Abstract
Human immunodeficiency virus type 1 (HIV-1) Gag is the main structural protein driving assembly and release of virions from infected cells. Gag alone is capable of self-assembly in vitro, but host factors have been shown to play a role in efficient viral replication and particle morphogenesis within the living cell. In a series of affinity purification experiments, we identified the cellular protein Lyric to be an HIV-1 Gag-interacting protein. Lyric was previously described to be an HIV-inducible gene and is involved in various signaling pathways. Gag interacts with endogenous Lyric via its matrix (MA) and nucleocapsid (NC) domains. This interaction requires Gag multimerization and Lyric amino acids 101 to 289. Endogenous Lyric is incorporated into HIV-1 virions and is cleaved by the viral protease. Gag-Lyric interaction was also observed for murine leukemia virus and equine infectious anemia virus, suggesting that it represents a conserved feature among retroviruses. Expression of the Gag binding domain of Lyric increased Gag expression levels and viral infectivity, whereas expression of a Lyric mutant lacking the Gag binding site resulted in lower Gag expression and decreased viral infectivity. The results of the current study identify Lyric to be a cellular interaction partner of HIV-1 Gag and hint at a potential role in regulating infectivity. Further experiments are needed to elucidate the precise role of this interaction.
INTRODUCTION
Human immunodeficiency virus 1 (HIV-1) assembly and budding at the plasma membrane lead to formation of immature particles with an incomplete spherical protein shell underneath the viral membrane. This process is mainly driven by the Gag polyprotein Pr55, comprising matrix (MA), capsid (CA), nucleocapsid (NC), and p6 domains as well as the spacer peptides SP1 and SP2 between CA and NC and between NC and p6, respectively.
Distinct functions during assembly have been ascribed to individual Gag domains: the membrane-binding domain in MA consists of an N-terminal myristic acid and a cluster of basic amino acids. Gag-Gag interaction is mainly mediated by the CA domain and enhanced by RNA binding to the NC domain, while the C-terminal p6 domain promotes viral release. Although Gag is capable of self-assembly in vitro, it is assumed that, in the environment provided by a living cell, the formation of new HIV particles results from complex interactions of virus and host factors (5, 22). Host cell factors potentially involved in HIV morphogenesis were predominantly identified using various assays to be Gag-interacting proteins. These included yeast two-hybrid assay screening, copurification with Gag, and identification as constituents of extracellular virions. Examples with proven functional importance are cyclophilin A, which interacts with the CA domain of Gag and is incorporated into HIV-1 (19, 34, 53), and the Tsg101 and Alix components of the endosomal sorting complex required for transport (ESCRT) machinery, which are recruited by short peptide motifs in the p6 domain of Gag and are involved in particle release (5). Various other proteins have been identified to be Gag interaction partners, but few defined functions have been described for these interactions. These include, e.g., actin (39), Staufen-1 (12, 35), RNA helicase A (45), and many other proteins (41). This list is being expanded by the results of several genome-wide small interfering RNA (siRNA) screens which led to the discovery of a large set of potential host factors involved in HIV-1 replication (7). Most of these factors have not been validated to date, however, and their mechanistic role in viral replication, if any, is unknown. Furthermore, most screening projects have primarily addressed viral entry and genome replication and have not always included the stages of viral assembly and release. Accordingly, a search for additional host factors by screening approaches and by biochemical characterization of Gag-binding proteins still appears to be warranted.
In the study described here, we performed a series of independent affinity purification experiments to identify previously unknown cellular interaction partners of HIV-1 Gag. Tandem affinity purification (TAP), affinity purifications with GFP-Trap A and green fluorescent protein (GFP) microbeads, and a stable isotope labeling by amino acids in cell culture (SILAC)/quantitative mass spectrometry (MS) approach yielded a large number of potential Gag interaction partners. The only cellular protein invariably detected in all affinity purification experiments and also identified to be a Gag-interacting protein in SILAC/MS analysis was the protein Lyric (lysine-rich carcinoembryonic antigen-related cell adhesion molecule [CEACAM] coisolated). Lyric, also named astrocyte-elevated gene 1 (AEG-1) or metadherin, is a ubiquitously expressed 64-kDa protein with several isoforms due to differential splicing which differ in their intracellular localization (54). Lyric was initially identified to be an HIV-1-inducible gene in astrocytes and has been suggested to act in a positive-feedback loop promoting HIV replication (27). Lyric has also been implicated in HIV-associated neuropathy (18, 27). It is involved in various signaling pathways, including the Ras, NF-κB, PI3K/Akt, and Wnt/β-catenin pathways, and has been suggested to have antiapoptotic effects and to be involved in tumorigenesis (17, 27, 28, 31, 32, 60). We showed that Lyric interacts specifically with HIV-1 Gag and is incorporated into HIV-1 particles, where it is cleaved by the viral protease. This interaction is conserved among retroviruses. We subsequently determined the Gag and Lyric domains mediating their interaction and demonstrated that Lyric influences Gag expression levels and HIV infectivity. Possible mechanisms for a functional involvement of Lyric in HIV-1 replication are discussed.
MATERIALS AND METHODS
Plasmids.
The proviral plasmid pNL4-3 (3), pCHIVEGFP carrying enhanced GFP (eGFP) in the MA domain (36), and pGag-EGFP carrying eGFP at the C terminus of a Rev-independent Gag (25) have been described previously. pEGFPc1 was purchased from Invitrogen (Carlsbad, CA). To generate pCTAP, a C-terminal TAP tag (43) was inserted into pcDNA3 Zeo (−) (Invitrogen); to generate pGagCTAP, rev-independent Gag (48) was cloned into pCTAP. Truncated Gag expression constructs were cloned by PCR amplification of the desired Gag portions with flanking BamHI and XbaI sites using pGag-EGFP as a template. The PCR products were cloned into the corresponding sites of pcDNA3 (Invitrogen) and validated by sequencing. pcDNA G2V Gag has been described previously (26). The ZWT-p6 and ZIL-p6 constructs contain codons 247 to 280 of the GCN4 zipper in the position of the NC-p6 domains in HIV-1 gag. ZIL-p6 has both the a and the d positions of the coiled coil heptad repeats mutated to isoleucine (2). pNL43-315a represents a proviral HIV-1 construct with all 15 lysine and arginine codons in NC replaced by alanine codons (13, 40). pcDNA monomer Gag is a Gag expression vector carrying mutations in CA (M39A and W184A/185A) and NC (15 substitutions of alanine for basic residues, as described above) and has been shown to give rise to a multimerization-incompetent Gag protein (16). pNLA-1 is a derivative of pNL4-3 which lacks the gag and pol genes but expresses all other HIV-1 genes (50). The pNL4-3 variant harboring the PR mutation D25A has been described previously (29). Murine leukemia virus (MLV) Gag-yellow fluorescent protein (YFP) had been cloned by inserting the MLV gag open reading frame into pEYFP-Nl (49). pCI EIAV Gag-EGFP is an expression vector for equine infectious anemia virus (EIAV) Gag with a C-terminal GFP fusion (52). pLTR GFP is a derivative of the retroviral vector pSTITCH (58), with the expression of GFP driven by the retroviral Moloney MLV long terminal repeat (LTR). pFLAG Lyric (rat Lyric cDNA in pFLAG-cytomegalovirus virus [CMV] 5a) (4) and Lyric/AEG-1 truncation constructs in pcDNA3.1 Hygro with a C-terminal hemagglutinin (HA) tag (pcDNA N2 HA, pcDNA N3 HA, pcDNA C5 HA C5, pcDNA N4 HA) have been described previously (47). Cloning of pFLAG Lyric codons 101 to 289 was performed by PCR amplification of the respective region from pFLAG Lyric, digestion with EcoRI and BamHI (New England BioLabs, Ipswich, MA), and insertion into pFLAG-CMV 5a. For cloning of pFLAG Lyric lacking the putative Gag interaction domain, codons 107 to 204 (pFLAG Lyric Δ107-204), two PCR products spanning codons 1 to 106 and 205 to 582 were generated, with a subsequent PCR amplifying the purified overlapping fragments. The PCR product was digested with EcoRI and BamHI and ligated into pFLAG-CMV 5a. The correctness of all constructs was verified by sequence analysis; primer sequences are available upon request.
Cell culture and transfection.
293T cells were grown in Dulbecco's modified Eagle's medium (DMEM), and MT-4 cells (24) were kept in RPMI 1640 medium. Both media were supplemented with 10% heat-inactivated fetal calf serum, penicillin, streptomycin, 4 mM glutamine, and 10 mM HEPES. For SILAC analysis, 293T cells were grown in DMEM minus arginine and lysine (Pierce Thermo Fisher Scientific, Bonn, Germany) supplemented with 10% dialyzed fetal calf serum and penicillin-streptomycin. l-Arginine (84 μg/ml; Pierce) and l-lysine (146 μg/ml lysine; Sigma-Aldrich, St. Louis, MO) were added to the light isotope medium, while [13C6]l-lysine·2HCl and [13C6, 15N4]l-arginine-HCl (Pierce) were added to the heavy isotope medium at the same concentrations. For transfection, 293T cells were seeded 24 h prior to transfection at a density of 5 × 105 in six-well plates. Transfection of 2 μg DNA per well was performed with 4 μl Fugene HD transfection reagent (Roche Diagnostics, Mannheim, Germany) in a total volume of 100 μl serum-free DMEM according to the manufacturer's instructions.
HIV-1 particle preparation and analysis of viral infectivity.
Culture media were harvested and cleared 48 h after transfection of 293T cells; particles were collected by ultracentrifugation for 90 min at 130,000 × g and 4°C through a 20% (wt/wt) sucrose cushion and resuspended in phosphate-buffered saline (PBS). Cells were scraped from the plates, washed with PBS, and lysed by boiling in sodium dodecyl sulfate (SDS) sample buffer. Alternatively, MT-4 cells were infected with HIV-1 by coculture of infected and uninfected MT-4 cells (59), and HIV-1 was purified by sequential centrifugation through a sucrose cushion and banding on an Optiprep gradient (Axis-Shield, Oslo, Norway) as described previously (15). The visible virus fraction was collected and concentrated by centrifugation. To analyze HIV-1 infectivity, 5 × 103 TZM cells per well were seeded in a 96-well plate. After 24 h, infections were carried out by adding cleared tissue culture supernatant from transfected 293T cells. After 48 h, cells were lysed and HIV Tat-driven luciferase activity was measured using the Steady-Glo assay (Promega, Mannheim, Germany) according to the manufacturer's recommendations. Quantification of CA antigen by p24 enzyme-linked immunosorbent assay was performed as described previously (29).
Affinity purification.
Tandem affinity purification was adapted from the procedure of Gingras et al. (23). Sixteen 10-cm plates of 293T cells each were transfected with pGagCTAP or pCTAP. Cells were harvested 48 h after transfection, washed, and resuspended in 1 ml of ice-cold TAP lysis buffer (10% glycerol, 50 mM HEPES-KOH, pH 8.0, 100 mM KCl, 2 mM EDTA, 0.1% Igepal CA-630 detergent, 2 mM dithiothreitol [DTT], 1× protease inhibitor cocktail [Sigma], 10 mM NaF, 0.25 mM NaOVO3) per plate. After 30 min incubation on ice, two freeze-thaw cycles were performed, cell debris was removed by centrifugation, and 100 μl IgG Sepharose (GE Healthcare Bio-Sciences AB; Uppsala, Sweden) washed thrice in TAP lysis buffer was added. After 4 h at 4°C, beads were washed thrice in TAP lysis buffer and thrice in TEV buffer (10 mM HEPES-KOH, pH 8.0, 150 mM NaCl, 0.1% NP-40, 0.5 mM EDTA, 1 mM DTT). Sepharose was spun down at 800 × g for 2 min, resuspended in 300 μl TEV buffer, and incubated with 100 U TEV protease (Invitrogen) overnight at 4°C. Subsequently, IgG Sepharose was removed by centrifugation, and the supernatant was diluted with 3 volumes of calmodulin binding buffer (10 mM β-mercaptoethanol, 10 mM HEPES-KOH, pH 8.0, 150 mM NaCl, 1 mM magnesium acetate, 1 mM imidazole, 2 mM CaCl2) and adjusted to 7.5 mM CaCl2. Following incubation with 100 μl calmodulin Sepharose (GE Healthcare Bio-Sciences) at 4°C for 90 min, the Sepharose was collected by centrifugation and washed thrice in calmodulin binding buffer and twice in calmodulin rinsing buffer (50 mM ammonium bicarbonate, pH 8.0, 75 mM NaCl, 1 mM magnesium acetate, 1 mM imidazole, 2 mM CaCl2), and bound proteins were eluted in 50 μl calmodulin elution buffer (50 mM ammonium bicarbonate, pH 8.0, 25 mM EGTA). Purification with GFP-Trap A was adapted from the method of Rothbauer et al. (44). 293T cells were transfected with pCHIVEGFP, pGag-EGFP, or pEGFPc1 and harvested in 500 μl immunoprecipitation (IP) buffer (10 mM Tris HCl, pH 7.5, 150 mM NaCl, 0.5 mM EDTA) per well 24 h after transfection. Cells were lysed by 6 serial passages through a 21-gauge needle, cell debris was removed, and the supernatant was incubated with 100 μl GFP-Trap A (ChromoTek, Martinsried, Germany) for 2 h at 4°C. Subsequently, GFP-Trap A was collected by centrifugation, washed twice with 500 μl IP buffer, and resuspended in 50 μl SDS sample buffer, and bound proteins were eluted by incubation at 95°C for 10 min. For affinity purification with anti-GFP microbeads, cells were lysed in 1 ml of IP buffer as described above. The lysate was incubated with 50 μl of anti-GFP magnetic beads (Miltenyi Biotech, Bergisch Gladbach, Germany) on ice for 30 min and loaded onto a μcolumn (Miltenyi Biotech) in a μMACS separator (Miltenyi Biotech), washed five times with 200 μl IP buffer, and finally, eluted with 50 μl SDS elution buffer.
Protein identification by mass spectrometry.
For TAP- and GFP-based screens, proteins were separated by SDS-PAGE and the whole lane was cut in pieces of about 4 mm in length. Gel pieces were reduced with DTT, alkylated with iodoacetamide, and digested with trypsin (10) using a Digest pro MS liquid handling system (Intavis AG, Cologne, Germany). Following digestion, tryptic peptides were extracted from the gel pieces with 50% acetonitrile–0.1% trifluoroacetic acid (TFA), concentrated nearly to dryness in a SpeedVac vacuum centrifuge, and diluted to a total volume of 30 μl with 0.1% TFA. Twenty-five microliters of the sample was analyzed by a nano-high-pressure liquid chromatography system (Dionex, Amsterdam, Netherlands) coupled to an electron spray ionization (ESI) LTQ Orbitrap mass spectrometer (Thermo Fisher). The samples were loaded on a C18 trapping column (Inertsil; LC Packings, Amsterdam, Netherlands) with a flow rate of 10 μl/min 0.1% TFA. Peptides were eluted and separated on an analytical column (75 μm by 150 mm) packed with Inertsil 3-μm C18 material (LC Packings) with a flow rate of 200 nl/min in a gradient of buffer A (0.1% formic acid) and buffer B (0.1% formic acid, acetonitrile): 0 to 6 min with 3% buffer B, 6 to 60 min with 3 to 40% buffer B, and 60 to 65 min with 60 to 90% buffer B. The column was connected with a nano-electrospray ionization emitter (New Objective, Woburn, MA). One thousand five hundred volts was applied via liquid junction. One survey scan (resolution, 60,000; resolution corresponds to m/z ratio) was followed by 5 information-dependent product ion scans in the LTQ mass spectrometer. Only doubly and triply charged ions were selected for fragmentation. Tandem mass spectra were extracted by the Mascot Daemon client application without grouping or smoothing and analyzed using Mascot software (version 2.2.04; Matrix Science). Mascot was set up to search the International Protein Index (IPI) human protein database (version 3.48), using trypsin as protease, a fragment ion mass tolerance of 0.20 Da, and a parent ion tolerance of 4.0 ppm. The iodoacetamide derivative of cysteine was specified in Mascot as a fixed modification. Deamidation of asparagine, oxidation of methionine, and phosphorylation of serine and threonine were specified in Mascot as variable modifications. Only protein hits with a probability (P) value of <0.05 for a random match were listed.
SILAC-based quantitative mass spectrometry.
For SILAC, DMEM deficient in Arg and Lys was used (Pierce SILAC quantification kit; Thermo Fisher Scientific, Bonn, Germany) as described previously (37). 293T cells grown in heavy isotope medium were transfected with pCHIVEGFP, and 293T cells grown in light isotope medium were transfected with pEGFPc1. Cells were lysed 24 h after transfection, as described above. The protein content of cell lysates was determined using the Bradford assay (6), and equal protein amounts of both lysates were subjected to affinity purification with anti-GFP magnetic beads as described above. The columns were washed five times with IP buffer and twice with IP washing buffer (150 mM NaCl, 1% Igepal CA-630 detergent, 0.5% sodium deoxycholate, 0.1% SDS, 50 mM Tris HCl, pH 8.0) and, finally, eluted with 50 μl SDS elution buffer (50 mM Tris HCl [pH 6.8], 50 mM DTT, 1% SDS, 1 mM EDTA, 0.005% bromophenol blue, 10% glycerol). Equal volumes of eluates were combined and separated on a 12.5% SDS-polyacrylamide gel. After separation, the gel was stained with Coomassie brilliant blue (Imperial protein stain; Pierce), and 2-mm slices were excised and subjected to mass spectrometry. Tryptic digestion and protein identification and quantification were performed as recently described (30). In brief, after tryptic in-gel digestion, the extracted peptide solution was taken to dryness under vacuum and samples were reconstituted in 6 μl of 0.1% (vol/vol) TFA, 5% (vol/vol) acetonitrile in water. Liquid chromatography (LC)-tandem MS (MS/MS) analyses were performed on an LTQ Orbitrap XL mass spectrometer (Thermo Scientific) equipped with an Eksigent 2D nanoflow LC system (Axel Semrau GmbH, Sprockhovel, Germany). Separations were performed on a capillary column (Atlantis dC18, 3 μm, 100 A, 150 mm by 75 μm [inner diameter]; Waters, Milford, MA) at an eluent flow rate of 250 nl/min using a linear gradient of 0 to 40% mobile phase B (0.1% formic acid in acetonitrile) in 50 min. Mobile phase A was 0.1% (vol/vol) formic acid in water. Mass spectra were acquired in a data-dependent mode with one MS survey scan (with a resolution of 60,000) in the Orbitrap mass spectrometer and MS/MS scans of the five most intense precursor ions in the LTQ mass spectrometer. The MS survey range was m/z 350 to 1,500. The dynamic exclusion time (for precursor ions) was set to 120 s, and automatic gain control was set to 3 × 106 and 20,000 for Orbitrap MS and LTQ MS/MS scans, respectively. Identification and quantification of proteins were carried out with version 1.0.12.31 of the MaxQuant software package (14). Generated peak lists (msm files) were submitted to a Mascot search engine (version 2.2; Matrix Science Ltd., London, United Kingdom) and searched against the IPI human protein database (version 3.52). The mass tolerances of precursor and sequence ions were set to 7 ppm and 0.3 Da, respectively. Methionine oxidation and the acrylamide modification of cysteine were used as variable modifications. False discovery rates were <1%, on the basis of matches to reversed sequences in the concatenated target-decoy database.
Coimmunoprecipitations, SDS-PAGE, and immunoblot analysis.
293T cells transfected with the respective plasmids were harvested in IP buffer 24 h after transfection and lysed as described above. Cleared cell lysates were subjected to immunoprecipitation with antibodies against Lyric (Abcam 45338; Abcam, Cambridge, United Kingdom) or the FLAG epitope tag (clone M2; Sigma), followed by 1 h of incubation with magnetic protein G beads (Dynabeads; Invitrogen). Beads were washed twice with IP buffer and eluted with preheated (95°C) SDS sample buffer for 5 min at 95°C. Protein samples were resolved on 12.5% low-cross-linked (200:1 acrylamide-bisacrylamide) polyacrylamide gels, transferred onto a nitrocellulose membrane, and probed with sheep anti-CA (1:5,000), rabbit anti-Lyric (1:1,000; Abcam 45338), M2 anti-FLAG (1:4,000; F3165; Sigma), mouse anti-HA (1:2,000; H3663; Sigma), monoclonal anti-actin (1:5,000; Santa Cruz), or anti-GFP (1:1,000; Santa Cruz Biotechnology, Santa Cruz, CA). Monoclonal antibody against HIV-1 gp41 (1:1,000) derived from cell culture media of Chessie-8 cells was originally described elsewhere (1) and obtained from the NIH AIDS Research and Reference Reagent Program. Secondary antibodies (rabbit anti-sheep, goat anti-rabbit, rabbit anti-mouse; 1:10,000) coupled with Alexa 700/800 fluorescent dyes were used for detection and quantification of band intensities with an Odyssey 10 infrared imaging system (Li-Cor Biosciences, Lincoln, NE) as suggested by the manufacturer.
RESULTS
HIV-1 Gag interacts with the cellular protein Lyric. (i) Affinity purification screens identify Gag interaction candidates.
To identify cellular interaction partners of HIV-1 Gag, we performed a series of independent affinity purification experiments applying tandem affinity purification (TAP), GFP nanotrap, and immunoaffinity purification with anti-GFP microbeads followed by mass spectrometry. HIV-1 Gag with a C-terminal TAP tag was used for TAP purification, while HIV-1 Gag with a GFP domain internally fused either to the MA domain (36) or at the C terminus of Gag (25) was used for anti-GFP purification. The unfused TAP tag or GFP served as a control. A total of five affinity purification experiments were performed, including experiments with GFP-Trap A and GFP microbeads against Gag with an internal or C-terminal GFP domain and TAP tag affinity purification. A large number of potential Gag interaction partners were identified, but only a few cellular proteins scored in three or more of these experiments (Table 1). Lyric was the only potential Gag interaction partner that was consistently detected in all five experiments. Since affinity purification is prone to false-positive results due to nonspecific binding of proteins to the affinity matrix or carryover of high-abundance proteins, we performed quantitative mass spectrometry in combination with stable isotope labeling by amino acids in cell culture (SILAC) to distinguish specific from nonspecific binders (37, 57). In the SILAC approach, two cell populations are generated. One population is grown in medium in which natural essential amino acids, typically arginine and lysine, are replaced by 13C6 or 15N4 derivatives. The other cell population serves as a negative control for identification of proteins binding nonspecifically to the bait tag or the affinity matrix. By comparing the MS signal intensities of heavy and light isotopes in the tryptic peptides identified by MS/MS, it is possible to distinguish from which cell population the peptides derive. Here we used a combination of affinity pull-down and SILAC-based quantitative mass spectrometry to perform an unbiased interaction screen for HIV-1 Gag, with GFP-expressing cells serving as a negative control. Proteins identified on the basis of at least three tryptic peptides and with SILAC ratios of >5.0 were considered potential interaction partners. Lyric was enriched over 50-fold compared to the GFP control and was among the top-ranked candidates (Table 1). On the basis of these consistent results, Lyric was considered the strongest candidate for a Gag-interacting protein and was therefore further evaluated. SILAC data for the other proteins identified in 3 or more affinity purifications are also presented in Table 1.
Table 1.
HIV-1 Gag interaction candidates identified in affinity purification screens
| Affinity purification screena | Gene symbol | Name | H/L ratiob | Peptide count |
|---|---|---|---|---|
| 5/5 | MTDHc | Lyric | 50.2 | 13 |
| 4/5 | C1orf25c | Chromosome 1 open reading frame 25 | 23.1 | 11 |
| MOV10c | Moloney murine leukemia virus 10 homolog | 22.7 | 17 | |
| SRP14c | Signal recognition particle, 14 kDa | 16.2 | 8 | |
| PRPF4 | PRP4 pre-mRNA processing factor 4 homolog | 3.3 | 12 | |
| CDC5L | CDC5 cell division cycle 5-like | 2.2 | 19 | |
| SFRS7c | Splicing factor, arginine/serine-rich 7, 35 kDa | 18.8 | 5 | |
| SFRS3c | Splicing factor, arginine/serine rich 3 | 13.9 | 4 | |
| 3/5 | STAU1c | Staufen, RNA binding protein, homolog 1 | 56.7 | 16 |
| ZC3HAV1c | Zinc finger CCCH type, antiviral 1 | 55.0 | 33 | |
| DARSc | Aspartyl-tRNA synthetase | 32.1 | 18 | |
| LARSc | Leucyl-tRNA synthetase | 27.5 | 6 | |
| GTPBP4c | GTP binding protein 4 | 21.4 | 14 | |
| ABCF1c | ATP-binding cassette, subfamily F (GCN20), member 1 | 6.2 | 15 | |
| MYBBP1Ac | MYB binding protein (P160) 1a | 18.0 | 9 | |
| BAT2D1c | BAT2 domain containing 1 | 24.7 | 18 | |
| NAT10c | N-Acetyltransferase 10 (GCN5 related) | 20.1 | 18 | |
| TOP1c | Topoisomerase (DNA) I | 12.6 | 42 | |
| SFRS9c | Splicing factor, arginine/serine rich 9 | 14.2 | 6 | |
| POP1c | Processing of precursor 1, RNase P/MRP subunit | 35.7 | 7 | |
| EXOSC10c | Exosome component 10 | 27.0 | 9 | |
| EXOSC6 | Exosome component 6 | 22.5 | 1 | |
| EXOSC5 | Exosome component 5 | 8.2 | 1 | |
| IK | IK cytokine, downregulator of HLA II | 1.0 | 2 | |
| TBL2 | Transducin (beta)-like 2 | 7.6 | 1 | |
| SRPK1 | SFRS protein kinase 1 | 2.5 | 16 | |
| SFRS2c | Splicing factor, arginine/serine rich 2 | 5.2 | 3 | |
| LARP1c | La ribonucleoprotein domain family, member 1 | 16.5 | 4 | |
| RBM14 | RNA binding motif protein 14 | 2.8 | 9 | |
| PRPF3 | PRP3 pre-mRNA processing factor 3 homolog | 3.0 | 16 | |
| TROVE2 | TROVE domain family, member 2 | 0.4 | 5 |
5/5, 4/5, and 3/5, HIV-1 Gag interaction candidates identified in 5, 4, or 3 of 5 affinity purification screens, respectively.
H/L, heavy chain/light chain ratio; peptide count from SILAC experiment.
SILAC criteria were significant (H/L ratio > 5; ≥3 peptides identified).
(ii) Coimmunoprecipitations confirm screening result.
To validate the interaction between HIV-1 Gag and Lyric, coimmunoprecipitations were performed using antisera directed against GFP following transfection of 293T cells with expression vectors for GFP-tagged HIV-1 Gag or GFP only. Endogenous Lyric was readily detected in the eluate from Gag-expressing cells but was completely absent in the eluate from GFP-expressing cells (Fig. 1 A, lanes 3 and 4). To confirm this result, we performed the IP experiment in the opposite direction, by precipitating endogenous Lyric with specific antisera. Untagged Gag was detected by immunoblot analysis. Even though Gag input was higher in the negative control (Fig. 1B, lanes 1 and 3), an isotypic IgG failed to precipitate Gag, while a specific signal was obtained for anti-Lyric antisera (Fig. 1B, lanes 2 and 4).
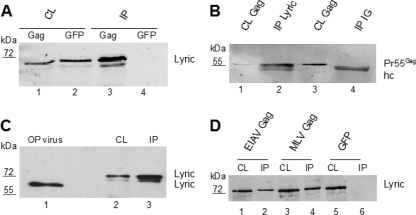
Fig. 1.
Coimmunoprecipitation and virion incorporation of Lyric. (A) Lysates from 293T cells transfected with either pCHIVEGFP or pEGFPc1 were subjected to immunoprecipitations using anti-GFP microbeads, followed by immunoblotting with anti-Lyric antibody. Lane 1, cell lysate (CL) of cells transfected with pCHIVEGFP; lane 2, lysate of cells transfected with pEGFPc1; lane 3, IP eluate from cells transfected with pCHIVEGFP; lane 4, IP eluate from cells transfected with pEGFPc1. Cell lysate, 10 μl of 500 μl total; IP, 15 μl of 50 μl total. Molecular mass standards and the position of Lyric are shown. (B) Lysates of 293T cells transfected with pcDNA Gag were subjected to immunoprecipitation with either anti-Lyric antibody or a control IgG, followed by immunoblotting with an antibody directed against the CA domain of Gag. Lane 1, lysate of transfected cells, input for anti-Lyric IP; lane 2, eluate following anti-Lyric IP; lane 3, lysate of transfected cells, input for IgG control; lane 4, eluate following IP with IgG control. Cell lysate, 10 μl of 500 μl total; IP, 15 μl of 50 μl total. The additional band at approximately 50 kDa corresponds to the IgG heavy chain (hc). (C) HIV-1 particles equivalent to 2 μg CA antigen produced from infected MT4 cells and purified through an Optiprep gradient (OP; lane 1), eluates following IP of cell lysates from infected MT-4 cells (lane 2), and cell lysates of 3 × 105 infected MT-4 cells (lane 3) were subjected to immunoblot analysis with anti-Lyric antibody. Note the different mobility of Lyric from cell lysate and purified virions. (D) Lysates of 293T cells transfected with the indicated retroviral Gag constructs were either directly analyzed by immunoblotting with antiserum against Lyric (CL) or subjected to immunoprecipitation with anti-GFP microbeads, followed by immunoblotting (IP). Cell lysate, 15 μl of 1 ml total; IP, 15 μl of 50 μl total.
(iii) Lyric is incorporated into viral particles and cleaved by HIV-1 PR.
Gag-interacting proteins are often incorporated into HIV-1 particles, and virion incorporation and enrichment have been considered indicators of true interaction (12, 19). To address whether Lyric is incorporated into HIV-1, virions were purified from infected MT-4 cells by velocity gradient centrifugation and analyzed by silver staining and immunoblotting. A distinct signal for Lyric was detected in highly purified HIV-1 (Fig. 1C, lane 1). Quantifying the amount of CA by comparison with a standard (see Fig. S1 in the supplemental material) allowed estimation of the relative concentration of Lyric in virions and cell lysates, assuming an average virion radius of 66 nm (9) and an average MT-4 cell radius of 10 μm. In Fig. 1C, extracts from 3 × 105 infected MT-4 cells were loaded in lane 3 and gave a weaker Lyric signal than the one observed for the extract of 2 × 1010 HIV-1 particles loaded in lane 1. This corresponds to an at least 6-fold enrichment of Lyric in virions compared to cell extracts. Lyric in the virion exhibited a faster mobility in SDS gels than Lyric obtained from cell lysates or obtained by coimmunoprecipitations, with apparent molecular masses of ∼65 kDa in the virion and ∼80 kDa in the cell (Fig. 1C). The predicted molecular mass of Lyric is 64 kDa, and the higher molecular mass of cellular Lyric has been attributed to ubiquitinylation or sumoylation of intracellular Lyric (54). The faster-migrating protein in the virion may therefore be due to a lack of posttranslational modification or to cleavage of virion-associated Lyric by the viral PR. To address this question, wild-type HIV-1 and a variant carrying an active site mutation in PR were produced by cotransfection with a Lyric variant carrying a C-terminal Flag tag. Lysates and highly purified virus preparations were probed with antisera against Flag and Lyric, respectively (see Fig. S1 in the supplemental material). Neither a Flag nor a Lyric signal was detected in a parallel preparation from cells transfected with Lyric-Flag alone (without an HIV-1 expression plasmid), indicating the absence of cellular vesicles or debris carrying Lyric (see Fig. S1 in the supplemental material). Only a very weak Flag signal was observed for wild-type virus, whereas a distinct Flag signal at the position of cellular Lyric was found for PR-deficient HIV-1. Reprobing with Lyric antisera revealed Lyric signals in both preparations, but they had different mobilities. Lyric in wild-type HIV-1 again exhibited a slower mobility than cellular Lyric, while Lyric in PR-defective virions comigrated with cellular Lyric (see Fig. S1 in the supplemental material). These results indicate that following incorporation into wild-type HIV-1, Lyric is cleaved at its C terminus by the viral PR, leading to its faster mobility and loss of the C-terminal Flag epitope.
(iv) The Gag-Lyric interaction is also found for other retroviruses.
The Gag polyproteins of different retroviruses exhibit only limited sequence homology but share structural homology (8, 56). To determine whether Lyric also interacts with Gag proteins of other retroviruses, we performed parallel coimmunoprecipitation experiments of endogenous Lyric with GFP-tagged Gag proteins of HIV-1, equine infectious anemia virus (EIAV), and murine leukemia virus (MLV); GFP-expressing cells served as a negative control. Endogenous Lyric clearly interacted with all three retroviral Gag polyproteins, suggesting a conserved interaction among retroviruses (Fig. 1D).
HIV-1 Gag determinants required for the interaction with Lyric. (i) MA and NC domains contribute to Gag-Lyric interaction.
In order to map the Gag domain(s) responsible for interaction with Lyric, coimmunoprecipitations were performed using a panel of HIV-1 Gag truncation variants lacking individual domains. Endogenous Lyric was precipitated with specific antiserum, and associated Gag proteins were identified by immunoblotting against CA. The domain organization of HIV-1 Gag is shown in Fig. 2 A. MA-NC, lacking the C-terminal SP2-p6 region of Gag, interacted with Lyric with similar efficiency as full-length Gag (Fig. 2B, lane 2). In contrast, CA-NC, also lacking the N-terminal MA domain of Gag, did not coprecipitate with Lyric (Fig. 2B, lane 4), indicating that the MA domain is needed for this interaction. MA-SP1, lacking the NC domain, in addition to the C-terminal SP2-p6 region, also failed to coprecipitate with endogenous Lyric (Fig. 2B, lane 6), suggesting that both the MA and NC domains contribute to this interaction.
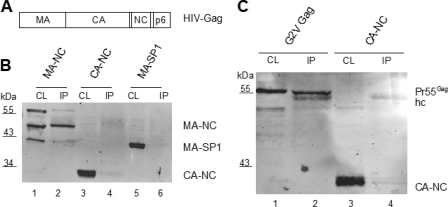
Fig. 2.
Mapping of Lyric interaction domains in HIV-1 Gag. (A) Domain organization of HIV-1 Gag. (B) Lysates of 293T cells transfected with the indicated pcDNA Gag-derived constructs were either directly analyzed by immunoblotting with antiserum against CA (CL) or subjected to immunoprecipitation with anti-Lyric antiserum, followed by immunoblotting (IP). Lanes 1 and 2, transfection of pcDNA MA-NC (49 kDa); lanes 3 and 4, transfection of pcDNA CA-NC (32 kDa); lanes 5 and 6, transfection of pcDNA MA-SP1 (42 kDa). The additional band at ∼65 kDa in lane 1 corresponds to an unidentified product and was not reproducible. (C) Lysates of 293T cells transfected with the indicated Gag constructs and pFLAG Lyric were either directly analyzed by immunoblotting with anti-CA (CL) or subjected to immunoprecipitation with anti-FLAG antibody, followed by immunoblot analysis (IP). Lanes 1 and 2, transfection of pcDNA Gag G2V, lanes 3 and 4, transfection of pcDNA CA-NC. Lysate, 10 μl of 500 μl total; IP, 15 μl of 50 μl total. The additional band due to cross-reaction with the IgG heavy chain is indicated (hc).
(ii) Lyric interaction is independent of Gag membrane association.
Since MA mainly serves to direct Gag to the cellular membrane and membrane association requires its N-terminal myristoylation, we made use of a Gag variant carrying a G2V mutation in the myristic acid acceptor amino acid (21). Membrane binding of this variant is reduced to 2% compared to wild-type Gag (26), and virion release is almost completely abolished, with Gag proteins largely being cytosolic. However, a lack of Gag myristoylation had no effect on coimmunoprecipitation with Lyric, indicating that the Gag-Lyric interaction is independent of membrane association (Fig. 2C; compare lanes 2 and 4).
(iii) Importance of the Gag NC domain for Lyric interaction.
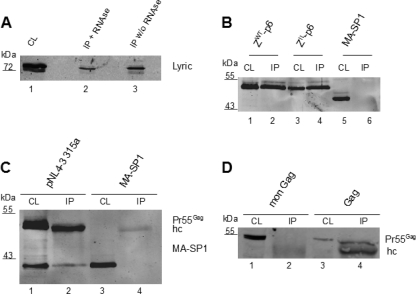
The NC domain of Gag is responsible for viral RNA encapsidation and functions as a nucleic acid chaperone (42). Two zinc finger motifs in NC mediate binding of HIV-1 genomic RNA in a sequence-specific manner, while basic residues account for nonspecific RNA binding (20, 22). To determine whether the Lyric-Gag interaction is dependent on NC binding to RNA, we performed experiments applying RNase treatment or using a Gag variant lacking the basic NC residues required for RNA binding. Lysates of transfected cells expressing GFP-fused Gag were either left untreated or treated with RNase. Gag was subsequently immunoprecipitated with anti-GFP microbeads. Endogenous Lyric was coprecipitated in both cases, but the signal intensity appeared to be slightly weaker for the treated sample (Fig. 3 A, lanes 2 and 3). To gain further insight into a potential role of NC-RNA interaction in Lyric binding, we performed coimmunoprecipitations from cells expressing a Gag variant, where all 15 basic residues in NC had been replaced by alanine. This RNA-binding-deficient Gag protein coprecipitated with Lyric at a similar efficiency as wild-type Gag (Fig. 3C, lane 2), while Gag lacking the NC-p6 domain did not coprecipitate (Fig. 3C, lane 4). These data suggest that the Gag-Lyric interaction is independent of its nucleic acid binding capacity but requires other features of NC. The NC domain of Gag can be replaced by the leucine zipper domain of the Saccharomyces cerevisiae transcription factor GCN4, facilitating protein interaction and thus substituting for the assembly function of NC (2). Virus-like particle formation in this case is independent of RNA-protein interactions but mediated exclusively by protein-protein interactions. A GCN4 zipper variant with isoleucine substitutions at eight residues forming trimers instead of dimers has also been shown to functionally substitute for NC in virus assembly (2). To examine whether the specific NC domain of HIV-1 Gag or its capacity to assemble into larger macromolecular structures is required for Gag-Lyric interaction, we performed coimmunoprecipitations of the respective proteins with Lyric. Both constructs with GCN4 zippers substituting for the NC domain of Gag were efficiently coprecipitated with Lyric, while truncated Gag lacking the NC domain was not precipitated (Fig. 3B). These results suggest that Gag multimerization, but not the NC domain itself, is important for Lyric binding.
Fig. 3.
Importance of Gag NC domain for Lyric interaction. (A) Lysates of 293T cells transfected with pCHIVEGFP were subjected to immunoprecipitation with anti-GFP microbeads in the presence or absence of RNase (60 μg/ml, 15 min at room temperature), followed by immunoblotting with anti-Lyric antibody. (B) Lysates of 293T cells transfected with the indicated Gag constructs and pFLAG Lyric were subjected to immunoprecipitation with anti-FLAG antibody, followed by immunoblotting with anti-CA. Lanes 1 and 2, pcDNA ZW T-p6; lanes 3 and 4, pcDNA ZI L-p6; lanes 5 and 6, pcDNA MA-SP1. Cell lysate, 10 μl of 500 μl total; IP, 15 μl of 50 μl total. (C) Lysates (CL) of 293T cells cotransfected with pNL43-315a (lanes 1 and 2) or pcDNA MA-SP1 (lanes 3 and 4) and pFLAG Lyric were subjected to immunoprecipitation (IP) with anti-FLAG antibody, followed by immunoblotting with anti-CA. The CA-reactive product in lanes 1 and 2 corresponds to a Gag processing intermediate, which may be recovered by anti-Lyric due to partial incorporation into the Gag lattice. Cell lysate, 10 μl of 500 μl total; IP, 15 μl of 50 μl total. (D) Lysates of 293T cells cotransfected with an expression vector for monomeric (mon) Gag (lanes 1 and 2) or wild-type Gag (lanes 3 and 4) and pFLAG Lyric were subjected to immunoprecipitation with anti-FLAG antibody, followed by immunoblotting with anti-CA. Cell lysate, 10 μl of 500 μl total; IP, 15 μl of 50 μl total. Additional bands in IPs representing IgG heavy chains are marked (hc). Expression and immunoprecipitation of Lyric-FLAG were verified by immunoblotting with anti-Lyric antibody (data not shown).
(iv) Gag multimerization is a prerequisite for interaction with Lyric.
To directly address whether Gag multimerization is a prerequisite for interaction with Lyric, we made use of a recently described Gag variant which was reported to be defective in multimerization. This variant carries mutations in MA and CA (M39A, W184A, and M185A), in addition to the described substitution of basic residues in NC, and has been shown to be monomeric when expressed in tissue culture (16). Immunoprecipitation of Lyric from cells expressing this variant failed to coprecipitate the monomeric Gag protein (Fig. 3D, lanes 2 and 4), supporting the notion that Gag multimerization is needed for its interaction with Lyric.
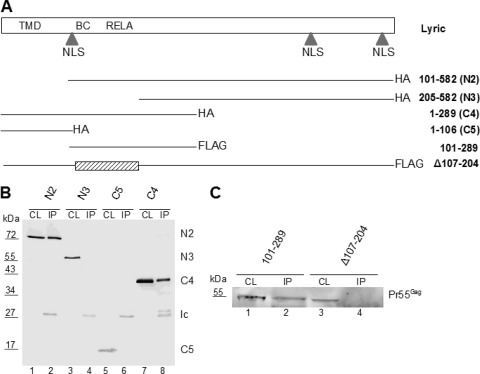
Lyric regions required for HIV-1 Gag interaction.
To map the Lyric domain responsible for Gag interaction, we employed a panel of truncation mutants for coimmunoprecipitation with HIV-1 Gag. These constructs give rise to C-terminally truncated Lyric proteins terminating after amino acid 513, 404, 356, 289, or 106 of Lyric (C1, C2, C3, C4, and C5, respectively) or N-terminally truncated Lyric molecules beginning at amino acid 71, 101, 205, or 232 of Lyric (N1, N2, N3, and N4, respectively) (Fig. 4 A). All truncated proteins carried an HA tag which was used to detect the respective protein following coimmunoprecipitation with a GFP-tagged HIV-1 Gag protein. Lyric was readily detected in the eluate following coimmunoprecipitation with Gag in the case of constructs C1-C4 and N1-N2 but not for constructs C5 and N3-N4. Figure 4B shows the results of coimmunoprecipitations of Lyric N2 and N3 (lanes 1 to 4) and C5 and C4 (lanes 5 to 8), revealing Gag interactions for a truncated Lyric protein ending at amino acid 289 (C4; Fig. 4B, lane 8) but not at amino acid 106 (C5; lane 6) and for Lyric encompassing amino acids 101 to 582 (N2; Fig. 4B, lane 2) but not 205 to 582 (N3; lane 4). Taken together, these results suggest that amino acids 101 to 289 of Lyric may be sufficient for HIV-1 Gag binding, while the Lyric region between amino acids 107 and 204 appears to be essential. To test this hypothesis directly, we constructed expression vectors for a Lyric variant lacking the putative Gag interaction domain (pFLAG Lyric Δ107-204) and for the putative Lyric interaction domain only (pFLAG Lyric 101-289). Their binding properties were verified by coimmunoprecipitation with HIV-1 Gag, yielding efficient binding of Lyric 101-289 (Fig. 4C, lane 2) but no binding of Lyric Δ107-204 (Fig. 4C, lane 4).
Fig. 4.
Mapping of Gag interaction domain in Lyric. (A) Schematic depiction of Lyric organization (top) and expression vectors for truncated Lyric. TMD, transmembrane domain (amino acids 49 to 69); NLS, nuclear localization signal (amino acids 78 to 130, 415 to 486, 546 to 582); BC, domain interacting with BCCIPα (amino acids 72 to 169); RELA, domain interacting with p65/RelA (amino acids 101 to 205); HA, HA tag. (B) Lysates of 293T cells (CL) transfected with pCHIVEGFP and the indicated Lyric constructs were subjected to immunoprecipitation (IP) with anti-GFP microbeads, followed by immunoblotting with anti-HA. Lanes 1 and 2, pcDNA N2 HA; lanes 3 and 4, pcDNA N3 HA; lanes 5 and 6, pcDNA C5 HA; lanes 7 and 8, pcDNA C4 HA. lc, immunoglobulin light chain. (C) Lysates of 293T cells transfected with pcDNA Gag and the indicated Lyric constructs were subjected to immunoprecipitation with anti-FLAG antibody, followed by immunoblotting with anti-CA. Expression and immunoprecipitation of Lyric variants were verified by Western blotting with anti-Lyric antibody (data not shown).
Effects of Lyric on HIV-1 protein expression and viral infectivity.
Having confirmed the interaction between HIV-1 Gag and Lyric, we aimed at investigating a possible functional relevance. Our efforts to achieve siRNA-mediated knockdown of Lyric were not successful, most likely because of a long half-life of the protein (only a <40% reduction of protein levels, despite an 85% reduction in mRNA; data not shown). We therefore made use of the Lyric variants described above. Expression vectors for wild-type Lyric, its Gag-binding domain, and the deletion variant lacking the binding domain were cotransfected with the full-length HIV-1 plasmid pNL4-3. After 48 h, cell lysates and tissue culture supernatants were harvested and analyzed regarding protein expression, particle release, and infectivity. Data from three independent experiments are shown in Fig. 5. Immunoblot analysis of extracts from transfected cells revealed approximately equal expression of full-length Lyric and the variant lacking the Gag-binding domain, while expression of only the Gag-binding domain yielded a 3-fold higher expression level (Fig. 5A, top). Gag protein expression levels were affected by the coexpression of Lyric variants. Cotransfection of an expression plasmid for full-length Lyric led to a 3-fold decrease in Gag expression compared to cells transfected with the empty vector, and cotransfection of the Lyric variant lacking the Gag-binding domain led to a further 2-fold decrease in Gag expression (Fig. 5A, middle). On the other hand, coexpression of only the Gag-binding domain of Lyric led to a small increase (∼1.5-fold) of Gag expression. These differences were less pronounced when particle release was analyzed (Fig. 5A, bottom; see Fig. S2 in the supplemental material), with quantification of extracellular antigen revealing approximately equal levels in the culture medium of cells transfected with expression vectors for full-length Lyric, the binding domain, or the empty vector control and a 2- to 3-fold reduction in the case of Lyric lacking the binding domain. Overexpression of full-length Lyric or the two truncated variants had a significant effect on HIV-1 infectivity (Fig. 5B), despite only minor differences in particle release. HIV-1 from cells overexpressing full-length Lyric showed an approximately 3-fold decrease in infectivity compared to the vector control, while HIV-1 from cells overexpressing the Gag-binding domain exhibited an approximately 4-fold increase in infectivity compared to the vector control and a 13-fold increase compared to full-length Lyric. These differences are not due to differences in particle production (compare Fig. 5A and Fig. S2 in the supplemental material), indicating that wild-type Lyric and the Gag-binding domain affect HIV-1 infectivity in opposite directions. HIV-1 from cells overexpressing Lyric lacking the binding domain showed a further decrease in infectivity compared to wild-type Lyric, but this effect was mostly due to the reduction in particle formation, with specific infectivity being almost equal in both cases.
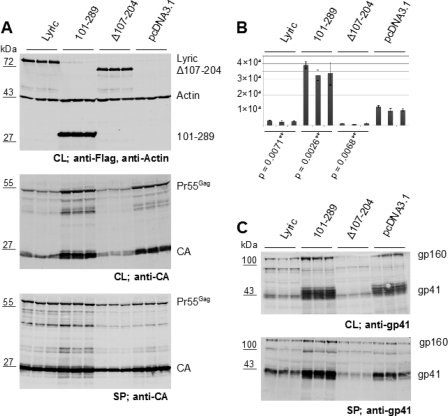
Fig. 5.
Influence of Lyric on HIV protein expression and infectivity. 293T cells were cotransfected with the full-length HIV-1 plasmid pNL4-3 and the constructs indicated on top of the figure. At 48 h after transfection, cell lysates (CL) and particles recovered from tissue culture supernatants by centrifugation through a sucrose cushion (SP) were analyzed by immunoblotting. Intensities of specific products were quantified with a Licor system. The results of three independent experiments are shown. (A) Cell lysates (top and middle) and particulate fractions (bottom) analyzed with antibodies against Flag and actin (top) or HIV-1 CA (middle and bottom). (B) Infectivity of tissue culture media from the transfection experiments shown in panel A. Equal volumes of cleared culture media were used to infect TZM cells, and Tat-dependent luciferase activity was measured in relative light units (RLU). Mean values of triplicate samples are shown; error bars indicate standard deviations. Two-tailed P values from paired Student's t test in relation to pCNDA3.1 are shown below. (C) Immunoblot analysis of cell lysates (top) and sucrose cushion (bottom) from the same transfections as in panel A with antisera against the HIV-1 glycoprotein gp41.
Because of these differences in viral infectivity, expression and virion incorporation of the HIV-1 transmembrane glycoprotein gp41 were also analyzed. Expression of gp41 in cells cotransfected with an expression vector for the Gag-binding domain of Lyric was higher than that in cells transfected with the empty vector, while transfection with an expression vector for Lyric lacking the Gag-binding domain or for full-length Lyric yielded a 6-fold decrease in gp41 compared to cells transfected with the empty vector (Fig. 5C, top). This resulted in a 3-fold decrease in gp41 in virus particles in the case of Gag-binding-deficient Lyric, whereas no change in particle production was observed for full-length Lyric compared to empty vector (Fig. 5A, bottom). To determine whether the observed effects on protein expression are specific for HIV-1 proteins and whether they require the HIV-1 LTR promoter, we performed additional cotransfection experiments with full-length Lyric and its Gag-binding domain (see Fig. S3 in the supplemental material). These experiments confirmed the enhancing effect of the Gag-binding domain of Lyric on the expression of HIV-1 Gag and Env (see Fig. S3B and D in the supplemental material). The effect on HIV-1 Gag expression was independent of the HIV-1 LTR promoter and was also observed for a Gag-EGFP fusion protein expressed from the CMV promoter (see Fig. S3B in the supplemental material), while GFP expression was not affected by Lyric (see Fig. S3C in the supplemental material). To determine whether the Lyric effect on HIV-1 Env expression is Gag dependent, we made use of pNLA-1, a derivative of pNL4-3 which lacks the gag and pol genes but expresses all other HIV-1 proteins (50). Figure S3D in the supplemental material shows similar effects of full-length Lyric and the Gag-binding domain on HIV-1 Env protein expression independent of the presence or absence of HIV-1 Gag (compare lanes NL4-3 and NLA-1), indicating that this effect is independent of Gag binding by Lyric.
DISCUSSION
In this report, we have identified and validated the cellular protein Lyric/AEG-1 to be a novel interaction partner of HIV-1 Gag and mapped the binding domains of both Lyric and Gag. Lyric was the only cellular protein identified consistently in five independent affinity purification experiments, and specific interaction with HIV-1 Gag was verified by efficient coimmunoprecipitation using antibodies against either Lyric or Gag. Furthermore, Lyric was also identified to be a strong candidate for Gag interaction using a stable isotope labeling approach (SILAC) in combination with quantitative mass spectrometry, yielding a highly significant enrichment factor. Taken together, Lyric was the strongest candidate for a Gag interaction partner in our screens, and this was supported by detecting Lyric in purified HIV-1 preparations. Lyric was enriched in virions compared to cell lysates, and virion-associated Lyric was found to be cleaved at its C terminus by the viral PR, suggesting its specific incorporation. Lyric was also found to interact with the Gag proteins of two other retroviruses, indicating a conserved interaction. Several proteins previously reported to interact with HIV-1 Gag were also detected in our interaction screens and/or in the SILAC experiment, most notably, the protein Staufen (which was detected in 3 of 5 screens and which exhibited a strong score in the SILAC experiment), but Lyric was the only protein that was invariably observed in all experiments.
Mapping the Lyric-interacting region of Gag revealed that the MA and NC domains appear to be required, while neither of the two domains alone is sufficient. Surprisingly, neither membrane binding, the main function of MA, nor RNA interaction, the main function of NC, is required for Gag-Lyric interaction. Many reported interactions of Gag with RNA-binding proteins have been shown to be mediated by their common binding to nucleic acid in an NC-dependent way (11, 33). This is not the case for Lyric, however, since Lyric has not been described to bind RNA, Gag-Lyric interaction was not abolished by RNase treatment, and, most importantly, a Gag protein deficient in RNA binding still efficiently coprecipitated with Lyric. Furthermore, Lyric does not belong to a previously characterized group of nucleic acid binding proteins commonly found in pull-down experiments (55) and was not detected in a similar SILAC screen using a nucleic acid binding protein complex as bait (G. A. Müller, unpublished observation). Coimmunoprecipitation of Lyric with Gag variants with a leucine zipper substituting for the NC domain further revealed that NC itself is dispensable for Lyric interaction, while its role in driving Gag multimerization appears to be important. Accordingly, a monomeric Gag variant carrying mutations in MA and CA, in addition to the basic NC residues, failed to coprecipitate Lyric. Taken together, these results suggest that the Gag-Lyric complex is formed by direct or indirect protein interactions involving an as yet unknown binding motif in the MA-CA region of Gag and binding requires Gag multimerization. This may indicate that Lyric recognizes the multimeric immature Gag lattice or that complex formation requires multiple interactions for stable binding.
The apparent requirement for a multimeric structure is not without precedent among Gag interaction partners: the cellular protein hp68 has been reported to transiently bind to HIV-1 assembly intermediates, while it is released once assembly is completed (61), but this interaction is RNA dependent. Direct binding to a protein lattice has been postulated for the restriction factor Trim5α, which appears to interact with incoming HIV capsids, leading to their premature disassembly and loss of infectivity (51). However, interaction in this case involves the mature CA lattice, while Lyric appears to bind the immature Gag lattice. To gain further insight into the Gag-Lyric interaction, we attempted mapping of the Gag-binding domain in Lyric (GagBD). Previous studies had shown that Lyric is involved in modulation of cellular signaling and proliferation, with an apparent deregulation in malignant gliomas and several other tumors and derived cell lines (4, 27, 47). These studies also identified putative functional domains in Lyric, including three potential nuclear localization signals, a predicted transmembrane domain, and an N-terminal LXXLL motif that has been described to mediate the interaction of transcriptional coactivators with transcription factors (reviewed in reference 46). In addition, Lyric contains a domain interacting with the p65 (RelA) subunit of NF-κB (47), and it interacts with the cell cycle regulator BRCA2 and CDKN1A-interacting protein (BCCIPα), downregulating its expression by targeting it for degradation (4). Our mapping studies indicated that the region encompassing amino acids 107 to 204 of Lyric is essential for Gag binding and the region encompassing amino acids 101 to 289 is sufficient. This region overlaps with the previously characterized binding domains for the NF-κB p65 subunit and BCCIPα and includes one of the putative nuclear localization signals (4, 47, 54).
Overexpression of only the binding domain of known interaction factors often has a dominant negative effect on the partner's function by competing for the interaction site. We therefore made use of our mapping studies to test whether overexpression of full-length Lyric or its GagBD influences HIV-1 morphogenesis and infectivity. We observed that overexpression of the GagBD led to a slight enhancement of HIV-1 Gag and Env protein expression and to a 4-fold increase in viral specific infectivity (defined as the number of infectious units per virus antigen). Overexpression of full-length Lyric or a variant lacking the GagBD had the opposite effect, reducing HIV-1-specific infectivity approximately 3-fold. It appears to be unlikely that these effects are caused by overexpression artifacts, since the GagBD was expressed at larger amounts than full-length Lyric in transfected cells. These results may be interpreted to suggest that Lyric has a negative impact on the production of infectious HIV-1 and thus acts as an inhibitory factor; this inhibition would be competed by overexpression of the GagBD. It should be noted, however, that the repressive effect was also observed for Lyric lacking GagBD, and more detailed studies of Lyric expression levels in relevant target cells and determination of concentration-dependent differences of Lyric effects will be needed to clarify this issue. The increased incorporation of HIV-1 glycoproteins into viral particles in the presence of the Lyric GagBD may point to Env incorporation as a relevant mechanism of action for increased infectivity. This effect may be indirect, however, since Env incorporation correlated with a Lyric-dependent increase of cellular Env expression. Furthermore, a similar increase in glycoprotein expression was also observed for an HIV-1 construct lacking the entire gag-pol region. Taken together, our results indicate opposite effects of full-length Lyric and its GagBD on HIV-1 infectivity and viral protein expression, but these effects appear to be at least partly independent of Lyric-Gag interaction.
Besides Lyric affecting Gag and the production of infectious HIV-1 (in a positive or negative way), this interaction may also have a role in modulating Lyric function in infected cells. Gag is the major viral protein in the late phase of the replication cycle and may conceivably compete for cellular Lyric binding partners or act as a sink for endogenous Lyric. Lyric has been reported to be induced upon HIV-1 infection (18, 27), and this effect could be counterbalanced in the later phases through increased Gag production. This may influence cellular proliferation and/or signaling, most notably by affecting the NF-κB and BCCIPα pathways since the binding sites for these factors overlap with GagBD. BCCIPα has been reported to bind the cell cycle regulator p21 WAF/CIP1 and enhance p21-mediated inhibition of Cdk2, thus stalling cell cycle progression (38). This could be overcome by Gag-Lyric interaction. Further studies on the Gag-dependent modulation of these pathways will be needed to clarify a potential influence of the Gag-Lyric interaction on infected cell activation and/or proliferation.
The results of the current study identify Lyric to be a cellular interaction partner of HIV-1 Gag and hint at a potential role in regulating infectivity, but further experiments are needed to elucidate the precise role of this interaction.
Supplementary Material
ACKNOWLEDGMENTS
We thank J. Reichert, P. Ihrig, and J. Lechner (BZH) and T. Bosserhoff, M. Ellis, and T. Ruppert (ZMBH) for mass spectrometry analysis. We owe special thanks to S. Sohr for helpful discussions. We thank U. Rothbauer (ChromoTek, Martinsried, Germany) for providing GFP-Trap A. We thank M. Resh, A. Rein, A. Aldovini, P. Bates, D.-E. Britt, D. Sarkar, and P. Spearman for kindly providing plasmids. We thank B. Glass for excellent technical assistance and C. Jost for critical reading of the manuscript.
This work was supported in part by a grant from the Deutsche Forschungsgemeinschaft (SFB638; A9). C.E.E. is a fellow of the M.D./Ph.D. program of the Faculty of Medicine and the Faculty of Biosciences at the University of Heidelberg and a member of the Hartmut Hoffmann-Berling International Graduate School of Molecular and Cellular Biology (HBIGS). H.-G.K. is an investigator of the CellNetworks Cluster of Excellence (EXC81).
Footnotes
Supplemental material for this article may be found at http://jvi.asm.org/.
Published ahead of print on 28 September 2011.
REFERENCES
- 1. Abacioglu Y. H., et al. 1994. Epitope mapping and topology of baculovirus-expressed HIV-1 gp160 determined with a panel of murine monoclonal antibodies. AIDS Res. Hum. Retroviruses 10:371–381 [DOI] [PubMed] [Google Scholar]
- 2. Accola M. A., Strack B., Göttlinger H. G. 2000. Efficient particle production by minimal Gag constructs which retain the carboxy-terminal domain of human immunodeficiency virus type 1 capsid-p2 and a late assembly domain. J. Virol. 74:5395–5402 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Adachi A., et al. 1986. Production of acquired immunodeficiency syndrome-associated retrovirus in human and nonhuman cells transfected with an infectious molecular clone. J. Virol. 59:284–291 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Ash S. C., Yang D. Q., Britt D. E. 2008. LYRIC/AEG-1 overexpression modulates BCCIPalpha protein levels in prostate tumor cells. Biochem. Biophys. Res. Commun. 371:333–338 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Bieniasz P. D. 2009. The cell biology of HIV virion genesis. Cell Host Microbe 5:550–558 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Bradford M. M. 1976. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 72:248–254 [DOI] [PubMed] [Google Scholar]
- 7. Bushman F. D., et al. 2009. Host cell factors in HIV replication: meta-analysis of genome-wide studies. PLoS Pathog. 5:e1000437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Campos-Olivas R., Newman J. L., Summers M. F. 2000. Solution structure and dynamics of the Rous sarcoma virus capsid protein and comparison with capsid proteins of other retroviruses. J. Mol. Biol. 296:633–649 [DOI] [PubMed] [Google Scholar]
- 9. Carlson L. A., et al. 2008. Three-dimensional analysis of budding sites and released virus suggests a revised model for HIV-1 morphogenesis. Cell Host Microbe 4:592–599 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Catrein I., Herrmann R., Bosserhoff A., Ruppert T. 2005. Experimental proof for a signal peptidase I like activity in Mycoplasma pneumoniae, but absence of a gene encoding a conserved bacterial type I SPase. FEBS J. 272:2892–2900 [DOI] [PubMed] [Google Scholar]
- 11. Châtel-Chaix L., Boulay K., Mouland A. J., DesGroseillers L. 2008. The host protein Staufen1 interacts with the Pr55Gag zinc fingers and regulates HIV-1 assembly via its N-terminus. Retrovirology 5:41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Châtel-Chaix L., et al. 2004. Identification of Staufen in the human immunodeficiency virus type 1 Gag ribonucleoprotein complex and a role in generating infectious viral particles. Mol. Cell. Biol. 24:2637–2648 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Cimarelli A., Sandin S., Höglund S., Luban J. 2000. Basic residues in human immunodeficiency virus type 1 nucleocapsid promote virion assembly via interaction with RNA. J. Virol. 74:3046–3057 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Cox J., Mann M. 2008. MaxQuant enables high peptide identification rates, individualized p.p.b. -range mass accuracies and proteome-wide protein quantification. Nat. Biotechnol. 26:1367–1372 [DOI] [PubMed] [Google Scholar]
- 15. Dettenhofer M., Yu X. F. 1999. Highly purified human immunodeficiency virus type 1 reveals a virtual absence of Vif in virions. J. Virol. 73:1460–1467 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Dou J., et al. 2009. Characterization of a myristoylated, monomeric HIV Gag protein. Virology 387:341–352 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Emdad L., et al. 2009. Astrocyte elevated gene-1 (AEG-1) functions as an oncogene and regulates angiogenesis. Proc. Natl. Acad. Sci. U. S. A. 106:21300–21305 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Emdad L., et al. 2006. Activation of the nuclear factor kappaB pathway by astrocyte elevated gene-1: implications for tumor progression and metastasis. Cancer Res. 66:1509–1516 [DOI] [PubMed] [Google Scholar]
- 19. Franke E. K., Yuan H. E., Luban J. 1994. Specific incorporation of cyclophilin A into HIV-1 virions. Nature 372:359–362 [DOI] [PubMed] [Google Scholar]
- 20. Freed E. O. 2001. HIV-1 replication. Somat. Cell Mol. Genet. 26:13–33 [DOI] [PubMed] [Google Scholar]
- 21. Freed E. O., Orenstein J. M., Buckler-White A. J., Martin M. A. 1994. Single amino acid changes in the human immunodeficiency virus type 1 matrix protein block virus particle production. J. Virol. 68:5311–5320 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Ganser-Pornillos B. K., Yeager M., Sundquist W. I. 2008. The structural biology of HIV assembly. Curr. Opin. Struct. Biol. 18:203–217 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Gingras A. C., et al. 2005. A novel, evolutionarily conserved protein phosphatase complex involved in cisplatin sensitivity. Mol. Cell. Proteomics 4:1725–1740 [DOI] [PubMed] [Google Scholar]
- 24. Harada S., Koyanagi Y., Yamamoto N. 1985. Infection of HTLV-III/LAV in HTLV-I-carrying cells MT-2 and MT-4 and application in a plaque assay. Science 229:563–566 [DOI] [PubMed] [Google Scholar]
- 25. Hermida-Matsumoto L., Resh M. D. 2000. Localization of human immunodeficiency virus type 1 Gag and Env at the plasma membrane by confocal imaging. J. Virol. 74:8670–8679 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Jäger S., Gottwein E., Kräusslich H. G. 2007. Ubiquitination of human immunodeficiency virus type 1 Gag is highly dependent on Gag membrane association. J. Virol. 81:9193–9201 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Kang D. C., et al. 2005. Cloning and characterization of HIV-1-inducible astrocyte elevated gene-1, AEG-1. Gene 353:8–15 [DOI] [PubMed] [Google Scholar]
- 28. Kikuno N., et al. 2007. Knockdown of astrocyte-elevated gene-1 inhibits prostate cancer progression through upregulation of FOXO3a activity. Oncogene 26:7647–7655 [DOI] [PubMed] [Google Scholar]
- 29. Konvalinka J., et al. 1995. An active-site mutation in the human immunodeficiency virus type 1 proteinase (PR) causes reduced PR activity and loss of PR-mediated cytotoxicity without apparent effect on virus maturation and infectivity. J. Virol. 69:7180–7186 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Lange S., Sylvester M., Schümann M., Freund C., Krause E. 2010. Identification of phosphorylation-dependent interaction partners of the adapter protein ADAP using quantitative mass spectrometry: SILAC vs (18)O-labeling. J. Proteome Res. 9:4113–4122 [DOI] [PubMed] [Google Scholar]
- 31. Lee S. G., Su Z. Z., Emdad L., Sarkar D., Fisher P. B. 2006. Astrocyte elevated gene-1 (AEG-1) is a target gene of oncogenic Ha-ras requiring phosphatidylinositol 3-kinase and c-Myc. Proc. Natl. Acad. Sci. U. S. A. 103:17390–17395 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Lee S. G., et al. 2008. Astrocyte elevated gene-1 activates cell survival pathways through PI3K-Akt signaling. Oncogene 27:1114–1121 [DOI] [PubMed] [Google Scholar]
- 33. Lingappa J. R., Dooher J. E., Newman M. A., Kiser P. K., Klein K. C. 2006. Basic residues in the nucleocapsid domain of Gag are required for interaction of HIV-1 gag with ABCE1 (HP68), a cellular protein important for HIV-1 capsid assembly. J. Biol. Chem. 281:3773–3784 [DOI] [PubMed] [Google Scholar]
- 34. Luban J., Bossolt K. L., Franke E. K., Kalpana G. V., Goff S. P. 1993. Human immunodeficiency virus type 1 Gag protein binds to cyclophilins A and B. Cell 73:1067–1078 [DOI] [PubMed] [Google Scholar]
- 35. Mouland A. J., et al. 2000. The double-stranded RNA-binding protein Staufen is incorporated in human immunodeficiency virus type 1: evidence for a role in genomic RNA encapsidation. J. Virol. 74:5441–5451 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Müller B., et al. 2004. Construction and characterization of a fluorescently labeled infectious human immunodeficiency virus type 1 derivative. J. Virol. 78:10803–10813 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Ong S. E., et al. 2002. Stable isotope labeling by amino acids in cell culture, SILAC, as a simple and accurate approach to expression proteomics. Mol. Cell. Proteomics 1:376–386 [DOI] [PubMed] [Google Scholar]
- 38. Ono T., et al. 2000. TOK-1, a novel p21Cip1-binding protein that cooperatively enhances p21-dependent inhibitory activity toward CDK2 kinase. J. Biol. Chem. 275:31145–31154 [DOI] [PubMed] [Google Scholar]
- 39. Ott D. E., et al. 1996. Cytoskeletal proteins inside human immunodeficiency virus type 1 virions. J. Virol. 70:7734–7743 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Poon D. T., Wu J., Aldovini A. 1996. Charged amino acid residues of human immunodeficiency virus type 1 nucleocapsid p7 protein involved in RNA packaging and infectivity. J. Virol. 70:6607–6616 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Ptak R. G., et al. 2008. Cataloguing the HIV type 1 human protein interaction network. AIDS Res. Hum. Retroviruses 24:1497–1502 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Rein A. 2010. Nucleic acid chaperone activity of retroviral Gag proteins. RNA Biol. 7:61–66 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Rigaut G., et al. 1999. A generic protein purification method for protein complex characterization and proteome exploration. Nat. Biotechnol. 17:1030–1032 [DOI] [PubMed] [Google Scholar]
- 44. Rothbauer U., et al. 2008. A versatile nanotrap for biochemical and functional studies with fluorescent fusion proteins. Mol. Cell. Proteomics 7:282–289 [DOI] [PubMed] [Google Scholar]
- 45. Roy B. B., et al. 2006. Association of RNA helicase a with human immunodeficiency virus type 1 particles. J. Biol. Chem. 281:12625–12635 [DOI] [PubMed] [Google Scholar]
- 46. Sarkar D., et al. 2009. Astrocyte elevated gene-1: far more than just a gene regulated in astrocytes. Cancer Res. 69:8529–8535 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Sarkar D., et al. 2008. Molecular basis of nuclear factor-kappaB activation by astrocyte elevated gene-1. Cancer Res. 68:1478–1484 [DOI] [PubMed] [Google Scholar]
- 48. Schneider R., Campbell M., Nasioulas G., Felber B. K., Pavlakis G. N. 1997. Inactivation of the human immunodeficiency virus type 1 inhibitory elements allows Rev-independent expression of Gag and Gag/protease and particle formation. J. Virol. 71:4892–4903 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Sherer N. M., et al. 2003. Visualization of retroviral replication in living cells reveals budding into multivesicular bodies. Traffic 4:785–801 [DOI] [PubMed] [Google Scholar]
- 50. Strebel K., et al. 1987. The HIV ‘A’ (sor) gene product is essential for virus infectivity. Nature 328:728–730 [DOI] [PubMed] [Google Scholar]
- 51. Stremlau M., et al. 2004. The cytoplasmic body component TRIM5alpha restricts HIV-1 infection in Old World monkeys. Nature 427:848–853 [DOI] [PubMed] [Google Scholar]
- 52. Tanzi G. O., Piefer A. J., Bates P. 2003. Equine infectious anemia virus utilizes host vesicular protein sorting machinery during particle release. J. Virol. 77:8440–8447 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Thali M., et al. 1994. Functional association of cyclophilin A with HIV-1 virions. Nature 372:363–365 [DOI] [PubMed] [Google Scholar]
- 54. Thirkettle H. J., et al. 2009. LYRIC/AEG-1 is targeted to different subcellular compartments by ubiquitinylation and intrinsic nuclear localization signals. Clin. Cancer Res. 15:3003–3013 [DOI] [PubMed] [Google Scholar]
- 55. Trinkle-Mulcahy L., et al. 2008. Identifying specific protein interaction partners using quantitative mass spectrometry and bead proteomes. J. Cell Biol. 183:223–239 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Turner B. G., Summers M. F. 1999. Structural biology of HIV. J. Mol. Biol. 285:1–32 [DOI] [PubMed] [Google Scholar]
- 57. Vermeulen M., Hubner N. C., Mann M. 2008. High confidence determination of specific protein-protein interactions using quantitative mass spectrometry. Curr. Opin. Biotechnol. 19:331–337 [DOI] [PubMed] [Google Scholar]
- 58. Weijtens M. E., Willemsen R. A., Hart E. H., Bolhuis R. L. 1998. A retroviral vector system ‘STITCH’ in combination with an optimized single chain antibody chimeric receptor gene structure allows efficient gene transduction and expression in human T lymphocytes. Gene Ther. 5:1195–1203 [DOI] [PubMed] [Google Scholar]
- 59. Welker R., Hohenberg H., Tessmer U., Huckhagel C., Kräusslich H. G. 2000. Biochemical and structural analysis of isolated mature cores of human immunodeficiency virus type 1. J. Virol. 74:1168–1177 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Yoo B. K., et al. 2009. Astrocyte elevated gene-1 regulates hepatocellular carcinoma development and progression. J. Clin. Invest. 119:465–477 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Zimmerman C., et al. 2002. Identification of a host protein essential for assembly of immature HIV-1 capsids. Nature 415:88–92 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.