Abstract
Objective:
Muscle-specific receptor tyrosine kinase (MuSK) antibody-positive myasthenia gravis (MG) accounts for 5%–15% of autoimmune MG. MuSK mediates the agrin-signaling pathway and also anchors the collagenic tail subunit (ColQ) of acetylcholinesterase (AChE). The exact molecular target of MuSK–immunoglobulin G (IgG), however, remains elusive. As acetylcholine receptor (AChR) deficiency is typically mild and as cholinesterase inhibitors are generally ineffective, we asked if MuSK-IgG interferes with binding of ColQ to MuSK.
Methods:
We used 3 assays: in vitro overlay of the human ColQ-tailed AChE to muscle sections of Colq−/− mice; in vitro plate-binding assay to quantitate binding of MuSK to ColQ and to LRP4; and passive transfer of MuSK-IgG to mice.
Results:
The in vitro overlay assay revealed that MuSK-IgG blocks binding of ColQ to the neuromuscular junction. The in vitro plate-binding assay showed that MuSK-IgG exerts a dose-dependent block of MuSK binding to ColQ by but not to LRP4. Passive transfer of MuSK-IgG to mice reduced the size and density of ColQ to ∼10% of controls and had a lesser effect on the size and density of AChR and MuSK.
Conclusions:
As lack of ColQ compromises agrin-mediated AChR clustering in Colq−/− mice, a similar mechanism may lead to AChR deficiency in MuSK-MG patients. Our experiments also predict partial AChE deficiency in MuSK-MG patients, but AChE is not reduced in biopsied NMJs. In humans, binding of ColQ to MuSK may be dispensable for clustering ColQ, but is required for facilitating AChR clustering. Further studies will be required to elucidate the basis of this paradox.
During development of the neuromuscular junction (NMJ), neural agrin released from the nerve terminal binds to the postsynaptic transmembrane protein LRP4.1,2 Dimerized LRP4 forms a heterotetramer with the dimerized muscle-specific receptor tyrosine kinase (MuSK).3 MuSK together with Dok-7 promotes clustering of acetylcholine receptor (AChR) on the junctional folds by rapsyn.4 The clustering effect of MuSK is mediated by distinct pathways involving Rho GTPase.5
At the NMJ, 3 tetramers of catalytic subunits of acetylcholinesterase (AChE) are linked to ColQ, the triple helical collagenic subunit.6 ColQ-tailed AChE is anchored to the synaptic basal lamina by 2 mechanisms: 2 sets of heparan sulfate proteoglycan residues in the collagen domain of ColQ7 bind to heparin sulfate proteoglycans, such as perlecan8; and the C-terminal domain of ColQ binds to MuSK.9
Five percent to 15% of patients with myasthenia gravis (MG) carry antibodies against MuSK (MuSK–immunoglobulin G [IgG]).10,11 MuSK-MG patients respond favorably to immunotherapy, but usually do not respond to, or are even worsened by, cholinesterase inhibitors.12–15 Anti-AChR antibodies comprise IgG1 and IgG3 moieties that bind complement whereas anti-MuSK antibodies are largely IgG4 that do not activate complement, and complement deposits at the NMJ are sparse.16–18 However, the exact target of MuSK-IgG remains elusive. We therefore examined an effect of MuSK-IgG on an interaction between ColQ and MuSK by in vitro and in vivo assays, and found that MuSK-IgG blocks this interaction.
METHODS
Patients.
We obtained serum from 4 MuSK-MG patients (patients 1–4) and a patient with limb-girdle muscular dystrophy as a control (control 1). We obtained 10 mL peripheral blood from patients 1, 3, 4, and control and residual plasmapheresis fluid from patient 2. We also obtained expired fresh-frozen plasma (control 2) from Dr. Isao Takahashi at the Aichi Red Cross Blood Center with institutional approval. We used sera of patient 2 and control 2 for all the experiments, and sera of patients 1, 3, 4, and control 1 only for the in vitro overlay and in vitro plate binding assays because only small amounts of sera were available from these patients.
Ages and genders of patients 1–4 were a 48-year-old woman, a 30-year-old woman, a 59-year-old man, and a 45-year-old woman, respectively. The titers of anti-MuSK antibodies of patients 1–3 were 22.0 nM, 11.2 nM, and 0.12 nM, respectively (normal <0.01 nM). Patient 4 was positive for anti-MuSK antibody, but the titer was not determined.
Standard protocol approvals, registrations, and patient consents.
We performed all human studies under the institutional review board approvals of the Nagoya University Graduate School of Medicine and the Mayo Clinic, and obtained written informed consents from each patient and a control. We also obtained approvals of the Colq−/− mice studies and the passive IgG transfer studies by the Animal Care and Use Committee of the Nagoya University.
Plasmids.
We previously made CMV-based mammalian expression vectors, pTargeT-COLQ and pTargeT-ACHE.19 To generate hMuSKect-myc, we cloned the extracellular domain (aa 1–393) of human MUSK cDNA (Open Biosystems) into a mammalian expression vector pAPtag-5 (GenHunter) at the NheI and XhoI sites upstream of a myc epitope. For hLRP4N-FLAG, we cloned the extracellular domain (aa 1–1722) of human LRP4 cDNA (Open Biosystems) into the HindIII and XbaI sites upstream of a 3xFLAG epitope of a mammalian expression vector p3XFLAG-CMV-14 (Sigma Aldrich).
Preparation of recombinant human ColQ-tailed AChE.
We prepared human ColQ-tailed AChE for in vitro overlay assay and for in vitro plate-binding assay. Both pTargeT-COLQ and pTargeT-ACHE were transfected into HEK293 cells in a 10-cm dish using the calcium phosphate method as described elsewhere.20 We extracted proteins from the cells in Tris-HCl buffer (50 mM Tris-HCl [pH 7.0], 0.5% Triton X-100, 0.2 mM EDTA, leupeptin [2 μg/mL], and pepstatin [1 μg/mL]) containing 1 M NaCl, and diluted the extracts containing ColQ-tailed AChE in Tris-HCl buffer containing 0.2 M NaCl and loaded onto the HiTrap Heparin HP columns (GE Healthcare). We washed the columns with 5 volumes of Tris-HCl buffer containing 0.2 M NaCl, and eluted ColQ-tailed AChE with Tris-HCl buffer containing 1 M NaCl. We concentrated the eluate with an Amicon Ultra-4 Centrifugal Filter (50K) (Millipore) to 12-Ellman units per mL. The units were normalized with the Torpedo-derived AChE (C2888, Sigma-Aldrich).
Preparation of hMuSKect-myc and hLRP4N-FLAG proteins.
We prepared hMuSKect-myc and hLRP4N-FLAG for in vitro plate-binding assays. We introduced a construct carrying either hMuSKect-myc or hLRP4N-FLAG into HEK293 cells in a 10-cm dish using the calcium phosphate method as above. We purified the hMuSKect-myc with the c-myc-Tagged Protein Mild Purification Kit version 2 (MBL), and purified the hLRP4N-FLAG with the Anti-DYKDDDDK-tag Antibody Beads (Wako). We detected purified hMuSKect-myc and hLRP4N-FLAG by anti-myc antibody (9E10, Abcam) and anti-FLAG antibody (M2, Sigma-Aldrich), respectively (data not shown), and also detected hMuSKect-myc by sodium dodecyl sulfate–polyacrylamide gel electrophoresis (SDS-PAGE) followed by protein staining with the Oriole Fluorescent Gel Stain (Bio-Rad).
Purification of plasma IgG.
We purified IgG as described elsewhere21 with minor modifications. We adjusted plasma to pH 8.0 with 1 M NaOH. While stirring 1 volume of plasma, we slowly added 3.5 volumes of 0.4% rivanol (Tokyo Chemical Industries) in water for 30 minutes. We left the solution overnight at RT, and removed a tenacious yellow precipitate. After filtering the supernatant through Whatman no. 1 paper to remove residual precipitates, we added 8 g of activated charcoal (Wako Chemicals) for 100 mL of the IgG solution and incubated overnight at 4°C to remove rivanol. We then slowly added an equal amount of saturated ammonium sulfate, and again incubated overnight at RT to precipitate crude IgG. We centrifuged the solution at 3,000 × g for 30 minutes, and added saline to the precipitate to form a slurry, which was then transferred to a dialysis tube (Spectra/Por MWCO 50,000, Spectrum Laboratories). We dialyzed the solution in saline at 4°C for 3 hours, followed by dialysis in PBS at 4°C for 2 hours and then overnight. We removed residual charcoals by filtering through a 0.22-μm Millex-GP filter (Millipore), and concentrated IgG using Amicon Ultra 50K (Millipore). We confirmed purity of isolated IgG by 6% SDS-PAGE under a nonreducing condition. We also reduced IgG in 4% 2-mercaptoethanol and fractionated the heavy and light chains by 10% SDS-PAGE.
Incubation of purified IgG to a muscle section of Colq−/− mice.
We prepared 10-μm-thick sections of quadriceps muscles of Colq−/− mice22 with a Leica CW3050–4 cryostat at −20°C. We blocked nonspecific binding of a muscle section with the blocking buffer that contained 5% sheep serum in PBS at RT for 2 hours. We suspended the purified IgG in the blocking buffer at 50 μg/mL, and overlaid it on a muscle section at 4°C overnight. We detected human IgG by FITC-labeled anti-human IgG antibody (02–10-06, KPL), and AChR by Alexa594-labeled α-bungarotoxin (Molecular Probes).
In vitro overlay assay.
The overlay binding method was essentially as previously described.23 We overlaid 600 μg IgG of patients at 4°C overnight before adding 120-milli-Ellman units of ColQ-tailed AChE.
In vitro plate-binding assay for quantifying ColQ-MuSK interaction.
We coated the Maxi-Sorp Immuno Plate (Nunc) with 0.15 μg of purified hMuSKect-myc at 4°C overnight and then incubated it with a blocking buffer that contained 50 mM Tris-HCl (pH 7.4), 0.5% BSA, 0.5% ovalbumin, and 0.5 M NaCl at RT for 1 hour. We incubated the wells with 1 pg to 100 μg of IgG of controls 1 and 2 and patients 1–4 at 4°C for 6 hours. We added 0.12-Ellman units of ColQ-tailed AChE as described above. We then quantified the bound ColQ-tailed AChE by the Ellman method in the presence of 5 × 10–5 M ethopropazine.19 Each time before we moved to the next step, we washed the plate 3 times with PBS.
In vitro plate-binding assay for quantifying LRP4-MuSK interaction.
We coated the Maxi-Sorp Immuno Plate with 0.15 μg of purified hMuSKect-myc as described above, and then blocked with 1% BSA in PBS at RT for 1 hour. We incubated the wells with 1 pg to 100 μg of IgG of control 2 and patient 2 at 4°C for 6 hours. We added 0.12 μg of purified hLRP4N-FLAG on each well at RT for 2 hours. We then quantified the bound hLRP4N-FALG by anti-FLAG-HRP using the TMB substrate kit (Pierce). Again, between each step, we washed the plates 3 times with PBS.
Passive transfer of human IgG to mice.
We made passive transfer model mice as described elsewhere.24 We intraperitoneally injected 40 mg IgG of control 2 and patient 2 into 6-week-old female C57BL/6J mice every day for 15 days. We sterilized IgG with a 0.22-μm filter (Millipore) and dissolved it in 400 μL PBS. The mice were killed on day 16 under deep anesthesia. To suppress any active immune response to the human protein,25 we injected 300 mg/kg of cyclophosphamide monohydrate (10 mg/mL in 0.9% NaCl) intraperitoneally 24 hours after the first IgG injection. We also injected IgG of patient 2 into 2 additional mice to confirm consistency, and analyzed a representative mouse in detail. We detected AChR by Alexa594-labeled α-bungarotoxin (Molecular Probes), ColQ by 1:100 of a newly raised anti-ColQ antibody (figure e-1 on the Neurology® Web site at www.neurology.org), and MuSK by 1:100 of anti-MuSK antibody (C-19, Santa Cruz). We quantified signals by the BZ-9000 microscope (Keyence) equipped with the Dynamic Cell Count software BZ-H1C (Keyence).
RESULTS
MuSK-IgG recognizes NMJ of a muscle section of Colq−/− mouse.
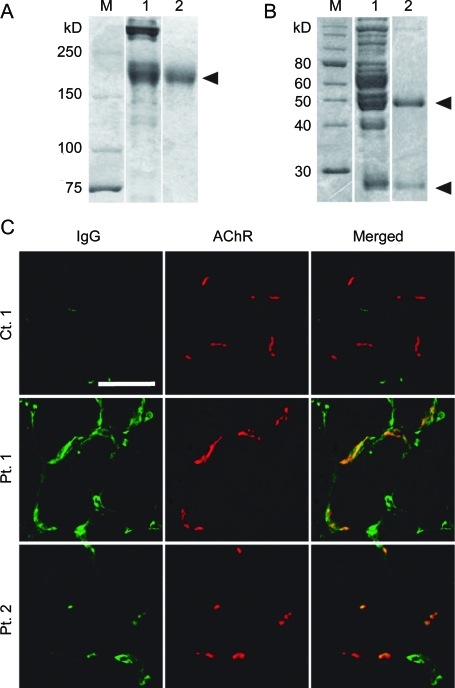
We first confirmed that human MuSK-IgG recognizes the mouse NMJ. We isolated IgG from serum of MuSK-MG patients and confirmed the purity of IgG by Coomassie staining of nonreducing (figure 1A) and reducing (figure 1B) SDS-PAGEs. We then overlaid MuSK-IgG on quadriceps muscle sections of Colq−/− mice.22 IgG of control 1 was not bound to the NMJ, whereas IgGs of patients 1 and 2 colocalized to the NMJs (figure 1C). Human MuSK-IgG thus has the potential to bind to the mouse NMJ.
Figure 1. Muscle-specific receptor tyrosine kinase (MuSK)–immunoglobulin G (IgG) recognizes the neuromuscular junction (NMJ) of Colq−/− mice.
Nonreducing (A) and reducing (B) sodium dodecyl sulfate–polyacrylamide gel electrophoresis of serum proteins and purified IgG of patient 1. Gels are stained with Coomassie brilliant blue. M = molecular weight markers; 1 = serum before purification; 2 = purified IgG. Arrowheads point to IgG of 160 kD (A), as well as the heavy (50 kD) and light (25 kD) chains of IgG (B). (C) In vitro overlay of purified IgG on a 10-μm skeletal muscle section of Colq−/− mice. IgG is visualized with FITC-labeled antihuman IgG and acetylcholine receptor with Alexa594-labeled α-bungarotoxin. Scale bar = 50 μm.
In vitro overlay assay discloses that MuSK-IgG blocks binding of ColQ-tailed AChE to the NMJ of a muscle section of Colq−/− mouse.
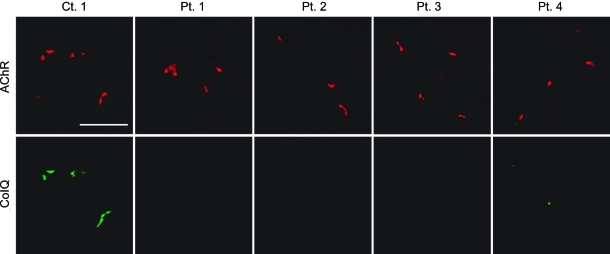
We previously demonstrated that the purified recombinant human ColQ-tailed AChE protein complex could bind to sections of the frog NMJs23 and the mouse NMJs (in preparation) in vitro. Using the in vitro overlay assay, we next examined whether MuSK-IgG blocks anchoring of ColQ-tailed AChE to the mouse NMJs. We incubated a muscle section of Colq−/− mice with MuSK-IgG overnight at 4°C and overlaid human ColQ-tailed AChE followed by histologic visualization of ColQ and AChR (figure 2). In the presence of IgG of control 1, ColQ was colocalized with AChRs, whereas, in the presence of 4 MuSK-IgGs, no ColQ signal was observed at the NMJs.
Figure 2. In vitro overlay assays.
Purified recombinant collagen Q (ColQ)-tailed acetylcholinesterase (AChE) was overlaid on a 10-μm quadriceps muscle section of Colq−/− mice in the presence of the indicated purified muscle-specific receptor tyrosine kinase–immunoglobulin G. ColQ is stained with anti-ColQ antibody and acetylcholine receptor (AChR) with Alexa594-labeled α-bungarotoxin. Scale bar = 50 μm.
In vitro plate-binding assay shows that MuSK-IgG blocks binding of ColQ-tailed AChE but not of LRP4 to MuSK.
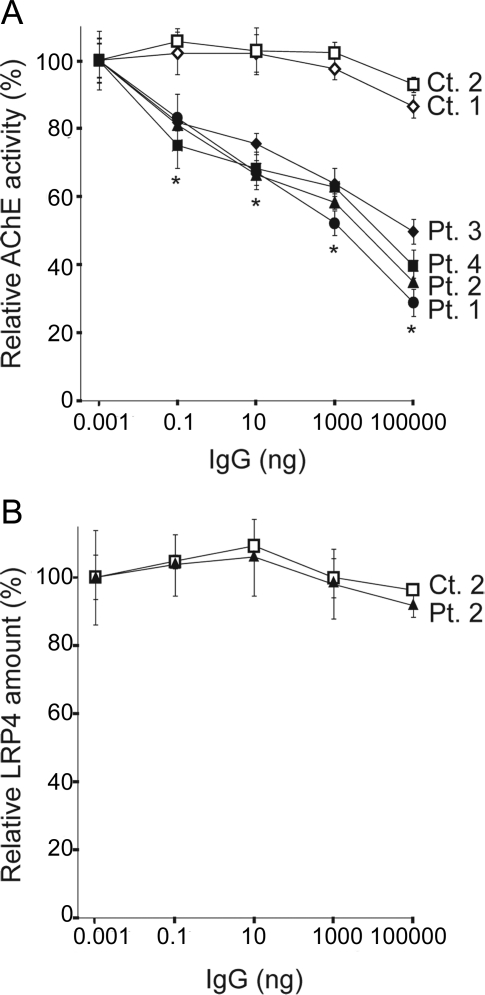
We next quantified an effect of MuSK-IgG on an interaction of human ColQ and human MuSK by an in vitro plate-binding assay. We synthesized and purified the myc-tagged extracellular domain of human MuSK (hMuSKect-myc). We then incubated an hMuSKect-coated plate with variable concentrations of control IgG or MuSK-IgG, and added a fixed amount of the purified recombinant human ColQ-tailed AChE. In 2 controls, AChE remained bound even in the presence of 100 μg of IgG, whereas in 4 MuSK-MG patients the numbers of bound AChE were proportionally decreased with increasing amounts of the patient's IgG (figure 3A).
Figure 3. In vitro plate-binding assays.
(A) Increasing amounts of muscle-specific receptor tyrosine kinase (MuSK)–immunoglobulin G (IgG) block binding of the purified recombinant collagen Q (ColQ)-tailed acetylcholinesterase (AChE) to the extracellular domain of human MuSK that is coated on a 96-well plate. Bound ColQ-tailed AChE is quantified by AChE activity. AChE activities are normalized for that at 1 pg IgG of each sample. Mean and SEM of 3 experiments are plotted. *p < 0.01 between controls and patients. (B) MuSK-IgG does not block binding of the purified FLAG-tagged extracellular domain of human LRP4 (LRP4N-FLAG) to MuSK that is coated on a 96-well plate. Bound LRP4N-FLAG is quantified with anti-FLAG-HRP. HRP activities are normalized for that at 1 pg IgG of each sample. Mean and SEM of 3 experiments are plotted.
We also examined the effect of MuSK-IgG on the interaction between the extracellular domain of MuSK and LRP4. We found that even at 100 μg IgG of control 2 or patient 2 did not block binding of LRP4 to MuSK (figure 3B).
Passive transfer model exhibits reduced ColQ signals at the NMJs.
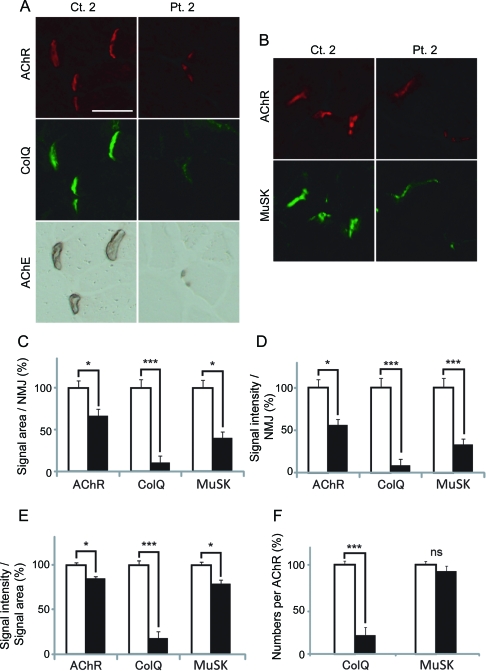
As described in the introduction, active and passive immunization of model animals reveals reduction of AChRs at the NMJs,24,26–29 but an effect of MuSK-IgG on ColQ-tailed AChE has not been examined to date. We thus injected IgG of control 2 and patient 2 for 14 days to C57BL/6J female mice and visualized the expression of AChR, ColQ, MuSK, and AChE in quadriceps muscle sections. Signal intensities of ColQ and AChE were markedly attenuated, but the AChR and MuSK signal intensities were only moderately reduced (figure 4,A and B). Quantitative analysis of the fluorescence signals revealed that signal areas (figure 4C), intensities (figure 4D), and densities (figure 4E) of ColQ in mice injected with patient 2 IgG were significantly reduced. Conversely, signal areas (figure 4C), intensities (figure 4D), and densities (figure 4E) of AChR were only moderately reduced. Similarly, the same parameters of the MuSK signal were moderately reduced (figure 4, C, D, and E). Moderate reductions of the areas and intensities of AChR and MuSK signals are likely due to reduced sizes of the NMJs, because the densities of AChR and MuSK were only marginally affected. In addition, whereas the number of MuSK per AChR remained essentially the same, the number of ColQ per AChR was prominently reduced (figure 4F). To summarize, MuSK-IgG compromised anchoring of ColQ-tailed AChE and had a less prominent effect on the expression of MuSK and AChR.
Figure 4. Passive transfer of muscle-specific receptor tyrosine kinase (MuSK)–immunoglobulin G (IgG) of control 2 and patient 2 to C57BL/6J mice.
(A, B) Quadriceps muscle sections of mice injected with IgG of control 2 or patient 2 are stained for acetylcholine receptor (AChR) by Alexa594-labeled α-bungarotoxin, collagen Q (ColQ) and MuSK by immunostaining, acetylcholinesterase (AChE) by cytochemical staining. Scale bar = 40 μm. Signal areas (C), intensities (D), and densities (intensity/area) (E) of the indicted molecules per neuromuscular junction (NMJ) are shown in mean and SEM. (F) Densities of ColQ and MuSK are normalized for the density of AChR to estimate the number of ColQ and MuSK per AChR. For AChR, ColQ, and MuSK, we analyzed 44 NMJs of control 2 and 23 NMJs of patient 2. For MuSK, we analyzed 82 NMJs of control 2 and 42 NMJs of patient 2. Areas and intensities are quantified by the BZ-9000 microscope (Keyence). Open and closed bars represent control 2 and patient 2, respectively. *p < 0.05, ***p < 0.001. NS = not significant.
DISCUSSION
Molecular basis of MuSK-MG has been examined in cultured cells30,31 as well as in active28,29 and passive24,26,27 immunization models. Application of MuSK-MG antibodies to TE671 muscle cells induces inhibition of cell proliferation and secondarily leads to downregulation of AChR and rapsyn.30 Similarly, MuSK-MG antibodies have no or minimal effect on the cell surface expression of AChR in TE671 and C2C12 muscle cells.31 Conversely, mice29 and rabbits28 immunized with recombinant MuSK develop myasthenic symptoms and NMJ AChR deficiency. Similarly, injection of MuSK-IgG into mice reduces the number of AChRs at the NMJ to 22% of controls, compromises the apposition of the presynaptic and postsynaptic components of the NMJ,24 and reduces muscle contractility.27 A recent report demonstrates that MuSK-IgG enhances internalization of MuSK from plasma membrane, which leads to progressive dispersal of postsynaptic AChRs by disruption of the MuSK scaffold and not by disruption of the agrin/LRP4/MuSK signaling pathway.26 To summarize, MuSK-IgG does not reduce AChR expression in cultured cells, but active and passive immunization of model animals results in AChR deficiency, which is not likely due to blocking of the agrin/LRP4/MuSK pathway. Our findings that MuSK-IgG blocks binding of ColQ but not of LRP4 to MuSK are consistent with these findings. In myotubes of Colq−/− mice, the number of membrane-bound MuSK is prominently reduced, and agrin-mediated phosphorylation of the AChR β subunit and the subsequent clustering of AChR are reduced to 30%–50% of the wild type.32 Thus, compromised clustering of AChRs at the NMJs in some MuSK-MG patients could result from blocking of ColQ binding to MuSK but not from blocking of LRP4 binding to MuSK.
Although our results predict endplate AChE deficiency in MuSK-MG patients, we found no AChE deficiency in intercostal muscles of one reported33 and two unreported cases of MuSK-MG. In vitro microelectrode studies showed a normal EPP decay time constant.34 In the 3 MuSK-MG patients observed by us, the MEPC decay times were shorter than normal, normal, and 2-fold prolonged33 compared to controls. Thus, our biopsy findings do not indicate that MuSK-MG patients have endplate AChE deficiency. There are 2 plausible explanations for the apparently contradicting observation on the human biopsies and the in vitro and in vivo studies. First, MuSK-IgG does not block binding of ColQ-tailed AChE to the NMJ to a detectable extent in the patients. ColQ is localized to the synaptic basal lamina via 2 mechanisms: one is by binding to heparin sulfate proteoglycans including perlecan,7,8 and the other is by binding to MuSK.9 We previously reported that both mechanisms are required for in vitro anchoring of human ColQ to the frog NMJ.23 Reduced clustering of ColQ in our passive transfer model suggests that ColQ needs to bind to at least MuSK in mice. However, binding of ColQ to MuSK is dispensable for clustering ColQ in humans, but is required for facilitating AChR clustering.32 Second, AChE could be deficient in severely affected muscles but not in the biopsied intercostal muscles. However, the respiratory functions of the patients who had intercostal muscle biopsies were severely compromised. Expression levels of MuSK35 and ColQ36 were reported to be different between slow- and fast-twitch muscles in model animals. In active37 and passive26 immunization models, slow-twitch diaphragm was more severely affected than fast-twitch tibialis anterior and intercostal muscles. Similar uneven distributions of affected muscles are reported in MuSK-MG patients.12 Further studies will be required to elucidate the basis of the discrepant observations between mice and humans.
Supplementary Material
ACKNOWLEDGMENT
The authors thank Kenji Otsuka for technical assistance and the patients who participated in this study.
GLOSSARY
- AChE
acetylcholinesterase
- AChR
acetylcholine receptor
- ColQ
collagen Q
- IgG
immunoglobulin G
- LRP4
low-density lipoprotein receptor-related protein 4
- MG
myasthenia gravis
- MuSK
muscle-specific receptor tyrosine kinase
- NMJ
neuromuscular junction
- SDS-PAGE
sodium dodecyl sulfate–polyacrylamide gel electrophoresis
Footnotes
Supplemental data at www.neurology.org
Editorial, page 1783
AUTHOR CONTRIBUTIONS
Dr. Kawakami designed and conducted experiments and wrote the paper. Dr. Ito designed and conducted experiments. Dr. Hirayama conducted experiments. Dr. Sahashi diagnosed a patient and conceived the study. Dr. Ohkawara designed experiments. Dr. Masuda designed experiments. Dr. Nishida diagnosed a patient and conceived the study. Dr. Mabuchi diagnosed a patient and conceived the study. Dr. Engel diagnosed a patient, conceived studies, designed experiments, and wrote the paper. Dr. Ohno conceived study, designed experiments, and wrote the paper.
DISCLOSURE
Dr. Kawakami, Dr. Ito, Dr. Hirayama, Dr. Sahashi, Dr. Ohkawara, Dr. Masuda, Dr. Nishida, and Dr. Mabuchi report no disclosures. Dr. Engel serves as an Associate Editor of Neurology®; receives publishing royalties for Myology 3rd ed. (McGraw-Hill, 2004); and has received research support from the NIH and the Muscular Dystrophy Association. Dr. Ohno has received Grants-in-Aids from the Japan Society for the Promotion of Science, the Ministry of Health, Labour and Welfare, and the Japan Science and Technology Agency.
REFERENCES
- 1. Kim N, Stiegler AL, Cameron TO, et al. Lrp4 is a receptor for Agrin and forms a complex with MuSK. Cell 2008;135:334–342 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Zhang B, Luo S, Wang Q, Suzuki T, Xiong WC, Mei L. LRP4 serves as a coreceptor of agrin. Neuron 2008;60:285–297 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Dechiara TM, Bowen DC, Valenzuela DM, et al. The receptor tyrosine kinase MuSK is required for neuromuscular junction formation in vivo. Cell 1996;85:501–512 [DOI] [PubMed] [Google Scholar]
- 4. Okada K, Inoue A, Okada M, et al. The muscle protein Dok-7 is essential for neuromuscular synaptogenesis. Science 2006;312:1802–1805 [DOI] [PubMed] [Google Scholar]
- 5. Wu H, Xiong WC, Mei L. To build a synapse: signaling pathways in neuromuscular junction assembly. Development 2010;137:1017–1033 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Krejci E, Thomine S, Boschetti N, Legay C, Sketelj J, Massoulié J. The mammalian gene of acetylcholinesterase-associated collagen. J Biol Chem 1997;272:22840–22847 [DOI] [PubMed] [Google Scholar]
- 7. Deprez P, Inestrosa NC, Krejci E. Two different heparin-binding domains in the triple-helical domain of ColQ, the collagen tail subunit of synaptic acetylcholinesterase. J Biol Chem 2003;278:23233–23242 [DOI] [PubMed] [Google Scholar]
- 8. Peng HB, Xie H, Rossi SG, Rotundo RL. Acetylcholinesterase clustering at the neuromuscular junction involves perlecan and dystroglycan. J Cell Biol 1999;145:911–921 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Cartaud A, Strochlic L, Guerra M, et al. MuSK is required for anchoring acetylcholinesterase at the neuromuscular junction. J Cell Biol 2004;165:505–515 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Farrugia ME, Vincent A. Autoimmune mediated neuromuscular junction defects. Curr Opin Neurol 2010;23:489–495 [DOI] [PubMed] [Google Scholar]
- 11. Farrugia ME, Robson MD, Clover L, et al. MRI and clinical studies of facial and bulbar muscle involvement in MuSK antibody-associated myasthenia gravis. Brain 2006;129:1481–1492 [DOI] [PubMed] [Google Scholar]
- 12. Evoli A, Tonali PA, Padua L, et al. Clinical correlates with anti-MuSK antibodies in generalized seronegative myasthenia gravis. Brain 2003;126:2304–2311 [DOI] [PubMed] [Google Scholar]
- 13. Sanders DB, El-Salem K, Massey JM, McConville J, Vincent A. Clinical aspects of MuSK antibody positive seronegative MG. Neurology 2003;60:1978–1980 [DOI] [PubMed] [Google Scholar]
- 14. Hatanaka Y, Hemmi S, Morgan MB, et al. Nonresponsiveness to anticholinesterase agents in patients with MuSK-antibody-positive MG. Neurology 2005;65:1508–1509 [DOI] [PubMed] [Google Scholar]
- 15. Pasnoor M, Wolfe GI, Nations S, et al. Clinical findings in MuSK-antibody positive myasthenia gravis: a U.S. experience. Muscle Nerve 2010;41:370–374 [DOI] [PubMed] [Google Scholar]
- 16. McConville J, Farrugia ME, Beeson D, et al. Detection and characterization of MuSK antibodies in seronegative myasthenia gravis. Ann Neurol 2004;55:580–584 [DOI] [PubMed] [Google Scholar]
- 17. Shiraishi H, Motomura M, Yoshimura T, et al. Acetylcholine receptors loss and postsynaptic damage in MuSK antibody-positive myasthenia gravis. Ann Neurol 2005;57:289–293 [DOI] [PubMed] [Google Scholar]
- 18. Niks EH, van Leeuwen Y, Leite MI, et al. Clinical fluctuations in MuSK myasthenia gravis are related to antigen-specific IgG4 instead of IgG1. J Neuroimmunol 2008;195:151–156 [DOI] [PubMed] [Google Scholar]
- 19. Ohno K, Brengman J, Tsujino A, Engel AG. Human endplate acetylcholinesterase deficiency caused by mutations in the collagen-like tail subunit (ColQ) of the asymmetric enzyme. Proc Natl Acad Sci USA 1998;95:9654–9659 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Okada T, Shimazaki K, Nomoto T, et al. Adeno-associated viral vector-mediated gene therapy of ischemia-induced neuronal death. Methods Enzymol 2002;346:378–393 [DOI] [PubMed] [Google Scholar]
- 21. Horejsi J, Smetana R. The isolation of gamma globulin from blood-serum by rivanol. Acta Med Scand 1956;155:65–70 [DOI] [PubMed] [Google Scholar]
- 22. Feng G, Krejci E, Molgo J, Cunningham JM, Massoulie J, Sanes JR. Genetic analysis of collagen Q: roles in acetylcholinesterase and butyrylcholinesterase assembly and in synaptic structure and function. J Cell Biol 1999;144:1349–1360 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Kimbell LM, Ohno K, Engel AG, Rotundo RL. C-terminal and heparin-binding domains of collagenic tail subunit are both essential for anchoring acetylcholinesterase at the synapse. J Biol Chem 2004;279:10997–11005 [DOI] [PubMed] [Google Scholar]
- 24. Cole RN, Reddel SW, Gervasio OL, Phillips WD. Anti-MuSK patient antibodies disrupt the mouse neuromuscular junction. Ann Neurol 2008;63:782–789 [DOI] [PubMed] [Google Scholar]
- 25. Toyka KV, Drachman DB, Griffin DE, et al. Myasthenia gravis: study of humoral immune mechanisms by passive transfer to mice. N Engl J Med 1977;296:125–131 [DOI] [PubMed] [Google Scholar]
- 26. Cole RN, Ghazanfari N, Ngo ST, Gervasio OL, Reddel SW, Phillips WD. Patient autoantibodies deplete postsynaptic muscle-specific kinase leading to disassembly of the ACh receptor scaffold and myasthenia gravis in mice. J Physiol 2010;588:3217–3229 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. ter Beek WP, Martinez-Martinez P, Losen M, et al. The effect of plasma from muscle-specific tyrosine kinase myasthenia patients on regenerating endplates. Am J Pathol 2009;175:1536–1544 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Shigemoto K, Kubo S, Maruyama N, et al. Induction of myasthenia by immunization against muscle-specific kinase. J Clin Invest 2006;116:1016–1024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Jha S, Xu K, Maruta T, et al. Myasthenia gravis induced in mice by immunization with the recombinant extracellular domain of rat muscle-specific kinase (MuSK). J Neuroimmunol 2006;175:107–117 [DOI] [PubMed] [Google Scholar]
- 30. Boneva N, Frenkian-Cuvelier M, Bidault J, Brenner T, Berrih-Aknin S. Major pathogenic effects of anti-MuSK antibodies in myasthenia gravis. J Neuroimmunol 2006;177:119–131 [DOI] [PubMed] [Google Scholar]
- 31. Farrugia ME, Bonifati DM, Clover L, Cossins J, Beeson D, Vincent A. Effect of sera from AChR-antibody negative myasthenia gravis patients on AChR and MuSK in cell cultures. J Neuroimmunol 2007;185:136–144 [DOI] [PubMed] [Google Scholar]
- 32. Sigoillot SM, Bourgeois F, Lambergeon M, Strochlic L, Legay C. ColQ controls postsynaptic differentiation at the neuromuscular junction. J Neurosci 2010;30:13–23 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Selcen D, Fukuda T, Shen X-M, Engel AG. Are MuSK antibodies the primary cause of myasthenic symptoms? Neurology 2004;62:1945–1950 [DOI] [PubMed] [Google Scholar]
- 34. Niks EH, Kuks JB, Wokke JH, et al. Pre- and postsynaptic neuromuscular junction abnormalities in musk myasthenia. Muscle Nerve 2010;42:283. [DOI] [PubMed] [Google Scholar]
- 35. Punga AR, Maj M, Lin S, Meinen S, Ruegg MA. MuSK levels differ between adult skeletal muscles and influence postsynaptic plasticity. Eur J Neurosci 2011;33:890–898 [DOI] [PubMed] [Google Scholar]
- 36. Krejci E, Legay C, Thomine S, Sketelj J, Massoulie J. Differences in expression of acetylcholinesterase and collagen Q control the distribution and oligomerization of the collagen-tailed forms in fast and slow muscles. J Neurosci 1999;19:10672–10679 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Xu K, Jha S, Hoch W, Dryer SE. Delayed synapsing muscles are more severely affected in an experimental model of MuSK-induced myasthenia gravis. Neuroscience 2006;143:655–659 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.