Abstract
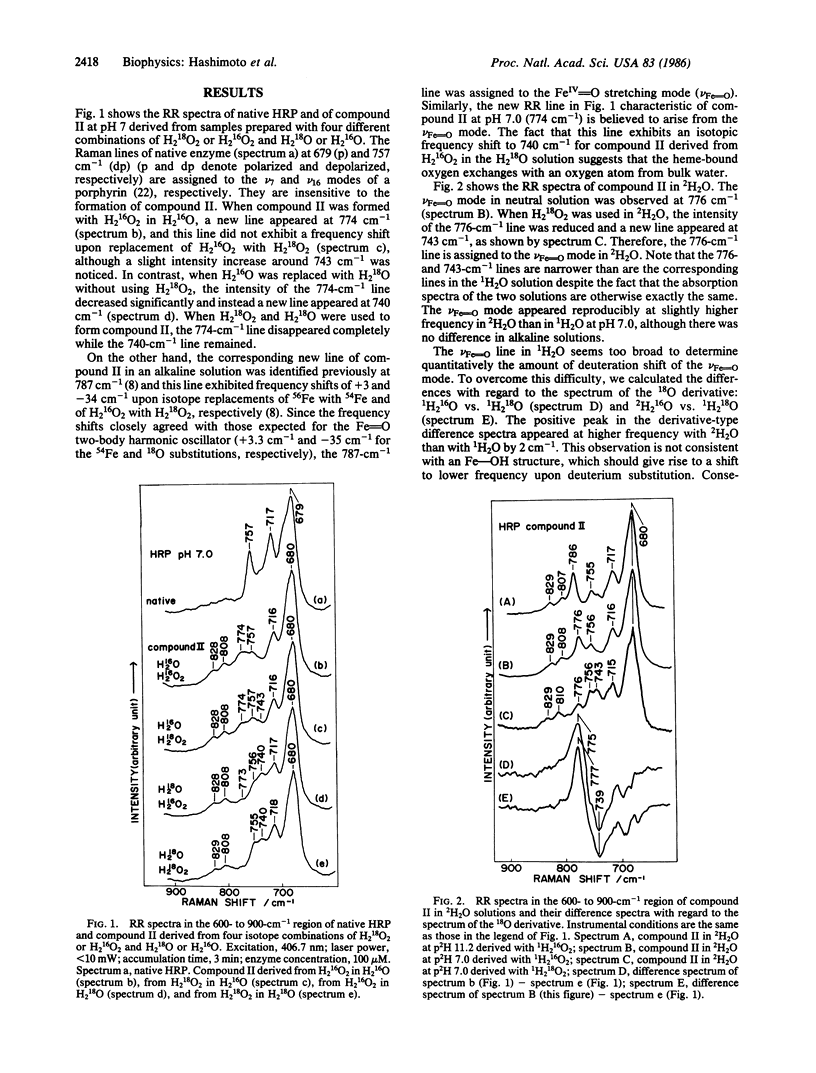
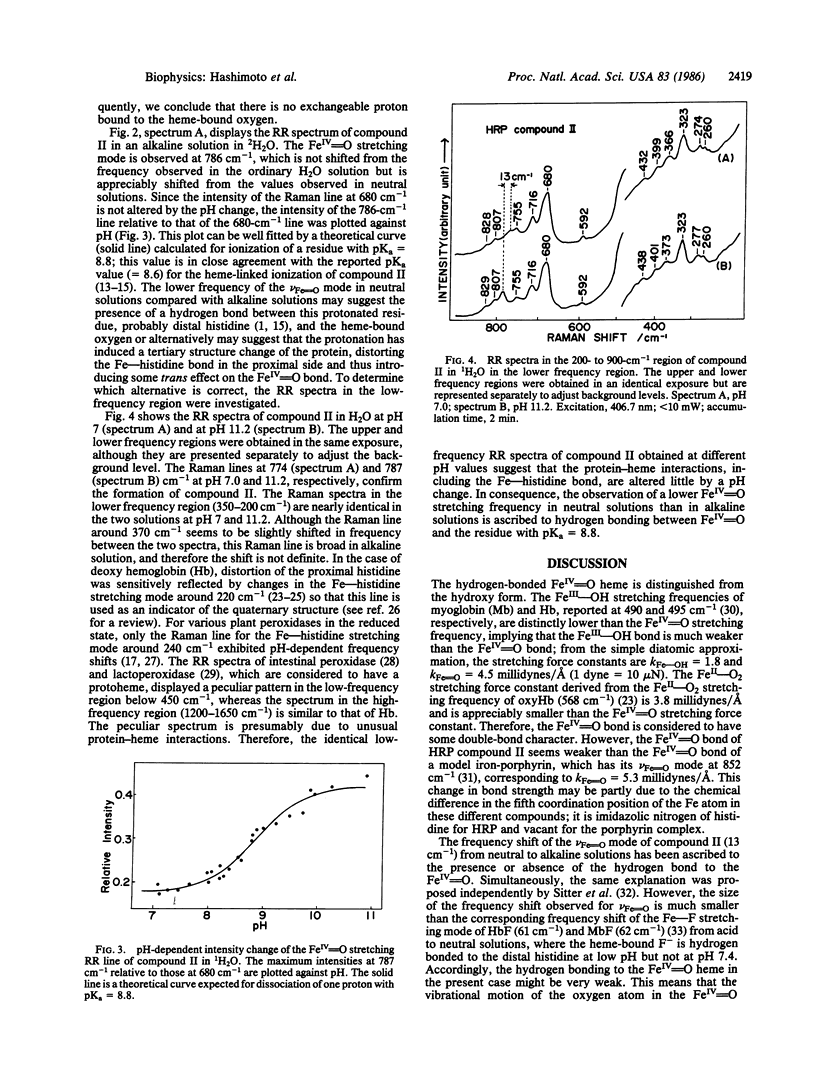
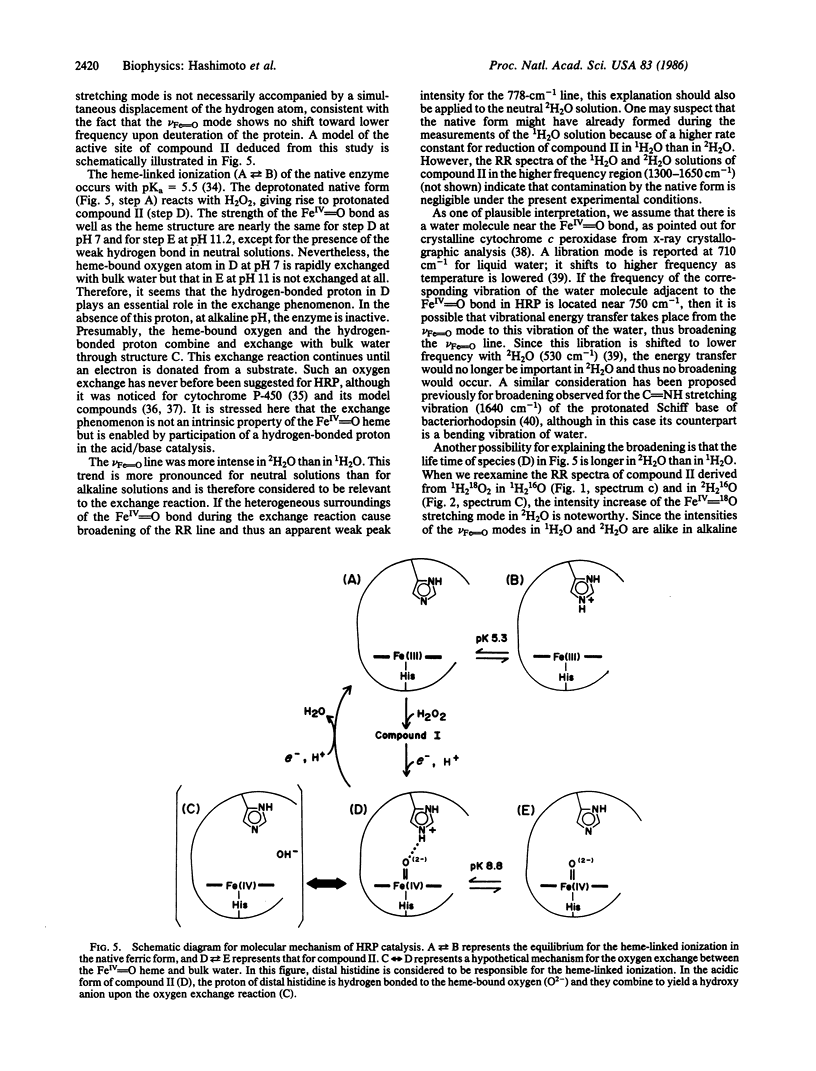
Raman spectroscopic studies of compound II of horseradish peroxidase show that the oxygen atom in the FeIV = O group of the heme is rapidly exchanged in H2O at pH 7.0 but not in an alkaline solution (pH 11.0). This conclusion is based on studies of shift in the FeIV = O stretching mode of compound II in H2(18)O; further studies show that the FeIV = O heme is hydrogen-bonded to an amino acid residue of the protein in neutral solutions but not in the alkaline solution. Deprotonation of this residue takes place with the midpoint pH at 8.8 and accordingly corresponds to the so-called heme-linked ionization. It is concluded that this hydrogen-bonded proton plays an important part in the oxygen exchange mechanism. From this it seems clear that this hydrogen-bonded proton has an essential role in the acid/base catalysis of this enzyme and that alkaline deactivation of this enzyme can be attributed to the lack of a hydrogen-bonded proton at high pH.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Asher S. A., Adams M. L., Schuster T. M. Resonance Raman and absorption spectroscopic detection of distal histidine--fluoride interactions in human methemoglobin fluoride and sperm whale metmyoglobin fluoride: measurements of distal histidine ionization constants. Biochemistry. 1981 Jun 9;20(12):3339–3346. doi: 10.1021/bi00515a004. [DOI] [PubMed] [Google Scholar]
- Asher S. A., Schuster T. M. Resonance Raman examination of axial ligand bonding and spin-state equilibria in metmyoglobin hydroxide and other heme derivatives. Biochemistry. 1979 Nov 27;18(24):5377–5387. doi: 10.1021/bi00591a019. [DOI] [PubMed] [Google Scholar]
- Critchlow J. E., Dunford H. B. Studies on horseradish peroxidase. X. The mechanism of the oxidation of p-cresol, ferrocyanide, and iodide by compound II. J Biol Chem. 1972 Jun 25;247(12):3714–3725. [PubMed] [Google Scholar]
- Desbois A., Lutz M., Banerjee R. Resonance Raman spectra of deoxyhemoproteins. Heme structure in relation to dioxygen binding. Biochim Biophys Acta. 1981 Dec 29;671(2):177–183. doi: 10.1016/0005-2795(81)90132-x. [DOI] [PubMed] [Google Scholar]
- Finzel B. C., Poulos T. L., Kraut J. Crystal structure of yeast cytochrome c peroxidase refined at 1.7-A resolution. J Biol Chem. 1984 Nov 10;259(21):13027–13036. [PubMed] [Google Scholar]
- HARBURY H. A. Oxidation-reduction potentials of horseradish peroxidase. J Biol Chem. 1957 Apr;225(2):1009–1024. [PubMed] [Google Scholar]
- Hasinoff B. B., Dunford H. B. Kinetics of the oxidation of ferrocyanide by horseradish peroxidase compounds I and II. Biochemistry. 1970 Dec 8;9(25):4930–4939. doi: 10.1021/bi00827a015. [DOI] [PubMed] [Google Scholar]
- Hayashi Y., Yamazaki I. Heme-linked ionization in compounds I and II of horseradish peroxidases A2 and C. Arch Biochem Biophys. 1978 Oct;190(2):446–453. doi: 10.1016/0003-9861(78)90297-7. [DOI] [PubMed] [Google Scholar]
- Kimura S., Yamazaki I. Comparisons between hog intestinal peroxidase and bovine lactoperoxidase-compound I formation and inhibition by benzhydroxamic acid. Arch Biochem Biophys. 1979 Dec;198(2):580–588. doi: 10.1016/0003-9861(79)90534-4. [DOI] [PubMed] [Google Scholar]
- Kimura S., Yamazaki I., Kitagawa T. Unusual low-frequency resonance Raman spectra of heme observed for hog intestinal peroxidase and its derivatives. Biochemistry. 1981 Aug 4;20(16):4632–4638. doi: 10.1021/bi00519a018. [DOI] [PubMed] [Google Scholar]
- Kitagawa T., Hashimoto S., Teraoka J., Nakamura S., Yajima H., Hosoya T. Distinct heme-substrate interactions of lactoperoxidase probed by resonance Raman spectroscopy: difference between animal and plant peroxidases. Biochemistry. 1983 Jun 7;22(12):2788–2792. doi: 10.1021/bi00281a003. [DOI] [PubMed] [Google Scholar]
- Moss T. H., Ehrenberg A., Bearden A. J. Mössbauer spectroscopic evidence for the electronic configuration of iron in horseradish peroxidase and its peroxide derivatives. Biochemistry. 1969 Oct;8(10):4159–4162. doi: 10.1021/bi00838a037. [DOI] [PubMed] [Google Scholar]
- Nagai K., Kitagawa T., Morimoto H. Quaternary structures and low frequency molecular vibrations of haems of deoxy and oxyhaemoglobin studied by resonance raman scattering. J Mol Biol. 1980 Jan 25;136(3):271–289. doi: 10.1016/0022-2836(80)90374-5. [DOI] [PubMed] [Google Scholar]
- Nordblom G. D., White R. E., Coon M. J. Studies on hydroperoxide-dependent substrate hydroxylation by purified liver microsomal cytochrome P-450. Arch Biochem Biophys. 1976 Aug;175(2):524–533. doi: 10.1016/0003-9861(76)90541-5. [DOI] [PubMed] [Google Scholar]
- Ondrias M. R., Rousseau D. L., Shelnutt J. A., Simon S. R. Quaternary-transformation-induced changes at the heme in deoxyhemoglobins. Biochemistry. 1982 Jul 6;21(14):3428–3437. doi: 10.1021/bi00257a028. [DOI] [PubMed] [Google Scholar]
- Penner-Hahn J. E., McMurry T. J., Renner M., Latos-Grazynsky L., Eble K. S., Davis I. M., Balch A. L., Groves J. T., Dawson J. H., Hodgson K. O. X-ray absorption spectroscopic studies of high valent iron porphyrins. Horseradish peroxidase compounds I and II and synthetic models. J Biol Chem. 1983 Nov 10;258(21):12761–12764. [PubMed] [Google Scholar]
- Sitter A. J., Reczek C. M., Terner J. Heme-linked ionization of horseradish peroxidase compound II monitored by the resonance Raman Fe(IV)=O stretching vibration. J Biol Chem. 1985 Jun 25;260(12):7515–7522. [PubMed] [Google Scholar]
- Tamura M., Asakura T., Yonetani T. Heme-modification studies on horseradish peroxidase. Biochim Biophys Acta. 1972 May 12;268(2):292–304. doi: 10.1016/0005-2744(72)90324-5. [DOI] [PubMed] [Google Scholar]
- Teraoka J., Kitagawa T. Resonance Raman study of the heme-linked ionization in reduced horseradish peroxidase. Biochem Biophys Res Commun. 1980 Apr 14;93(3):694–700. doi: 10.1016/0006-291x(80)91133-x. [DOI] [PubMed] [Google Scholar]
- Teraoka J., Kitagawa T. Structural implication of the heme-linked ionization of horseradish peroxidase probed by the Fe-histidine stretching Raman line. J Biol Chem. 1981 Apr 25;256(8):3969–3977. [PubMed] [Google Scholar]
- Yamada H., Makino R., Yamazaki I. Effects of 2,4-substituents of deuteropheme upon redox potentials of horseradish peroxidases. Arch Biochem Biophys. 1975 Jul;169(1):344–353. doi: 10.1016/0003-9861(75)90350-1. [DOI] [PubMed] [Google Scholar]
- Yamazaki I., Araiso T., Hayashi Y., Yamada H., Makino R. Analysis of acid-base properties of peroxidase and myoglobin. Adv Biophys. 1978;11:249–281. [PubMed] [Google Scholar]
- Yamazaki I., Tamura M., Nakajima R. Horseradish peroxidase C. Mol Cell Biochem. 1981 Nov 13;40(3):143–153. doi: 10.1007/BF00224608. [DOI] [PubMed] [Google Scholar]


