Abstract
In plants, sensitive and selective mechanisms have evolved to perceive and respond to light and gravity. We investigated the effects of microgravity on the growth and development of Arabidopsis thaliana (ecotype Landsberg) in a spaceflight experiment. These studies were performed with the Biological Research in Canisters (BRIC) hardware system in the middeck region of the space shuttle during mission STS-131 in April 2010. Seedlings were grown on nutrient agar in Petri dishes in BRIC hardware under dark conditions and then fixed in flight with paraformaldehyde, glutaraldehyde, or RNAlater. Although the long-term objective was to study the role of the actin cytoskeleton in gravity perception, in this article we focus on the analysis of morphology of seedlings that developed in microgravity. While previous spaceflight studies noted deleterious morphological effects due to the accumulation of ethylene gas, no such effects were observed in seedlings grown with the BRIC system. Seed germination was 89% in the spaceflight experiment and 91% in the ground control, and seedlings grew equally well in both conditions. However, roots of space-grown seedlings exhibited a significant difference (compared to the ground controls) in overall growth patterns in that they skewed to one direction. In addition, a greater number of adventitious roots formed from the axis of the hypocotyls in the flight-grown plants. Our hypothesis is that an endogenous response in plants causes the roots to skew and that this default growth response is largely masked by the normal 1 g conditions on Earth. Key Words: Gravity—Multicellular life—Spacecraft experiments—Spaceflight. Astrobiology 11, 787–797.
1. Introduction
Plants have been an important part of space biology investigations over the past few decades for two main reasons. First, plants likely will be a key component of bioregenerative life-support systems as generators of oxygen and as a food source for astronauts on long-term space missions (Ferl et al., 2002; Ferl and Paul, 2010). Second, the spaceflight environment can serve as a unique laboratory for the study of the fundamental processes underlying plant growth and development (Wolverton and Kiss, 2009; Millar et al., 2010). In the present study, we used the microgravity environment on the space shuttle in low Earth orbit to study the development of seedlings of the model plant Arabidopsis thaliana.
Gravity has been a ubiquitous and unidirectional signal throughout the evolution of life on Earth and has provided a directional cue by which plants organize their body plans (Palmieri and Kiss, 2006). Throughout their entire life cycle, plants use gravity to orient and coordinate their growth in order to maximize access to light, water, and nutrients. In germinating seedlings, gravity is important for orienting the plant so that shoots grow upward and roots grow downward.
Plants sense and respond to gravity through the process of gravitropism, the directed growth in response to this stimulus (Blancaflor and Masson, 2003). Gravitropism can be divided into three temporal phases: perception, transduction, and response. Gravity perception or sensing occurs in specialized cells (i.e., statocytes) in the roots and shoots of all flowering plants (Kiss, 2000). The putative gravity sensors are amyloplasts: dense, starch-filled organelles that are located exclusively in statocytes and move within the cell in response to gravity (Saito et al., 2005).
During the second phase of gravitropism, signal transduction, the dissipation of the potential energy of statolithic amyloplasts results in the production of chemical signals that ultimately trigger a growth response (Morita, 2010). Many subcellular structures have been implicated in gravity signal transduction, including the vacuole, endoplasmic reticulum, and the cytoskeleton (Blancaflor and Masson, 2003). The final (i.e., response) phase of gravitropism is characterized by directed growth in response to gravity (Perrin et al., 2005). This growth response is elicited by auxin concentration gradients that form across reoriented organs such that more of this hormone is present in the lower portion, as compared to the upper portion of the organs.
Several studies have used the microgravity environment aboard orbiting spacecraft to identify downstream elements in signal transduction (reviewed in Correll and Kiss, 2008). An experiment with lentil roots was performed to identify the role of the actin cytoskeleton in amyloplast movement in microgravity (Driss-Ecole et al., 2000). Other ground-based studies with drugs that disrupt the actin cytoskeleton have also demonstrated that the cytoskeleton is involved in gravity responses, although results from these studies are often conflicting and depend on the organ, plant species, drug dosage, and experimental conditions (Palmieri and Kiss, 2005).
We performed spaceflight experiments to study the effects of microgravity on the structure and organization of the actin cytoskeleton in plants. These studies were performed on space shuttle Discovery during mission STS-131 in April 2010 by utilizing the Biological Research in Canisters (BRIC) hardware system, which was placed in the middeck region of the orbiter (Kern et al., 1999). The specific objectives were to investigate the role of the cytoskeleton in statocytes in microgravity by cytological methods and to study effects of microgravity on actin cytoskeleton–related gene expression by gene profiling. However, in this paper, we will first focus on (1) the implementation of the BRIC system to study the development of Arabidopsis seedlings during spaceflight and (2) the analysis of the overall morphology of the root system of seedlings that developed in microgravity. Based on our data, we propose that an endogenous response in seedlings causes the roots to skew toward one direction and that this default growth response is largely masked by the normal 1 g conditions on Earth.
2. Materials and Methods
2.1. Spaceflight hardware
Seedlings of Arabidopsis thaliana (ecotype Landsberg) were grown on nutrient agar in Petri dishes in the BRIC hardware system (Fig. 1). This hardware was developed at NASA's Kennedy Space Center, and many of the detailed specifications have been described in previous technical publications (Kern et al., 1999; Wells et al., 2001). In each Petri Dish Fixation Unit (PDFU) of the BRIC, specimens first were grown in one chamber with a single Petri dish followed by an in-flight fixation with fluids from another chamber (Fig. 1A). All components of the PDFU were autoclaved to ensure axenic conditions of the samples.
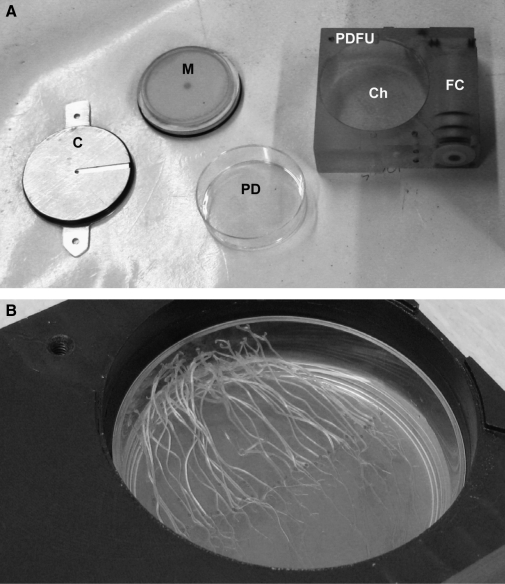
FIG. 1.
Petri Dish Fixation Unit (PDFU) overview. (A) An individual PDFU as used during the BRIC-16 project on space shuttle mission STS-131. The lower half of a standard polystyrene Petri dish (PD), with dimensions of 60×15 mm, is placed into a PDFU, which has a chamber (Ch) for the dish and a fluid chamber (FC) filled with fixative. The Petri dish is directly covered with a manifold (M), and an additional cover (C) is attached to the PDFU polycarbonate body via a set of screws. (B) Arabidopsis seedlings grown on nutrient agar in a PDFU in darkness in a ground control. The covers have been removed to show that seedlings exhibited vigorous growth.
Standard laboratory Petri dishes (60×15 mm) were placed into a PDFU with a polycarbonate body. Only the lower half of the Petri dish was used; the top cover was discarded and replaced by a PDFU manifold (with a mesh), on top of which was placed a PDFU cover (Fig. 1A). The PDFU fluid chamber was filled with one of three fixatives as described below. The fixation and specimen chambers were separated by a check valve to prevent early fixation of the biological material, and each sealed PDFU provided two levels of containment as defined by NASA standards. Figure 1B illustrates a ground control in which seedlings are growing in a Petri dish with the PDFU manifold and cover removed.
In general, six PDFUs can be placed into one BRIC unit (Fig. 2A), which is an anodized aluminum container (total mass=1.8 kg) with one level of containment (Wells et al., 2001). However, in the BRIC-16 project (April 2010), there were five PDFUs per BRIC, and the sixth slot had a battery-operated temperature recorder termed HOBO (Kern et al., 1999). Following the flight and ground controls, temperature data were downloaded from the HOBO data recorder.
FIG. 2.
BRIC hardware overview. (A) A BRIC unit shown with the cover (C) on and off. The BRIC is an anodized aluminum container and houses six PDFUs. (B) The actuator tool (AT) is used by the astronauts in flight to release fixatives from the fluid chamber to the Petri dish chamber. (C) During the BRIC-16 project, eight BRIC units (labeled A–H) were placed into foam inserts that fit into a standard half-middeck locker on the space shuttle. Scale: length of a BRIC unit is 17.3 cm.
An actuator tool (Fig. 2B) was used by the astronauts to terminate the experiment in flight by fluid fixation. While it was possible to inject two types of fluids, in BRIC-16, we used the entire fluid chamber (volume=13 mL) for a single fixation. Eight BRIC units were placed into a tray (Fig. 2C), which fit into the volume of one-half of a standard middeck locker of the space shuttle.
2.2. Characteristics of the spaceflight experiment
The BRIC-16 experiment was performed on space shuttle mission STS-131 on the orbiter Discovery, which was launched on 5 April 2010 and landed at Kennedy Space Center on 20 April 2010 (total mission elapsed time=15 days, 2 h, 47 min). The BRIC payload in a similar configuration with the PDFUs had flown on missions STS-87 in November–December 1997 (Kern and Sack, 1999) and STS-107 in January–February 2003 (Kern et al., 2005). Although Discovery was docked to the International Space Station during the STS-131 mission, the BRIC-16 experiment was performed in the middeck area of the space shuttle.
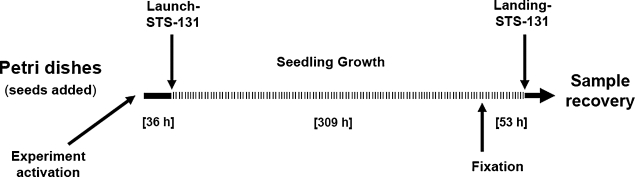
The time line of the spaceflight experiment as performed is shown in Fig. 3. The experiment was done in darkness. Petri dishes with seeds on a nutrient agar were prepared at the Space Life Sciences (SLS) laboratory at Kennedy Space Center (FL, USA) approximately 36 h before the launch of STS-131, and the BRIC units were integrated into Discovery 23 h before the launch. During the pre-launch period (including the time in the orbiter on the launch pad), the BRIC units were held so that the surface of the agar (with seeds) was maintained in a vertical orientation at 22°C. Seedlings developed for approximately 309 h in microgravity, at which time the experiment was terminated by fixation in one of three fluid solutions: paraformaldehyde, glutaraldehyde, and RNAlater. Samples remained in the fluids for 53 h until landing (which was delayed for 24 h due to poor weather conditions at the Kennedy Space Center). Samples remained in fixatives for an additional 9.5 h following the landing of Discovery.
FIG. 3.
The time line of the BRIC-16 experiments as performed on mission STS-131. Seeds were sown onto a nutrient agar in Petri dishes on the ground (experiment activation), and dishes were placed into PDFUs, which then were placed into the BRIC units. Seeds imbibed for 36 h on the ground prior to launch of Discovery; during this time the BRIC units were held so that the agar surfaces (with seeds) were maintained in a vertical orientation. Seedlings developed for 309 h in microgravity and then were fixed in flight in an aldehyde or RNAlater. The fixed specimens remained in microgravity for 53 h prior to recovery on the ground after the landing of Discovery at the Kennedy Space Center. Solid lines=1 g, and the dashed line=microgravity. For the ground control (which was delayed by 24 h relative to flight), the BRIC units were held so that the agar surfaces were maintained in a vertical orientation.
The primary ground control was done in identical hardware with the same time line as the flight experiment in an incubator at 22°C with controlled humidity at the SLS laboratory at Kennedy Space Center. There was a 24 h delay relative to the spaceflight experiment due to the difficulty (i.e., a resource limitation) of building the flight and ground hardware with seeds simultaneously. The BRIC units in the ground control were placed so that the surface of the agar was maintained in a vertical orientation throughout the time course of the experiment. An additional ground control was performed in which seedlings were grown in 60×15 mm Petri dishes with an open atmosphere in darkness in standard laboratory conditions rather than in the sealed PDFU.
2.3. Preflight preparation
At the SLS laboratory, seeds of Arabidopsis thaliana (ecotype Landsberg) were surface sterilized in 70% (v/v) ethanol (with 1 drop Triton X-100 per 100 mL) for 5 min, followed by two 1 min rinses in 95% (v/v) ethanol. An additional wash with 1 drop Triton X-100 in 100 mL nanopure water was followed by four final rinses in water. Prepared seeds were stored in water at 4°C for up to 2 days prior to sowing on agar. Under sterile conditions within a laminar flow hood, 40 seeds in one row were sown into 60×15 mm Petri dishes with 1.2% (w/v) agar, containing one-half–strength Murashige and Skoog salts with 1% (w/v) sucrose and 1 mM MES at pH 5.5. During the sowing process, the quality of each seed was assessed by using a stereomicroscope, and damaged seeds were discarded. Seeds were not oriented (with respect to the micropyle) but were randomly placed on the surface of the nutrient agar. Petri dishes then were wrapped in Parafilm and placed in a sterile box in darkness at 4°C before integration into the BRIC-PDFU flight hardware (Fig. 1). An identical set of Petri dishes with seeds was prepared for the ground control. Both flight and ground experiments were performed in darkness.
Our group had 13 PDFUs flown in microgravity (Table 1). In terms of fixation fluids (13 mL per PDFU), the following three solutions were used: (1) 4% (v/v) glutaraldehyde in 100 mM Sörensen's phosphate buffer at pH 7.2; (2) 3% (v/v) paraformaldehyde with 300 μM MBS (100 mL) in PME buffer (100 mM Pipes, 8 mM MgSO4, 20 mM EGTA) at pH 6.9; (3) RNAlater (Ambion, Austin, TX, USA). Seedlings in these fixatives were prepared for analysis by using (1) transmission electron microscopy, (2) confocal microscopy, and (3) gene profiling, respectively.
Table 1.
Seed Germination and Seedling Length in the Ground Control and the Spaceflight Experiment on STS-131
| PDFU | Ground Seed germination | Flight (%) |
|---|---|---|
| 1 | 95.0 | 77.5 |
| 2 | 72.5 | 90.0 |
| 3 | 80.0 | 95.0 |
| 4 | 92.5 | 90.0 |
| 5 | 100.0 | 87.5 |
| 6 | 85.0 | 82.5 |
| 7 | 85.0 | 97.5 |
| 8 | 100.0 | 77.5 |
| 9 | 85.0 | 82.5 |
| 10 | 95.0 | 95.0 |
| 11 | 97.5 | 95.0 |
| 12 | 87.5 | 95.0 |
| 13 | 100.0 | 92.5 |
| Mean | 90.9 | 89.0 |
| N | 520 | 520 |
| Seedling length (mm±SE) | ||
|---|---|---|
| Mean | 47.4±1.5 | 47.2±1.9 |
| N | 29 | 31 |
Germination in both the 13 individual PDFUs and the mean are reported, as well as the mean length of seedlings. Forty seeds were placed in each Petri dish per PDFU. A small subset of the seedlings was randomly selected and removed from Petri dishes to make detailed length measurements. N=sample size; SE=standard error.
2.4. Postflight recovery of samples and processing
Following in-flight fixation, samples remained in the fixatives for 53 h (in microgravity) plus an additional 9.5 h on the ground. The BRIC PDFUs were opened in the SLS laboratory by NASA staff; then the specimens in Petri dishes were returned to the investigators. Samples were photographed immediately in the Petri dishes retrieved from the PDFUs (and then placed into fresh fixatives). From these images, we gathered information on seed germination, growth, and seedling morphology. A small subset of the specimens were removed from the original Petri dishes for detailed length measurements with Image Pro Plus (Media Cybernetics, Bethesda, MD, USA). Each parameter obtained from flight and ground samples was compared with a Student t test by using Sigma Plot 11.0. If the data were not normally distributed, a Mann-Whitney rank sum test was performed.
Following photography, samples were transported to our home laboratory for further processing. Results from the cytological and gene profiling studies will be reported in future publications; the methods used were similar to those utilized in our previously published papers (Guisinger and Kiss, 1999; Yamamoto and Kiss, 2002; Stimpson et al., 2009).
3. Results
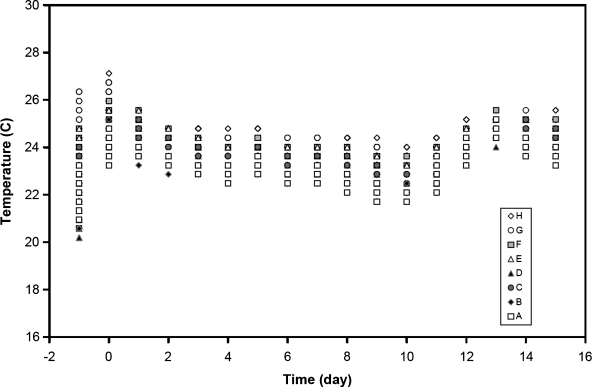
The temperature of the eight individual BRIC units (Fig. 4) was monitored throughout the course of the spaceflight experiment on STS-131 (Fig. 3). While there was some variation among the eight units, the temperature was within 22–25°C during the growth phase of the seedlings, and these temperature ranges were favorable for the growth of seedlings of Arabidopsis thaliana. The temperature range was somewhat greater (i.e., 20–27°C) prior to launch of the space shuttle when seeds were imbibing, but this broader range did not have a deleterious effect on seed germination (Table 1).
FIG. 4.
The temperature profile of individual BRIC units in microgravity as determined with a HOBO temperature recorder during the flight of STS-131. The individual BRIC units were labeled A–H.
Seed germination was 90.9% for ground and 89.0% for flight samples (Fig. 5, Table 1). The mean seedling length was not significantly different (P>0.05) when comparing ground and flight conditions (Table 1). Seedlings in both flight and ground controls exhibited an etiolated appearance (Fig. 5) that is typical for plants grown in darkness (i.e., elongated hypocotyls).
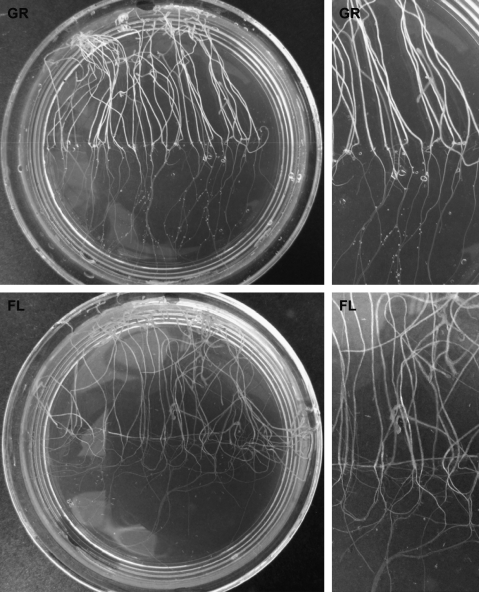
FIG. 5.
Photographs of fixed seedlings following the ground (GR) control and flight (FL) experiment in the BRIC PDFUs. In both types of samples, the seedlings exhibited vigorous growth. In the FL samples, the roots showed an extreme skew to the left of the Petri dish (when viewed through the lid of the Petri dish). While there was a slight skew to the left in the GR control, the magnitude of skewing to the left was much greater in roots of FL seedlings. In the GR control, the gravity vector is toward the bottom of the photographs. Scale: the diameter of the Petri dish is 60 mm.
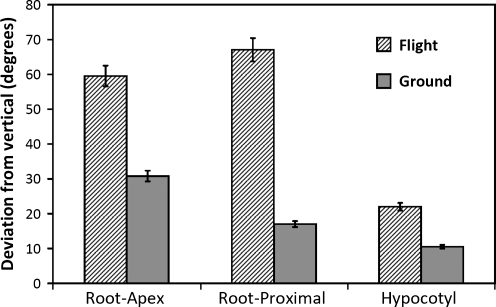
Several major morphological differences in seedlings were observed in flight seedlings compared to those in the ground controls (Fig. 5). First, there was a significant difference in the overall growth patterns of roots. In the flight samples, the roots showed an extreme skew to one direction of the Petri dish. This skew can be considered to the left when the seedlings are viewed through the lid of the Petri dish (Rutherford and Masson, 1996). While there was a slight skew to the left in the ground control, the magnitude of the left skewing of the roots from flight seedlings was much greater (Fig. 5). The skew was quantified (Fig. 6) by considering the angle of the root apex and the angle in the proximal root (5 mm from the root-hypocotyl junction). Both types of root angles were significantly greater (P<0.05) in the seedlings from the flight samples compared to the ground controls. In addition, while the overall magnitude of skewing was less in hypocotyls compared to roots, there still was a greater skew in hypocotyls of space-grown seedlings compared to those of the ground control samples (Fig. 6).
FIG. 6.
Quantification of root and hypocotyl skewing (to the left) in flight and ground seedlings. The skew was measured by mean angles of the root apex, proximal root (5 mm from the root-hypocotyl junction), and the proximal hypocotyl region. Angles were significantly greater (P<0.05) in the seedlings from the flight samples compared to the ground controls. Bars=standard error. For flight samples, N was 56 to 74. For ground samples, N was 62 to 105.
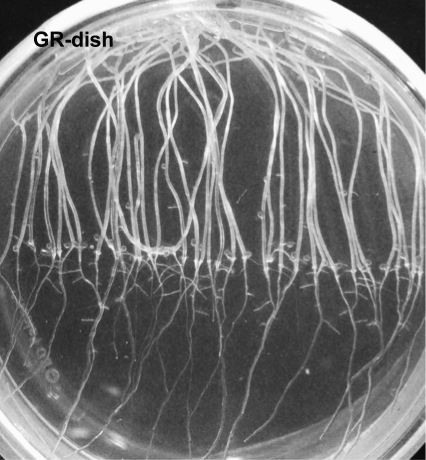
An additional control was performed to determine the effect of growing seedlings in the closed BRIC PDFU (Fig. 7). In this control experiment, seedlings were grown in Petri dishes in darkness in standard laboratory conditions with an open atmosphere (i.e., not in the sealed PDFU). In this control (Fig. 7), seed germination, seedling length, and root morphology were similar to these parameters in the ground control in which seedlings were in a BRIC PDFU (Fig. 5).
FIG. 7.
Photographs of living seedlings in an additional ground control (GR-dish), which were grown in Petri dishes in darkness in standard laboratory conditions with an open atmosphere. The seedlings are comparable to the samples in the PDFU ground control (Fig. 5) in that they exhibited similar growth and the roots showed a slight skew to the left of the Petri dish. The diameter of the Petri dish is 60 mm, and the gravity vector is toward the bottom of the image. (Note that this image is of living seedlings, whereas seedlings shown in Fig. 5 have been fixed.)
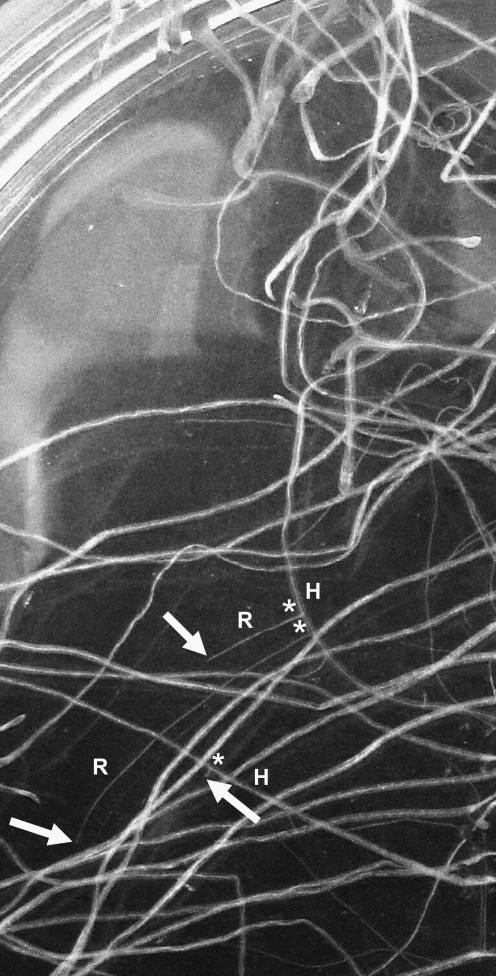
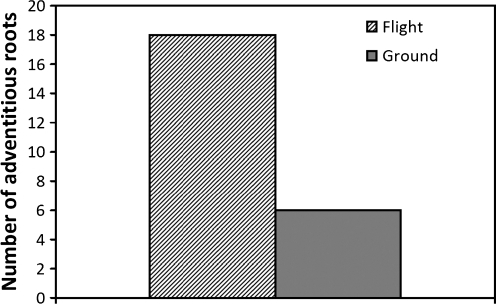
Another major morphological difference in flight versus ground samples was the system of adventitious roots that developed from the axis of the hypocotyl. Adventitious roots form from shoot tissues, not from another parental root as do lateral roots (Sorin et al., 2005). Flight seedlings that developed in microgravity had adventitious roots derived from the hypocotyl (Fig. 8). While six adventitious roots were detected in the ground 1 g control samples, we observed 18 adventitious roots in the spaceflight-grown seedlings in microgravity (Fig. 9). Due to some displacement of the hypocotyls of seedlings following the retrieval from the BRIC hardware, there may have been an undercount of the total number of adventitious roots from both flight and ground control specimens. However, this potential undercount was approximately equal for ground and flight samples, and there clearly were a greater number of adventitious roots in the spaceflight-grown seedlings.
FIG. 8.
Photographs of adventitious roots (R) from seedlings fixed during the flight experiment. These adventitious roots developed from the hypocotyl (H). Asterisks indicate the origin of the root from the hypocotyl axis, and the arrows point to the root tip.
FIG. 9.
The total number of adventitious roots observed in all flight and ground seedlings from fixed samples.
4. Discussion
4.1. Seedlings of Arabidopsis thaliana exhibited vigorous growth in the BRIC spaceflight hardware
Seedlings of Arabidopsis thaliana grown on a nutrient agar in the BRIC hardware in darkness exhibited good seed germination as well as vigorous growth during our spaceflight experiments in the shuttle middeck on mission STS-131 (Fig. 5). The BRIC payload (Figs. 1 and 2) in a similar configuration had been used on missions STS-87 and STS-107 (Kern and Sack, 1999; Kern et al., 2005). However, in these experiments, light was provided from a red LED, and cultures of the moss Ceratodon purpureus were grown in the BRIC spaceflight hardware (Kern et al., 1999). Because of these key differences, prior to the BRIC-16 project, it was not unequivocally known whether the BRIC-PDFU system could be successfully used for growth and development studies of the model plant Arabidopsis thaliana in spaceflight experiments, particularly under dark-grown conditions.
Due to mission constraints, power was not available to the BRIC tray in the middeck locker on the space shuttle. Thus, it was important to verify that our seeds could germinate in complete darkness. Previous studies had shown that there are differences in light requirements for seed germination among ecotypes of Arabidopsis (Penfield and King, 2009), and variations in growth rates among Arabidopsis ecotypes in microgravity conditions also were noted (Kiss et al., 2000). Preflight testing in our laboratory and during the Payload Verification Test at the SLS laboratory at Kennedy Space Center demonstrated that the ecotype Landsberg readily germinated on nutrient agar in complete darkness. However, in the BRIC-16 experiment as configured during STS-131, it was important not to use other common Arabidopsis ecotypes such as Columbia, which have a light requirement for optimal germination (Penfield and King, 2009).
In Arabidopsis thaliana, seed germination of greater than 80% is considered very good (Botto et al., 1996). In our BRIC-16 experiment, overall seed germination for the ground control was 90.9%; and for the spaceflight experiment, germination was 89.0%. Thus, we consider these germination values (N=520 each for ground and flight experiments) to be excellent. Seedling length, which was not significantly different (P>0.05) in flight and ground samples (Table 1), indicated vigorous growth in the BRIC spaceflight hardware.
It is likely that the radicle emerged from most of the seeds in a 36 h period prior to launch of the spacecraft in the spaceflight experiment (Fig. 3). While it is important to note that seeds from both ground and flight samples received 1 g for 36 h prior to launch, in the space specimens, the majority of the development time (309 h) was in microgravity.
An additional control was performed to determine the effect of growing seedlings in the closed BRIC PDFU. In this control experiment, seedlings were grown in Petri dishes in darkness in standard laboratory conditions (i.e., not in the sealed PDFU). In this control (Fig. 7), seed germination, seedling length, and root morphology were similar to these parameters in the ground control in which seedlings were in a BRIC PDFU (Fig. 5).
Another potential concern of growing Arabidopsis seedlings in the BRIC PDFUs was the biological effect of accumulation of cabin gases. In particular, ethylene gas has been shown to alter the structure of plants grown in spaceflight hardware (Guisinger and Kiss, 1999). Ethylene induces the characteristic triple response in seedlings, which includes thickening and shortening of hypocotyls with an exaggerated apical hook, which is very distinctive in dark-grown seedlings of Arabidopsis (Kiss et al., 1999). In the present study, we did not observe any of the morphological features associated with elevated ethylene in the ground control or in the spaceflight-grown seedlings. In addition, there were no significant morphological differences between the seedlings in the ground control in the (sealed) PDFU and the control in which seedlings were grown in standard Petri dishes. Thus, we are convinced there were no ethylene effects since Arabidopsis with its classic triple response provides a particularly sensitive bioassay for even low amounts of ethylene (Kiss et al., 1999).
4.2. Exaggerated skewing of roots and increased adventitious root formation occurred in microgravity
Roots of Arabidopsis seedlings grown on agar surfaces can show two distinct growth patterns, which include skewing (also termed slanting) to one direction of the Petri plate and waving or undulate growth (Oliva and Dunand, 2007). Typically, the Petri plate needs to be tilted from the vertical for the expression of the skewing phenomenon in roots (Rutherford and Masson, 1996; Mullen et al., 1998). Some variation in the skewing growth pattern occurs among ecotypes of Arabidopsis. For instance, roots of the Landsberg and Wassilewskija strains show a much more distinct skewing compared to the roots of the Columbia strain (Rutherford and Masson, 1996). The age of the seedling and the concentration of agar on the Petri plate may also alter the skewing pattern (Migliaccio and Piconese, 2001).
What causes this distinct skewing growth in Arabidopsis roots? One hypothesis is that skewing results from an interaction between circumnutation, an endogenous pattern of oscillatory growth in plant organs (Kiss, 2009), and gravitropism, directed growth in response to gravity (Simmons et al., 1995; Mullen et al., 1998). Others also have suggested that thigmotropism, directed growth in response to tactile stimuli, plays a role in the skewing growth of roots (Mullen et al., 1998; Olivia and Dunand, 2007). According to this latter hypothesis, when the root tip runs into the agar surface, it experiences a touch stress that causes a change of direction that ultimately leads to one-sided slanting growth or skewing of the roots.
In our spaceflight studies on STS-131, we found that roots of the seedlings grown in microgravity had an increased slant or skew to the left compared to the ground control (Fig. 5). While some degree of skew was noted in the control, the skew was greatly exaggerated in the flight-grown plants (Fig. 6). Interestingly, the roots did not exhibit random growth in the spaceflight experiment as has been reported by others (Johnsson et al., 1996; Mortley et al., 2008). Thus, our data suggest that there is an endogenous response in seedlings that causes the roots to skew toward one direction and that this endogenous, default growth response is largely masked by the normal 1 g conditions on Earth.
Previous investigators had shown that protonemata of the moss Ceratodon purpureus exhibited nonrandom spiral growth in microgravity, which is similar to our observation that an endogenous response is masked by gravity (Kern et al., 2005). In a different spaceflight experiment, these workers also noted that amyloplasts were grouped into subapical clusters in the moss protonemata in the flight samples as another nonrandom growth response (Kern et al., 2001).
We also found that there were a greater number of adventitious roots from hypocotyls of seedlings grown in microgravity compared to the ground controls (Figs. 8 and 9). Adventitious roots develop from the pericycle, a site of intense mitotic activity. Since previous spaceflight studies have shown that cells in microgravity have an enhanced proliferation rate (Matía et al., 2010), it is possible that microgravity induced an increase in mitosis in the pericycle, which led to the expression of a larger number of adventitious roots in the space-grown plants compared to the control. Taken together, the previous and present studies suggest that the growth of plants in microgravity induces alterations in essential cellular functions that may be related to cell cycle regulation (reviewed in Wolverton and Kiss, 2009).
4.3. Conclusions and future prospects
To date, two major conclusions can be drawn from our spaceflight studies on STS-131. First, seedlings of Arabidopsis thaliana can be successfully grown with the BRIC hardware system and then fixed in flight by using fluids such as aldehydes and RNAlater. Second, plants have a novel, endogenous growth pattern that is largely masked in normal 1 g conditions on Earth based on our observations of roots of plants in microgravity exhibiting a developmental pattern that is not apparent in ground controls.
We currently are analyzing the cytological features, with a focus on the interaction between the cytoskeleton and statolithic amyloplasts, of seedlings grown in microgravity by light and electron microcopy. In addition, studies on microgravity effects on actin cytoskeleton–related gene expression via microarray analyses also are in progress. The overarching goal is to directly correlate results from cytological investigations and gene profiling in order to understand the nature of the actin cytoskeleton in mechanisms of gravity perception. More broadly and beyond these experiments, the success of this project to date demonstrates that the modified BRIC system can be used by other investigators to study the cellular and molecular responses to microgravity of the model plant Arabidopsis on platforms such as the International Space Station (Wolverton and Kiss, 2009).
Acknowledgments
Financial support was provided by NASA through grant NNX10AF44G. We thank our fellow BRIC-16 principal investigators Elison Blancaflor, Jin Nakashima, Anna-Lisa Paul, and Rob Ferl for helpful discussions of the experiments. Jin Nakashima also aided in the photography of samples postflight. A spaceflight project involves a very large team effort, and we thank the staff at the SLS laboratory at Kennedy Space Center, especially Howard Levine, David Cox, Christopher Comstock, Kimberly Slater, David Reed, Susan Manning-Roach, and Stacy Engel. In addition, special thanks go to astronaut Stephanie Wilson for her work with BRIC-16 during flight operations on mission STS-131.
Abbreviations
BRIC, Biological Research in Canisters; PDFU, Petri Dish Fixation Unit; SLS, Space Life Sciences.
References
- Blancaflor E. Masson P.H. Plant gravitropism. Unraveling the ups and downs of a complex process. Plant Physiol. 2003;133:1677–1690. doi: 10.1104/pp.103.032169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Botto F. Sanchez R.A. Whitelam G.C. Casal J.J. Phytochrome A mediates the promotion of seed germination by very low fluences of light and canopy shade light in Arabidopsis. Plant Physiol. 1996;110:439–444. doi: 10.1104/pp.110.2.439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Correll M.J. Kiss J.Z. Space-based research on plant tropisms. In: Gilroy S., editor; Masson P.H., editor. Plant Tropisms. Blackwell; Ames, IA: 2008. pp. 161–182. [Google Scholar]
- Driss-Ecole D. Vassy J. Rembur J. Guivarc'h A. Prouteau M. Dewitte W. Perbal G. Immunolocalization of actin in root statocytes of Lens culinaris L. J Exp Bot. 2000;51:521–528. doi: 10.1093/jexbot/51.344.521. [DOI] [PubMed] [Google Scholar]
- Ferl R.J. Paul A.L. Lunar plant biology—a review of the Apollo era. Astrobiology. 2010;10:261–274. doi: 10.1089/ast.2009.0417. [DOI] [PubMed] [Google Scholar]
- Ferl R.J. Wheeler R. Levine H.G. Paul A.-L. Plants in space. Curr Opin Plant Biol. 2002;5:258–263. doi: 10.1016/s1369-5266(02)00254-6. [DOI] [PubMed] [Google Scholar]
- Guisinger M.M. Kiss J.Z. The influence of microgravity and spaceflight on columella cell ultrastructure in starch-deficient mutants of Arabidopsis. Am J Bot. 1999;86:1357–1366. [PubMed] [Google Scholar]
- Johnsson A. Karlsson C. Chapman D.K. Braseth J.D. Iversen T.-H. Dynamics of root growth in microgravity. J Biotechnol. 1996;47:155–165. doi: 10.1016/0168-1656(96)01381-8. [DOI] [PubMed] [Google Scholar]
- Kern V.D. Sack F.D. Irradiance dependent regulation of gravitropism by red light in protonemata of the moss Ceratodon purpureus. Planta. 1999;209:299–307. doi: 10.1007/s004250050636. [DOI] [PubMed] [Google Scholar]
- Kern V.D. Sack F.D. White N.J. Anderson K. Wells W. Martin C. Spaceflight hardware allowing unilateral irradiation and chemical fixation in Petri dishes. Adv Space Res. 1999;24:775–778. doi: 10.1016/s0273-1177(99)00412-3. [DOI] [PubMed] [Google Scholar]
- Kern V.D. Smith J.D. Schwuchow J.M. Sack F.D. Amyloplasts that sediment in protonemata of the moss Ceratodon purpureus are nonrandomly distributed in microgravity. Plant Physiol. 2001;125:2085–2094. doi: 10.1104/pp.125.4.2085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kern V.D. Schwuchow J.M. Reed D.W. Nadeau J.A. Lucas J. Skripnikov A. Sack F.D. Gravitropic moss cells default to spiral growth on the clinostat and in microgravity during spaceflight. Planta. 2005;221:149–157. doi: 10.1007/s00425-004-1467-3. [DOI] [PubMed] [Google Scholar]
- Kiss J.Z. Mechanisms of the early phases of plant gravitropism. CRC Crit Rev Plant Sci. 2000;19:551–573. doi: 10.1080/07352680091139295. [DOI] [PubMed] [Google Scholar]
- Kiss J.Z. Plants circling in outer space. New Phytol. 2009;182:555–557. doi: 10.1111/j.1469-8137.2009.02817.x. [DOI] [PubMed] [Google Scholar]
- Kiss J.Z. Edelmann R.E. Wood P.C. Gravitropism of hypocotyls of wild-type and starch-deficient Arabidopsis seedlings in spaceflight studies. Planta. 1999;209:96–103. doi: 10.1007/s004250050610. [DOI] [PubMed] [Google Scholar]
- Kiss J.Z. Brinckmann E. Brillouet C. Development and growth of several strains of Arabidopsis seedlings in microgravity. Int J Plant Sci. 2000;161:55–62. doi: 10.1086/314223. [DOI] [PubMed] [Google Scholar]
- Matía I. González-Camacho F. Herranz R. Kiss J.Z. Gasset G. van Loon J. Marco R. Medina F.J. Plant cell proliferation and growth are altered by microgravity conditions in spaceflight. J Plant Physiol. 2010;167:184–193. doi: 10.1016/j.jplph.2009.08.012. [DOI] [PubMed] [Google Scholar]
- Migliaccio F. Piconese S. Spiralizations and tropisms in Arabidopsis roots. Trends Plant Sci. 2001;6:561–565. doi: 10.1016/s1360-1385(01)02152-5. [DOI] [PubMed] [Google Scholar]
- Millar K.D.L. Kumar P. Correll M.J. Mullen J.L. Hangarter R.P. Edelmann R.E. Kiss J.Z. A novel phototropic response to red light is revealed in microgravity. New Phytol. 2010;186:648–656. doi: 10.1111/j.1469-8137.2010.03211.x. [DOI] [PubMed] [Google Scholar]
- Morita M.T. Directional gravity sensing in gravitropism. Annu Rev Plant Biol. 2010;61:705–720. doi: 10.1146/annurev.arplant.043008.092042. [DOI] [PubMed] [Google Scholar]
- Mortley D.G. Bonsi C.K. Hill W.A. Morris C.E. Williams C.S. Davis C.F. Williams J.W. Levine L.H. Petersen B.V. Wheeler R.M. Influence of microgravity environment on root growth, soluble sugars, and starch concentration of sweet potato stem cuttings. J Am Soc Hortic Sci. 2008;133:327–332. [PMC free article] [PubMed] [Google Scholar]
- Mullen J.L. Turk E. Johnson K. Wolverton C. Ishikawa H. Simmons C. Soll D. Evans M.L. Root-growth behavior of the Arabidopsis mutant rgr1. Roles of gravitropism and circumnutation in the waving/coiling phenomenon. Plant Physiol. 1998;118:1139–1145. doi: 10.1104/pp.118.4.1139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oliva M. Dunand C. Waving and skewing: how gravity and the surface of growth media affect root development in Arabidopsis. New Phytol. 2007;176:37–43. doi: 10.1111/j.1469-8137.2007.02184.x. [DOI] [PubMed] [Google Scholar]
- Palmieri M. Kiss J.Z. Disruption of the F-actin cytoskeleton limits statolith movement in Arabidopsis hypocotyls. J Exp Bot. 2005;56:2539–2550. doi: 10.1093/jxb/eri248. [DOI] [PubMed] [Google Scholar]
- Palmieri M. Kiss J.Z. The role of plastids in gravitropism. In: Wise R.R., editor; Hoober J.K., editor. The Structure and Function of Plastids. Springer; Dordrecht, the Netherlands: 2006. pp. 507–525. [Google Scholar]
- Penfield S. King J. Towards a systems biology approach to understanding seed dormancy and germination. Proc R Soc Lond B Biol Sci. 2009;276:3561–3569. doi: 10.1098/rspb.2009.0592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perrin R.M. Young L.-S. Narayana Murthy U.M. Harrison B.R. Wang Y. Will J.L. Masson P.H. Gravity signal transduction in primary roots. Ann Bot. 2005;96:737–743. doi: 10.1093/aob/mci227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rutherford R. Masson P.H. Arabidopsis thaliana sku mutant seedlings show exaggerated surface-dependent alteration in root growth vector. Plant Physiol. 1996;111:987–998. doi: 10.1104/pp.111.4.987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saito C. Morita M.T. Kato T. Tasaka M. Amyloplasts and vacuolar membrane dynamics in the living graviperceptive cell of the Arabidopsis inflorescence stem. Plant Cell. 2005;17:548–558. doi: 10.1105/tpc.104.026138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simmons C. Migliaccio F. Masson P. Caspar T. Soll D. A novel root gravitropism mutant of Arabidopsis thaliana exhibiting altered auxin physiology. Physiol Plant. 1995;93:790–798. [PubMed] [Google Scholar]
- Sorin C. Bussell J.D. Camus I. Ljung K. Kowalczyk M. Geiss G. McKhann H. Garcion C. Vaucheret H. Sandberg G. Bellini C. Auxin and light control of adventitious rooting in Arabidopsis require ARGONAUTE1. Plant Cell. 2005;17:1343–1359. doi: 10.1105/tpc.105.031625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stimpson A.J. Pereira R.S. Kiss J.Z. Correll M.J. Extraction and labeling methods for microarrays using small amounts of plant tissue. Physiol Plant. 2009;135:229–236. doi: 10.1111/j.1399-3054.2008.01191.x. [DOI] [PubMed] [Google Scholar]
- Wells B. Best M.D. McCray R.H. Levine H.G. A flight-rated Petri dish apparatus providing two stage fluid injection for aseptic biological investigations in space [paper No. 2001-01-2286]. 31st International Conference on Environmental Systems, SAE International; Warrendale, PA. 2001. [Google Scholar]
- Wolverton S.C. Kiss J.Z. An update on plant space biology. Gravit Space Biol Bull. 2009;22:13–20. [Google Scholar]
- Yamamoto K. Kiss J.Z. Disruption of the actin cytoskeleton results in the promotion of gravitropism in inflorescence stems and hypocotyls of Arabidopsis. Plant Physiol. 2002;128:669–681. doi: 10.1104/pp.010804. [DOI] [PMC free article] [PubMed] [Google Scholar]