Abstract
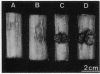
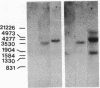
During tumor inception in crown gall disease, a portion of the tumor-inducing (Ti) plasmid, the transferred DNA (T-DNA), is integrated into the genome of the plant cell. Autonomous growth of the transformants requires expression of genes in the tmr and tms regions of the T-DNA, which code for enzymes concerned with biosynthesis of the plant growth hormones cytokinin and auxin, respectively. We show that a mutation of the Habituated leaf gene, Hl, of tobacco can compensate for a defective tmr locus in expression of the tumor phenotype. This provides evidence that a specific host-cell gene has an oncogenic function similar to tmr in the T-DNA.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Akiyoshi D. E., Klee H., Amasino R. M., Nester E. W., Gordon M. P. T-DNA of Agrobacterium tumefaciens encodes an enzyme of cytokinin biosynthesis. Proc Natl Acad Sci U S A. 1984 Oct;81(19):5994–5998. doi: 10.1073/pnas.81.19.5994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Akiyoshi D. E., Regier D. A., Jen G., Gordon M. P. Cloning and nucleotide sequence of the tzs gene from Agrobacterium tumefaciens strain T37. Nucleic Acids Res. 1985 Apr 25;13(8):2773–2788. doi: 10.1093/nar/13.8.2773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Auerswald E. A., Ludwig G., Schaller H. Structural analysis of Tn5. Cold Spring Harb Symp Quant Biol. 1981;45(Pt 1):107–113. doi: 10.1101/sqb.1981.045.01.019. [DOI] [PubMed] [Google Scholar]
- BRAUN A. C. Plant tumors as an experimental model. Harvey Lect. 1960;56:191–210. [PubMed] [Google Scholar]
- BRAUN A. C. The activation of two growth-substance systems accompanying the conversion of normal to tumor cells in crown gall. Cancer Res. 1956 Jan;16(1):53–56. [PubMed] [Google Scholar]
- Barry G. F., Rogers S. G., Fraley R. T., Brand L. Identification of a cloned cytokinin biosynthetic gene. Proc Natl Acad Sci U S A. 1984 Aug;81(15):4776–4780. doi: 10.1073/pnas.81.15.4776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barton K. A., Binns A. N., Matzke A. J., Chilton M. D. Regeneration of intact tobacco plants containing full length copies of genetically engineered T-DNA, and transmission of T-DNA to R1 progeny. Cell. 1983 Apr;32(4):1033–1043. doi: 10.1016/0092-8674(83)90288-x. [DOI] [PubMed] [Google Scholar]
- Bevan M. W., Chilton M. D. T-DNA of the Agrobacterium Ti and Ri plasmids. Annu Rev Genet. 1982;16:357–384. doi: 10.1146/annurev.ge.16.120182.002041. [DOI] [PubMed] [Google Scholar]
- Braun A. C. A DEMONSTRATION OF THE RECOVERY OF THE CROWN-GALL TUMOR CELL WITH THE USE OF COMPLEX TUMORS OF SINGLE-CELL ORIGIN. Proc Natl Acad Sci U S A. 1959 Jul;45(7):932–938. doi: 10.1073/pnas.45.7.932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braun A. C. Plant tumors. Biochim Biophys Acta. 1978 Oct 27;516(2):167–191. doi: 10.1016/0304-419x(78)90007-0. [DOI] [PubMed] [Google Scholar]
- Braun A. C., Wood H. N. Suppression of the neoplastic state with the acquisition of specialized functions in cells, tissues, and organs of crown gall teratomas of tobacco. Proc Natl Acad Sci U S A. 1976 Feb;73(2):496–500. doi: 10.1073/pnas.73.2.496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fraley R. T., Rogers S. G., Horsch R. B., Sanders P. R., Flick J. S., Adams S. P., Bittner M. L., Brand L. A., Fink C. L., Fry J. S. Expression of bacterial genes in plant cells. Proc Natl Acad Sci U S A. 1983 Aug;80(15):4803–4807. doi: 10.1073/pnas.80.15.4803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldberg S. B., Flick J. S., Rogers S. G. Nucleotide sequence of the tmr locus of Agrobacterium tumefaciens pTi T37 T-DNA. Nucleic Acids Res. 1984 Jun 11;12(11):4665–4677. doi: 10.1093/nar/12.11.4665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halpaap-Wood K., Horning E. C., Horning M. G. The effect of phenobarbital and beta-naphthoflavone induction on the metabolism of biphenyl in the rat and mouse. Drug Metab Dispos. 1981 Mar-Apr;9(2):97–102. [PubMed] [Google Scholar]
- Heldin C. H., Westermark B. Growth factors: mechanism of action and relation to oncogenes. Cell. 1984 May;37(1):9–20. doi: 10.1016/0092-8674(84)90296-4. [DOI] [PubMed] [Google Scholar]
- Hepburn A. G., Clarke L. E., Blundy K. S., White J. Nopaline Ti-plasmid, pTiT37, T-DNA insertions into a flax genome. J Mol Appl Genet. 1983;2(2):211–224. [PubMed] [Google Scholar]
- Hoekema A., de Pater B. S., Fellinger A. J., Hooykaas P. J., Schilperoort R. A. The limited host range of an Agrobacterium tumefaciens strain extended by a cytokinin gene from a wide host range T-region. EMBO J. 1984 Dec 20;3(13):3043–3047. doi: 10.1002/j.1460-2075.1984.tb02255.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matzke A. J., Chilton M. D. Site-specific insertion of genes into T-DNA of the Agrobacterium tumor-inducing plasmid: an approach to genetic engineering of higher plant cells. J Mol Appl Genet. 1981;1(1):39–49. [PubMed] [Google Scholar]
- Meins F., Jr, Binns A. Epigenetic variation of cultured somatic cells: evidence for gradual changes in the requirement for factors promoting cell division. Proc Natl Acad Sci U S A. 1977 Jul;74(7):2928–2932. doi: 10.1073/pnas.74.7.2928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murray M. G., Thompson W. F. Rapid isolation of high molecular weight plant DNA. Nucleic Acids Res. 1980 Oct 10;8(19):4321–4325. doi: 10.1093/nar/8.19.4321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ralston R., Bishop J. M. The protein products of the myc and myb oncogenes and adenovirus E1a are structurally related. Nature. 1983 Dec 22;306(5945):803–806. doi: 10.1038/306803a0. [DOI] [PubMed] [Google Scholar]
- Rigby P. W., Dieckmann M., Rhodes C., Berg P. Labeling deoxyribonucleic acid to high specific activity in vitro by nick translation with DNA polymerase I. J Mol Biol. 1977 Jun 15;113(1):237–251. doi: 10.1016/0022-2836(77)90052-3. [DOI] [PubMed] [Google Scholar]
- Rothstein S. J., Jorgensen R. A., Postle K., Reznikoff W. S. The inverted repeats of Tn5 are functionally different. Cell. 1980 Mar;19(3):795–805. doi: 10.1016/s0092-8674(80)80055-9. [DOI] [PubMed] [Google Scholar]
- SKOOG F., MILLER C. O. Chemical regulation of growth and organ formation in plant tissues cultured in vitro. Symp Soc Exp Biol. 1957;11:118–130. [PubMed] [Google Scholar]
- Schröder G., Waffenschmidt S., Weiler E. W., Schröder J. The T-region of Ti plasmids codes for an enzyme synthesizing indole-3-acetic acid. Eur J Biochem. 1984 Jan 16;138(2):387–391. doi: 10.1111/j.1432-1033.1984.tb07927.x. [DOI] [PubMed] [Google Scholar]
- Southern E. M. Detection of specific sequences among DNA fragments separated by gel electrophoresis. J Mol Biol. 1975 Nov 5;98(3):503–517. doi: 10.1016/s0022-2836(75)80083-0. [DOI] [PubMed] [Google Scholar]
- Thomashow L. S., Reeves S., Thomashow M. F. Crown gall oncogenesis: evidence that a T-DNA gene from the Agrobacterium Ti plasmid pTiA6 encodes an enzyme that catalyzes synthesis of indoleacetic acid. Proc Natl Acad Sci U S A. 1984 Aug;81(16):5071–5075. doi: 10.1073/pnas.81.16.5071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turgeon R., Wood H. N., Braun A. C. Studies on the recovery of crown gall tumor cells. Proc Natl Acad Sci U S A. 1976 Oct;73(10):3562–3564. doi: 10.1073/pnas.73.10.3562. [DOI] [PMC free article] [PubMed] [Google Scholar]