Abstract
Sexual reproduction in Volvocine algae coevolved with the acquisition of multicellularity. Unicellular genera such as Chlamydomonas and small colonial genera from this group have classical mating types with equal-sized gametes, while larger multicellular genera such as Volvox have differentiated males and females that produce sperm and eggs respectively. Newly available sequence from the Volvox and Chlamydomonas genomes and mating loci open up the potential to investigate how sex-determining regions co-evolve with major changes in development and sexual reproduction. The expanded size and sequence divergence between the male and female haplotypes of the Volvox mating locus (MT) provide insights into how the colonial Volvocine algae might have evolved sexual dimorphism, but also raise questions about why the putative ancestral-like MT locus in Chlamydomonas shows less divergence between haplotypes than expected.
Introduction
The diversity of eukaryotic sex determination systems is unparalleled in biology. However, underlying this diversity are common themes that have appeared and reappeared suggesting that similar dynamics and constraints shape the evolution of sex. One such theme is oogamy (i.e. sperm-egg mating) which is a near universal strategy for gamete production in multicellular eukaryotes, but relatively uncommon in unicellular species. A second theme is the emergence of sex chromosomes: cytologically or genetically distinct chromosomes that govern sexual differentiation [1,2]. Previously these two themes have been investigated separately because sex chromosomes are usually found in species with well-established oogamous mating systems. However, Volvocine algae present an opportunity to understand how sex chromosomes coevolved with the isogamy-oogamy transition that is embodied by the differences between Chlamydomonas reinhardtii, an isogamous unicellular species, and its cousin Volvox carteri, an oogamous multicellular species. Newly available sequence information from the sex determining loci of Chlamydomonas and Volvox paves the way for uncovering the molecular origins of the isogamy to oogamy transition, and suggests a means by which the Volvox mating locus acquired key properties of a sex chromosome.
Volvcine algae: a living snapshot of morphological diversity
Volvocine algae are a sub-group of chlorophytes (green algae) that have well-characterized reproductive cycles [3,4]. They are haploid and can reproduce asexually through mitosis as their main means of proliferation, but they also have a sexual cycle in which gametes mate to form a diploid zygotic spore. Upon germination the spore undergoes meiosis to regenerate haploid vegetative progeny (Fig. 1). This life history pattern is similar to that of many other facultatively sexual eukaryotic microbes.
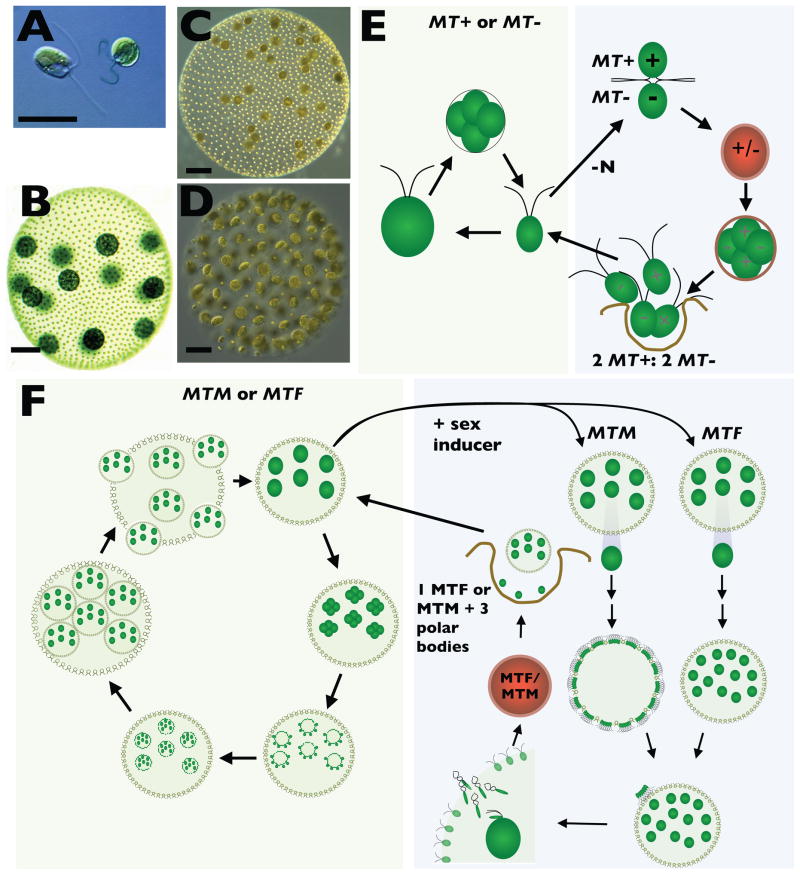
Figure 1.
A. Two Chlamydomonas cells. Scale bar is 10 μM. B. Vegetative Volvox spheroid. C. Sexual female Volvox spheroid. D. Sexual Male Volvox spheroid. Scale bar is 50 μM for panels B–D. E. Schematic of Chlamydomonas life cycle. Left side depicts the vegetative reproductive cycle of growth, and division by multiple fission. Right side depicts mating, diploid MT+/MT− zygotic spore formation, meiosis and hatching to produce four haploid progeny. F. Schematic of the Volvox life cycle. Left side depicts key stages in the vegetative reproductive cycle. Starting in the upper right are a mature spheroid, cleavage stage embryo, pre-inversion embryo, inverted juvenile, expanding juvenile, and hatching stage. Right side depicts the sexual cycle. Pre-cleavage gonidia from males and females undergo modified development to produce sperm packet bearing male spheroids and egg bearing female spheroids. Sperm travel as a packet, attach to a female, dissociate, enter, and fertilize eggs to form a diploid MTF/MTM zygotic spore. Meiosis and germination produce a single haploid vegetative progeny (either male or female) and three polar bodies.
Chlamydomonas and its unicellular cousins are a taxonomic outgroup to the colonial Volvocine species that are thought to have evolved multicelluarity through successive innovations [5,6]. Among these innovations are anisogamy and oogamy: The simpler colonial genera such as Chlamydomonas, Gonium, and Pandorina make equal-sized gametes (isogamy), while the larger-sized genera (Eudorina, Pleodorina, Volvox) are anisogamous or oogamous [7]. Gamete size is not the only sex-related trait that is modified in colonial species (Table I), but it is the most obvious and has parallels in other multicellular lineages. Our knowledge of Volvocine algal sexual cycles comes largely from work on two well-developed model species, Chlamydomonas reinhardtii and Volvox carteri.
Table 1.
Comparison of Chlamydomonas reinhardtii and Volvox carteri sexual cycles
| Chlamydomonas reinhardtii | Volvox carteri |
|---|---|
| Nitrogen starvation is signal for gametogenesis | Diffusible sex inducer protein is signal for gametogenesis |
| Isogamous (equal sized gametes) | Oogamous (eggs and sperm) |
| All vegetative cells can differentiate into gametes directly | Eggs and sperm packets formed by only a subset of cells during sexual spheroid embryogenesis |
| Gametes can de-differentiate back to vegetative cells | Eggs can de-differentiate. Sperm are terminally differentiated |
| Gametes are free-swimming single cells that find each other by chance | Motile sperm packets must find a sexual female spheroid, gain entry, dissociate, and fertilize eggs |
| Zygotes undergo meiosis to form tetrads (all four products viable) | One large meiotic product survives; 3 small polar bodies are non-viable |
| Uniparental inheritance of chloroplast genome from MT+ parent and mitochondrial genome from MT− parent | Uniparental inheritance of both chloroplast and mitochondrial genomes from female parent |
Chlamydomonas reinhardtii: Sex and the single cell
The sex life of Chlamydomonas is typical for a eukaryotic unicellular organism that alternates between vegetative and sexual reproduction--though it should be kept in mind that sex did stop evolving in Chlamydomonas since the Volvocine radiation began ~200 Mya [8*]. Recent reviews cover sex and mating in Chlamydomonas in detail [9,10], so the following description is brief. In nutrient replete conditions Chlamydomonas reproduces asexually using a modified mitotic cycle called multiple fission (Fig. 1A, 1E)[11]. Sex is triggered environmentally by nitrogen starvation–N) that induces differentiation of vegetative cells into mating-competent gametes of two types, plus and minus, that are morphologically similar to their vegetative parents. However, unlike vegetative cells, gametes of each genetically determined mating type express a specialized set of cell-type specific genes that allow them to mate. Gamete fusion triggers additional developmental changes that lead to formation of a dormant and environmentally resistant diploid zygote spore [12*]. Upon return to favorable conditions the spore will undergo meiosis and produce four viable haploid progeny.
Volvox carteri: Sex meets development
Volvox carteri is among the most developmentally complex Volvocine algae and has evolved a number of innovations including specialized reproductive and somatic cell types and embryonic patterning [13]. Mitotically reproducing vegetative colonies of both Volvox sexes contain two separate cell types: ~2000 sterile flagellated somatic cells that are arranged around the periphery and provide motility, and ~16 large vegetative reproductive cells called gonidia that lie inside the spheroid (Fig. 1B). Both cell types are embedded in a clear secreted extracellular matrix (ECM) that occupies most of the spheroid volume. When mature, each gonidial cell undergoes 12 or 13 embryonic divisions followed by a morphogenetic process called inversion to produce a miniature juvenile colony with a full complement of newly formed gonidial and somatic precursors. The juvenile colony will grow and eventually hatch from its mother colony to complete the vegetative reproductive cycle (Fig. 1F). The number of gonidia in each adult spheroid is set by the timing and placement of asymmetric cell divisions during embryogenesis [14]. In vegetative embryos of both sexes (male and female) asymmetric cell division occurs in the anterior cells of the embryo at cycle 6 (32 → 64 cell stage) and typically produces 16 large gonidial precursors that are destined to become the next generation of reproductive cells.
Unlike Chlamydomonas where –N induces gametogenesis, sexual reproduction in Volvox is triggered by a species-specific diffusible glycoprotein called sex-inducer [15]. When exposed to sex-inducer, the gonidia within vegetative males and females respond with altered cleavage patterns to produce modified embryos that mature into adults containing sexual germ cells (Fig. 1C, 1D, 1F) [16]. Anterior cells in embryos of sexually induced females cleave asymmetrically at cycle 7 (64 → 128 cell stage) and produce sexual juvenile spheroids with 32–48 large egg precursors and ~2000 sexual somatic cells. In sexually induced males all the cells divide asymmetrically at cycle 7 (128 → 256 cell stage) and produce sexual juvenile spheroids with 128 sexual male somatic cells and 128 large androgonidia. A day later each androgonidia divides six or seven more times into sperm packets containing 64–128 small sperm. The events leading to fertilization are not well studied, but involve sperm packets swimming as a unit to a female sexual spheroid, breaking into individual sperm cells, entering the female ECM through a fertilization pore, and finally fusing with an egg cell to initiate zygote development. The innovations that gave rise to the Volvox sexual development cycle are controlled by its mating locus that is described below.
MT: the master regulator of sex in Volvocine algae
In most species of Volvocine algae sex is controlled by a haploid mating locus (MT) that encodes two mating types or sexes. Chlamydomonas MT has two haplotypes (MT+) and (MT−) that specify plus and minus differentiation respectively and which reside near one telomere of chromosome VI (Fig. 2)[17]. Although MT segregates as a single Mendelian trait, it is a complex, multigenic locus. The core of the two Chlamydomonas MT haplotypes is termed the R (rearranged) domain and encompasses 200–300 kb. Within the R domain are genes involved in sex determination, cell-cell recognition, zygote maturation, and organelle inheritance [9,10]. The R domain was previously defined by restriction mapping [17,18], but it has since been revised based on full sequence information from both haplotypes [19**]. This sequencing revealed a new 30 kb region of MT+ termed SRL (scavenger receptor like) and is one of at least two regions of MT+ that appear to have been acquired by autosomal translocation/duplication events which may be a means of adding new genetic material to MT. Indeed, one gene of unknown function, MTA1, acquired a gamete specific expression pattern as a result of translocating into MT+ [18].
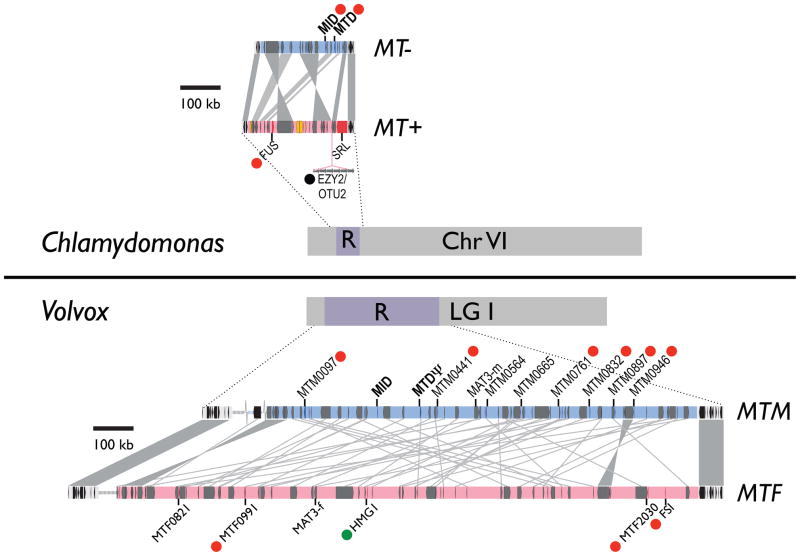
Figure 2.
Schematic of the Chlamydomonas and Volvox mating type chromosomes and mating loci. The rearranged (R) domain and its relative location on each chromosome is labeled. Above and below the chromosomal schematics are expanded versions of MT from each species with the R domain for each haplotype shown in red or blue and genes overlaid in gray. EZY2/OTU2 are in a tandem repeat region. Several sex-limited genes are shown for each species, with vegetative expression depicted by a green dot, sexual expression by a red dot, and zygotic expression by a black dot [18,19**].
A handful of MT genes are sex-limited, meaning that they are found in only one of the two mating types (Fig 2). Sexual differentiation in Chlamydomonas is largely controlled by a single sex-limited gene, MID (minus dominance) that resides in MT− and encodes a RWP-RK family putative transcription factor [20]. Presence or absence of MID determines minus or plus differentiation, respectively. Intriguingly, sex-limited MID homologs have been identified in other Volvocine species including Gonium pectorale (GpMID in MT−), Pleodorina starrii (PsMID in males), and Volvox carteri (VcMID in males) [19**,21,22*]. Thus MID appears to be conserved and may play a role in sexual differentiation throughout the lineage, though the situation in Volvox is unclear since MID mRNA is expressed in vegetative spheroids and is not sex-regulated [19**].
Other sex-limited genes in MT also contribute to the sexual cycle. MTD is a MT− gene whose product augments MID expression [23,24]. FUS1 is a MT+ gene whose product is required for plasma membrane fusion with minus gametes [25,26]. Additional sex-regulated processes including uniparental organelle DNA inheritance and zygote development may be controlled by other MT genes such as the EZY2/OTU2 cluster, but the functions of most of these additional genes have yet to be established [10,18].
In addition to sex-limited genes, a dozen or more shared genes (i.e. those with a copy in both MT+ and MT−) are in the R domain and are in small syntenic blocks that are rearranged between the two mating types [17]. This configuration suppresses recombination and helps maintain linkage disequilibrium of the sex-limited genes. However, most of the shared genes have predicted functions that are not directly related to sex (e.g. primary metabolism) [18,19**]. This arrangement, therefore, generates a potential fitness cost and raises questions about the dynamics that maintain non-sex-related genes in the R domain (see below). Moreover, the non-recombining nature of MT compared with autosomes is expected to result in some distinct features including rapid evolution, divergence of shared genes, and accumulation of transposons and repeats [27]. At the population level MT should be subject to selective sweeps and therefore exhibit low intra-haplotype diversity (i.e. between isolates of the same mating type), but also exhibit high inter-haplotype diversity (between MT+ and MT−) due to lack of recombination. Chlamydomonas MT displays some of these properties [28], but has yet to be described fully at the population level. Nonetheless, the two MT haplotypes are not differentiated to the degree that might be expected after 200 MY of blocked recombination [19**,29]. This apparently discordant property of Chlamydomonas MT stands in contrast to Volvox MT that shows a high degree of sex-linked differentiation.
Volvox MT: a nascent sex chromosome?
The two mating type haplotypes of Volvox are designated MTF (female) and MTM (male). Volvox MT is near a presumed telomere on Linkage Group I that is syntenic with the Chlamydomonas MT locus on chromosome VI, though there is little if any micro-synteny between their respective R domains (Fig. 2)[19**]. The global stability of the location of MT on equivalent linkage groups in both species was unanticipated because sex-determining regions often translocate or undergo inter-chromosomal fusions, neither of which appears to have occurred in these two species [2]. On the horizon are more sequencing projects for Volvocine algae that will establish whether the relative location of MT is truly stable.
Location notwithstanding, Volvox MT has properties that are strikingly different from that of Chlamydomonas MT, and these attributes may provide clues about the evolution of sexual dimorphism in this lineage [19**]. While the entire Volvox genome is ~17% larger than that of Chlamydomonas (138 vs. 118 Mb; due to a slightly higher average repeat density in Volvox), the Volvox MT R domain is four or five times larger than that of Chlamydomonas and encompasses >1 Mb [19**,30**,31]. The genetic content of Volvox MT is also higher than that of Chlamydomonas MT with >50 identifiable protein coding genes. However, the overall protein coding gene density in Volvox MT is atypically low (about half of that in autosomal regions) and its repeat density is about three times higher than on the autosomes. These properties distinguish Volvox MT from Chlamydomonas MT and bring it onto the doorstep of becoming a differentiated haploid sex chromosome. The dynamics that may have led to this relative expansion are discussed below.
Genetic innovation in Volvox MT
Like Chlamydomonas MT, Volvox has sex-limited protein coding genes (10 male, 5 female) but only three of them have homology to known proteins, two of which are sex-limited Chlamydomonas MT genes [19**]. VcMID in MTM is a male gene, but unlike the case in Chlamydomonas, its expression is not sex-regulated. If VcMID is involved in sex-determination it will likely be in collaboration with other genes. A second male gene, vcMTD, encodes a protein with partial similarity to the Chlamydomonas and Gonium MT− gene MTD, but vcMTD has numerous premature termination codons in its message indicating that it is a likely pseudogene. Finally, a Volvox female gene, HMG1, encodes a putative HMG-box DNA binding protein. This gene is intriguing since HMG domain proteins are involved in sex determination in fungi and animals [32,33]. HMG1 has no ortholog in Chlamydomonas, though there are other HMG-box paralogs in both species [19**]. HMG1 message levels decrease in sexual versus vegetative females making it a possible negative regulator of female sexual or zygotic functions. The remaining twelve sex-limited genes in Volvox are unique: they encode proteins without any identifiable homologs anywhere, and intriguingly, most of them have mRNAs that are induced during sexual differentiation suggestive of a function for their encoded proteins during the sexual cycle [19**] (Fig. 2). A growing arsenal of molecular genetic tools for Volvox will enable future work aimed at assigning functions to these novel sex-limited genes [34].
A second striking difference between Volvox and Chlamydomonas MT is in the degree of divergence between shared genes from opposite sexes. While shared genes in Chlamydomonas MT exhibit minor polymorphisms between MT+ and MT− alleles, shared genes in MTM and MTF are diverged to the point where many do not recognizably belong to the same species. Neutral and non-neutral polymorphisms in shared Volvox MT genes are up to two orders of magnitude higher than those for shared genes in Chlamydomonas, and this divergence extends through speciation events that have generated male and female lineages for genes in each MT haplotype [19**]. Thus, in some sense nearly every gene in the expanded Volvox MT locus is sex-limited due to extreme divergence from its allele in the opposite sex. This situation opens the door for shared genes in the two Volvox haplotypes to become masculinized and feminized in expression or function, and evidence for such sex-specific diversification exists. For example, the MAT3 locus that is predicted to encode a key cell cycle and cell size regulator in Volvox is highly dimorphic between males and females, and also undergoes sex-inducer regulated alternative splicing in both sexes [19**,35,36]. The potential now exists to identify how Volvox MT genes such as MAT3 and others contribute to the evolution of an oogamous mating system.
Comparative evolution of Chlamydomonas and Volvox MT: insights and enigmas
There are many fundamental biological differences between Chlamydomonas and Volvox that are not directly related to sex, yet the MT locus is the only genomic region that stands out as being substantially different between the two species [19**,30**]. The dimorphic male-female structure of Volvox MT in some sense mirrors the sexually dimorphic gamete differentiation program that evolved in this lineage, and suggests a connection between MT size and an expanded genetic control program for sex in Volvox. Starting from an isogamous ancestor with a smaller MT region, successive inversions could add new genes into the R domain, and these inversions could then be fixed in the population if they contribute to gamete fitness (Fig. 3A)[27]. For example, the pressure for oogamy in a new colonial species could be resolved by incorporation of a gamete size control gene into MT that could then evolve in tight linkage with the sex determination locus to promote differential gamete size in males versus females [37]. There is evidence of stepwise sequence addition in Volvox MT that supports this ratcheting model [19**]. In addition, as mentioned above, a candidate size regulator, MAT3, was incorporated into MT and appears to have diverged in a manner that supports a role in gamete differentiation.
Figure 3.
A. Model for expansion of Volvox MT. The R domain is red or blue and the autosomal region is green. SD represents a sex determining gene, and A and B are flanking genes. Autosomal inversions adjacent to the existing R domain will block recombination and allow differentiation of the formerly autosomal A/a and B/b loci in linkage with mating haplotype. Sexually antagonistic alleles B and b (shaded red and blue) can be fixed if they contribute to fitness of their respective mating types [19**]. B. Expected divergence patterns for MT if no recombination occurred in either the Chlamydomonas or Volvox lineages. Light red and blue represent less diverged regions while darker red and blue represent more diverged regions. More recently acquired regions of Volvox MT should be less diverged, while the older regions should have divergence similar to that seen in Chlamydomonas. This divergence pattern is not observed (see text). C. Mating type resetting can occur if a mid mutation and FUS1 gene end up on the same chromosome to generate a neo-MT+ haplotype that is highly similar to MT−. D. Mating type sequence homogenization can occur through gene conversion between MT+ and MT− shared genes.
However, the ratcheting model of MT expansion in Volvox does not explain the puzzling observation that the equally old or older MT locus from Chlamydomonas reinhardtii looks far more youthful with respect to genetic divergence than does MT from the younger species, Volvox carteri. If recombination between MT haplotypes did not occur in either lineage since divergence, then Chlamydomonas MT+ and MT− genes should be at least as diverged from each other as those from Volvox MTM and MTF (Fig. 3B)[29].
One resolution comes if MT has undergone at least one round of collapse/reformation in the lineage that gave rise to Chlamydomonas reinhardtii (Fig. 1C). In this scenario Chlamydomonas MT may actually be younger than Volvox MT. For example a Chlamydomonas MT− mid mutant can mate effectively as a plus strain with the addition of just one MT+ limited gene, FUS1 [25]. It is, therefore, possible for a new plus strain to form from a mutant mid MT− parent. Such a strain would be nearly isogenic to its MT− partner, thus resetting the sequence clock for MT divergence. A similar resetting model has been proposed to explain the youthfulness of sex chromosomes in lower vertebrates [38,39].
A second explanation for how shared genes in Chlamydomonas MT might retain their relative youth is through gene conversion (Fig. 1E). If this occurred on a regular basis it would act to homogenize the shared genes in the locus and effectively “erase” their divergence. Interestingly, evidence for rare X-Y gene conversion events exists in the cat lineage [40].
The above models might explain the relative youth of Chlamydomonas MT, but do not explain why Volvox MT has not behaved similarly and remained homogenous. With respect to the collapse/reformation model, Volvox carteri and its recent kin might be at a late stage in the MT aging cycle or have become so genetically complex that collapse and reformation are not feasible. It is also possible that above a certain size threshold the dynamics of MT change so that genetic exchange between the two haplotypes cannot occur. Once past the gene conversion size threshold, MT allelic divergence in the Volvox lineage would be expected to accelerate and potentially enter a feedback cycle of further expansion, divergence and recombination suppression (Fig. 3A).
A third perspective that needs to be incorporated into thinking about MT size is population genetics. The efficiency of natural selection is influenced by population size [41]; and a larger, slower growing organism such as Volvox is expected to have a smaller effective population size (Ne) than that of a unicellular organism like Chlamydomonas. If mutation rates are not significantly different between the two species, then lower Ne in Volvox is predicted to result in decreased polymorphism rates and increased genome size. These predictions are generally upheld for autosomal nuclear and organellar genes [42]. However, the overall size of the Volvox genome is only modestly larger than that of Chlamydomonas (~140 vs. ~120 Mb) and does not match the five-fold change in size of Volvox MT relative to Chlamydomonas MT [19**,30**]. Thus, the differences between the MT loci of the two species are likely due to a combination of population genetic effects, differential recombination rates, and sexual selection. New sequencing projects for Volvocine algae and their MT loci along with population genetic studies will help refine questions related to MT divergence and test competing hypotheses about its evolution.
The Volvox sexual cycle: back to the future
Sequence information alone cannot answer the question of how sexual dimorphism evolved in Volvocine algae. Previous genetic screens identified many potentially informative mutants that affect Volvox sexual development [43], but the technology was not available at that time to identify the affected genes. With the Volvox genome and mating locus sequence completed the stage is set to begin dissecting the Volvox sexual cycle and to identify sources of innovation in the Volvox sex determination pathway. Future studies with Volvocine algae promise to shed new light on the origins of gender and on the fascinating evolutionary tango between sex chromosomes and developmental diversity that is exemplified by this group.
Highlights.
Volvocine algae include unicellular Chlamydomonas and multicellular Volvox
Chlamydomonas is isogamous while Volvox is oogamous
The Volvox mating locus (MT) is expanded relative to Chlamydomonas MT
A model for MT expansion driven by sexual selection is presented
Models for unexpectedly low divergence of Chlamydomonas MT haplotypes are presented
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Fraser JA, Heitman J. Chromosomal sex-determining regions in animals, plants and fungi. Current Opinion in Genetics & Development. 2005;15:645–651. doi: 10.1016/j.gde.2005.09.002. [DOI] [PubMed] [Google Scholar]
- 2.Charlesworth B. The evolution of chromosomal sex determination. Novartis Found Symp. 2002;244:207–219. [PubMed] [Google Scholar]
- 3.Nozaki H. Morphology and evolution of sexual reproduction in the Volvocaceae (Chlorophyta) J Plant Res. 1996;109:353–361. [Google Scholar]
- 4.Goodenough U, Armbrust E, Campbell A, Ferris P. Molecular genetics of sexuality in Chlamydomonas. Annual Reviews of Plant Physiology and Plant Molecular Biology. 1995;46:21–44. [Google Scholar]
- 5.Kirk DL. A twelve-step program for evolving multicellularity and a division of labor. Bioessays. 2005;27:299–310. doi: 10.1002/bies.20197. [DOI] [PubMed] [Google Scholar]
- 6.Herron MD, Michod RE. Evolution of complexity in the Volvocine algae: Transitions in individuality through Darwin’s eye. Evolution. 2008;62:436–451. doi: 10.1111/j.1558-5646.2007.00304.x. [DOI] [PubMed] [Google Scholar]
- 7.Nozaki H. Origin and evolution of the colonial Volvocales (Chlorophyceae) as inferred from multiple, chloroplast gene sequences. Molecular Phylogenetics and Evolution. 2000;17:256–268. doi: 10.1006/mpev.2000.0831. [DOI] [PubMed] [Google Scholar]
- *8.Herron M, Hackett J, Aylward F, Michod R. Triassic origin and early radiation of multicellular volvocine algae. Proc Natl Acad Sci USA. 2009;106:3524–3528. doi: 10.1073/pnas.0811205106. The authors use fossil evidence to estimate the divergence time of the Volvocine lineage at ~200 million years, a date that is significantly older than earlier estimates of <100 million years. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Goodenough U, Lin H, Lee J-H. Sex determination in Chlamydomonas. Seminars in Cell & Developmental Biology. 2007;18:350–361. doi: 10.1016/j.semcdb.2007.02.006. [DOI] [PubMed] [Google Scholar]
- 10.Nishimura Y. Uniparental inheritance of cpDNA and the genetic control of sexual differentiation in Chlamydomonas reinhardtii. J Plant Res. 2010;123:149–162. doi: 10.1007/s10265-009-0292-y. [DOI] [PubMed] [Google Scholar]
- 11.Umen JG. The elusive sizer. Current Opinion in Cell Biology. 2005;17:435–441. doi: 10.1016/j.ceb.2005.06.001. [DOI] [PubMed] [Google Scholar]
- *12.Lee J-H, Lin H, Joo S, Goodenough U. Early sexual origins of homeoprotein heterodimerization and evolution of the plant KNOX/BELL family. Cell. 2008;133:829–840. doi: 10.1016/j.cell.2008.04.028. The basis for diploid zygote development in Chlamydomonas was found to be heterodimerization of conserved homeodomain proteins from plus and minus. Homologs of these homeoproteins are also involved in plant development. [DOI] [PubMed] [Google Scholar]
- 13.Nishii I, Miller SM. Volvox: simple steps to developmental complexity? Current Opinion in Plant Biology. 2010;13:646–653. doi: 10.1016/j.pbi.2010.10.005. [DOI] [PubMed] [Google Scholar]
- 14.Kirk D. Volvox. Current Biology. 2004;14:R599–R600. doi: 10.1016/j.cub.2004.07.034. [DOI] [PubMed] [Google Scholar]
- 15.Starr R, Jaenicke L. Purification and characterization of the hormone initiating sexual morphogenesis in Volvox carteri f. nagariensis Iyengar. Proc Natl Acad Sci USA. 1974;71:1050–1054. doi: 10.1073/pnas.71.4.1050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hallmann A, Godl K, Wenzl S, Sumper M. The highly efficient sex-inducing pheromone system of Volvox. Trends in Microbiology. 1998;6:185–189. doi: 10.1016/s0966-842x(98)01234-7. [DOI] [PubMed] [Google Scholar]
- 17.Ferris PJ, Goodenough UW. The mating-type locus of Chlamydomonas reinhardtii contains highly rearranged DNA sequences. Cell. 1994;76:1135–1145. doi: 10.1016/0092-8674(94)90389-1. [DOI] [PubMed] [Google Scholar]
- 18.Ferris PJ, Armbrust EV, Goodenough UW. Genetic structure of the mating-type locus of Chlamydomonas reinhardtii. Genetics. 2002;160:181–200. doi: 10.1093/genetics/160.1.181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- **19.Ferris P, Olson BJSC, De Hoff PL, Douglass S, Casero D, Prochnik S, Geng S, Rai R, Grimwood J, Schmutz J, et al. Evolution of an expanded sex-determining locus in Volvox. Science. 2010;328:351–354. doi: 10.1126/science.1186222. The authors identified, cloned, sequenced and characterized the Volvox mating locus as well as the MT− haplotype of Chlamydomonas. The unexpected expansion and evolutionary diversification of Volvox MT stand in contrast to the Chlamydomonas MT locus and suggest a process of sexual cooption that led to the establishment of oogamy in the Volvox lineage. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ferris PJ, Goodenough UW. Mating type in Chlamydomonas is specified by mid, the minus-dominance gene. Genetics. 1997;146:859–869. doi: 10.1093/genetics/146.3.859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hamaji T, Ferris PJ, Coleman AW, Waffenschmidt S, Takahashi F, Nishii I, Nozaki H. Identification of the minus-dominance gene ortholog in the mating-type locus of Gonium pectorale. Genetics. 2008;178:283–294. doi: 10.1534/genetics.107.078618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- *22.Nozaki H, Mori T, Misumi O, Matsunaga S, Kuroiwa T. Males evolved from the dominant isogametic mating type. Curr Biol. 2006;16:R1018–1020. doi: 10.1016/j.cub.2006.11.019. The authors identified Mid as a male-limited gene in the oogamous Volvocine alga Pleodorina starii. This finding genetically connects males to the minus mating type in the unicellular ancestor of Volvcine algae. [DOI] [PubMed] [Google Scholar]
- 23.Lin H, Goodenough UW. Gametogenesis in the Chlamydomonas reinhardtii minus mating type is controlled by two genes, MID and MTD1. Genetics. 2007;176:913–925. doi: 10.1534/genetics.106.066167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hamaji T, Ferris PJ, Nishii I, Nozaki H. Identification of the minus mating-type specific gene MTD1 from Gonium pectorale (Volvocales, Chlorophyta) J Phycol. 2009;45:1310–1314. doi: 10.1111/j.1529-8817.2009.00744.x. [DOI] [PubMed] [Google Scholar]
- 25.Ferris PJ, Woessner JP, Goodenough UW. A sex recognition glycoprotein is encoded by the plus mating-type gene fus1 of Chlamydomonas reinhardtii. Mol Biol Cell. 1996;7:1235–1248. doi: 10.1091/mbc.7.8.1235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Misamore MJ, Gupta S, Snell WJ. The Chlamydomonas Fus1 protein is present on the mating type plus fusion organelle and required for a critical membrane adhesion event during fusion with minus gametes. Mol Biol Cell. 2003;14:2530–2542. doi: 10.1091/mbc.E02-12-0790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bull J. Sex chromosomes in haploid dioecy: A unique contrast to Muller’s theory for diploid dioecy. The American Naturalist. 1978;112:245–250. [Google Scholar]
- 28.Ferris PJ, Pavlovic C, Fabry S, Goodenough UW. Rapid evolution of sex-related genes in Chlamydomonas. Proc Natl Acad Sci USA. 1997;94:8634–8639. doi: 10.1073/pnas.94.16.8634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Charlesworth D, Charlesworth B. Evolutionary biology: The origins of two sexes. Current Biology. 2010;20:R519–R521. doi: 10.1016/j.cub.2010.05.015. [DOI] [PubMed] [Google Scholar]
- **30.Prochnik SE, Umen J, Nedelcu AM, Hallmann A, Miller SM, Nishii I, Ferris P, Kuo A, Mitros T, Fritz-Laylin LK, et al. Genomic analysis of organismal complexity in the multicellular green alga Volvox carteri. Science. 2010;329:223–226. doi: 10.1126/science.1188800. The sequence of the Volvox carteri genome revealed a high degree of similarity to that of Chlamydomonas reinhardtii. This finding suggests that developmental complexity need not involve major expansions or innovations in protein coding gene capacity. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Merchant SS, Prochnik SE, Vallon O, Harris EH, Karpowicz SJ, Witman GB, Terry A, Salamov A, Fritz-Laylin LK, Marechal-Drouard L, et al. The Chlamydomonas genome reveals the evolution of key animal and plant functions. Science. 2007;318:245–250. doi: 10.1126/science.1143609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Idnurm A, Walton FJ, Floyd A, Heitman J. Identification of the sex genes in an early diverged fungus. Nature. 2008;451:193–196. doi: 10.1038/nature06453. [DOI] [PubMed] [Google Scholar]
- 33.Waters PD, Wallis MC, Marshall Graves JA. Mammalian sex--origin and evolution of the Y chromosome and SRY. Seminars in Cell & Developmental Biology. 2007;18:389–400. doi: 10.1016/j.semcdb.2007.02.007. [DOI] [PubMed] [Google Scholar]
- 34.Hallmann A. Evolution of reproductive development in the volvocine algae. Sex Plant Reprod. 2010:1–16. doi: 10.1007/s00497-010-0158-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kianianmomeni A, Nematollahi G, Hallmann A. A gender-specific retinoblastoma-related protein in Volvox carteri implies a role for the retinoblastoma protein family in sexual development. Plant Cell. 2008;20:2399–2419. doi: 10.1105/tpc.107.057836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Umen JG. Control of cell division by a retinoblastoma protein homolog in Chlamydomonas. Genes & Development. 2001;15:1652–1661. doi: 10.1101/gad.892101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Charlesworth B. The population genetics of anisogamy. Journal of Theoretical Biology. 1978;73:347–357. doi: 10.1016/0022-5193(78)90195-9. [DOI] [PubMed] [Google Scholar]
- 38.Perrin N. Sex reversal: A fountain of youth for sex chromosomes? Evolution. 2009;63:3043–3049. doi: 10.1111/j.1558-5646.2009.00837.x. [DOI] [PubMed] [Google Scholar]
- 39.Stöck M, Horn A, Grossen C, Lindtke D, Sermier R, Betto-Colliard C, Dufresnes C, Bonjour E, Dumas Z, Luquet E, et al. Ever-young sex chromosomes in European tree frogs. PLoS Biol. 2011;9:e1001062. doi: 10.1371/journal.pbio.1001062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Pecon Slattery J, Sanner-Wachter L, O’Brien SJ. Novel gene conversion between X-Y homologues located in the nonrecombining region of the Y chromosome in Felidae (Mammalia) Proc Natl Acad Sci USA. 2000;97:5307–5312. doi: 10.1073/pnas.97.10.5307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Lynch M, Conery JS. The origins of genome complexity. Science. 2003;302:1401–1404. doi: 10.1126/science.1089370. [DOI] [PubMed] [Google Scholar]
- 42.Smith DR, Lee RW. Low nucleotide diversity for the expanded organelle and nuclear genomes of Volvox carteri supports the mutational-hazard hypothesis. Molecular Biology and Evolution. 2010;27:2244–2256. doi: 10.1093/molbev/msq110. [DOI] [PubMed] [Google Scholar]
- 43.Callahan AM, Huskey RJ. Genetic control of sexual development in Volvox. Dev Biol. 1980;80:419–435. doi: 10.1016/0012-1606(80)90416-9. [DOI] [PubMed] [Google Scholar]