTAGCTTTCTTCGGCTTGTGAGCGTTAG 3′ |
| Flu1 | 5′ CTAGCTACCCATACGACGTCCCAGACTACGCTTAAC 3′ |
| Flu2 | 5′ TCGAGTTAAGCGTAGTCTGGGACGTCGTATGGGTAG 3′ |
| fur.1254.BseRI | 5′ AGAACAGAGGAGGGAAAGTATATGTCAGACAA 3′ |
| furV-R | 5′ GGAAACAGCTATGACCATG 3′ |
| Nor-16S-F | 5′ AGGTGTGGCTTTCGGAGCTAAC 3′ |
| Nor-16S-R | 5′ CCACCATTACGTGCTGGCAAAC 3′ |
| Nor-ompT-F2 | 5′ GGTGATGCACTGATTG 3′ |
| Nor-ompT-R2 | 5′ AACCCAGAGTTGTACC 3′ |
| Nor-ToxR-BF | 5′ TGGCCCAACGTCCAAACGAG 3′ |
| Nor-ToxR-BR | 5′ TGGCTGATGAAGGCACACTG 3′ |
| EMSA oligonucleotides | |
| SOD.for.flu | 5′ 6-FAM-TTGTTAATGATATTAATTATCATTAACAT 3′ |
| SOD.rev | 5′ ATGTTAATGATAATTAATATCATTAACAA 3′ |
| OMPT.for.flu | 5′ 6-FAM-CATGAAATAAATGTAATTTATTGAATTTTAAGG 3′ |
| OMPT.rev | 5′ CCTTAAAATTCAATAAATTACATTTATTTCATG 3′ |
| CONTROL.for.flu | 5′ 6-FAM-CGAGTTTTAAGTTATTTACTGTAAGTAAAGTA 3′ |
| CONTROL.rev | 5′ GTACTTTACTTACAGTAAATAACTTAAAACTCG 3′ |
To generate a toxR mutant in V. cholerae, primer sets ToxR1/ToxR2 and ToxR3/ToxR4 were used to amplify overlapping fragments. Primers ToxR1 and ToxR4 were used to amplify the overlap PCR product, which then was A-tailed and ligated into pGEM-T Easy to create plasmid pGEM-ToxR. A HincII fragment of pUC4K (Pharmacia) containing the kanamycin resistance gene was ligated into the SmaI site of pGEM-ToxR to create pGEM-ToxR-kn. The NotI fragment of pGEM-ToxR-kn containing the inactivated toxR gene was ligated to NotI-digested pCVD442N to form pCVD-ToxR. pCVD-ToxR was transferred into V. cholerae strain N16961, and allelic exchange was carried out as described above. Mutants were verified by PCR using primer sets flanking the toxR gene, ToxRV1/ToxRV2 and ToxRV3/ToxRV4.
The N16961 fur mutant was constructed by allelic exchange using suicide vector pAMS12 as described previously (30).
The E. coli fur mutant strain EWE1008, used for the production of recombinant V. cholerae Fur protein, was constructed by the bacteriophage P1 transduction of the fur::kan mutation from the Keio collection mutant JWK0669-1 (2) into W3110.
Construction of HA-Fur expression plasmid.
The V. cholerae fur gene with 3′ NheI and XhoI sites was amplified from Lou15 DNA using Pfx polymerase (Invitrogen) and the primers fur.745 and fur.nhe.xho.rev, and the resulting fragment was cloned into the SmaI site of pWKS30 (44) to make pVcfurNX1. The hemagglutinin (HA) tag primers Flu1 and Flu2 were annealed with each other and inserted into pVcfurNX1 that had been cleaved at the 3′ end of the fur gene with NheI and XhoI. The fur gene from the resulting plasmid, pVcfurflu3, was amplified with fur.1254.BseRI and the vector reverse primer furV-R, which inserted a BseRI site at the 5′ end of the gene. The resulting fragment was digested with BseRI and SalI and inserted into BseRI/XhoI-digested pQE-2 (Qiagen) to yield pQVcFurFlu1.
SDS-PAGE.
Cells were grown overnight in LB medium, diluted 1:100 into EZ-RDM with or without iron supplementation, and grown to stationary phase. Equal numbers of cells (as determined by optical density) were centrifuged, and the pellets were resuspended in 100 μl Tris buffer (10 mM Tris, pH 8.0, and 10 mM MgCl2) and 100 μl 2× Laemmli sample buffer (18) (10% β-mercaptoethanol, 6% [wt/vol] SDS, 20% glycerol, 0.002% bromophenol blue). The samples were boiled for 5 min and then loaded onto an SDS-polyacrylamide gel. Following electrophoresis, the gels were stained with Coomassie brilliant blue or transferred to nitrocellulose membranes for Western blot analysis. The blot was probed with anti-Omp antibodies (generously provided by Johnny W. Peterson, University of Texas Medical Branch, Galveston, TX) or anti-ToxR antibodies (generously provided by Ronald Taylor, Dartmouth Medical School, Hanover, NH), followed by goat anti-rabbit horseradish peroxidase-conjugated secondary antibodies (Perkin Elmer Life Sciences). OmpT or ToxR protein was detected by developing the blot using Western Lighting chemiluminescence reagent plus (Perkin Elmer Life Sciences).
Dot blot analysis.
To determine the relative amounts of mRNA in each strain under the indicated conditions, cultures were grown overnight in LB medium, diluted 1:100 into fresh EZ-RDM, and grown for 4 h. The culture then was divided, and FeSO4 was added to a final concentration of 5 μM to one culture. After incubation for an additional hour, the optical densities of the cultures were measured, and equal numbers of cells were harvested for RNA isolation as described above. The concentration of total RNA was measured, and equal amounts of RNA were spotted onto a positively charged nylon membrane (BrightStar Plus; Ambion) and UV cross-linked (GS Gene Cross-linker UV chamber; Bio-Rad). Membranes were incubated with DNA probes labeled using the BrightStar psoralen-biotin nonisotopic labeling kit (Ambion) per the manufacturer's instructions. The probes were generated with the following primers: Nor-16S-F/Nor-16S-R (rrsA), Nor-ompT-F2/Nor-ompT-R2 (ompT), and Nor-ToxR-BR/Nor-ToxR-BF (toxR). The BrightStar Biodetect nonisotopic detection kit (Ambion) was used to visualize the biotinylated probes after hybridization. The rrsA probe was used to determine that equal amounts of RNA were spotted onto the membrane.
Purification of Fur-HA.
A 1-ml overnight culture of E. coli fur mutant strain EWE1008 containing pQVcFurFlu1 was added to 100 ml of LB containing ampicillin and incubated at 37°C with aeration. Once the cells reached mid-log phase, the expression of fur was induced with 0.5 mM isopropyl-β-d-thiogalactopyranoside (IPTG), and the culture was grown for another 2 h. Cells were pelleted at 10,000 × g for 10 min at 4°C and stored at −20°C until needed. The cells were resuspended in lysis buffer (20 mM Tris-HCl, pH 7.5, 100 mM NaCl, 10% glycerol) and lysed via sonication using three 15-s bursts with 1 min of cooling between bursts. Fur-HA was purified using anti-HA affinity matrix according to the manufacturer's protocol (Roche, Indianapolis, IN).
Protein samples were dialyzed against 20 mM Tris-HCl, pH 7.5, 100 mM NaCl, 10% glycerol and concentrated using Microcon YM-30 columns according to the manufacturer's protocol (Millipore Corporation, Bedford, MA). The concentrations of protein samples were determined using the Bradford assay (Bio-Rad, Hercules, CA).
EMSA.
Electrophoretic mobility shift assays (EMSA) were performed as described by Watnick et al. (45) with the following modifications. Purified Fur-HA was added to a reaction mixture containing 6-carboxyfluorescein (6-FAM)-labeled, double-stranded DNA oligonucleotide (Table 2), 20 mM Tris-HCl at pH 7.5, 50 mM NaCl, 200 μM MnCl2, 100 μg/ml bovine serum albumin (BSA), 10% glycerol, and 20 μg/ml salmon sperm DNA, where indicated. The reaction mixtures were incubated at room temperature for 20 min before being loaded onto a 5% nondenaturing polyacrylamide gel.
RESULTS
Iron increases OmpT levels.
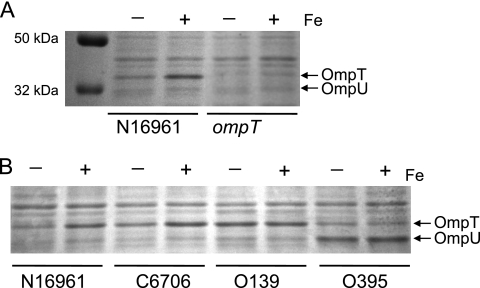
In studies of iron metabolism by V. cholerae, we noted differences in levels of outer membrane proteins as a function of the amount of available iron. To examine this in more detail, V. cholerae N16961 was grown in defined medium with or without added iron, and the proteins were separated by SDS-PAGE. The outer membrane porins OmpT (40 kDa) and OmpU (38 kDa) both were detected, but OmpT levels were higher in cells grown in high iron than in those grown without added iron (Fig. 1). The loss of this band in an ompT mutant confirmed that the protein induced by iron was OmpT (Fig. 1A). The increase in OmpT levels in the presence of iron also was seen in the El Tor strain C6706 but was not apparent in the classical strain O395 or in an O139 strain (Fig. 1B), suggesting that the iron regulation of OmpT is associated with El Tor strains. In contrast to the other strains, O395 produced more OmpU than OmpT in the defined medium (Fig. 1B), and this was not dependent upon iron levels.
Fig. 1.
Effect of iron on V. cholerae porin levels. (A) N16961 and the ompT mutant (SAC116) were grown in T medium with (+) or without (−) 5 μM iron. Proteins were separated on SDS-10% polyacrylamide gel and stained with Coomassie blue. Positions of the major outer membrane proteins are indicated at the right. (B) Strains N16961, C6706, 0395, and O139 were grown and the proteins analyzed as described for panel A.
Increased OmpT in the presence of iron requires Fur but not RyhB.
In V. cholerae, positive regulation by iron is frequently the result of Fe-Fur repressing the expression of the negative regulator sRNA RyhB (5, 30). To determine whether the increased amount of OmpT produced in cells grown in the presence of iron was controlled by Fur and RyhB, mutants defective in each of these genes were constructed in the N16961 background, and the effect of these mutations on the level of OmpT was determined (Fig. 2). No increase in OmpT levels was observed in the N16961 fur mutant in the presence of iron (Fig. 2A), showing that Fur is required for the effect of iron on OmpT levels. Supplying fur on a plasmid restored the iron-dependent increase in OmpT, indicating that the phenotype of the fur mutant is due only to the loss of fur (Fig. 2B). In the ryhB mutant, however, the increase in OmpT in the presence of iron was the same as that in the wild-type strain, indicating that RyhB is not required (Fig. 2A). Thus, Fur exerts positive regulation by a mechanism that is independent of RyhB.
Fig. 2.
Fur, but not RyhB, is required for increased OmpT in the presence of iron. (A) Wild-type N16961 and the isogenic ompT (SAC116), ryhB (ARM711), and fur (ARM713) mutants were grown overnight in EZ-RDM with (+) or without (−) 5 μM iron. Proteins from equivalent numbers of cells were separated on SDS-10% polyacrylamide gel (upper panel) and subjected to Western blot analysis with a polyclonal antiserum against OmpT and OmpU, which is indicated by arrows (lower panel). (B) Wild-type N16961, the fur mutant (ARM713) carrying the empty cloning vector pWKS30, and the fur mutant complemented with the fur gene (ARM713/pAMF1) were grown with (+) or without (−) 5 μM iron, and proteins from equivalent numbers of cells were separated on SDS-10% polyacrylamide.
Iron increases the amount of OmpT mRNA.
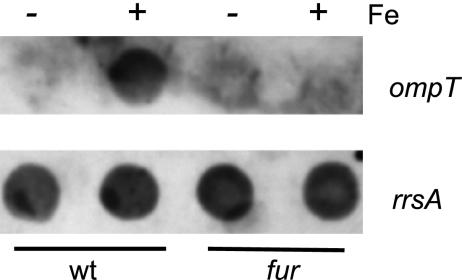
Since Fur is a transcriptional regulator, it was likely that Fur regulates ompT at the level of transcription. A dot blot assay was performed in which RNA isolated from cells grown with or without added iron was hybridized with an ompT probe (Fig. 3). Higher levels of ompT mRNA were present in wild-type cells grown in the presence of iron. As expected based on the protein levels, the fur mutant did not show increased ompT expression when grown in high iron.
Fig. 3.
OmpT is regulated by Fur at the mRNA level. Dot blot analysis of equal amounts of RNA harvested from wild-type (N16961) and fur mutant (ARM713) cells grown in EZ-RDM with (+) or without (−) 5 μM FeSO4. RNA samples were probed with an ompT probe (upper panel), and a duplicate blot was probed with an rrsA probe (lower panel) to ensure equal amounts of RNA were present in the samples.
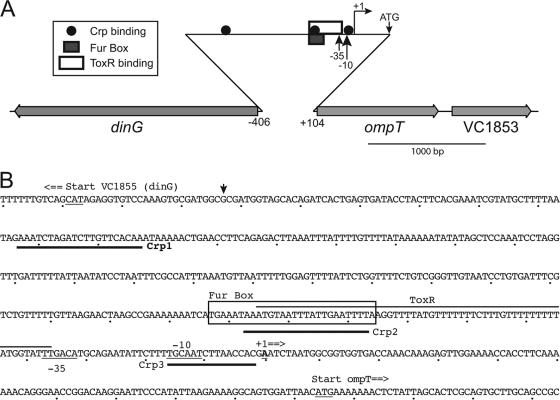
Fur binds DNA upstream of ompT.
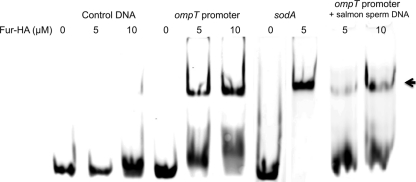
The ompT promoter sequence was examined to identify possible mechanisms for enhanced expression in the presence of iron (Fig. 4). A possible Fur box is located in the ompT promoter at approximately −90, overlapping the −85 site shown to bind both ToxR and CRP in DNA binding assays (23) (Fig. 4). To determine whether Fur binds this putative Fur box upstream of OmpT, electrophoretic mobility shift assays were performed (Fig. 5). V. cholerae Fur was affinity purified from cells expressing HA-tagged Fur, and its activity was confirmed by showing its binding to a known Fur box in the sodA promoter (36, 39). The purified Fur was added to labeled double-stranded DNA encompassing the putative Fur box in the ompT promoter. A gel mobility shift was observed in the presence of Fur, and increasing the amount of Fur protein increased the proportion of DNA with reduced mobility (Fig. 5). The shift was not as complete as that observed with the sodA Fur box sequence, suggesting that the ompT promoter has a lower affinity for Fur than the sodA Fur-binding site. The binding appeared to be specific, as no shift was seen when the sequence of the oligonucleotide was changed to eliminate the conserved nucleotides in the putative Fur box (control lanes), and nonspecific DNA (salmon sperm DNA) did not prevent binding (Fig. 5). The sequence of the ompT promoter, including the putative Fur box, is the same in both classical and El Tor strains (Fig. 4b), indicating that factors other than the Fur box are responsible for the difference in the iron regulation of ompT expression in the two biotypes.
Fig. 4.
(A) Map of the V. cholerae ompT gene region. The locations and the direction of transcription are indicated by the gray arrows. The approximate locations of the transcription factor binding sites (22, 23) are shown with the indicated symbols. The small arrows indicate the −35, −10, and transcription start sites for the ompT promoter, as well as the location of the translational start ATG. (B) DNA sequence of the ompT-dinG intergenic region. The locations of the three CRP binding sites are underlined in boldface, there is a line over the ToxR binding site, and the Fur box is enclosed in a box. The −35, −10, and transcriptional start sites of the ompT promoter are underlined. The sequence shown is for the V. cholerae El Tor strain N16961. This sequence differs from the sequence of the classical strain O395 at a single position, which is indicated by the arrowhead. The translational start sites for dinG and ompT also are shown. The dots below the sequence represent 10 bp.
Fig. 5.
Fur binding to the ompT promoter region. The 5% nondenaturing gel shows an electrophoretic mobility shift assay with 0.1 μM FAM-labeled, double-stranded (ds) oligonucleotide incubated with 0, 5, or 10 μM Fur-HA. The ds oligonucleotides used in the assay are specified at the top of each gel. The control oligonucleotide is the reverse ompT sequence with modifications so that it does not contain the highly conserved sequence of the putative Fur binding site. The ompT promoter oligonucleotide contains sequence upstream of ompT, including the possible Fur binding site. The sodA promoter oligonucleotide contains a known Fur box (31). Salmon sperm DNA was added as a nonspecific competitor. DNA bound by Fur-HA is indicated by the arrow.
The effect of iron on OmpT is independent of carbon source and ToxR.
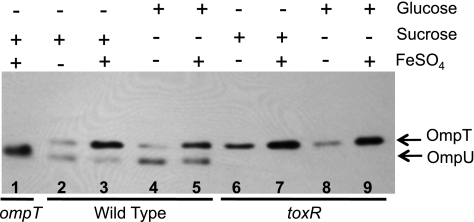
The binding of Fur to the ompT promoter indicates that it is acting directly on ompT, either alone or through interactions with other regulatory proteins, such as CRP and ToxR. If Fur is influencing the cAMP-CRP-dependent activation of ompT, then the loss of cAMP or CRP should eliminate the iron-dependent increase in OmpT. Because cya (adenylate cyclase) or crp (CRP) mutations are pleiotropic, we reduced cAMP levels by growing the cells in minimal medium with glucose instead of sucrose as the carbon source. In the wild-type genetic background, there was a small decrease in the overall amount of OmpT produced in cells grown in the presence of glucose compared to levels for those grown in sucrose (Fig. 6, compare lanes 2 and 4 and lanes 3 and 5), which is consistent with diminished cAMP-CRP-dependent ompT activation in the presence of glucose. However, glucose did not affect the iron-dependent increase in OmpT, suggesting that Fur is not regulating ompT levels by altering the binding of cAMP-CRP to the promoter. The amount of OmpT was similarly reduced when the toxR mutant was grown in the presence of glucose (Fig. 6, lane 6) compared to growth with sucrose (Fig. 6, lane 8), which is consistent with previous reports that ToxR is not required for the effect of glucose on ompT expression (23). Interestingly, OmpU levels are increased in the presence of glucose (Fig. 6, lanes 2 and 4), which may indicate an inhibitory effect of CRP on ompU.
Fig. 6.
Glucose and ToxR do not affect iron-dependent activation of ompT. N16961 (lanes 2 to 5) and the toxR mutant, SAC119, (lanes 6 to 9) were grown overnight in T medium with added sucrose or glucose and with or without 5 μM FeSO4. The ompT mutant (SAC116) is shown in lane 1 for comparison. Equal numbers of cells from each strain were electrophoresed on a 10% SDS-polyacrylamide gel and subjected to Western analysis using rabbit polyclonal antiserum against the porins.
ToxR represses the transcription of ompT by binding specific sites in the ompT promoter (Fig. 4). To test the possibility that the binding of Fe-Fur increases ompT expression by interfering with the binding of ToxR, the effect of iron on OmpT levels was determined in a toxR mutant. An iron-dependent increase in the amount of OmpT produced was evident in the toxR mutant (Fig. 6, lanes 6 to 9), showing that Fe-Fur does not increase OmpT levels by alleviating repression by ToxR.
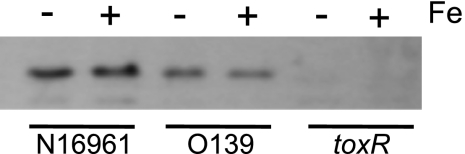
Iron does not affect toxR expression.
ToxR was not required for the iron effect on OmpT synthesis (Fig. 6, lanes 6 to 8), but because ToxR plays a dominant role in controlling OmpT levels, we determined whether iron exerts any effects on the regulation of toxR. If growth in medium containing iron decreases the levels of ToxR, this could contribute to the increase in OmpT levels. However, iron had no effect on ToxR levels in N16961 or in O139 (Fig. 7). Thus, the effect of iron on ompT expression is not linked to the modulation of ToxR production.
Fig. 7.
Iron does not affect ToxR synthesis. Wild-type N16961 and O139 and the N16961 toxR mutant, SAC119, were grown in T medium with or without 5 μM FeSO4. Equal numbers of cells from each strain were electrophoresed on a 10% SDS-polyacrylamide gel and subjected to Western analysis using rabbit polyclonal antiserum against ToxR.
DISCUSSION
V. cholerae has approximately 10 major outer membrane proteins (17). Several of these, including OmpT, OmpU, and OmpS, have been shown to function as porins (3, 10, 19), which permit the diffusion of small molecules across the outer membrane. The two most abundant porins in V. cholerae are OmpT and OmpU (3, 17). OmpU has a larger effective radius (0.55 nm) than OmpT (0.43 nm) (10), but it is less permeable to some molecules, including bile.
The relative proportion of the porins in the V. cholerae outer membrane is influenced by environmental signals, such as carbon source, pH, osmolarity, and bile (23, 37, 42). In nature, V. cholerae is found in aquatic environments, often in association with other organisms or in biofilms, while during human infections it colonizes the small intestine. Each of these environments contains different nutrients and inhibitory substances, and V. cholerae must be able to express the appropriate porins to adapt to these different conditions. The regulation of porin composition allows the bacterium to control the small molecules that cross the membrane barrier in these varied habitats.
The regulation of the expression of these porins occurs at both the transcriptional and posttranscriptional levels. The transcription of ompT is repressed by ToxR, which activates the expression of ompU. ompT transcription is increased by the carbon source signal cAMP-CRP (23), while a small RNA, VrrA, acts posttranscriptionally to reduce expression. VrrA binds to the 5′ untranslated region of the ompT mRNA and inhibits translation (42). vrrA expression requires the membrane stress sigma factor σE (41). Thus, VrrA may be responsible for the decreased OmpT levels and increased OmpU that occurs in response to acid stress (28).
The importance of regulating the porin composition of V. cholerae is best characterized with respect to the infection of the mammalian intestine, where bile resistance may be important for optimal colonization. An ompT mutant that has OmpU as the major porin exhibits wild-type resistance to bile and antimicrobial peptides, while an ompU mutant is more sensitive (26, 37). A strain engineered to express ompT rather than ompU in response to ToxR was sensitive to bile salts in vitro and had a reduced ability to colonize the infant mouse intestine (38). These data are consistent with a shift from OmpT to OmpU synthesis, promoting the colonization of the host by reducing sensitivity to bile and antimicrobial peptides. In fact, OmpU, but not OmpT, was detected consistently in the proteomic analysis of vibrios isolated from stools of cholera patients (20). The interaction of specific bile compounds, such as deoxycholate, with the barrel of OmpT may serve a signaling role, indicating the entry of V. cholerae into the small intestine (46) and promoting the switch to OmpU synthesis.
Here, we show that the expression of ompT is responsive to the level of iron in the environment, and the induction of ompT by iron requires Fur. Although most iron regulation in V. cholerae is negative, both RyhB-dependent and RyhB-independent positive regulation has been observed (5, 30, 31). The positive regulation of ompT by iron and Fur is independent of RyhB and is likely due to Fe-Fur binding to the Fur box upstream of ompT. CRP and ToxR, the other known transcription factors for ompT, also bind to the region encompassed by the Fur box, and a model in which Fur binding interferes with ToxR binding or promotes CRP binding was considered. Competition between Fur and a negative regulator has been shown to induce the expression of the ferritin gene ftnA in E. coli. Fur prevents the H-NS silencing of ftnA by competing with H-NS for binding to sequences upstream of the promoter (34). However, the effect of Fur on ompT requires neither CRP nor ToxR. This indicates that the effect of Fur is not due to interference with the binding of these factors, although our data do not exclude the possibility that Fur interferes with the binding of an unidentified repressor of ompT expression. It is possible that Fur bound to the ompT promoter interacts directly with RNA polymerase, or it may alter the supercoiling or bending of the DNA to promote transcription. It is interesting that the putative Fur box in the ompT promoter is located in a position upstream of the −10 and −35 sites similar to that of the Fur box in N. meningitidis norB, a nitric oxide reductase gene that, like ompT, is positively regulated by iron and Fur (7, 16). This suggests that Fur bound to sites upstream of the polymerase binding site allows the enhanced transcription of the gene, while Fur sites that overlap the RNA polymerase recognition site mediate the repression of gene expression.
The significance of the iron regulation of a porin is not clear. It is possible that some porins serve as a portal for iron in environments containing free iron or iron loosely associated with anions. V. cholerae has at least two iron transport systems that transport free ferric (Fbp) and ferrous (Feo) iron across the cytoplasmic membrane (48), and no system has yet been identified for transporting the iron across the outer membrane to the periplasm for access to these transporters. If OmpT serves as a portal for free iron, then the modulation of its expression by Fur would help provide iron to the Feo and Fbp systems in environments where iron is not strongly chelated. This may be more common in the marine environment than in the host, where iron is tightly withheld from bacteria. The low free-iron levels in the host, combined with the repressing effects of ToxR, would reduce ompT expression in vivo while increasing the expression of high-affinity iron transporters, such as siderophores and heme receptors.
ACKNOWLEDGMENTS
We thank John Peterson and Ron Taylor for generously sharing antibodies. The National BioResource Project (NIG, Japan) provided the E. coli fur mutant used for the construction of EWE1008.
This work was funded by grants R56AI050669 and R01AI091957 from the National Institute of Allergy and Infectious Diseases.
Footnotes
Published ahead of print on 30 September 2011.
REFERENCES
- 1. Andrews S. C., Robinson A. K., Rodríguez-Quiñones F. 2003. Bacterial iron homeostasis. FEMS Microbiol. Rev. 27:215–237 [DOI] [PubMed] [Google Scholar]
- 2. Baba T., et al. 2006. Construction of Escherichia coli K-12 in-frame, single-gene knockout mutants: the Keio collection. Mol. Syst. Biol. 2:2006.0008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Chakrabarti S. R., Chaudhuri K., Sen K., Das J. 1996. Porins of Vibrio cholerae: purification and characterization of OmpU. J. Bacteriol. 178:524–530 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Childers B. M., Klose K. E. 2007. Regulation of virulence in Vibrio cholerae: the ToxR regulon. Future Microbiol. 2:335–344 [DOI] [PubMed] [Google Scholar]
- 5. Davis B. M., Quinones M., Pratt J., Ding Y., Waldor M. K. 2005. Characterization of the small untranslated RNA RyhB and its regulon in Vibrio cholerae. J. Bacteriol. 187:4005–4014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Delany I., Spohn G., Rappuoli R., Scarlato V. 2001. The Fur repressor controls transcription of iron-activated and -repressed genes in Helicobacter pylori. Mol. Microbiol. 42:1297–1309 [DOI] [PubMed] [Google Scholar]
- 7. Delany I., Rappuoli R., Scarlato V. 2004. Fur functions as an activator and as a repressor of putative virulence genes in Neisseria meningitidis. Mol. Microbiol. 52:1081–1090 [DOI] [PubMed] [Google Scholar]
- 8. DiRita V. J. 1992. Co-ordinate expression of virulence genes by ToxR in Vibrio cholerae. Mol. Microbiol. 6:451–458 [DOI] [PubMed] [Google Scholar]
- 9. DiRita V. J., Parsot C., Jander G., Mekalanos J. J. 1991. Regulatory cascade controls virulence in Vibrio cholerae. Proc. Natl. Acad. Sci. U. S. A. 88:5403–5407 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Duret G., Delcour A. H. 2010. Size and dynamics of the Vibrio cholerae porins OmpU and OmpT probed by polymer exclusion. Biophys. J. 98:1820–1829 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Escolar L., Pérez-Martín J., de Lorenzo V. 1999. Opening the iron box: transcriptional metalloregulation by the Fur protein. J. Bacteriol. 181:6223–6229 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Figurski D. H., Helinski D. R. 1979. Replication of an origin-containing derivative of plasmid RK2 dependent on a plasmid function provided in trans. Proc. Natl. Acad. Sci. U. S. A. 76:1648–1652 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Hantke K. 1984. Cloning of the repressor protein gene of iron-regulated systems in Escherichia coli K12. Mol. Gen. Genet. 197:337–341 [DOI] [PubMed] [Google Scholar]
- 14. Hantke K. 1981. Regulation of ferric iron transport in Escherichia coli K12: isolation of a constitutive mutant. Mol. Gen. Genet. 182:288–292 [DOI] [PubMed] [Google Scholar]
- 15. Hill C. W., Harnish B. W. 1981. Inversions between ribosomal RNA genes of Escherichia coli. Proc. Natl. Acad. Sci. U. S. A. 78:7069–7072 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Isabella V., et al. 2008. cis- and trans-acting elements involved in regulation of norB (norZ), the gene encoding nitric oxide reductase in Neisseria gonorrhoeae. Microbiology 154:226–239 [DOI] [PubMed] [Google Scholar]
- 17. Kelley J. T., Parker C. D. 1981. Identification and preliminary characterization of Vibrio cholerae outer membrane proteins. J. Bacteriol. 145:1018–1024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Laemmli U. K. 1970. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature 227:680–685 [DOI] [PubMed] [Google Scholar]
- 19. Lång H., Palva E. T. 1993. The ompS gene of Vibrio cholerae encodes a growth-phase-dependent maltoporin. Mol. Microbiol. 10:891–901 [DOI] [PubMed] [Google Scholar]
- 20. LaRocque R. C., et al. 2008. Proteomic analysis of Vibrio cholerae in human stool. Infect. Immun. 76:4145–4151 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Lee J.-W., Helmann J. D. 2007. Functional specialization within the Fur family of metalloregulators. Biometals 20:485–499 [DOI] [PubMed] [Google Scholar]
- 22. Li C. C., Crawford J. A., DiRita V. J., Kaper J. B. 2000. Molecular cloning and transcriptional regulation of ompT, a ToxR-repressed gene in Vibrio cholerae. Mol. Microbiol. 35:189–203 [DOI] [PubMed] [Google Scholar]
- 23. Li C. C., Merrell D. S., Camilli A., Kaper J. B. 2002. ToxR interferes with CRP-dependent transcriptional activation of ompT in Vibrio cholerae. Mol. Microbiol. 43:1577–1589 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Massé E., Gottesman S. 2002. A small RNA regulates the expression of genes involved in iron metabolism in Escherichia coli. Proc. Natl. Acad. Sci. U. S. A. 99:4620–4625 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Massé E., Salvail H., Desnoyers G., Arguin M. 2007. Small RNAs controlling iron metabolism. Curr. Opin. Microbiol. 10:140–145 [DOI] [PubMed] [Google Scholar]
- 26. Mathur J., Waldor M. K. 2004. The Vibrio cholerae ToxR-regulated porin OmpU confers resistance to antimicrobial peptides. Infect. Immun. 72:3577–3583 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Mekalanos J. J., et al. 1983. Cholera toxin genes: nucleotide sequence, deletion analysis and vaccine development. Nature 306:551–557 [DOI] [PubMed] [Google Scholar]
- 28. Merrell D. S., Bailey C., Kaper J. B., Camilli A. 2001. The ToxR-mediated organic acid tolerance response of Vibrio cholerae requires OmpU. J. Bacteriol. 183:2746–2754 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Mey A. R., Payne S. M. 2001. Haem utilization in Vibrio cholerae involves multiple TonB-dependent haem receptors. Mol. Microbiol. 42:835–849 [DOI] [PubMed] [Google Scholar]
- 30. Mey A. R., Craig S. A., Payne S. M. 2005. Characterization of Vibrio cholerae RyhB: the RyhB regulon and role of ryhB in biofilm formation. Infect. Immun. 73:5706–5719 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Mey A. R., Wyckoff E. E., Kanukurthy V., Fisher C. R., Payne S. M. 2005. Iron and fur regulation in Vibrio cholerae and the role of fur in virulence. Infect. Immun. 73:8167–8178 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Miller V. L., Mekalanos J. J. 1988. A novel suicide vector and its use in construction of insertion mutations: osmoregulation of outer membrane proteins and virulence determinants in Vibrio cholerae requires toxR. J. Bacteriol. 170:2575–2583 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Miller V. L., Mekalanos J. J. 1984. Synthesis of cholera toxin is positively regulated at the transcriptional level by toxR. Proc. Natl. Acad. Sci. U. S. A. 81:3471–3475 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Nandal A., et al. 2010. Induction of the ferritin gene (ftnA) of Escherichia coli by Fe2+–Fur is mediated by reversal of H-NS silencing and is RyhB independent. Mol. Microbiol. 75:637–657 [DOI] [PubMed] [Google Scholar]
- 35. Neidhardt F. C., Bloch P. L., Smith D. F. 1974. Culture medium for enterobacteria. J. Bacteriol. 119:736–747 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Niederhoffer E. C., Naranjo C. M., Bradley K. L., Fee J. A. 1990. Control of Escherichia coli superoxide dismutase (sodA and sodB) genes by the ferric uptake regulation (fur) locus. J. Bacteriol. 172:1930–1938 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Provenzano D., Schuhmacher D. A., Barker J. L., Klose K. E. 2000. The virulence regulatory protein ToxR mediates enhanced bile resistance in Vibrio cholerae and other pathogenic Vibrio species. Infect. Immun. 68:1491–1497 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Provenzano D., Klose K. E. 2000. Altered expression of the ToxR-regulated porins OmpU and OmpT diminishes Vibrio cholerae bile resistance, virulence factor expression, and intestinal colonization. Proc. Natl. Acad. Sci. U. S. A. 97:10220–10224 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Sheikh M. A., Taylor G. L. 2009. Crystal structure of the Vibrio cholerae ferric uptake regulator (Fur) reveals insights into metal co-ordination. Mol. Microbiol. 72:1208–1220 [DOI] [PubMed] [Google Scholar]
- 40. Simon E. H., Tessman I. 1963. Thymidine-requiring mutants of phage T4. Proc. Natl. Acad. Sci. U. S. A. 50:526–532 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Song T., et al. 2008. A new Vibrio cholerae sRNA modulates colonization and affects release of outer membrane vesicles. Mol. Microbiol. 70:100–111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Song T., Sabharwal D., Wai S. N. 2010. VrrA mediates Hfq-dependent regulation of OmpT synthesis in Vibrio cholerae. J. Mol. Biol. 400:682–688 [DOI] [PubMed] [Google Scholar]
- 43. Taylor R. K., Miller V. L., Furlong D. B., Mekalanos J. J. 1987. Use of phoA gene fusions to identify a pilus colonization factor coordinately regulated with cholera toxin. Proc. Natl. Acad. Sci. U. S. A. 84:2833–2837 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Wang R. F., Kushner S. R. 1991. Construction of versatile low-copy-number vectors for cloning, sequencing and gene expression in Escherichia coli. Gene 100:195–199 [PubMed] [Google Scholar]
- 45. Watnick P. I., Butterton J. R., Calderwood S. B. 1998. The interaction of the Vibrio cholerae transcription factors, Fur and IrgB, with the overlapping promoters of two virulence genes, irgA and irgB. Gene 209:65–70 [DOI] [PubMed] [Google Scholar]
- 46. Wibbenmeyer J. A., Provenzano D., Landry C. F., Klose K. E., Delcour A. H. 2002. Vibrio cholerae OmpU and OmpT porins are differentially affected by bile. Infect. Immun. 70:121–126 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Wyckoff E. E., et al. 1998. Structure of the Shigella dysenteriae haem transport locus and its phylogenetic distribution in enteric bacteria. Mol. Microbiol. 28:1139–1152 [DOI] [PubMed] [Google Scholar]
- 48. Wyckoff E. E., Mey A. R., Leimbach A., Fisher C. F., Payne S. M. 2006. Characterization of ferric and ferrous iron transport systems in Vibrio cholerae. J. Bacteriol. 188:6515–6523 [DOI] [PMC free article] [PubMed] [Google Scholar]