Abstract
Yeast eIF1 inhibits initiation at non-AUG triplets, but it was unknown whether it also discriminates against AUGs in suboptimal context. As in other eukaryotes, the yeast gene encoding eIF1 (SUI1) contains an AUG in poor context, which could underlie translational autoregulation. Previously, eIF1 mutations were identified that increase initiation at UUG codons (Sui− phenotype), and we obtained mutations with the opposite phenotype of suppressing UUG initiation (Ssu− phenotype). Remarkably, Sui− mutations in eukaryotic translation initiation factor 1 (eIF1), eIF1A, and eIF2β all increase SUI1 expression in a manner diminished by introducing the optimal context at the SUI1 AUG, whereas Ssu− mutations in eIF1 and eIF1A decrease SUI1 expression with the native, but not optimal, context present. Therefore, discrimination against weak context depends on specific residues in eIFs 1, 1A, and 2β that also impede selection of non-AUGs, suggesting that context nucleotides and AUG act coordinately to stabilize the preinitiation complex. Although eIF1 autoregulates by discriminating against poor context in yeast and mammals, this mechanism does not prevent eIF1 overproduction in yeast, accounting for the hyperaccuracy phenotype afforded by SUI1 overexpression.
INTRODUCTION
Bacterial translation initiation factor 3 (IF3) promotes the fidelity of initiation at AUG codons by discriminating against non-AUG triplets as start sites (17, 26, 40, 45). This discriminatory function forms the basis for IF3's ability to negatively autoregulate translation of its mRNA, which initiates with an AUU start codon (5, 6). IF3 also destabilizes initiation complexes formed on AUG codons at the 5′ ends of leaderless mRNAs (47), which lack the Shine-Dalgarno sequence that stabilizes mRNA association with the small (30S) ribosomal subunit at the AUG codon.
In eukaryotes, the 43S preinitiation complex (PIC), harboring the eIF2-GTP-Met-tRNAiMet ternary complex (TC) and various other eIFs, attaches to the capped 5′ end of the mRNA and identifies the AUG codon by scanning the mRNA leader base-by-base for complementarity with the anticodon of Met-tRNAiMet. Efficient initiation is influenced by the sequence immediately upstream from the AUG, but it is unclear how this sequence context is recognized or regulates AUG selection (20, 36). The functional counterpart of IF3 in eukaryotes appears to be eIF1 (Sui1 in yeast). eIF1 and IF3 occupy analogous locations on the platform of the small ribosomal subunit (11, 27, 38) and, similar to IF3, eIF1 blocks formation of stable 48S PICs at near-cognate start codons (20, 36). eIF1/Sui1 and eIF1A (Tif11 in yeast) cooperate to promote an open conformation of the 40S subunit (34) thought to be conducive to scanning (35), and eIF1 also blocks the final step of GTP hydrolysis by the TC, the release of Pi from eIF2-GDP-Pi, until an AUG enters the P site (1). AUG recognition triggers dissociation of eIF1 from the 40S subunit (30), enabling Pi release and stabilizing a closed conformation of the 40S subunit that is incompatible with scanning.
Similar to the effects of IF3 mutations in bacteria, hypomorphic mutations in yeast eIF1 (an essential protein) increase initiation from UUG codons in vivo (12, 49), i.e., the Sui− phenotype, whereas overexpressing wild-type (WT) eIF1 suppresses UUG initiation in mutants with Sui− substitutions in other initiation factors, i.e., the Ssu− phenotype (41, 48). Interestingly, Sui− mutations in eIF1 generally weaken its affinity for the 40S and enable inappropriate eIF1 dissociation and Pi release from eIF2-GDP-Pi at non-AUG codons. Furthermore, a mutation in the N-terminal tail (NTT) of eIF1A (17-21) that suppresses UUG initiation in vivo retards eIF1 dissociation on start codon recognition (8). Thus, the rate of eIF1 dissociation and Pi release are critical determinants of AUG recognition. Suppression of the increased UUG initiation frequency in Sui− mutants by eIF1 overexpression can be understood as the consequence of impeding rearrangement of the PIC from the eIF1-bound, scanning conformation to the closed, scanning-arrested state lacking eIF1 in the absence of an AUG-anticodon match, with a UUG in the P site.
It was reported recently that the genes encoding eIF1 in diverse eukaryotes contain an AUG context that deviates significantly from that found at highly expressed genes (22, 31). In particular, eIF1 genes generally contain pyrimidines rather than purines at the −3 position, shown by Kozak to be the most critical contextual determinant of AUG selection in mammals (24). Using a reconstituted in vitro system, it was demonstrated that mammalian eIF1 can discriminate against AUGs in suboptimal context in addition to preventing recognition of non-AUG codons (35), and it was envisioned that context nucleotides help to stabilize the closed conformation of the PIC that is competent for start codon recognition (37). As first proposed by Ivanov et al. (22), its ability to discriminate against poor AUG context would allow mammalian eIF1 to negatively autoregulate translation by discriminating against the poor context of its start codon and, while our work was under way, these researchers provided strong evidence that this autoregulatory mechanism indeed operates in mammalian cells.
Adenines at positions −3 to −1 relative to AUG are highly preferred among genes in Saccharomyces species (44), and there is evidence that A−3-A−2-(A/G)−1-AUG is the optimal context for initiation in Saccharomyces cerevisiae (7). Saccharomyces species resemble other eukaryotes in displaying poor context for the AUG codon at the eIF1 gene (SUI1), C−3-G−2-U−1-AUG, which matches at −3 and −2 a highly unfavorable sequence context identified in S. cerevisiae, of C−3-G−2-C−1-AUG (7). However, it was unknown whether eIF1 autoregulates translation in yeast, or whether other eIFs participate in evaluating AUG context in any eukaryotic cells. In this report, we show that Sui− and Ssu− substitutions in eIF1/Sui1 suppress and exacerbate, respectively, the deleterious effect of poor AUG context on eIF1 expression. We extend this finding to include Sui− and Ssu− substitutions in eIF1A and eIF2β (Sui3 in yeast) and a suboptimal context that is even less functional than that present at native SUI1. These findings indicate that eIF1 autoregulates translation in yeast and that discrimination against poor context in vivo depends on specific domains and residues in eIF1, eIF1A, and eIF2β that also function in stringent selection of AUG codons. This strongly supports the notion that a favorable context and AUG triplet cooperate to promote the rearrangement from an open, scanning conformation to a closed, initiation-competent state of the PIC.
MATERIALS AND METHODS
Yeast strain constructions.
To generate strains PMY30 through PMY51 and PMY98, strain JCY03 [MATaura3-52 leu2-3 leu2-112 trp1Δ-63 his4-301 (ACG) sui1Δ::hisG p1200 (sc URA3 SUI1)] was transformed to Leu+ with single-copy (sc) or high-copy-number (hc) LEU2 plasmids harboring the appropriate SUI1 alleles on SC-L medium, and the resident SUI1+ URA3 plasmid (p1200) was evicted by selecting for growth on 5-fluoorotic acid (5-FOA) medium. The plasmids and yeast strains discussed in the present study are listed in Tables 1 and 2, respectively.
Table 1.
Plasmids used in this study
| Plasmid | Descriptiona | Source or reference |
|---|---|---|
| YCplac111 | sc LEU2 cloning vector | 16 |
| YEplac181 | hc LEU2 cloning vector | 16 |
| YCplac22 | sc TRP1 cloning vector | 16 |
| p1200 | sc URA3 SUI1 in YCp50 | 49 |
| pCFB03 | sc LEU2 His-SUI1 in YCplac111 | 8 |
| pJCB101 | sc LEU2SUI1 in YCplac111 | This study |
| pCFB04 | hc LEU2SUI1 in YEplac181 | 8 |
| pPMB01 | sc LEU2sui1-K56E in YCplac111 | This study |
| pPMB02 | sc LEU2sui1-K60E in YCplac111 | This study |
| pPMB03 | sc LEU2sui1-L96P in YCplac111 | This study |
| pPMB30 | hc LEU2 sui1-L96P in YEplac181 | This study |
| p367 | sc URA3HIS4(ATG)-lacZ | 13 |
| p391 | sc URA3 HIS4(TTG)-lacZ | 13 |
| p4281/YCpTIF5-G31R-W | sc TRP1 TIF5-G31R in YCplac22 | 48 |
| p4280/YCpSUI3-S264Y-W | sc TRP1 SUI3-S264Y in YCplac22 | 48 |
| pPMB04 | sc LEU2sui1-T15A in YCplac111 | This study |
| pPMB05 | sc LEU2sui1-E48V in YCplac111 | This study |
| pPMB06 | sc LEU2sui1-L51F in YCplac111 | This study |
| pPMB07 | sc LEU2sui1-D61G in YCplac111 | This study |
| pPMB08 | sc LEU2sui1-Q84H in YCplac111 | This study |
| pPMB09 | sc LEU2sui1-E48V,L51F in YCplac111 | This study |
| pPMB10 | sc LEU2SUI1-opt in YCplac111 | This study |
| pPMB11 | hc LEU2SUI1-opt in YEplac181 | This study |
| pPMB12 | sc LEU2sui1-K56E-opt in YCplac111 | This study |
| pPMB13 | sc LEU2sui1-K60E-opt in YCplac111 | This study |
| pPMB14 | sc LEU2sui1-L96P-opt in YCplac111 | This study |
| pPMB15 | sc LEU2sui1-T15A-opt in YCplac111 | This study |
| pPMB16 | sc LEU2sui1-E48V-opt in YCplac111 | This study |
| pPMB17 | sc LEU2sui1-L51F-opt in YCplac111 | This study |
| pPMB18 | sc LEU2sui1-D61G-opt in YCplac111 | This study |
| pPMB19 | sc LEU2sui1-Q84H-opt in YCplac111 | This study |
| pPMB20 | sc LEU2sui1-E48V,L51F-opt in YCplac111 | This study |
| pPMB21 | sc TRP1 SUI1 in YCplac22 | This study |
| pPMB22 | sc TRP1 SUI1-opt in YCplac22 | This study |
| p4450 | sc TRP1 SUI3 in YCplac22 | C. Fekete |
| pDSO9 | sc LEU2 TIF11 in YCplac111 | 9 |
| p4552 | sc LEU2TIF11-NDSDG17-21AAAAA in YCplac111 | 14 |
| pAS23 | sc LEU2TIF11-FGFESDE121-127AAAAAAA,FEFGN131-135FAAAA in YCplac111 | 41 |
| p4119/TIF5-FL TRP1 | sc TRP1 TIF5-FL in YCplac22 | K. Asano |
| p164 | sc URA3GCN4 in YCp50 | 19 |
| p180 | sc URA3GCN4-lacZ in YCp50 | 19 |
| pPMB23 | sc URA3 SUI1-GCN4 in p164 | This study |
| pPMB24 | sc URA3 SUI1-lacZ in p164 | This study |
| pPMB25 | sc URA3 SUI1-opt-lacZ in p164 | This study |
| pHQ1303 | hc URA3GCN4 in YEplac195 | 50 |
| pPMB26 | hc URA3 SUI1-lacZ in pHQ1303 | This study |
| pPMB27 | hcURA3 SUI1-opt-lacZ in pHQ1303 | This study |
| pPMB28 | sc URA3 SUI1UUU-lacZ in p164 | This study |
hc, high copy number; sc, single copy.
Table 2.
Yeast strains used in this study
| Strain | Genotypea | Source or reference |
|---|---|---|
| JCY03 | MATaura3-52 leu2-3 leu2-112 trp1Δ-63 his4-301(ACG) sui1Δ::hisG p1200 (sc URA3SUI1) | 8 |
| PMY30 | MATaura3-52 leu2-3 leu2-112 trp1Δ-63 his4-301(ACG) sui1Δ::hisG pJCB101 (sc LEU2SUI1) | This study |
| PMY31 | MATaura3-52 leu2-3 leu2-112 trp1Δ-63 his4-301(ACG) sui1Δ::hisG pPMB01 (sc LEU2sui1-K56E) | This study |
| PMY32 | MATaura3-52 leu2-3 leu2-112 trp1Δ-63 his4-301(ACG) sui1Δ::hisG pPMB02 (sc LEU2sui1-K60E) | This study |
| PMY33 | MATaura3-52 leu2-3 leu2-112 trp1Δ-63 his4-301(ACG) sui1Δ::hisG pPMB03 (sc LEU2sui1-L96P) | This study |
| PMY98 | MATaura3-52 leu2-3 leu2-112 trp1Δ-63 his4-301(ACG) sui1Δ::hisG pPMB30 (hc LEU2sui1-L96P) | This study |
| PMY34 | MATaura3-52 leu2-3 leu2-112 trp1Δ-63 his4-301(ACG) sui1Δ::hisG pPMB04 (sc LEU2sui1-T15A) | This study |
| PMY35 | MATaura3-52 leu2-3 leu2-112 trp1Δ-63 his4-301(ACG) sui1Δ::hisG pPMB05 (sc LEU2sui1-E48V) | This study |
| PMY36 | MATaura3-52 leu2-3 leu2-112 trp1Δ-63 his4-301(ACG) sui1Δ::hisG pPMB06 (sc LEU2sui1-L51F) | This study |
| PMY37 | MATaura3-52 leu2-3 leu2-112 trp1Δ-63 his4-301(ACG) sui1Δ::hisG pPMB07 (sc LEU2sui1-D61G) | This study |
| PMY38 | MATaura3-52 leu2-3 leu2-112 trp1Δ-63 his4-301(ACG) sui1Δ::hisG pPMB08 (sc LEU2sui1-Q84H) | This study |
| PMY39 | MATaura3-52 leu2-3 leu2-112 trp1Δ-63 his4-301(ACG) sui1Δ::hisG pPMB09 (sc LEU2sui1-E48V,L51F) | This study |
| PMY40 | MATaura3-52 leu2-3 leu2-112 trp1Δ-63 his4-301(ACG) sui1Δ::hisG pCFB04 (hc LEU2SUI1) | This study |
| PMY41 | MATaura3-52 leu2-3 leu2-112 trp1Δ-63 his4-301(ACG) sui1Δ::hisG pPMB10 (sc LEU2SUI1-opt) | This study |
| PMY42 | MATaura3-52 leu2-3 leu2-112 trp1Δ-63 his4-301(ACG) sui1Δ::hisG pPMB12 (sc LEU2sui1-K56E-opt) | This study |
| PMY43 | MATaura3-52 leu2-3 leu2-112 trp1Δ-63 his4-301(ACG) sui1Δ::hisG pPMB13 (sc LEU2sui1-K60E-opt) | This study |
| PMY44 | MATaura3-52 leu2-3 leu2-112 trp1Δ-63 his4-301(ACG) sui1Δ::hisG pPMB14 (sc LEU2sui1-L96P-opt) | This study |
| PMY45 | MATaura3-52 leu2-3 leu2-112 trp1Δ-63 his4-301(ACG) sui1Δ::hisG pPMB15 (sc LEU2sui1-T15A-opt) | This study |
| PMY46 | MATaura3-52 leu2-3 leu2-112 trp1Δ-63 his4-301(ACG) sui1Δ::hisG pPMB16 (sc LEU2sui1-E48V-opt) | This study |
| PMY47 | MATaura3-52 leu2-3 leu2-112 trp1Δ-63 his4-301(ACG) sui1Δ::hisG pPMB17 (sc LEU2sui1-L51F-opt) | This study |
| PMY48 | MATaura3-52 leu2-3 leu2-112 trp1Δ-63 his4-301(ACG) sui1Δ::hisG pPMB18 (sc LEU2sui1-D61G-opt) | This study |
| PMY49 | MATaura3-52 leu2-3 leu2-112 trp1Δ-63 his4-301(ACG) sui1Δ::hisG pPMB19 (sc LEU2sui1-Q84H-opt) | This study |
| PMY50 | MATaura3-52 leu2-3 leu2-112 trp1Δ-63 his4-301(ACG) sui1Δ::hisG pPMB20 (sc LEU2sui1-E48V,L51F-opt) | This study |
| PMY51 | MATaura3-52 leu2-3 leu2-112 trp1Δ-63 his4-301(ACG) sui1Δ::hisG pPMB11 (hc LEU2SUI1-opt) | This study |
| PMY01 | MATaura3-52 leu2-3 leu2-112 trp1Δ-63 his4-301(ACG) sui1Δ::hisG kanMX6:PGAL1-TIF5 p1200 (sc URA3SUI1) | This study |
| PMY02 | MATaura3-52 leu2-3 leu2-112 trp1Δ-63 his4-301(ACG) sui1Δ::hisG kanMX6:PGAL1-SUI3 p1200 (sc URA3SUI1) | This study |
| PMY03 | MATaura3-52 leu2-3 leu2-112 trp1Δ-63 his4-301(ACG) sui1Δ::hisG kanMX6:PGAL1-TIF11 p1200 (sc URA3SUI1) | This study |
| PMY04 | MATaura3-52 leu2-3 leu2-112 trp1Δ-63 his4-301(ACG) sui1Δ::hisG kanMX6:PGAL1-TIF5 p1200 (sc URA3SUI1) p4281 (sc TRP1 TIF5-G31R) | This study |
| PMY52 | MATaura3-52 leu2-3 leu2-112 trp1Δ-63 his4-301(ACG) sui1Δ::hisG kanMX6:PGAL1-TIF5 p4281 (sc TRP1 TIF5-G31R) pJCB101 (sc LEU2SUI1) | This study |
| PMY53 | MATaura3-52 leu2-3 leu2-112 trp1Δ-63 his4-301(ACG) sui1Δ::hisG kanMX6:PGAL1-TIF5 p4281 (sc TRP1 TIF5-G31R) pPMB04 (sc LEU2sui1-T15A) | This study |
| PMY54 | MATaura3-52 leu2-3 leu2-112 trp1Δ-63 his4-301(ACG) sui1Δ::hisG kanMX6:PGAL1-TIF5 p4281 (sc TRP1 TIF5-G31R) pPMB05 (sc LEU2sui1-E48V) | This study |
| PMY55 | MATaura3-52 leu2-3 leu2-112 trp1Δ-63 his4-301(ACG) sui1Δ::hisG kanMX6:PGAL1-TIF5 p4281 (sc TRP1 TIF5-G31R) pPMB06 (sc LEU2sui1-L51F) | This study |
| PMY56 | MATaura3-52 leu2-3 leu2-112 trp1Δ-63 his4-301(ACG) sui1Δ::hisG kanMX6:PGAL1-TIF5 p4281 (sc TRP1 TIF5-G31R) pPMB07 (sc LEU2sui1-D61G) | This study |
| PMY57 | MATaura3-52 leu2-3 leu2-112 trp1Δ-63 his4-301(ACG) sui1Δ::hisG kanMX6:PGAL1-TIF5 p4281 (sc TRP, TIF5-G31R) pPMB08 (sc LEU2sui1-Q84H) | This study |
| PMY58 | MATaura3-52 leu2-3 leu2-112 trp1Δ-63 his4-301(AUU) sui1Δ::hisG kanMX6:PGAL1-TIF5 p4281 (sc TRP1 TIF5-G31R) pPMB09 (sc LEU2sui1-E48V,L51F) | This study |
| PMY59 | MATaura3-52 leu2-3 leu2-112 trp1Δ-63 his4-301(ACG) sui1Δ::hisG kanMX6:PGAL1-TIF5 p4281 (sc TRP1 TIF5-G31R) pCFB04 (hc LEU2SUI1) | This study |
| JCY04 | MATaura3-52 leu2-3 leu2-112 trp1Δ-63 his4-301(ACG)-myc10::KanMX sui1Δ::hisG p1200 (sc URA3SUI1) | 33 |
| JCY806 | MATaura3-52 leu2-3 leu2-112 trp1Δ-63 his4-301(ACG)-myc10::KanMX sui1Δ::hisG p4281 (sc TRP1 TIF5-G31R) pJCB101 (sc LEU2SUI1) | This study |
| JCY807 | MATaura3-52 leu2-3 leu2-112 trp1Δ-63 his4-301(ACG)-myc10::KanMX sui1Δ::hisG p4281 (sc TRP1, TIF5-G31R) pPMB04 (sc LEU2sui1-T15A) | This study |
| JCY808 | MATaura3-52 leu2-3 leu2-112 trp1Δ-63 his4-301(ACG)-myc10::KanMX sui1Δ::hisG p4281 (sc TRP1 TIF5-G31R) pPMB05 (sc LEU2sui1-E48V) | This study |
| PMY60 | MATaura3-52 leu2-3 leu2-112 trp1Δ-63 his4-301(ACG)-myc10::KanMX sui1Δ::hisG p4281 (sc TRP1, TIF5-G31R) pPMB06 (sc LEU2sui1-L51F) | This study |
| JCY809 | MATaura3-52 leu2-3 leu2-112 trp1Δ-63 his4-301(ACG)-myc10::KanMX sui1Δ::hisG p4281 (sc TRP1 TIF5-G31R) pPMB07 (sc LEU2sui1-D61G) | This study |
| JCY810 | MATaura3-52 leu2-3 leu2-112 trp1Δ-63 his4-301(ACG)-myc10::KanMX sui1Δ::hisG p4281 (sc TRP1 TIF5-G31R) pPMB08 (sc LEU2sui1-Q84H) | This study |
| PMY61 | MATaura3-52 leu2-3 leu2-112 trp1Δ-63 his4-301(ACG)-myc10::KanMX sui1Δ::hisG p4281 (sc TRP1 TIF5-G31R) pPMB09 (sc LEU2sui1-E48V,L51F) | This study |
| PMY62 | MATaura3-52 leu2-3 leu2-112 trp1Δ-63 his4-301(ACG)-myc10::KanMX sui1Δ::hisG p4281 (sc TRP1 TIF5-G31R) pCFB04 (hc LEU2SUI1) | This study |
| H466 | MATαhis1-29 gcn2-101 gcn3-101 ino1 ura3-52 | A. Hinnebusch |
| PMY16 | MATaura3-52 leu2-3 leu2-112 trp1Δ-63 HIS4-myc10::KanMX sui1Δ::hisG p1200 (sc URA3SUI1) | This study |
| PMY63 | MATaura3-52 leu2-3 leu2-112 trp1Δ-63 HIS4-myc10::KanMX sui1Δ::hisG p4281 (sc TRP1 TIF5-G31R) pJCB101 (sc LEU2SUI1) | This study |
| PMY64 | MATaura3-52 leu2-3 leu2-112 trp1Δ-63 HIS4-myc10::KanMX sui1Δ::hisG p4281 (sc TRP1, TIF5-G31R) pPMB04 (sc LEU2sui1-T15A) | This study |
| PMY65 | MATaura3-52 leu2-3 leu2-112 trp1Δ-63 HIS4-myc10::KanMX sui1Δ::hisG p4281 (sc TRP1 TIF5-G31R) pPMB05 (sc LEU2sui1-E48V) | This study |
| PMY66 | MATaura3-52 leu2-3 leu2-112 trp1Δ-63 HIS4-myc10::KanMX sui1Δ::hisG p4281 (sc TRP1, TIF5-G31R) pPMB06 (sc LEU2sui1-L51F) | This study |
| PMY67 | MATaura3-52 leu2-3 leu2-112 trp1Δ-63 HIS4-myc10::KanMX sui1Δ::hisG p4281 (sc TRP1 TIF5-G31R) pPMB07 (sc LEU2sui1-D61G) | This study |
| PMY68 | MATaura3-52 leu2-3 leu2-112 trp1Δ-63 HIS4-myc10::KanMX sui1Δ::hisG p4281 (sc TRP1 TIF5-G31R) pPMB08 (sc LEU2sui1-Q84H) | This study |
| PMY69 | MATaura3-52 leu2-3 leu2-112 trp1Δ-63 HIS4-myc10::KanMX sui1Δ::hisG p4281 (sc TRP1 TIF5-G31R) pPMB09 (sc LEU2sui1-E48V,L51F) | This study |
| PMY70 | MATaura3-52 leu2-3 leu2-112 trp1Δ-63 HIS4-myc10::KanMX sui1Δ::hisG p4281 (sc TRP1 TIF5-G31R) pCFB04 (hc LEU2SUI1) | This study |
| PMY71 | MATaura3-52 leu2-3 leu2-112 trp1Δ-63 his4-301(ACG) sui1Δ::hisG p4280 (sc TRP1 SUI3-2) pJCB101(sc LEU2SUI1) | This study |
| PMY72 | MATaura3-52 leu2-3 leu2-112 trp1Δ-63 his4-301(ACG) sui1Δ::hisG p4280 (sc TRP1 SUI3-2) pPMB04 (sc LEU2sui1-T15A) | This study |
| PMY73 | MATaura3-52 leu2-3 leu2-112 trp1Δ-63 his4-301(ACG) sui1Δ::hisG p4280 (sc TRP1 SUI3-2) pPMB05 (sc LEU2sui1-E48V) | This study |
| PMY74 | MATaura3-52 leu2-3 leu2-112 trp1Δ-63 his4-301(ACG) sui1Δ::hisG p4280 (sc TRP1 SUI3-2) pPMB06 (sc LEU2sui1-L51F) | This study |
| PMY75 | MATaura3-52 leu2-3 leu2-112 trp1Δ-63 his4-301(ACG) sui1Δ::hisG p4280 (sc TRP1 SUI3-2) pPMB07 (sc LEU2sui1-D61G) | This study |
| PMY76 | MATaura3-52 leu2-3 leu2-112 trp1Δ-63 his4-301(ACG) sui1Δ::hisG p4280 (sc TRP1 SUI3-2) pPMB08 (sc LEU2sui1-Q84H) | This study |
| PMY77 | MATaura3-52 leu2-3 leu2-112 trp1Δ-63 his4-301(ACG) sui1Δ::hisG p4280 (sc TRP1 SUI3-2) pPMB09 (sc LEU2sui1-E48V,L51F) | This study |
| PMY78 | MATaura3-52 leu2-3 leu2-112 trp1Δ-63 his4-301(ACG) sui1Δ::hisG p4280 (sc TRP1 SUI3-2) pCFB04 (hc LEU2SUI1) | This study |
| PMY79 | MATaura3-52 leu2-3 leu2-112 trp1Δ-63 his4-301(ACG) sui1Δ::hisG kanMX6:PGAL1-SUI3 p4450 (sc TRP1 SUI3) pJCB101 (sc LEU2SUI1) | This study |
| PMY80 | MATaura3-52 leu2-3 leu2-112 trp1Δ-63 his4-301(ACG) sui1Δ::hisG kanMX6:PGAL1-SUI3 p4450 (sc TRP1 SUI3) pPMB10 (sc LEU2SUI1-opt) | This study |
| PMY81 | MATaura3-52 leu2-3 leu2-112 trp1Δ-63 his4-303(ACG) sui1Δ::hisG kanMX6:PGAL1-SUI3 p4280 (sc TRP1 SUI3-2) pJCB101 (sc LEU2SUI1) | This study |
| PMY82 | MATaura3-52 leu2-3 leu2-112 trp1Δ-63 his4-301(ACG) sui1Δ::hisG kanMX6:PGAL1-SUI3 p4280 (sc TRP1 SUI3-2) pPMB10 (sc LEU2SUI1-opt) | This study |
| PMY83 | MATaura3-52 leu2-3 leu2-112 trp1Δ-63 his4-301(ACG) sui1Δ::hisG kanMX6:PGAL1-TIF11 pDSO9 (sc LEU2 TIF11) pPMB21 (sc TRP1SUI1) | This study |
| PMY84 | MATaura3-52 leu2-3 leu2-112 trp1Δ-63 his4-301(ACG) sui1Δ::hisG kanMX6:PGAL1-TIF11 pDSO9 (sc LEU2 TIF11) pPMB22 (sc TRP1SUI1-opt) | This study |
| PMY85 | MATaura3-52 leu2-3 leu2-112 trp1Δ-63 his4-301(ACG) sui1Δ::hisG kanMX6:PGAL1-TIF11 p4552 (sc LEU2TIF11-NDSDG17-21AAAAA) pPMB21 (sc TRP1SUI1) | This study |
| PMY86 | MATaura3-52 leu2-3 leu2-112 trp1Δ-63 his4-301(ACG) sui1Δ::hisG kanMX6:PGAL1-TIF11 p4552 (sc LEU2TIF11-NDSDG17-21AAAAA) pPMB22 (sc TRP1SUI1-opt) | This study |
| PMY87 | MATaura3-52 leu2-3 leu2-112 trp1Δ-63 his4-301(ACG) sui1Δ::hisG kanMX6:PGAL1-TIF11 pAS23 (sc LEU2TIF11-FGFESDE121-127AAAAAAA,FEFGN131-135FAAAA) pPMB21 (sc TRP1SUI1) | This study |
| PMY88 | MATaura3-52 leu2-3 leu2-112 trp1Δ-63 his4-301(ACG) sui1Δ::hisG kanMX6:PGAL1-TIF11 pAS23 (sc LEU2TIF11-FGFESDE121-127AAAAAAA,FEFGN131-135FAAAA) pPMB22 (sc TRP1SUI1-opt) | This study |
| PMY89 | MATaura3-52 leu2-3 leu2-112 trp1Δ-63 his4-301(ACG) sui1Δ::hisG kanMX6:PGAL1-TIF5 p4119 (sc TRP1 TIF5-FL) pJCB101 (sc LEU2SUI1) | This study |
| PMY90 | MATaura3-52 leu2-3 leu2-112 trp1Δ-63 his4-301(ACG) sui1Δ::hisG kanMX6:PGAL1-TIF5 p4119 (sc TRP1 TIF5-FL) pPMB10 (sc LEU2SUI1-opt) | This study |
| PMY91 | MATaura3-52 leu2-3 leu2-112 trp1Δ-63 his4-301(ACG) sui1Δ::hisG kanMX6:PGAL1-TIF5 YCplac22 (sc TRP1) pJCB101 (sc LEU2SUI1) | This study |
| PMY92 | MATaura3-52 leu2-3 leu2-112 trp1Δ-63 his4-301(ACG) sui1Δ::hisG kanMX6:PGAL1-TIF5 YCplac22 (sc TRP1) pPMB10 (sc LEU2SUI1-opt) | This study |
| PMY93 | MATaura3-52 leu2-3 leu2-112 trp1Δ-63 his4-301(ACG) sui1Δ::hisG kanMX6:PGAL1-TIF5 p4281 (sc TRP1 TIF5-G31R) pPMB10 (sc LEU2SUI1-opt) | This study |
hc, high copy number; sc, single copy.
Strains PMY01, PMY02, and PMY03, in which TIF5, SUI3 or TIF11, respectively, are under the control of the GAL1 promoter, were generated from JCY03 by the one-step PCR strategy (28) selecting for resistance to kanamycin on rich medium containing galactose as carbon source (YPGal). Integration of the kanMX:PGAL1 promoter cassette at the correct chromosomal location was verified by PCR analysis of genomic DNA using the appropriate primers.
Strain PMY01 was transformed with an sc TRP1 plasmid containing SUI5 (p4281/YCpTIF5-G31R-W) on synthetic complete medium containing galactose as a carbon source and lacking tryptophan (SGal-W) to generate PMY04. To obtain strains PMY52 through PMY59, PMY04 was transformed with sc or hc LEU2 plasmids harboring the appropriate SUI1 alleles on SGal lacking leucine and tryptophan (SGal-LW), and p1200 was evicted on 5-FOA medium containing galactose as a carbon source (5-FOA/Gal).
Myc-tagging of HIS4 in strain PMY16 was constructed as follows. A PCR fragment containing a portion of HIS4 from 340 bp upstream to 437 bp downstream of the ATG was amplified from genomic DNA of H466 using the primers PM-18 (5′-GAGCATTGCGATACGATGGG-3′) and PM-19 (5′-CGGTCTGTACGTACTTCACC-3′). The PCR product was used to transform strain JCY04 to His+ on SC lacking uracil and histidine (SC-UH), and replacement of his4-301-myc10 with HIS4-myc10 was verified by PCR analysis of chromosomal DNA and sequencing using the appropriate primers.
To obtain strains JCY806 through JCY810, and PMY60 through PMY62, strain JCY04 was cotransformed with a sc TRP1 plasmid containing SUI5 (p4281/YCpTIF5-G31R-W) and sc or hc LEU2 plasmids harboring the appropriate SUI1 alleles on SC-LW medium, and p1200 was evicted on 5-FOA medium. The same procedure was followed to generate PMY63 through PMY70 except beginning with PMY16 rather than JCY04.
To produce strains PMY71 through PMY78, JCY03 was cotransformed with an sc TRP1 plasmid containing SUI3-2 (p4280/YCpSUI3-S264Y-W) and sc or hc LEU2 plasmids harboring the appropriate SUI1 alleles on SC-LW medium, and p1200 was evicted on 5-FOA medium.
Strains PMY79 through PMY82 were constructed by cotransforming PMY02 with an sc LEU2 plasmid containing SUI1 (pJCB101) or SUI1-opt (pPMB10) and with an sc TRP1 plasmid containing SUI3 (p4450) or SUI3-2 (p4280/YCpSUI3-S264Y-W), on SGal-LW, and p1200 was evicted on 5-FOA/Gal medium.
Strains PMY83 through PMY88 were generated by cotransforming PMY03 with an sc TRP1 plasmid harboring SUI1 (pPMB21) or SUI1-opt (pPMB22) and with an sc LEU2 plasmid containing TIF11 (pDSO9), tif11-17-21 (p4552), or tif11-SE1*, SE2*+F131 (pAS23) on SGal-LW, and p1200 was evicted on 5-FOA/Gal medium.
Strains PMY89 through PMY93 were produced by cotransforming PMY01 with an sc LEU2 plasmid containing SUI1 (pJCB101) or SUI1-opt (pPMB10) and with an sc TRP1 plasmid containing TIF5-FL (p4119/TIF5-FL TRP1) or SUI5 (p4281/YCpTIF5-G31R-W), or with empty TRP1 vector YCplac22, on SGal-LW, and p1200 was evicted on 5-FOA/Gal medium.
Plasmid constructions.
Plasmid pJCB101 was constructed by cloning a 0.9-kb BssHII-SacII fragment containing SUI1 from p1200 into similarly digested pCFB03. The construction of plasmids pPMB01 through pPMB08 is described in the next section.
pPMB30 was created by inserting a 1.6-kb HindIII-SacI fragment containing sui1-L96P from pPMB03 into the corresponding sites of YCplac181.
To construct pPMB09, containing sui1-E48V,L51F, the QuikChange site-directed mutagenesis system (Stratagene) was used with the primers PM-74 (5′-CCCAGAGGTATATGATTTTAAGAGAATTCTTAAGGTC-3′) and PM-75 (5′-GACCTTAAGAATTCTCTTAAAATCATATACCTCTGGG-3′), using pPMB05 as a template.
To introduce the optimal sequence context A−3-A−2-A−1-AUG into the corresponding sc or hc plasmids containing either SUI1 or different sui1 mutant alleles, the QuikChange site-directed mutagenesis system (Stratagene) was used with the primers PM-181 (5′-TATAGCTGAAGCAAATAAAATGTCCATTGAGAATC-3′) and PM-182 (5′-GATTCTCAATGGACATTTTATTTGCTTCAGCTATA-3′).
To construct pPMB24, an 812-bp SUI1 fragment containing 577 bp upstream and 212 bp downstream of the ATG was amplified by PCR from pJCB101 using primers PM-169 (5′- GTGAGCTACGCGTCGACAGATCTGAATCTATTCTGGAC-3′) and PM-170 (5′- CGCGGATCCTTGACAATGTTACCATTACATGC-3′) introducing a novel SalI site in the 5′ end and a BamHI site in the 3′ end of the fragment, digested with SalI and BamHI, and inserted between the corresponding sites in the GCN4 plasmid p164, generating pPMB23. A BamHI fragment containing lacZ was excised from p180 and inserted into the BamHI site of pPMB23 to produce pPMB24, harboring a SUI1-lacZ fusion construct that includes the promoter region and first 215 bp of the SUI1 open reading frame (ORF), the lacZ ORF, and GCN4 sequences extending from the BamHI site through the 3′ untranslated region to the EcoRI site 3′ of GCN4.
pPMB25 and pPMB28 were constructed by cloning the same 812-bp SalI/BamHI fragment containing SUI1 just described into pCR2.1-TOPO (Invitrogen). Site-directed mutagenesis was conducted with the QuikChange system by using primer pairs (i) PM-181 (5′-TATAGCTGAAGCAAATAAAATGTCCATTGAGAATC-3′) and PM-182 (5′-GATTCTCAATGGACATTTTATTTGCTTCAGCTATA-3′) to introduce the A−3-A−2-A−1-AUGcontext and (ii) PM-206 (5′-TATAGCTGAAGCAAATTTTATGTCCATTGAGAATC-3′) and PM-207 (5′-GATTCTCAATGGACATAAAATTTGCTTCAGCTATA-3′) to introduce the U−3-U−2-U−1-AUG context. The resulting PCR products were digested with BamHI and SalI and cloned into p164, and the corresponding SUI1-lacZ fusions, pPMB25 and pPMB28, were generated as described above for pPMB24.
To construct pPMB26 and pPMB27, an ∼4.1-kb SalI-KpnI fragment containing either SUI1-lacZ or SUI1-opt-lacZ from plasmids pPMB24 and pPMB25, respectively, was cloned between the corresponding sites of pHQ1303.
Selection of mutant SUI1 alleles.
Random mutagenesis of SUI1 was conducted by error-prone PCR using the SUI1 URA3 plasmid p1200 as a template with the primers JCO075 (5′-TGTACACTATGCATGCGCGT-3′) and JCO076 (5′-CCCCATGATAATGTACTCTCG-3′) and a GeneMorph II random mutagenesis kit (Stratagene). A 0.9-kb BssHII-SacII fragment from His-SUI1 plasmid pCFB03 was replaced with the resulting PCR products and ∼67,000 bacterial transformants were pooled. Plasmid DNA was prepared from the pool for screening in the appropriate yeast strains. To identify Sui− alleles, Leu+ yeast transformants of strain JCY03 were selected on SC-L and pooled, and dilutions were plated on SC-L supplemented with 5-FOA (to evict p1200) and 0.0015 mM histidine. Transformants with His+ phenotype were colony purified, and the resident plasmids were isolated.
Ssu− mutations were identified as suppressors of the lethality of SUI5, as follows. Leu+ Trp+ transformants of strain PMY04 were selected on SGal-LW and pooled, and dilutions were plated on SC-LW supplemented with 5-FOA. Transformants that grew in the presence of glucose were colony purified, and the resident plasmids were isolated.
For each selection, the ability of the purified plasmids to confer the relevant phenotypes was verified after transformation of the same yeast strain used to screen the mutant library, and the complete DNA sequence of the 0.9-kb BssHII-SacII fragment was determined to identify the mutations present in SUI1. In most cases, more than one mutation was present either in the promoter or in the SUI1 coding region. In identify the mutations that confer the relevant phenotypes, site-directed mutagenesis was conducted to generate SUI1 alleles with single mutations using the QuikChange system and the appropriate primers to generate the following plasmids: (i) pPMB01, primers PM-43 (5′-AAGAGAATTCTTGAGGTCCTAAAGA-3′) and PM-44 (5′-TCTTTAGGACCTCAAGAATTCTCTT-3′); (ii) pPMB02, primers PM-47 (5′-CTTAAGGTCCTAAAGGAGGACTTTGCATG-3′) and PM-48 (5′-CATGCAAAGTCCTCCTTTAGGACCTTAAG-3′); (iii) pPMB03, primers PM-138 (5′-GAATTTATGATCTCCCAACCGGGATTGCAAAAGAAG-3′) and PM-139 (5′-CTTCTTTTGCAATCCCGGTTGGGAGATCATAAATTC-3′); (iv) pPMB04, primers PM-23 (5′-CCTTTCGCCGACGCAGGAGACGACG-3′) and PM-24 (5′-CGTCGTCTCCTGCGTCGGCGAAAGG-3′); (v) pPMB05, primers PM-25 (5′-GCAAGGTGTCCCAGAGGTATATGATTTAAAGAGAA-3′) and PM-26 (5′-TTCTCTTTAAATCATATACCTCTGGGACACCTTGC-3′); (vi) pPMB06, primers PM-33 (5′-CCCAGAGGAATATGATTTTAAGAGAATTCTTAAGGTC-3′ and PM-34 (5′-GACCTTAAGAATTCTCTTAAAATCATATTCCTCTGGG-3′); (vii) pPMB07, primers PM-132 (5′-GTCCTAAAGAAGGGCTTTGCATGTAATG-3′) and PM-133(5′-CATTACATGCAAAGCCCTTCTTTAGGAC-3′); and (viii) pPMB08, primers PM-27 (5′-CAGTTGCAGGGTGACCATAGAGCAAAGGTTTGC-3′) and PM-28 (5′-GCAAACCTTTGCTCTATGGTCACCCTGCAACTG-3′).
Biochemical assays.
Assays of β-galactosidase activity in whole-cell extracts (WCEs) were performed as described previously (32). For Western analysis, WCEs were prepared by trichloroacetic acid extraction as previously described (39), and immunoblot analysis was conducted as described previously (33) with antibodies against eIF1 (48), eIF2Bε/Gcd6 (4) or myc epitope (Sigma). Enhanced chemiluminescence or the Odyssey infrared imaging system (Li-Cor) was used to visualize immune complexes, and signal intensities were quantified by densitometry using NIH ImageJ software or with the Odyssey application software, respectively.
For Northern analysis, RNA was extracted as previously described (42), resolved by electrophoresis in 1.2% agarose-4% formaldehyde gels, blotted onto positively charged nylon membranes (Roche), and immobilized with a UV Stratalinker 2400 (Stratagene). The blots were probed with a 1.6-kb HindIII-SacI fragment from plasmid pJCB101 containing the entire SUI1 ORF or with a 6.7-kb HindIII fragment containing the PYK1 coding sequence (encoding pyruvate kinase), radiolabeled using a random primed DNA labeling kit from Roche.
RESULTS
SUI1 Sui− mutations increase SUI1 expression by suppressing poor AUG context.
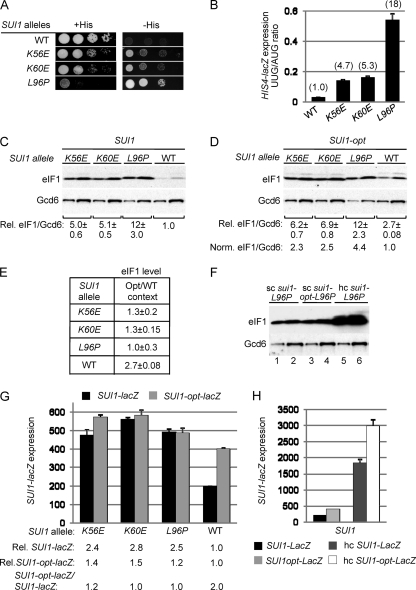
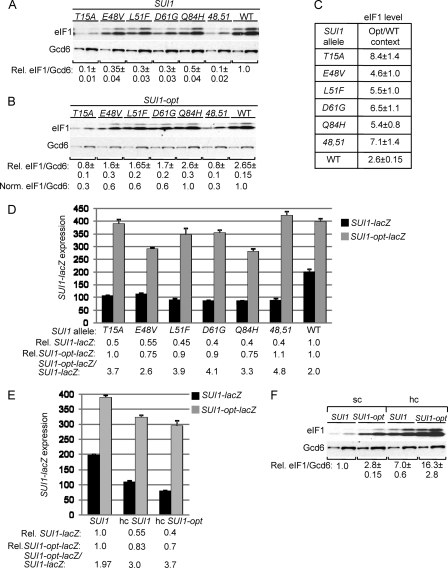
We recently conducted a random mutagenesis of SUI1, the structural gene for eIF1/Sui1, in an effort to saturate single amino acid replacements in eIF1 that would evoke strong Sui− phenotypes, increasing initiation at the third (UUG) codon in the mutant his4-301 mRNA lacking an AUG start codon. Such mutations restore the ability to grow on medium lacking histidine or containing only 1% of the normal histidine supplement (−His medium), conferring a His+ phenotype on his4-301 cells. The mutant SUI1 alleles were introduced on a LEU2 plasmid into a his4-301 sui1Δ strain harboring WT SUI1 on a URA3 plasmid, and the latter SUI1+ plasmid was evicted by counterselection on medium containing 5-FOA (3). Among the sui1 alleles that conferred a His+ phenotype, we chose three for detailed analysis, including two that generate Glu substitutions of Lys-56 or Lys-60 in helix α1 of eIF1, and the third producing a Pro substitution for Leu-96 in helix α2 (15). These mutant alleles are designated below as K56E, K60E, and L96P, respectively. As shown in Fig. 1A, compared to the SUI1+ strain, all three sui1 mutants exhibit increased growth on −His medium despite diminished growth on histidine-replete medium (+His), and the His+ phenotype is most pronounced for L96P (Fig. 1A). These mutants also display an increased frequency of UUG initiation, detected by assaying matched HIS4-lacZ fusions with AUG or UUG start codons. The UUG/AUG initiation ratio was increased by a factor of ∼5 to ∼18 above the WT level, with the greatest increase observed for L96P (Fig. 1B), confirming that all three strains are bona fide Sui− mutants.
Fig. 1.
SUI1 Sui− mutations increase SUI1 expression by suppressing poor AUG context. (A) Slg− and His+/Sui− phenotypes of derivatives of sui1Δ his4-301 strain JCY03 containing the indicated SUI1 alleles on sc plasmids determined by spotting serial 10-fold dilutions on synthetic complete medium lacking leucine (Leu) supplemented with 0.3 mM histidine (+His) or 0.003 mM His (−His) and incubating for 3 days (+His) or 7 days (−His) at 30°C. (B) Strains from panel A also harboring HIS4-lacZ reporter plasmids with an AUG (p367) or UUG (p391) start codon were cultured in synthetic dextrose minimal medium (SD) supplemented with His and tryptophan (Trp) at 30°C to an A600 of ∼1.0, and β-galactosidase activities (nmol of o-nitrophenyl β-d-galactopyranoside cleaved per min per mg) were measured in WCEs. The ratio of expression of the UUG versus AUG reporter was calculated for replicate experiments, and the means and standard errors of the mean (SEM [error bar]) were plotted. Numbers in parentheses are the means normalized to the WT value. (C to E) SUI1 Sui− mutations elevate eIF1 expression dependent on the SUI1 AUG context. Derivatives of sui1Δ strain JCY03 containing the indicated SUI1 alleles (C) or SUI1-opt alleles (D) were cultured in SD supplemented with His, Trp, and uracil (Ura) at 30°C to an A600 of ∼1.0, and WCEs were subjected to Western analysis using antibodies against eIF1/Sui1 or eIF2Bε/Gcd6 (analyzed as a loading control). Two different amounts of each extract differing by a factor of 2 were loaded in successive lanes. Signal intensities were quantified from replicate experiments, and mean eIF1/Gcd6 ratios were normalized to that obtained for WT SUI1 to yield the relative (Rel.) eIF1/Gcd6 values listed below the blots. In panel D, the Rel. eIF1/Gcd6 ratios were normalized to that obtained for WT SUI1-opt to yield the Norm. eIF1/Gcd6 values. In panel E, the ratios of mean eIF1/Gcd6 values for SUI1 versus SUI1-opt alleles were calculated for each strain. (F) Western analysis of JCY03 derivatives containing sc or hc plasmids with sui1-L96P (lanes 1 and 2 and lanes 5 and 6, respectively) or sc sui1-opt-L96P (lanes 3 and 4), conducted as in panels C and D. (G) Derivatives of JCY03 harboring the indicated sc SUI1 alleles and sc plasmids with SUI1-lacZ (pPMB24) or SUI1-opt-lacZ (pPMB25) were cultured and assayed for β-galactosidase activities as in panel B. Mean SUI1-lacZ or SUI1-opt-lacZ expression levels determined from replicate measurements were normalized to those for WT to yield the relative (Rel.) expression values listed below the histogram, and the ratio of mean SUI1-lacZ versus SUI1-opt-lacZ expression is given on the bottom line. (H) Transformants of strain PMY30 harboring sc plasmids with SUI1-lacZ (pPMB24) or SUI1-opt-lacZ (pPMB25) and hc plasmids with SUI1-lacZ (pPMB26) or SUI1-opt-lacZ (pPMB27) reporters were cultured and assayed for β-galactosidase activities as in panel G.
Because the Sui− phenotype generally results from a reduction in eIF1 function (8, 48, 49), it was important to demonstrate that the Sui− substitutions reduce eIF1 activity rather than its expression. Western analysis of WCEs revealed that the sui1 mutants actually contain ∼5- to ∼12-fold higher than WT levels of eIF1 (normalized to eIF2Bε subunit Gcd6), with the largest increase observed for the strongest Sui− mutant (L96P) (Fig. 1C).
The fact that eIF1 expression is elevated by these Sui− mutations is consistent with the possibility that they increase translation initiation from the SUI1 AUG codon by overcoming its poor sequence context. If so, the increased eIF1 expression should be dampened by replacing the poor context with the optimal context for initiation in yeast. As expected, replacing the poor context of the SUI1 start codon (C−3-G−2-U−1-AUG) with the optimal context (A−3-A−2-A−1-AUG), in the SUI1-opt allele, increased the eIF1 level in WT cells by a factor of 2.7 (cf. the WT lanes in Fig. 1C and D and the Opt/WT ratio for WT in Fig. 1E). Notably, introducing the optimal context dampened by a factor of 2 to 3 the increase in eIF1 expression conferred by the Sui− substitutions. For example, L96P increased expression of SUI1-opt by a factor of 4.4 (Fig. 1D, Norm. eIF1/Gcd6) compared to the 12-fold increase observed for SUI1 alleles with the WT context (Fig. 1C, Rel. eIF1/Gcd6). Moreover, the Sui− substitutions reduced or completely masked the stimulatory effect of introducing the optimal context on eIF1 expression, conferring Opt/WT ratios close to unity (Fig. 1E).
Importantly, the sui1-L96P allele on a high-copy (hc) plasmid produced a substantially higher level of eIF1 than that given by the sui1-opt-L96P allele in single copy (sc) (Fig. 1F, lanes 5 and 6 versus lanes 3 and 4), indicating that the lack of an increase in eIF1-L96P expression conferred by the optimal context (Fig. 1F, lanes 3 and 4 versus lanes 1 and 2) does not reflect saturation of Western signals achieved with anti-eIF1 antibodies or an unknown posttranslational mechanism that would block eIF1 overexpression. Furthermore, the fact that introducing the optimal AUG context dampens the effects of the Sui− mutations (Fig. 1E) makes it unlikely that they increase SUI1 transcription or stability of SUI1 mRNA as the means of increasing eIF1 expression. Together, these results support the idea that eIF1 Sui− mutations overcome the deleterious effect of poor context at the SUI1 AUG codon.
To provide independent evidence that eIF1 Sui− mutations suppress poor AUG context and do not increase SUI1 expression by stabilizing SUI1 mRNA, we examined their effects in trans on expression of SUI1-lacZ fusions containing the native, poor context or the optimal context described above (SUI1-opt-lacZ). Consistent with the Western analysis in Fig. 1C and D, expression of β-galactosidase from SUI1-opt-lacZ was 2-fold higher than that of SUI1-lacZ in WT cells (Fig. 1G, WT), reflecting the stimulatory effect of optimal context. Moreover, the sui1 alleles increased expression of the WT fusion by a factor of 2.4 to 2.8 (Fig. 1G, Rel. SUI1-lacZ) but produced increases of only 1.2- to 1.5-fold for the SUI1-opt-lacZ construct (Fig. 1G, Rel. SUI1-opt-lacZ). Importantly, introducing the optimal context increased β-galactosidase expression by factors of only 1.2 or less in the Sui− mutants compared to the 2-fold increase in WT cells (Fig. 1G, SUI1-opt-lacZ/SUI1-lacZ ratios). Introducing SUI1-opt-lacZ on an hc plasmid into WT cells increased the β-galactosidase activity by ∼8-fold (Fig. 1H), ruling out the possibility that fusion expression cannot substantially exceed that observed in the Sui− mutants. These findings support the conclusion that the eIF1 Sui− mutations increase SUI1 expression by overcoming the deleterious effect of poor AUG context on translation initiation.
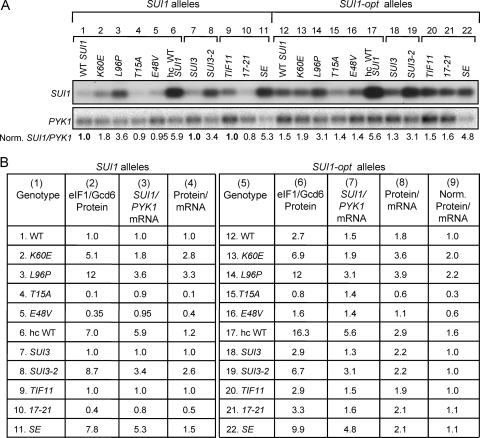
Finally, to provide direct evidence that the sui1 mutations increase translational efficiency, we compared their effects on the level of SUI1 mRNA, measured by Northern analysis, and the level of eIF1 measured by Western analysis. Although the K60E and L96P mutations conferred significant increases in SUI1 mRNA abundance (Fig. 2, SUI1 alleles, Norm. SUI1/PYK1 ratios), they evoked ∼3-fold greater increases in expression of eIF1 protein versus SUI1 mRNA (Fig. 2, SUI1 alleles, protein/mRNA ratios, WT versus K60E,L96P), thus confirming that they increase the translational efficiency of SUI1 mRNA. Introducing the optimal context into WT SUI1 also produced a moderate increase (∼50%) in the level of SUI1 mRNA, but the increase in eIF1 protein (2.7-fold) exceeded the increase in SUI1 mRNA (1.5) by a factor of 1.8 (Fig. 2, rows 1 and 12, protein/mRNA values), again confirming an increase in translational efficiency. After normalizing the eIF1 protein level for the corresponding SUI1 mRNA level, it can be seen that K60E and L96P produce smaller increases in this ratio for the SUI1-opt allele compared to WT SUI1 (Fig. 2, cf. rows 2 and 3, protein/mRNA, versus rows 13 and 14, Norm. Protein/mRNA), supporting the conclusion that the optimum context dampens the stimulatory effects of these Sui− mutations on the translational efficiency of SUI1 mRNA.
Fig. 2.
Comparison of effects of Sui− and Ssu− mutations in eIF1, eIF2β, and eIF1A on eIF1 protein and SUI1 mRNA levels. (A) Northern analysis of SUI1 mRNA in selected mutants. Lanes 1 to 6 and lanes 12 to 17 show results for strains described in Fig. 1C and D and in Fig. 5A, B, and F containing the indicated SUI1+ alleles (lanes 1 to 6) or SUI1-opt alleles (lanes 12 to 17) cultured as in Fig. 1C. Lanes 7 to 11 and lanes 18 to 22 show the results for strains described in Fig. 6A and C containing SUI1+ (lanes 7 to 11) or SUI1-opt (lanes 18 to 22) cultured as described in Fig. 6A. Total RNA was subjected to Northern analysis of SUI1 and PYK1 mRNAs, the hybridization signals were quantified with a Phosphorimager, and ratios of SUI1 to PYK1 mRNA were calculated and normalized to the ratio obtained for the corresponding strain, harboring WT SUI1, SUI3, and TIF11. The resulting “Norm. SUI1/PYK1” values are listed below the blot and also in columns 3 and 7 of panel B. The WT SUI1 reference strain is shown in lane 1 for the mutants examined in lanes 1 to 6 and lanes 12 to 17. The WT SUI1 SUI3 reference strain is shown in lane 7 for the mutants examined in lanes 7 and 8 and lanes 18 and 19. The WT SUI1 TIF11 reference strain is shown in lane 9 for the mutants examined in lanes 9 to 11 and lanes 20 to 22. (B) Comparison of eIF1 protein and SUI1 mRNA levels. For the strains analyzed in panel A, the appropriate “Rel. eIF1/Gcd6” values taken from Fig. 1C and D, from Fig. 5A, B, and F, and from Fig. 6A and C are listed in columns 2 and 6 (eIF1/Gcd6 Protein) for SUI1 and SUI1-opt alleles, respectively; the “Norm. SUI1/PYK1” values from panel A are listed in columns 3 and 7 (SUI1/PYK1 mRNA); the ratios of values in columns 2 and 3 are listed in column 4 (Protein/mRNA); the ratios of values in columns 6 and 7 are listed in column 8 (Protein/mRNA); and the values in column 8 normalized to the cognate WT ratios in rows 12, 18, or 20 are listed in column 9 (Norm. Protein/mRNA).
In these and other experiments below, we consistently observed that mutations in the SUI1 AUG context, or in various initiation factors, that alter expression of eIF1 protein also change SUI1 mRNA abundance in the same direction, but to a significantly smaller extent. In fact, it is well established that translation efficiency is a major determinant of mRNA stability in yeast, since mutations in various initiation factors or the AUG context of specific mRNAs will decrease mRNA stability in proportion to their deleterious effects on translation initiation (25, 43). Hence, the simplest interpretation of the changes in SUI1 mRNA abundance produced by mutations in initiation factors or AUG context is that they are the indirect consequences of changes in translational efficiency. As such, the eIF1 protein/SUI1 mRNA ratios calculated in Fig. 2 likely underestimate the differences in translational efficiency conferred by these mutations.
SUI1 Ssu− mutations reduce SUI1 expression by exacerbating poor context.
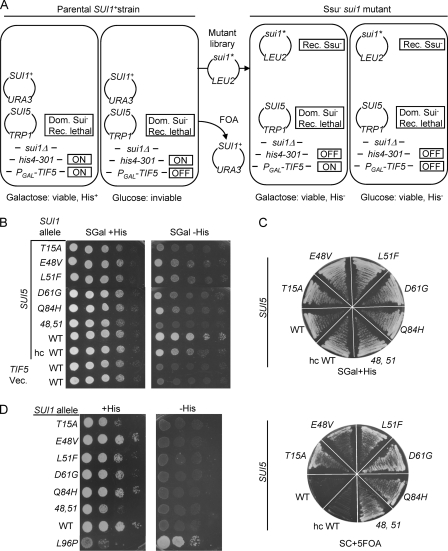
We also screened our mutagenized SUI1 plasmids for eIF1/Sui1 substitutions that would suppress the Sui− phenotypes of mutations in other eIFs, since this phenotype was not previously described for eIF1 mutants. Ssu− substitutions were of interest because they should increase the accuracy of initiation and reinstate the requirement for an AUG start codon for efficient initiation in Sui− mutants. It was of interest to determine whether such Ssu− substitutions would also impose a stronger requirement for optimal AUG context for efficient initiation.
Ssu− alleles of SUI1 were isolated by their ability to suppress the recessive lethality of the SUI5 mutation in eIF5 (Tif5 in yeast), the GTPase activating protein for eIF2. As diagrammed in Fig. 3A, we used a sui1Δ his4-301 strain containing plasmid-borne copies of SUI1+ and SUI5, plus a chromosomal TIF5 allele under the GAL1 promoter (PGAL-TIF5) that expresses WT eIF5 only on galactose medium. This strain can grow on galactose medium lacking histidine owing to the dominant Sui− phenotype of SUI5 and the complementation of its recessive lethality by PGAL1-TIF5; however, the strain cannot grow on glucose medium because PGAL1-TIF5 is repressed, and SUI5 is the only source of eIF5 under these conditions (Fig. 3A, left). Hence, we selected plasmids from the mutant library that, following eviction of the SUI1+ plasmid on 5-FOA medium, rescued the ability to grow on glucose medium (suppressing SUI5 lethality) and eliminated the His+ phenotype on galactose medium (suppressing the SUI5 Sui− phenotype) (Fig. 3A, right). We focused on five such sui1 alleles that introduce single amino acid substitutions into eIF1—T15A, E48V, L51F, D61G, and Q84H—and by site-directed mutagenesis generated a sixth allele that combines two of the mutations, E48V and L51F (abbreviated as “48,51”). The results in Fig. 3B (right) document that these sui1 alleles suppress the His+/Sui− phenotype of SUI5, while those in Fig. 3C (bottom) demonstrate cosuppression of SUI5 lethality. In the absence of SUI5, these sui1 alleles produce slight to moderate slow-growth phenotypes and, as expected, do not confer Sui− phenotypes on their own (Fig. 3D).
Fig. 3.
Isolation of Ssu− substitutions in eIF1. (A) Summary of genetic selection used to isolate Ssu− alleles of SUI1 as suppressors of the recessive lethality of SUI5. The relevant genotype, the expression levels of his4-301 and PGAL-TIF5 (ON or OFF), and the growth phenotypes on medium containing galactose or glucose as carbon source are indicated for the parental strain (two rectangles on the left) and for Ssu− sui1 mutants (two rectangles on the right). See the text for further details. (B) Ssu− phenotypes of derivatives of a sui1Δ his4-301 PGAL-TIF5 strain with episomal SUI5 (PMY04) harboring the indicated SUI1 alleles. Tenfold serial dilutions of these strains plus strains PMY89 and PMY91 containing episomal TIF5 (p4119) or empty vector, versus SUI5 (last two rows) were spotted on SGal+His and SGal-His (containing 0.003 mM His). (C) In the top panel, the same strains as in panel B were streaked on SC containing 2% galactose, 1% raffinose as a carbon source, lacking Leu and tryptophan (Trp), and supplemented with 0.3 mM His (SGal+His). In the bottom panel, strains were streaked on SC lacking Leu and Trp and supplemented with 5-FOA (SC + 5-FOA). (D) Phenotypes of SUI1 Ssu− mutations in the absence of SUI5. Derivatives of sui1Δ his4-301 strain JCY03 containing the indicated SUI1 alleles were analyzed as in Fig. 1A.
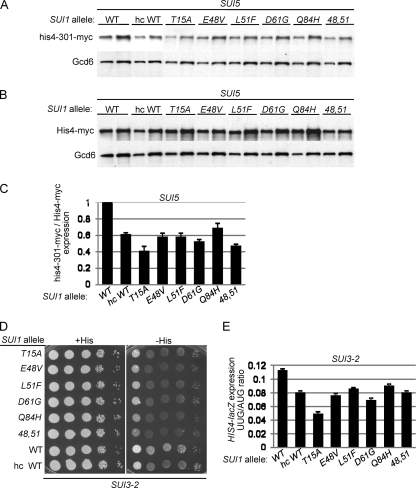
We verified the Ssu− phenotypes of these mutants by Western analysis of the myc-tagged version of the his4-301 product expressed in these strains. Expression of WT His4-myc was measured in parallel in isogenic strains harboring HIS4-myc (with AUG start codon) versus his4-301-myc (Fig. 4A and B). Quantification of the Western data revealed that, compared to the SUI1+ strain, all of the mutants display significantly reduced levels of his4-301-myc versus His4-myc (Fig. 4C), indicating a decreased UUG to AUG initiation ratio for HIS4 mRNA in the manner expected for Ssu− alleles. Note also that the mutants resemble the SUI1+ strain that overexpresses WT eIF1 from an hc SUI1+ plasmid (hc WT), which is known to confer an Ssu− phenotype (Fig. 4C) (48).
Fig. 4.
SUI1 Ssu− mutations reduce the HIS4 UUG/AUG initiation ratio in SUI5 and SUI3-2 cells. (A to C) Derivatives of sui1Δ his4-301-myc SUI5 (A) and sui1Δ HIS4-myc SUI5 (B) strains JCY04 and PMY16, respectively, harboring the indicated SUI1 alleles were cultured as in Fig. 1C, and WCEs were subjected to Western analysis with antibodies against myc epitope or Gcd6. Two different amounts of each extract differing by a factor of 2 were loaded in successive lanes. (C) Western signals from panels A and B were quantified, and the mean ratios of his4-301-myc to His4-myc (each normalized to Gcd6) are plotted with the SEM as error bars. (D) Derivatives of JCY03 containing episomal SUI3-2 (p4280/YCpSUI3-S264Y-W) and harboring the indicated SUI1 alleles were analyzed for Slg− and His+/Sui− phenotypes by spotting serial 10-fold dilutions on SC lacking Leu and Trp and supplemented with either 0.3 mM His (+His) or 0.003 mM His (−His). (E) Transformants of the SUI3-2 strains from panel D containing the AUG or UUG HIS4-lacZ reporters were analyzed as in Fig. 1B.
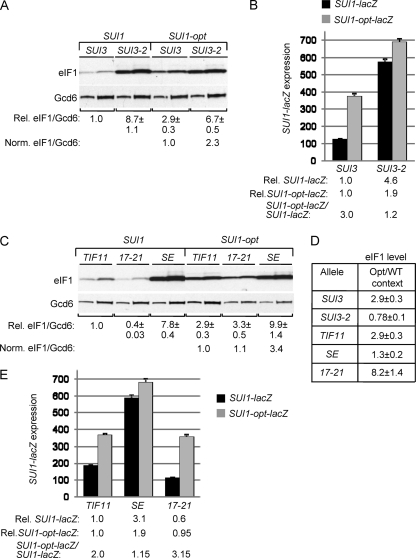
To confirm these last findings, we examined whether the sui1 Ssu− mutations also suppress the Sui− phenotype of the dominant eIF2β Sui− allele SUI3-2 (21). Indeed, all of the sui1 alleles resemble hc SUI1+ in suppressing the His+ phenotype of SUI3-2 (Fig. 4D) and in lowering the UUG/AUG initiation ratio for the matched HIS4-lacZ fusions in the SUI3-2 strain (Fig. 4E). The fact that they mitigate the Sui− phenotype of SUI3-2 confirms that all six sui1 alleles have Ssu− phenotypes.
Remarkably, all of the Ssu− sui1 alleles exhibit levels of eIF1 expression lower than that of the WT, with the T15A and 48,51 mutants decreasing expression the most, by a factor of ∼10 (Fig. 5A). These results suggest that the Ssu− substitutions exacerbate the effect of poor context at the SUI1 start codon. Supporting this interpretation, the reduction in eIF1 expression conferred by these sui1 mutations was substantially alleviated, or completely eliminated, by the presence of the optimal AUG context in the cognate SUI1-opt alleles (Fig. 5B). For example, whereas T15A and 48,51 reduced expression of WT SUI1 by ∼10-fold (Fig. 5A), they reduced expression of SUI1-opt by only ∼3-fold (Fig. 5B). Note also that whereas optimal context increases eIF1 expression from SUI1+ by a factor of 2.6, it provokes substantially larger increases, between 4.6- and 8.4-fold, in eIF1 expression from the Ssu− alleles (Fig. 5C), as expected if the latter exacerbate the effect of poor context at the SUI1 AUG codon. The fact that T15A and 48,51 produce considerably lower than WT levels of eIF1 even in the presence of the optimal context (Fig. 5B) might indicate that these substitutions destabilize eIF1 protein in addition to decreasing AUG recognition. Alternatively, another unknown feature of SUI1 mRNA might be suboptimal, and these Ssu− substitutions would discriminate against this hypothetical anti-determinant in addition to poor AUG context. For example, it was shown that coding sequences function coordinately with the AUG context to stimulate the efficiency of translation and stability of PGK1 mRNA in yeast (25).
Fig. 5.
SUI1 Ssu− mutations reduce SUI1 expression by exacerbating poor context. (A to C) SUI1 Ssu− mutations reduce eIF1 expression dependent on SUI1+ AUG context. Derivatives of JCY03 containing the indicated SUI1 alleles (A) or SUI1-opt alleles (B) were subjected to Western analysis as in Fig. 1C. Two different amounts of each extract differing by a factor of 2 were loaded in successive lanes. (C) Ratios of mean eIF1/Gcd6 values for SUI1 versus SUI1-opt alleles were calculated as in Fig. 1E. (D) Derivatives of JCY03 harboring the indicated SUI1 alleles and the SUI1-lacZ or SUI1-opt-lacZ reporter were analyzed for β-galactosidase activities as in Fig. 1G. (E) SUI1-lacZ and SUI1-opt-lacZ expression levels were determined in derivatives of JCY03 containing sc SUI1, hc SUI, or hc SUI1-opt, as indicated, as in Fig. 1G. (F) Western analysis of eIF1 expression in JCY03 derivatives containing the indicated sc or hc SUI1 alleles, conducted as in Fig. 1C.
To confirm the conclusions reached from Western analysis, we also examined the effects of the Ssu− mutations in trans on SUI1-lacZ expression. All of the mutations decrease expression of the WT fusion by factors of 2 to 2.5 (Fig. 5D, Rel. SUI1-lacZ) but, importantly, have considerably less effect on expression of the SUI1-opt-lacZ fusion (Fig. 5D, Rel. SUI1-opt-lacZ). Furthermore, whereas introducing the optimal context increases fusion gene expression by a factor of 2.0 in WT cells, it produces larger increases, between 2.6- and 4.8-fold, in the Ssu− mutants (Fig. 5D, SUI1-opt-lacZ/SUI1-lacZ). These findings support the conclusion that eIF1 Ssu− substitutions reduce SUI1 expression by exacerbating its poor AUG context.
Consistent with their deleterious effects on eIF1 expression, Northern analysis revealed that the T15A and E48V mutations also reduced the level of SUI1 mRNA. However, since the magnitude of the decrease in mRNA is less than the reduction in eIF1 protein levels evoked by these mutations, they confer protein/mRNA ratios of less than unity (Fig. 2, rows 4 and 5, Protein/mRNA), supporting the conclusion that the mutations reduce SUI1 translational efficiency. Moreover, the eIF1 protein/SUI1 mRNA ratios are reduced by the T15A and E48V mutations to a greater extent for SUI1 versus SUI1-opt alleles (Fig. 2, rows 4 and 5 [Protein/mRNA] versus rows 15 and 16 [Norm. Protein/mRNA]), supporting the idea that they reduce SUI1 translational efficiency by intensifying the negative effect of its native, poor AUG context.
eIF1 moderately autoregulates translation by exacerbating the effects of poor AUG context at SUI1.
As noted above, overexpressing WT eIF1 confers an Ssu− phenotype (41, 48). Consistent with this, hc SUI1+ phenocopies the sui1 Ssu− mutations and reduces expression of the WT SUI1-lacZ fusion by a factor of 2 but diminishes expression of SUI1-opt-lacZ by only ∼20% (Fig. 5E, SUI1 versus hc SUI1). The repression of SUI1-lacZ expression occurs in response to an ∼7-fold increase in eIF1 expression from hc SUI1 versus sc SUI1 (Fig. 5F). We noted that ∼2-fold greater eIF1 expression (16-fold above WT) was achieved with the hc version of SUI1-opt versus hc SUI1 (Fig. 5F), which is consistent with the 2-fold greater translational efficiency of SUI1-opt versus SUI1 determined above (Fig. 1C, D, and G and Fig. 2). Consistent with this, hc SUI1-opt conferred more extensive repression of SUI1-lacZ expression than did hc SUI1, plus an increased stimulatory effect of introducing the optimal context into the lacZ fusion (Fig. 5E, cf. SUI1-opt-lacZ/SUI1-lacZ ratios). These findings suggest that eIF1 negatively autoregulates its synthesis by exacerbating the deleterious effect of the poor AUG context in SUI1 mRNA when the eIF1 level increases in the cell. On the other hand, the autoregulation is not efficient enough to prevent considerable eIF1 overexpression and the attendant Ssu− phenotype in response to elevated SUI1 dosage. As shown below, this might reflect the fact that the native SUI1 context is not fully suboptimal.
Sui− mutations in eIF2β and eIF1A also suppress the poor AUG context at SUI1.
The results presented above indicate that Sui− substitutions in eIF1 that reduce its discrimination against UUG codons in HIS4 mRNA likewise reduce the deleterious effect of poor AUG context in SUI1 mRNA. We hypothesized that other factors involved in rejecting near-cognate start codons, including eIF2β, eIF1A, and eIF5, also function in rejecting poor AUG context. Accordingly, we sought to determine whether Sui− mutations in these factors provoke increased expression of SUI1 dependent on its poor context. To examine the effect of the Sui− eIF2β mutation SUI3-2, we generated strains with chromosomal SUI3+ under the GAL1 promoter and harboring plasmid-borne SUI3-2 or SUI3, which express only the plasmid-encoded S264Y mutant, or WT, eIF2β on glucose medium.
Remarkably, the SUI3-2 transformant displayed a strong, 8.7-fold increase in eIF1 expression from WT SUI1 but only a 2.3-fold increase in eIF1 expression from SUI1-opt (Fig. 6A). In addition, optimizing the context produced no increase in eIF1 expression in the SUI3-2 cells (Fig. 6D). Analysis of mRNA levels revealed that SUI3-2 conferred a 2.6-fold increase in the eIF1 protein/SUI1 mRNA ratio for SUI1+ but had almost no effect on this ratio for SUI1-opt (Fig. 2, row 8, Protein/mRNA versus row 19, Norm. Protein/mRNA), confirming a significant increase in translational efficiency exclusively for the suboptimal, native AUG context. The same conclusion emerged from analysis of the SUI1-lacZ fusions, as SUI3-2 provoked 4.6-fold higher expression of SUI1-lacZ, but only 1.9-fold higher expression of SUI1-opt-lacZ, relative to the levels observed in SUI3+ transformants (Fig. 6B). Moreover, introducing the optimal context produced only a slight (∼20%) increase in fusion gene expression in SUI3-2 cells, compared to the 3-fold higher expression seen in SUI3 cells (Fig. 6B). These findings indicate that SUI3-2 strongly suppresses the effect of poor context at SUI1. Thus, eIF2β (presumably in the context of the eIF2 holoprotein) discriminates against poor AUG context in addition to its known function in blocking non-AUG initiation.
Fig. 6.
Sui− mutations in eIF2β and eIF1A suppress the poor AUG context at SUI1. (A) Derivatives of sui1Δ PGAL-SUI3 strain PMY02 containing plasmid-borne SUI3 (p4450) or SUI3-2 (p4280) and either sc SUI1+ or sc SUI1-opt were cultured continuously in SD supplemented with His and Ura (with repression of chromosomal PGAL-SUI3) and subjected to Western analysis of eIF1 expression, as in Fig. 1C. Two different amounts of each extract differing by a factor of 2 were loaded in successive lanes. (B) Analysis of SUI1-lacZ and SUI1-opt-lacZ expression in the SUI1+ strains from panel A conducted as in Fig. 1G. (C) Western analysis of eIF1 in derivatives of sui1Δ PGAL-TIF11 strain PMY03 containing plasmid-borne TIF11+ (pDSO9), tif11-SE1*,SE2*+F131(pAS23), or tif11-17-21 (p4552), and either sc SUI1+ or sc SUI1-opt, conducted as in panel A. (D) Ratios of mean eIF1/Gcd6 values for SUI1 versus SUI1-opt alleles calculated for strains analyzed in panels A and C. (E) Analysis of SUI1-lacZ and SUI1-opt-lacZ expression in transformants of the strains from the SUI1+ strains from panel C, as in Fig. 1G.
To evaluate whether a Sui− substitution affecting eIF1A also overcomes poor context, we took the approach described above and examined transformants harboring the plasmid-borne eIF1A Sui− allele tif11-SE1*,SE2*+F131 (abbreviated SE below), or WT TIF11, in a strain where chromosomal TIF11+ is glucose repressible. The SE mutation, which impairs both scanning enhancer elements (SE1 and SE2) in the eIF1A C-terminal tail, greatly elevates the UUG/AUG ratio for the HIS4-lacZ fusion (41). We also examined the Ssu− mutation tif11-17-21, which introduces substitutions into the scanning inhibitory (SI) element in the N-terminal tail of eIF1A and lowers the UUG/AUG ratio in cells harboring SUI3-2 (14, 41).
Consistent with our findings on other Sui− mutations, the eIF1A SE mutation provoked an ∼8-fold increase in eIF1 expression from WT SUI1 compared to an ∼3-fold increase for the SUI1-opt allele (Fig. 6C), and introducing the optimal context increased eIF1 expression by only 1.3-fold in SE mutant cells compared to 2.9-fold in TIF11+ cells (Fig. 6D). We observed a more substantial increase in SUI1 mRNA in the SE mutant than in other Sui− mutants, so that the eIF1 protein/SUI1 mRNA ratio was only 50% higher in SE versus TIF11+ cells (Fig. 2, row 11, Protein/mRNA). However, the SE mutation produced a larger increase in expression of SUI1-lacZ versus SUI1-opt-lacZ, of 3.1- versus 1.9-fold, respectively, and introducing the optimal context increased SUI1-lacZ expression only slightly (by 15%) in the SE mutant compared to the 2-fold increase observed in WT cells (Fig. 6E). Taken together, the results indicate that the eIF1A SE mutation mitigates the deleterious effect of poor AUG context, as described above for Sui− mutations in eIF1 and eIF2β.
The eIF1A Ssu− mutation tif11-17-21 reduced the level of eIF1 expressed from SUI1 but not from sui1-opt (Fig. 6C) and increased the stimulatory effect of introducing optimal AUG context on eIF1 expression from ∼3-fold (in WT) to ∼8-fold (Fig. 6D). The 17-21 mutation also produced an obvious reduction in the eIF1 protein/SUI1 mRNA ratio for SUI1, but not SUI1-opt (Fig. 2, row 10, Protein/mRNA, versus row 21, Norm. Protein/mRNA), and it decreased expression of the SUI1-lacZ fusion but not that of SUI1-opt-lacZ (Fig. 6E). These results indicate that, like the Ssu− mutations in eIF1 discussed above, the eIF1A Ssu− mutation 17-21 confers a significant decrease in SUI1 translation that depends on its poor AUG context.
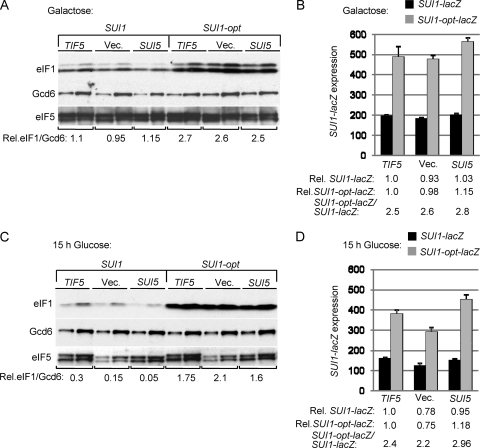
Finally, we examined the effect of the eIF5 Sui− substitution (G31R) encoded by the SUI5 allele of TIF5. As noted above, SUI5 is lethal as the only source of eIF5 but produces a dominant Sui− phenotype and a large increase in the UUG/AUG ratio in cells coexpressing WT eIF5 (21). Hence, we first examined the effect of SUI5 on eIF1 and SUI1-lacZ expression in a strain containing chromosomal PGAL-TIF5, and cultured cells on galactose to allow coexpression of WT eIF5 and attendant cell growth. Under these conditions, SUI5 produced little or no change in eIF1 expression from SUI1+ or SUI1-opt (Fig. 7A, Vec. versus SUI5 lanes), nor did it alter expression of SUI1-lacZ or SUI1-opt-lacZ (Fig. 7B). We repeated the analysis after shifting cells to glucose medium to repress eIF5 production from the chromosomal PGAL-TIF5 allele. Western analysis confirmed the expected reduction in total eIF5 level in cells harboring empty vector versus episomal SUI5 or TIF5+ (Fig. 7C, SUI1 lanes, eIF5 blot). Under these conditions, we observed a reduction in eIF1 expression in the cells harboring episomal SUI5 versus TIF5+ or empty vector in the SUI1+, but not SUI1-opt, strains (Fig. 7C), which could indicate that SUI5 differs from other Sui− mutants in exacerbating rather than suppressing the effect of poor context at SUI1. However, this effect was not observed when expression of the SUI1-lacZ and SUI1-opt-lacZ fusions were assayed under the same conditions (Fig. 7D). Similar results were obtained in a strain containing chromosomal TIF5+ instead of PGAL-TIF5 (data not shown). Thus, despite the fact that SUI5 elevates initiation from the near-cognate UUG start codon at HIS4 to confer a Sui− phenotype, it does not overcome the effect of poor context at SUI1 and thereby elevate eIF1 protein expression.
Fig. 7.
The eIF5 Sui− mutation SUI5 does not suppress poor AUG context at SUI1. (A and C). Western analysis of eIF1 expression in derivatives of sui1Δ PGAL-TIF5 strain PMY01 containing plasmid-borne TIF5-FL (p4119), empty vector (YCplac22) or SUI5 (p4281) and either sc SUI1+ or sc SUI1-opt, conducted as in Fig. 1C except that strains were cultured in synthetic minimal medium with 2% galactose as carbon source and histidine and uracil supplements (SGal+HU) (A) and then shifted to SD+HU for 15 h (C). Two different amounts of each extract differing by a factor of 2 were loaded in successive lanes. (B and D) Analysis of SUI1-lacZ and SUI1-opt-lacZ expression in transformants of SUI1+ strains from panels A and C, cultured as described there.
eIF1, eIF1A, and eIF2β discriminate generally against poor AUG context.
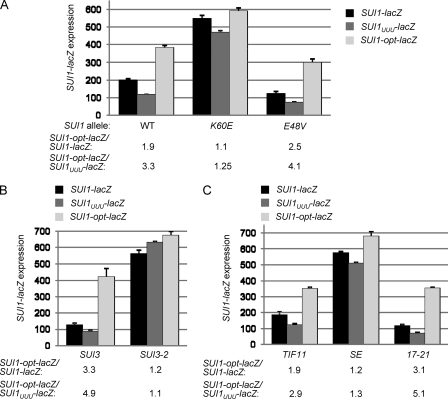
Although the context of WT SUI1 is expected to be unfavorable, we sought to determine whether Sui− mutations in eIF1, eIF2β, and eIF1A also discriminate against a different context that deviates strongly from the yeast optimum. Since previous studies in mammalian cells identified U−3-U−2-U−1-AUG as a highly unfavorable context (23), we introduced it into SUI1-lacZ, producing the SUI1-UUU-lacZ construct, and examined its effect on fusion expression in different mutants. Interestingly, the U−3-U−2-U−1-AUG context reduced fusion expression compared to the WT SUI1-lacZ construct, such that SUI1-UUU-lacZ is expressed at a level ∼3.5-fold below that of SUI1-opt-lacZ (Fig. 8A). Importantly, these differences in expression are nearly eliminated by Sui− mutations in eIF1 (K60E), eIF2β (SUI3-2), and eIF1A (SE), while the difference in expression between SUI1-UUU-lacZ and SUI1-opt-lacZ is exacerbated by the Ssu− mutations affecting eIF1 (E48V) and eIF1A (17-21) (Fig. 8). These findings demonstrate that the effects of these Sui− and Ssu− mutations are not restricted to the native, poor context at SUI1 and likely apply more generally to deviations from the optimal context. They also show that the native SUI1 context is not fully suboptimal, since the U−3-U−2-U−1-AUG context appears to be even less efficient.
Fig. 8.
Sui− and Ssu− mutations in eIF1, eIF2β and eIF1A modulate the deleterious effects of the U−3-U−2-U−1-AUG context. (A to C). Analysis of expression of reporter plasmids SUI1-lacZ (pMB24), SUI1UUU-lacZ (pMB28), and SUI1-opt-lacZ (pMB25) in derivatives of sui1Δ his4-301 strain JCY03 containing the indicated sc SUI1 alleles (A), derivatives of sui1Δ PGAL-SUI3 strain PMY02 containing plasmid-borne SUI3 (p4450) or SUI3-2 (p4280) and sc SUI1+ (pJCB101) (B), and derivatives of sui1Δ PGAL-TIF11 strain PMY03 containing plasmid-borne TIF11+ (pDSO9), tif11-SE1*, SE2*+F131 (pAS23), or tif11-17-21 (p4552), and sc SUI1+ (pJCB101) (C), as in Fig. 1G.
DISCUSSION
In this study, we have shown that eIF1/Sui1, eIF2β/Sui3, and eIF1A/Tif11 all participate in recognition of the sequence context of initiation codons in vivo and utilize specific residues or domains to discriminate against both poor sequence context and non-AUG start codons. As a result, alterations in these factors that increase (Sui−) or decrease (Ssu−) utilization of a UUG start codon at HIS4 also increase or decrease, respectively, initiation at the SUI1+ AUG codon in a manner that depends on its native, poor context. The Sui− and Ssu− mutations in eIF1, eIF2β, and eIF1A similarly suppress or exacerbate, respectively, the negative effect of the U−3-U−2-U−1-AUG context, suggesting that these factors act generally to regulate context effects in vivo. Overexpressing WT eIF1 reduces the initiation frequency at UUG start codons in various sui mutants (Ssu− phenotype) and, similarly, we found that eIF1 overexpression decreases initiation at the SUI1 AUG in the native, poor context. This enables eIF1 to negatively autoregulate its synthesis, by reducing SUI1 mRNA translation when the cellular concentration exceeds the native, steady-state level.
Among the key observations supporting the aforementioned conclusions is our finding that novel Sui− mutations in eIF1 and the previously described Sui− mutations in eIF2β (SUI3-2) and eIF1A (tif11-SE) all confer strong increases in eIF1 protein expression from SUI1+ but not from the SUI1-opt allele, the latter containing a perfect match to the yeast consensus context of A−3-A−2-A−1-AUG (44) that we found to elevate eIF1 abundance in otherwise WT cells. Furthermore, the same effects were observed for the corresponding SUI1-lacZ and SUI1-opt-lacZ fusions, where the effects of the eIF1 mutations, as well as those in eIF2β and eIF1A, are exerted in trans. Conversely, our novel Ssu− substitutions in eIF1, and the previously described Ssu− mutation 17-21 of eIF1A, more strongly repressed expression of SUI1+ compared to SUI1-opt. The fact that the optimum consensus in SUI1-opt masks the effects of these mutations on SUI1 expression constitutes strong genetic evidence that these mutations modulate SUI1 expression by suppressing (in Sui− mutants) or exacerbating (in Ssu− mutants) the deleterious effects of poor context on recognition of the SUI1 AUG codon.
The increase or decrease in eIF1 protein expression produced by the Sui− and Ssu− mutations, respectively, were accompanied by increases or decreases, respectively, in the level of SUI1 mRNA. It could be argued that the mutations have a direct effect on SUI1 transcription or mRNA stability rather than on translation initiation. This seems quite improbable, however, because the mutations affect factors or mRNA sequences with well-established functions in translation initiation, and the eIF mutations were originally selected by their ability to alter the stringency of AUG selection. Another possibility is that the observed changes in mRNA levels are elicited by the nonsense-mediated decay pathway (NMD). Owing to the poor context of the SUI1 start codon, a proportion of scanning PICs might bypass this AUG codon and initiate 100 nt downstream at an out-of-frame five-codon ORF, triggering NMD on translation termination. Introducing the optimal context or a Sui− mutation would suppress leaky scanning of the first AUG and block the NMD response, thus increasing SUI1 mRNA abundance, as we observed in these situations. Ssu− mutations, by contrast, would exacerbate the effect of poor context, increase leaky scanning of the first AUG codon and intensify the effect of NMD in lowering SUI1 mRNA abundance. At odds with this possibility, however, He et al. reported that SUI1 mRNA abundance is not altered by inactivation of NMD in different upf mutants (18). Thus, in view of previous findings that mRNA stability is coupled to translational efficiency in yeast (25, 43), the simplest interpretation of our findings seems to be that the changes in translational efficiency produced by the eIF mutations under study lead indirectly to alterations in mRNA degradation by a non-NMD mechanism.
Previous work led to the conclusion that eIF1 and eIF1A bind to the 40S subunit and stabilize an open conformation that is conducive to recruitment of TC and scanning, but incompatible with start codon selection. In the open conformation, the anticodon stem-loop (ASL) of Met-tRNAiMet occupies the P site in a way that enables inspection of successive triplets for base-pairing with the initiator anticodon without triggering downstream events in the pathway. Furthermore, while the GTP in a fraction of the 43S complexes is hydrolyzed, release of Pi from eIF2-GDP-Pi is blocked in the open complex. Entry of AUG in the P site triggers a series of events that are precipitated, or facilitated, by dissociation of eIF1 from the 40S subunit. These include rearrangement to a closed, scanning-incompatible 40S conformation, in which Met-tRNAiMet is fully accommodated and more tightly bound to the P site, plus release of Pi from eIF2-GDP-Pi to complete the GTP hydrolysis reaction.
Most Sui− mutations in eIF1 analyzed previously weaken its binding to the 40S and thereby promote rearrangement from the open to closed PIC conformation. Although the reduced 40S occupancy of a Sui− eIF1 mutant decreases the rate of TC loading, since this reaction occurs in the open 40S conformation, once TC is bound it can isomerize more readily to the fully accommodated mode of P-site binding in the absence of a perfect codon-anticodon match, e.g., at UUG codons, thus accounting for the Sui− phenotype (8, 33). We presume that the novel Sui− mutations in eIF1 described here similarly reduce its affinity for the 40S because they exhibit the hallmark of this class of eIF1 mutations, that their Sui− phenotypes are suppressed by overexpressing the mutant proteins (unpublished observations). It is thought that increasing the cellular concentration of eIF1 proteins harboring such Sui− substitutions overcomes their 40S binding defects by mass action, reversing premature eIF1 release at non-AUG codons. This is the same mechanism evoked to explain how overexpressing WT eIF1 suppresses the Sui− phenotypes of mutations in other eIFs (8, 48).
We found that the novel Ssu− mutations in eIF1 suppress the Sui− phenotypes of both SUI3-2 and SUI5 mutations in eIF2β and eIF5, respectively. Ssu− mutations in eIF1A with these properties have been shown to shift the equilibrium toward the open conformation (41), with increased retention of eIF1 on the 40S subunit (8), suppressing both the defect in TC loading to the open complex and the inappropriate rearrangement to the closed complex at UUG provoked by Sui− mutations in the eIF1A SE elements (41). The fact that the 17-21 Ssu− mutation in eIF1A also diminishes the Sui− and TC loading defects conferred by the SUI3-2 mutation in eIF2β (14, 41) suggests that SUI3-2 produces a Sui− phenotype, at least partly, by destabilizing TC binding to the open conformation (41). There is biochemical evidence that elevated GTP hydrolysis by the TC also plays a role (21), possibly shifting the equilibrium between eIF2-GTP and eIF2-GDP-Pi to the right and driving Pi release at non-AUG codons by mass action, or increasing the rate of Pi release directly. This latter defect of SUI3-2 could also be mitigated by the ability of the 17-21 mutation to stabilize the open conformation and retard eIF1 dissociation. Suppression of the eIF5 Sui− mutation SUI5 by 17-21 (14) can be explained similarly, since this eIF5 substitution both destabilizes the open conformation of the PIC (29) and accelerates GTP hydrolysis at UUG codons (21). Hence, we propose that the novel Ssu− substitutions in eIF1 described here likewise impede the open-to-closed transition of the PIC, possibly by retarding eIF1 dissociation, as a means of suppressing UUG initiation in cells harboring the SUI5 or SUI3-2 Sui− substitutions in eIF5 or eIF2β, respectively.
Our finding that Sui− substitutions in eIF1 and eIF1A increase utilization of AUG codons in poor context in addition to enhancing initiation at UUG codons can be readily understood in the context of the model for scanning and AUG recognition described above. As suggested previously by Pestova and coworkers (37), we envision that optimal context is another feature besides the perfect AUG-anticodon duplex that stabilizes the closed conformation of the PIC, such that a poor context will impede the rearrangement from open to closed conformation and provoke bypass of AUG codons (Fig. 9). This effect will be exacerbated by Ssu− mutations in eIF1 or eIF1A, which favor the open conformation, producing an even stronger bypass of AUGs with poor context. In contrast, by favoring the closed conformation, Sui− mutations will mitigate the destabilizing effect of poor context and restore recognition of AUGs in poor context.
Fig. 9.
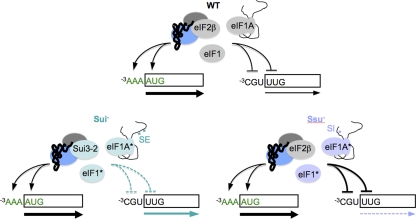
Specific domains or residues in eIF1, eIF1A and eIF2β discriminate against both poor AUG context and non-AUG start codons to maintain optimal initiation accuracy. In the WT, these initiation factors promote recognition of AUG start codons in optimal context (−3AAA) and impede recognition of initiation sites with poor context, such as the −3CGU sequence at SUI1, or with a non-AUG start codon such as UUG. Sui− mutations affecting any of these factors render initiation less accurate by diminishing antagonism of start sites with either poor context or a non-AUG start codon. Ssu− mutations affecting eIF1 or eIF1A render initiation hyperaccurate by increasing antagonism of start sites with either poor context or a non-AUG start codon.
It is conceivable that one or more of the initiation factors also plays a direct role in “reading” the sequence context of the start codon. However, the fact that the Sui− and Ssu− mutations we analyzed alter the efficiencies of initiation with either start codon mismatches or poor contexts favors the notion that eIF1, eIF1A, and eIF2β, together with AUG and a favorable context, all contribute to the formation or stability of the closed conformation as the means of promoting AUG recognition (Fig. 9) rather than interacting directly with context nucleotides. On the other hand, Pestova et al. reported that in reconstituted mammalian PICs, the −3 context nucleotide (when substituted with 4-thiouridine) could be cross-linked to eIF2α, and that replacing heterotrimeric eIF2 with the eIF2βγ heterodimer (lacking eIF2α) diminished the effect of good context in addition to reducing the efficiency of AUG recognition during 48S assembly (37). Thus, it is possible that interaction of eIF2α with the −3 base helps to stabilize the closed 40S conformation, or the P site binding of Met-tRNAiMet, as a way of promoting selection of AUG codons in good context.
In contrast to the Sui− mutations in eIF1, eIF1A, and eIF2β, the SUI5 mutation in eIF5 did not appear to suppress the poor context at SUI1, since the expression of eIF1 or SUI1-lacZ was not increased in SUI5 cells. This seems incompatible with the conclusion above that SUI5 destabilizes the open conformation of the PIC and accelerates GTP hydrolysis by the TC; however, it was shown previously that SUI5 efficiently rescues initiation with UUG but not with other near-cognates, including AUU, CUG, or GUG, as the HIS4 start codon (21). In addition, SUI5 stabilized the closed conformation of 48S PICs reconstituted with UUG but not AUU start codons (29). Thus, SUI5 might stabilize the closed complex only in response to the UUG-anticodon mismatch and be unable to compensate for the destabilizing effect of poor context. The SUI3-2 mutation in eIF2β, by contrast, increases initiation from nearly all functional near-cognate triplets in vivo (21, 46) in addition to its ability (shown here) to overcome poor context at SUI1.
It was demonstrated recently that eIF1 discriminates against poor AUG context in mammalian cells, such that eIF1 overexpression specifically repressed by a factor of ∼5 the expression of luciferase reporters lacking both critical residues of the optimal “Kozak” consensus (purine at −3 and G at +4) (24) and repressed by an order of magnitude a reporter gene containing the poor consensus found at the native gene encoding eIF1 (EIF1) (22). This repressive function underlies the robust autoregulation of EIF1 expression in mammalian cells. In comparing these results to ours, it appears that eIF1 is more effective in discriminating against poor context in mammalian cells than in yeast, as the SUI1-lacZ reporter with native context was repressed by only a factor of 2 in response to high-level eIF1 overexpression in yeast. As a consequence, eIF1 is substantially overexpressed in cells harboring the hc SUI1 plasmid (with the native, poor context), whereas overexpression of eIF1 in mammalian cells was achieved only by introducing additional copies of the EIF1 gene modified to contain the optimal Kozak consensus sequence (22). Moreover, replacing the native EIF1 context with the optimal Kozak consensus increased reporter expression by a factor of 4, while the comparable replacement in yeast increased SUI1-lacZ expression by only a factor of ∼2. These observations are consistent with previous results indicating that AUG context has a smaller effect on translation efficiency in yeast (2, 10) versus mammalian cells (23, 24). Nevertheless, it is now clear that in yeast, as in mammals, eIF1 can play an important regulatory role in modulating the expression of genes containing either a poor AUG context or a non-AUG start codon. Moreover, since the ability of eIF1 to autoregulate translation operates in fungi as well as mammals, it should be regarded as the conserved, eukaryotic equivalent of the autoregulatory mechanism first described for IF3 in bacteria.
ACKNOWLEDGMENTS
We thank Tom Dever and Jon Lorsch for many helpful suggestions and comments on the manuscript.
This study was supported in part by the Intramural Research Program of the National Institutes of Health and in part by International Human Frontiers of Science Program grant R6P0028.
Footnotes
Published ahead of print on 19 September 2011.
REFERENCES
- 1. Algire M. A., Maag D., Lorsch J. R. 2005. Pi release from eIF2, not GTP hydrolysis, is the step controlled by start-site selection during eukaryotic translation initiation. Mol. Cell 20: 251–262 [DOI] [PubMed] [Google Scholar]
- 2. Baim S. B., Sherman F. 1988. mRNA structures influencing translation in the yeast Saccharomyces cerevisiae. Mol. Cell. Biol. 8: 1591–1601 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Boeke J. D., Trueheart J., Natsoulis G., Fink G. R. 1987. 5-fluoroorotic acid as a selective agent in yeast molecular genetics. Methods Enzymol. 154: 164–175 [DOI] [PubMed] [Google Scholar]
- 4. Bushman J. L., Foiani M., Cigan A. M., Paddon C. J., Hinnebusch A. G. 1993. Guanine nucleotide exchange factor for eIF-2 in yeast: genetic and biochemical analysis of interactions between essential subunits GCD2, GCD6, and GCD7 and regulatory subunit GCN3. Mol. Cell. Biol. 13: 4618–4631 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Butler J. S., Springer M., Dondon J., Graffe M., Grunberg-Manago M. 1986. Escherichia coli protein synthesis initiation factor IF3 controls its own gene expression at the translational level in vivo. J. Mol. Biol. 192: 767–780 [DOI] [PubMed] [Google Scholar]
- 6. Butler J. S., Springer M., Grunberg-Manago M. 1987. AUU-to-AUG mutation in the initiator codon of the translation initiation factor IF3 abolishes translational autocontrol of its own gene (infC) in vivo. Proc. Natl. Acad. Sci. U. S. A. 84: 4022–4025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Chen S. J., Lin G., Chang K. J., Yeh L. S., Wang C. C. 2008. Translational efficiency of a non-AUG initiation codon is significantly affected by its sequence context in yeast. J. Biol. Chem. 283: 3173–3180 [DOI] [PubMed] [Google Scholar]
- 8. Cheung Y. N., et al. 2007. Dissociation of eIF1 from the 40S ribosomal subunit is a key step in start codon selection in vivo. Genes Dev. 21: 1217–1230 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Choi S. K., et al. 2000. Physical and functional interaction between the eukaryotic orthologs of prokaryotic translation initiation factors IF1 and IF2. Mol. Cell. Biol. 20: 7183–7191 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Cigan A. M., Pabich E. K., Donahue T. F. 1988. Mutational analysis of the HIS4 translational initiator region in Saccharomyces cerevisiae. Mol. Cell. Biol. 8: 2964–2975 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Dallas A., Noller H. F. 2001. Interaction of translation initiation factor 3 with the 30S ribosomal subunit. Mol. Cell 8: 855–864 [DOI] [PubMed] [Google Scholar]
- 12. Donahue T. 2000. Genetic approaches to translation initiation in Saccharomyces cerevisiae, p. 487–502 In Sonenberg N., Hershey J. W. B., Mathews M. B. (ed.), Translational control of gene expression. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, NY [Google Scholar]
- 13. Donahue T. F., Cigan A. M. 1988. Genetic selection for mutations that reduce or abolish ribosomal recognition of the HIS4 translational initiator region. Mol. Cell. Biol. 8: 2955–2963 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Fekete C. A., et al. 2007. N-and C-terminal residues of eIF1A have opposing effects on the fidelity of start codon selection. EMBO 26: 1602–1614 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Fletcher C. M., Pestova T. V., Hellen C. U. T., Wagner G. 1999. Structure and interactions of the translation initiation factor eIF1. EMBO J. 18: 2631–2639 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Gietz R. D., Sugino A. 1988. New yeast-Escherichia coli shuttle vectors constructed with in vitro mutagenized yeast genes lacking six-base pair restriction sites. Gene 74: 527–534 [DOI] [PubMed] [Google Scholar]
- 17. Hartz D., Binkley J., Hollingsworth T., Gold L. 1990. Domains of initiator tRNA and initiation codon crucial for initiator tRNA selection by Escherichia coli IF3. Genes Dev. 4: 1790–1800 [DOI] [PubMed] [Google Scholar]
- 18. He F., et al. 2003. Genome-wide analysis of mRNAs regulated by the nonsense-mediated and 5′ to 3′ mRNA decay pathways in yeast. Molecular Cell 12: 1439–1452 [DOI] [PubMed] [Google Scholar]
- 19. Hinnebusch A. G. 1985. A hierarchy of trans-acting factors modulate translation of an activator of amino acid biosynthetic genes in Saccharomyces cerevisiae. Mol. Cell. Biol. 5: 2349–2360 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Hinnebusch A. G., Dever T. E., Asano K. 2007. Mechanism of translation initiation in the yeast Saccharomyces cerevisiae, p. 225–268 In Mathews M., Sonenberg N., Hershey J. W. B. (ed.), Translational control in biology and medicine. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, NY [Google Scholar]
- 21. Huang H., Yoon H., Hannig E. M., Donahue T. F. 1997. GTP hydrolysis controls stringent selection of the AUG start codon during translation initiation in Saccharomyces cerevisiae. Genes Dev. 11: 2396–2413 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Ivanov I. P., Loughran G., Sachs M. S., Atkins J. F. 2010. Initiation context modulates autoregulation of eukaryotic translation initiation factor 1 (eIF1). Proc. Natl. Acad. Sci. U. S. A. 107: 18056–18060 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Kozak M. 1986. Point mutations define a sequence flanking the AUG initiator codon that modulates translation by eukaryotic ribosomes. Cell 44: 283–292 [DOI] [PubMed] [Google Scholar]
- 24. Kozak M. 1989. The scanning model for translation: an update. J. Cell Biol. 108: 229–241 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. LaGrandeur T., Parker R. 1999. The cis-acting sequences responsible for the differential decay of the unstable MFA2 and stable PGK1 transcripts in yeast include the context of the translational start codon. RNA 5: 420–433 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. La Teana A., Pon C. L., Gualerzi C. O. 1993. Translation of mRNAs with degenerate initiation triplet AUU displays high initiation factor 2 dependence and is subject to initiation factor 3 repression. Proc. Natl. Acad. Sci. U. S. A. 90: 4161–4165 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Lomakin I. B., Kolupaeva V. G., Marintchev A., Wagner G., Pestova T. V. 2003. Position of eukaryotic initiation factor eIF1 on the 40S ribosomal subunit determined by directed hydroxyl radical probing. Genes Dev. 17: 2786–2797 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Longtine M. S., et al. 1998. Additional modules for versatile and economical PCR-based gene deletion and modification in Saccharomyces cerevisiae. Yeast 14: 953–961 [DOI] [PubMed] [Google Scholar]
- 29. Maag D., Algire M. A., Lorsch J. R. 2006. Communication between eukaryotic translation initiation factors 5 and 1A within the ribosomal pre-initiation complex plays a role in start site selection. J. Mol. Biol. 356: 724–737 [DOI] [PubMed] [Google Scholar]
- 30. Maag D., Fekete C. A., Gryczynski Z., Lorsch J. R. 2005. A conformational change in the eukaryotic translation preinitiation complex and release of eIF1 signal recognition of the start codon. Mol. Cell 17: 265–275 [DOI] [PubMed] [Google Scholar]
- 31. Miyasaka H., Endo S., Shimizu H. 2010. Eukaryotic translation initiation factor 1 (eIF1), the inspector of good AUG context for translation initiation, has an extremely bad AUG context. J. Biosci. Bioeng. 109: 635–637 [DOI] [PubMed] [Google Scholar]
- 32. Moehle C. M., Hinnebusch A. G. 1991. Association of RAP1 binding sites with stringent control of ribosomal protein gene transcription in Saccharomyces cerevisiae. Mol. Cell. Biol. 11: 2723–2735 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Nanda J. S., et al. 2009. eIF1 controls multiple steps in start codon recognition during eukaryotic translation initiation. J. Mol. Biol. 394: 268–285 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Passmore L. A., et al. 2007. The eukaryotic translation initiation factors eIF1 and eIF1A induce an open conformation of the 40S ribosome. Mol. Cell 26: 41–50 [DOI] [PubMed] [Google Scholar]
- 35. Pestova T. V., Kolupaeva V. G. 2002. The roles of individual eukaryotic translation initiation factors in ribosomal scanning and initiation codon selection. Genes Dev. 16: 2906–2922 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Pestova T. V., Lorsch J. R., Hellen C. U. T. 2007. The mechanism of translation initiation in eukaryotes, p. 87–128 In Mathews M., Sonenberg N., Hershey J. W. B. (ed.), Translational control in biology and medicine. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, NY [Google Scholar]
- 37. Pisarev A. V., et al. 2006. Specific functional interactions of nucleotides at key −3 and +4 positions flanking the initiation codon with components of the mammalian 48S translation initiation complex. Genes Dev. 20: 624–636 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Rabl J., Leibundgut M., Ataide S. F., Haag A., Ban N. 2011. Crystal structure of the eukaryotic 40S ribosomal subunit in complex with initiation factor 1. Science 331: 730–736 [DOI] [PubMed] [Google Scholar]
- 39. Reid G. A., Schatz G. 1982. Import of proteins into mitochondria: yeast cells grown in the presence of carbonyl cyanide m-chlorophenylhydrazone accumulate massive amounts of some mitochondrial precursor polypeptides. J. Biol. Chem. 257: 13056–13061 [PubMed] [Google Scholar]
- 40. Sacerdot C., et al. 1996. The role of the AUU initiation codon in the negative feedback regulation of the gene for translation initiation factor IF3 in Escherichia coli. Mol. Microbiol. 21: 331–346 [DOI] [PubMed] [Google Scholar]
- 41. Saini A. K., Nanda J. S., Lorsch J. R., Hinnebusch A. G. 2010. Regulatory elements in eIF1A control the fidelity of start codon selection by modulating tRNAiMetbinding to the ribosome. Genes Dev. 24: 97–110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Schmitt M. E., Brown T. A., Trumpower B. L. 1990. A rapid and simple method for preparation of RNA from Saccharomyces cerevisiae. Nucleic Acids Res. 18: 3091–3092 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Schwartz D. C., Parker R. 1999. Mutations in translation initiation factors lead to increased rates of deadenylation and decapping of mRNAs in Saccharomyces cerevisiae. Mol. Cell. Biol. 19: 5247–5256 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Shabalina S. A., Ogurtsov A. Y., Rogozin I. B., Koonin E. V., Lipman D. J. 2004. Comparative analysis of orthologous eukaryotic mRNAs: potential hidden functional signals. Nucleic Acids Res. 32: 1774–1782 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Sussman J. K., Simons E. L., Simons R. W. 1996. Escherichia coli translation initiation factor 3 discriminates the initiation codon in vivo. Mol. Microbiol. 21: 347–360 [DOI] [PubMed] [Google Scholar]
- 46. Takacs J. E., et al. 2011. Identification of compounds that decrease the fidelity of start codon recognition by the eukaryotic translational machinery. RNA 17: 439–452 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Tedin K., et al. 1999. Translation initiation factor 3 antagonizes authentic start codon selection on leaderless mRNAs. Mol. Microbiol. 31: 67–77 [DOI] [PubMed] [Google Scholar]
- 48. Valasek L., Nielsen K. H., Zhang F., Fekete C. A., Hinnebusch A. G. 2004. Interactions of eukaryotic translation initiation factor 3 (eIF3) subunit NIP1/c with eIF1 and eIF5 promote preinitiation complex assembly and regulate start codon selection. Mol. Cell. Biol. 24: 9437–9455 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Yoon H. J., Donahue T. F. 1992. The sui1 suppressor locus in Saccharomyces cerevisiae encodes a translation factor that functions during tRNAiMetrecognition of the start codon. Mol. Cell. Biol. 12: 248–260 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Yoon S., Qiu H., Swanson M. J., Hinnebusch A. G. 2003. Recruitment of SWI/SNF by Gcn4p does not require Snf2p or Gcn5p but depends strongly on SWI/SNF integrity, SRB mediator, and SAGA. Mol. Cell. Biol. 23: 8829–8845 [DOI] [PMC free article] [PubMed] [Google Scholar]