To facilitate crosstrial comparisons and the understanding of resistance mechanisms, unifying definitions of trastuzumab resistance and trastuzumab refractoriness are provided. Mechanisms of resistance are reviewed.
Keywords: Breast cancer, ErbB-2, mTOR, PI3 kinase, AKT, Trastuzumab
Abstract
Human epidermal growth factor receptor (HER)-2+ breast cancer is a distinct molecular and clinical entity, the prognosis of which is improved by trastuzumab. However, primary resistance to trastuzumab is observed in >50% of patients with HER-2+ advanced breast cancer, and the majority of patients who initially respond to treatment eventually develop disease progression. To facilitate crosstrial comparisons and the understanding of resistance mechanisms, we propose a unifying definition of trastuzumab resistance as progression at first radiological reassessment at 8–12 weeks or within 3 months after first-line trastuzumab in the metastatic setting or new recurrences diagnosed during or within 12 months after adjuvant trastuzumab. In contrast, we define trastuzumab-refractory breast cancer as disease progression after two or more lines of trastuzumab-containing regimens that initially achieved disease response or stabilization at first radiological assessment. We review mechanisms of trastuzumab resistance mediated by p95HER-2 overexpression, phosphoinositide 3-kinase pathway activation, and signaling pathway activation driven by HER-3, epidermal growth factor receptor, and insulin-like growth factor 1 receptor. We distinguish in vitro from in vivo evidence, highlighting that most data describing trastuzumab resistance are derived from preclinical studies or small retrospective patient cohorts, and discuss targeted therapeutic approaches to overcome resistance. Prospective analysis through clinical trials with robust tissue collection procedures, prior to and following acquisition of resistance, integrated with next-generation tumor genome sequencing technologies, is identified as a priority area for development. The identification of predictive biomarkers is of paramount importance to optimize health economic costs and enhance stratification of anti-HER-2 targeted therapies.
Introduction
Human epidermal growth factor receptor (HER)-2+ breast cancer is a distinct molecular and clinical entity. HER-2, encoded by the HER-2 (ERBB2) gene, is overexpressed in 20%–30% of metastatic breast cancer (MBC) cases, wherein it plays a direct role in pathogenesis [1].
HER-2, together with HER-1, HER-3, and HER-4, are members of the human epidermal growth factor receptor family. HER-1, HER-3, and HER-4 can be activated by various ligands, which trigger conformational rearrangement of the receptor molecules to allow homo- or heterodimerization. The phosphoinositide 3-kinase (PI3K)–AKT and the RAS–RAF–mitogen-activated protein kinase (MAPK)/extracellular signal–related kinase kinase–MAPK pathways are two key downstream pathways [2]. HER-2 has no known ligand, and its dimerization domain is exposed for dimerization even in the inactive monomer state [2]. Because of this peculiar property, HER-2 amplification leads to increased signaling through both homodimerization and heterodimerization.
Prior to the advent of targeted therapy, HER-2+ disease was associated with shorter disease-free survival and overall survival (OS) times than with HER-2− disease [1]. Trastuzumab is a humanized recombinant monoclonal antibody that binds to the extracellular domain of HER-2. The introduction of trastuzumab into clinical practice has changed the landscape of the management and outcome of HER-2+ breast cancer. Blockade of HER-2 cleavage, inhibition of downstream intracellular signaling, antiangiogenesis effects, and recruitment of antibody-dependent cell-mediated immunity have all been implicated as its mechanisms of action [3]. Moreover, recent in vitro studies demonstrated that trastuzumab disrupts ligand-independent HER-2–HER-3–PI3K complex formation, thus inhibiting PI3K–AKT signaling [4].
Single-agent trastuzumab is associated with objective response rates (ORRs) of 19%–26% in the first-line treatment of HER-2–overexpressing MBC [5, 6]. The combination of chemotherapy with trastuzumab, compared with chemotherapy alone, resulted in a higher ORR (50% versus 32%; p < .001), longer time to tumor progression (TTP) (7.4 months versus 4.6 months; p < .001), and longer OS time (25.1 months versus 20.3 months; p = .01) in a landmark phase III first-line study [7].
Despite its robust activity, disease progression at the first radiological assessment, or primary resistance, was observed in >50% of patient with HER-2+ disease treated with single-agent trastuzumab [5, 6]. For patients who initially have sensitive disease, the majority eventually develop acquired resistance.
In the current literature, there is no standard definition for trastuzumab resistance or refractoriness; these terms are often used interchangeably. They carry variable meanings with different assessment time points across trials (Table 1). The lack of a standard definition has made crosstrial comparisons and the understanding of resistance mechanisms difficult. Tumors that progress after multiple lines of trastuzumab-containing treatment but have demonstrated sensitivity to anti–HER-2 approaches may have different resistance mechanisms than tumors that progress during first-line trastuzumab treatment. Such intrinsic resistance mechanisms may encompass a cohort of patients with disease that grows independently of HER-2 signaling, despite HER-2 amplification or overexpression. The current terminology does not clearly define such a distinction and may impede the discovery and implementation of effective biomarkers to predict appropriate therapeutic strategies following progression on trastuzumab.
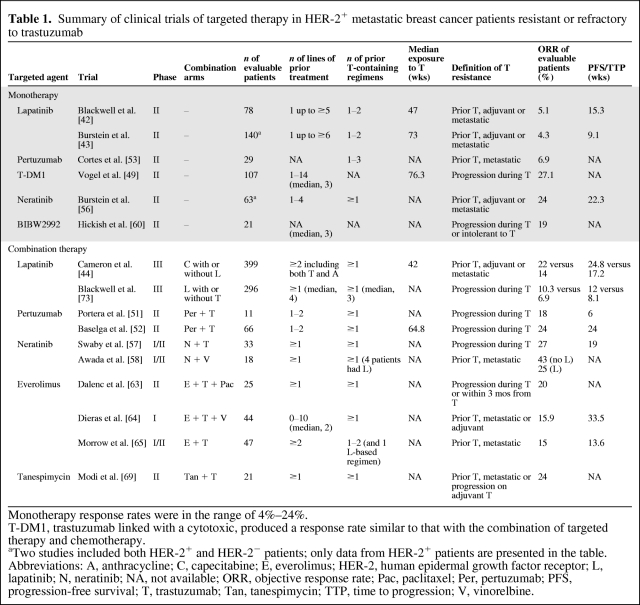
Table 1.
Summary of clinical trials of targeted therapy in HER-2+ metastatic breast cancer patients resistant or refractory to trastuzumab
Monotherapy response rates were in the range of 4%–24%.
T-DM1, trastuzumab linked with a cytotoxic, produced a response rate similar to that with the combination of targeted therapy and chemotherapy.
aTwo studies included both HER-2+ and HER-2− patients; only data from HER-2+ patients are presented in the table.
Abbreviations: A, anthracycline; C, capecitabine; E, everolimus; HER-2, human epidermal growth factor receptor; L, lapatinib; N, neratinib; NA, not available; ORR, objective response rate; Pac, paclitaxel; Per, pertuzumab; PFS, progression-free survival; T, trastuzumab; Tan, tanespimycin; TTP, time to progression; V, vinorelbine.
In this review, we first propose definitions of trastuzumab resistance and trastuzumab refractoriness, then explore possible mechanisms and HER-2–targeted therapeutics to circumvent resistance.
Materials and Methods
Data for this review were identified by searches of MEDLINE, Current Contents, PubMed, and references from relevant articles using the search terms “HER2,” “breast cancer,” and “trastuzumab resistance.” Abstracts and reports from meetings were included when directly relevant. Only articles published in English in 1980–2011 were included.
Definitions of Trastuzumab Resistance and Refractoriness
In a summary of recently published clinical trials (Table 1), we suggest clear and consistent definitions for HER-2+ breast cancers resistant or refractory to trastuzumab therapy that will serve as future benchmarks to implement molecularly driven biomarker discovery and validation clinical trial programs. Trastuzumab resistance is defined as progression at first radiological reassessment at 8–12 weeks or within 3 months after first-line trastuzumab with or without chemotherapy in the metastatic setting or new recurrences diagnosed during or within 12 months after adjuvant trastuzumab. Trastuzumab refractoriness may be defined as disease progression after two or more lines of trastuzumab-containing regimens that initially achieved disease response or stabilization at first radiological assessment. The time points and number of treatment lines stated in these definitions are based on those specified in the majority of published clinical trials (Table 1).
We suggest that the occurrence of brain metastases as the first and only site of progression after trastuzumab should not be regarded as the development of trastuzumab resistance. This phenomenon, associated with trastuzumab treatment both in the adjuvant [8, 9] and metastatic [10] settings, may be explained by the poor penetration of trastuzumab into the central nervous system (CNS) and, paradoxically, by its high efficacy to control extra-CNS trastuzumab-sensitive disease.
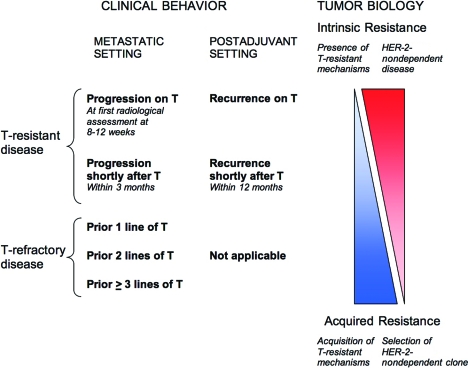
Furthermore, we propose a model delineating the relationship between trastuzumab-resistant or trastuzumab-refractory disease and intrinsic or acquired resistance (Fig. 1). In this model, the terms “trastuzumab resistance” and “trastuzumab refractoriness” describe the clinical observation of trastuzumab failure, whereas “intrinsic resistance” and “acquired resistance” reflect the underlying tumor biology. Intrinsic resistance plays a dominant role in trastuzumab-resistant disease, whereas acquired resistance is relatively more important in trastuzumab-refractory disease. Intratumor heterogeneity [11], known to occur in HER-2+ breast cancer, should also be considered within this definition, whereby both intrinsic and acquired resistance, with different dominance, may be important in defining clinical outcome. This model would be applicable to describe other anti–HER-2 therapies.
Figure 1.
Model illustrating the implications of trastuzumab-resistant or trastuzumab-refractory disease. Disease progression while on trastuzumab correlates with intrinsic or primary resistance, whereas trastuzumab-refractory disease correlates with acquired or secondary resistance. Clinical scenarios in between represent a spectrum. In the adjuvant setting, progression while on or within a short period from trastuzumab likewise implies intrinsic resistance, but disease relapse after prior trastuzumab is heterogeneous and can fit into anywhere in the spectrum.
Abbreviation: T, trastuzumab.
Molecular Mechanisms of Trastuzumab Resistance
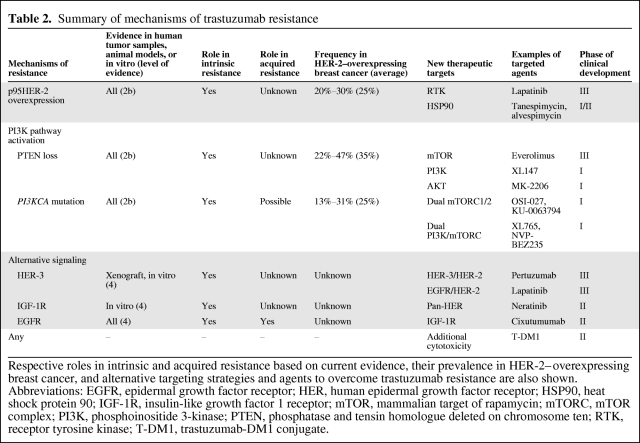
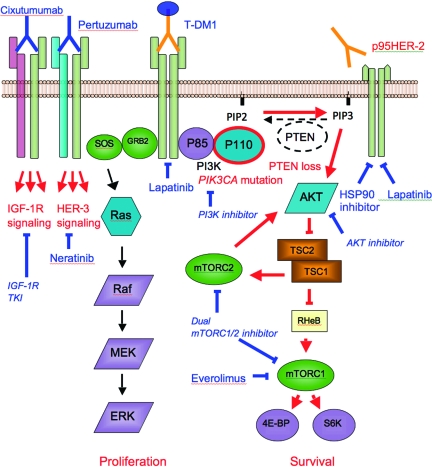
Understanding these mechanisms and their relevance to intrinsic and acquired resistance facilitates rational design of treatment strategies following disease progression on trastuzumab (Table 2 and Fig. 2).
Table 2.
Summary of mechanisms of trastuzumab resistance
Respective roles in intrinsic and acquired resistance based on current evidence, their prevalence in HER-2–overexpressing breast cancer, and alternative targeting strategies and agents to overcome trastuzumab resistance are also shown.
Abbreviations: EGFR, epidermal growth factor receptor; HER, human epidermal growth factor receptor; HSP90, heat shock protein 90; IGF-1R, insulin-like growth factor 1 receptor; mTOR, mammalian target of rapamycin; mTORC, mTOR complex; PI3K, phosphoinositide 3-kinase; PTEN, phosphatase and tensin homologue deleted on chromosome ten; RTK, receptor tyrosine kinase; T-DM1, trastuzumab-DM1 conjugate.
Figure 2.
Illustration of main mechanisms of trastuzumab resistance (in red) and targeted agents to circumvent resistance (in blue). Truncated HER-2 expression prevents binding of trastuzumab. Alternative signaling, such as through HER-3, can lead to continued downstream signaling. The PI3K–AKT pathway can be constitutively activated through loss of PTEN or PIK3CA mutations. Lapatinib inhibits downstream signaling of HER-2 and p95HER-2. T-DM1 provides additional cytotoxic activity. HSP90 inhibitors, such as tanespimycin, lead to degradation of p95HER-2. Pertuzumab prevents dimerization of HER-2 and HER-3. Cixutumumab inhibits IGF-1R. Everolimus is a mTORC1 inhibitor. Other investigational agents in early clinical development are also shown in italics.
Abbreviations: ERK, extracellular signal–related kinase; GRB2, growth factor receptor-bound protein 2; HER-2, human epidermal growth factor receptor; HSP90, heat shock protein 90; IGF-1R, insulin-like growth factor 1 receptor; MEK, mitogen-activated protein kinase/ERK kinase; mTORC1, mammalian target of rapamycin complex 1; mTORC2, mammalian target of rapamycin complex 2; PI3K, phosphoinositide 3-kinase; PIP2, phosphatidylinositol-bisphosphate; PIP3, phosphatidylinositol-trisphosphate; PTEN, phosphatase and tensin homologue deleted on chromosome ten; RHeB, Ras homolog enriched in brain; SOS; son of sevenless; T-DM1, trastuzumab–DM1 conjugate; TKI, tyrosine kinase inhibitor; TSC, tuberous sclerosis protein.
Expression of p95HER-2
The action of trastuzumab may be prevented in tumor cells that express p95HER-2, a truncated form of HER-2 that lacks the extracellular trastuzumab-binding domain while retaining downstream kinase activity [12]. In cultured breast cancer cells, p95HER-2 is generated by proteolytic shedding of the extracellular domain of the receptor [13]. In patients with advanced breast cancer, shed extracellular domain can be detected in the serum [14]. p95HER-2 can also be produced from an alternative RNA transcript that encodes the isoform lacking the extracellular domain [15]. Overexpression of this truncated form is found in 20%–30% HER-2–overexpressed human breast tumors [16]. Whereas the frequency of full-length HER-2 expression is similar in primary and metastatic human breast tumors, p95HER-2 expression is significantly higher in metastatic lymph nodes, suggesting its role in metastasis [16]. Moreover, p95HER-2 expression was correlated with worse a lower 5-year disease-specific survival rate in a retrospective cohort of patients who did not receive trastuzumab, and its prognostic power appeared to be independent of age and nodal or hormone receptor status [16].
p95HER-2 overexpression is associated with an inferior response to trastuzumab in xenografts and human breast tumors [12]. That retrospective analysis showed a significantly lower RR to trastuzumab and trastuzumab–chemotherapy combinations in patients expressing p95HER-2 than in patients with full-length HER-2 (11% versus 51.4%; p = .029). Recently, an assay to quantitatively determine p95HER-2 levels was developed, and high levels of p95HER-2 were found, retrospectively, to correlate with significantly shorter progression-free survival (PFS) and OS times with trastuzumab treatment in HER-2+ MBC patients [17] .
Cells expressing p95HER-2 were shown in vitro and in xenografts to be sensitive to lapatinib [12], which inhibits epidermal growth factor receptor (EGFR) and HER-2 intracellular tyrosine kinase domains. In a retrospective analysis of p95HER-2 expression in human breast tumor samples obtained in two lapatinib trials [18], the ORR and PFS interval following lapatinib therapy were independent of p95HER-2 status. Also, inhibition of heat shock protein 90 (HSP90) results in degradation of p95HER-2 [19] .
Constitutive Activation of the PI3K–AKT Pathway
A second mechanism of trastuzumab resistance is mediated through aberrant activation of the PI3K–AKT pathway, via loss-of-function mutations or loss of heterozygosity of phosphatase and tensin homologue deleted on chromosome ten (PTEN), which encodes a lipid phosphatase that negatively regulates PI3K [20], or by activating mutations of PIK3CA, which encodes the p110α subunit of PI3K [21]. In small retrospective cohorts, activation of the PI3K–AKT pathway was associated with a significantly poorer response to trastuzumab-containing therapies [21].
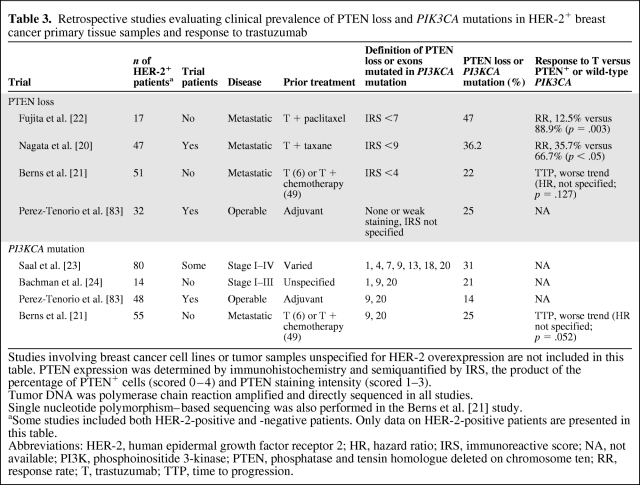
Retrospective studies investigating the prevalence of low PTEN expression [20–22] and PIK3CA mutations [21, 23, 24] in HER-2+ breast cancer patients are summarized in Table 3. Overall, the authors suggest that PTEN loss or PIK3CA mutations are not enriched in HER-2+ breast cancer.
Table 3.
Retrospective studies evaluating clinical prevalence of PTEN loss and PIK3CA mutations in HER-2+ breast cancer primary tissue samples and response to trastuzumab
Studies involving breast cancer cell lines or tumor samples unspecified for HER-2 overexpression are not included in this table. PTEN expression was determined by immunohistochemistry and semiquantified by IRS, the product of the percentage of PTEN+ cells (scored 0–4) and PTEN staining intensity (scored 1–3).
Tumor DNA was polymerase chain reaction amplified and directly sequenced in all studies.
Single nucleotide polymorphism–based sequencing was also performed in the Berns et al. [21] study.
aSome studies included both HER-2-positive and -negative patients. Only data on HER-2-positive patients are presented in this table.
Abbreviations: HER-2, human epidermal growth factor receptor 2; HR, hazard ratio; IRS, immunoreactive score; NA, not available; PI3K, phosphoinositide 3-kinase; PTEN, phosphatase and tensin homologue deleted on chromosome ten; RR, response rate; T, trastuzumab; TTP, time to progression.
The most common somatic PIK3CA mutations are the E545K and H1047R mutations in exon 9 and 20, respectively; both confer gain-of-function activity to HER-2–overexpressing breast cancer cell lines [25]. In vitro, H1047R was found to stimulate autocrine and paracrine HER family ligand production, which in turn enhances signaling through other receptors leading to further activation of AKT [25]. It was reported that ∼20% of patients with wild-type PIK3CA primary breast tumors demonstrated an exon 20 mutation in their metastasis, suggesting that the mutation may be acquired during tumor progression [26].
Trastuzumab resistance mediated through PTEN loss or PIK3CA mutation could be overcome by lapatinib, which reduced phosphorylated AKT levels in trastuzumab-resistant cell lines [27]. Also in vitro, the PI3K inhibitor BEZ235 reversed overexpression of the HER-3 ligand heregulin, which was upregulated in an HER-2–overexpressing PI3K-mutant breast cancer cell line, and in combination with lapatinib, completely inhibited cell growth [25]. Inhibition of mammalian target of rapamycin (mTOR) was shown to reverse trastuzumab resistance caused by PTEN loss in the preclinical setting [28].
Signaling Through Other Receptors
A third mechanism of resistance may occur through signaling by other receptors, especially HER-3, insulin-like growth factor 1 receptor (IGF-1R), and EGFR. HER-3 was found to be a critical coreceptor to maintain cell proliferation in HER-2–overexpressing cell lines, and inhibition of HER-3 signaling via blocking HER-2–HER-3 heterodimerization by pertuzumab induced rapid tumor regression of in vivo xenografts [29].
IGF-1R signaling antagonizes the trastuzumab-induced increase in the level of the cyclin-dependent kinase (CDK) inhibitor p27Kip1, which in turn promotes restoration of CDK2 activity, abrogating cell-cycle arrest in the G1 phase [30]. IGF-1R may also play a role in acquired trastuzumab resistance, and its inhibition improves the response to trastuzumab in vitro [31]. Crosstalk and heterodimerization of IGF-1R with HER-2, HER-3, or both are also observed in trastuzumab-resistant cells in vitro, whereas disruption of these heterodimers resensitizes these cells to trastuzumab [32, 33]. Lapatinib was found to be effective in vitro because it blocked the transactivation of EGFR and HER-2 by IGF-1R [34].
EGFR inhibition suppressed HER-2–driven signaling in HER-2–amplified breast cancer cell lines [35], and higher levels of phosphorylated EGFR and EGFR–HER-2 heterodimer were found in trastuzumab-resistant xenografts [36]. Recently, a correlative analysis of the Translational Breast Cancer Research Consortium TBCRC003 study suggested possible selection of EGFR amplification after trastuzumab treatment mediating trastuzumab resistance, and the benefit of lapatinib in these patients [37].
Other Mechanisms
Other mechanisms have been reported in laboratory models. The interaction of HER-2 and trastuzumab is hindered by expression of the membrane-associated glycoprotein mucin 4, which masks trastuzumab-binding epitopes in HER-2 without altering expression levels [38]. Transforming growth factor (TGF)-β induces secretion of HER ligands, promoting phosphorylation of EGFR, HER-2, and HER-3 [39]. Acquired trastuzumab resistance may result from autocrine production of TGF-α that initiates active EGFR–HER-2 heterodimers and impairs degradation of HER-2 [40]. Feedback upregulation of a disintegrin and metalloprotease (ADAM) proteases and subsequent release of HER ligands [41] have also been observed.
Clinical Development of Alternative HER-2–Targeted Agents
Various HER-2–targeted agents have mechanisms of action that impact known trastuzumab resistance pathways and produce an RR of 4%–27% as monotherapy after trastuzumab failure. Neratinib as a single agent or in combination with chemotherapy appears to produce the highest RR and longest TTP after trastuzumab failure, compared with other targeted agents (Table 1). Although definitive conclusions cannot be drawn based on crosstrial comparisons, these observations suggest that therapeutic strategies to circumvent trastuzumab resistance may rely on potent concomitant blockade of multiple HER family targets.
Lapatinib
Lapatinib is an oral small-molecule reversible dual tyrosine kinase inhibitor (TKI) of both EGFR and HER-2. In particular, lapatinib is active against trastuzumab-resistant cell lines because it inhibits IGF-1 signaling by reducing IGF-1R and HER-2 phosphorylation [34].
Phase II trials have shown ORRs of 4%–5% with lapatinib monotherapy in heavily pretreated MBC patients [42, 43]. In one of the trials, the ORR for the HER-2+ population was 4.3%, compared with 1.4% for the HER-2− group. No correlation was observed between EGFR expression and response to lapatinib. In a pivotal phase III study, HER-2+ MBC patients progressing on prior anthracycline-, taxane-, and trastuzumab-containing treatment were randomly assigned to a combination of capecitabine and lapatinib or capecitabine alone [44]. The median TTP was 6.2 months in the combination group and 4.3 months in the capecitabine group (p = .001), with a trend toward a longer OS duration. This led to the approval of lapatinib in combination with capecitabine for the treatment of HER-2–overexpressing MBC patients with prior anthracycline, taxane, and trastuzumab exposure.
First-line lapatinib monotherapy resulted in an ORR of 24% in a phase II study [45]. This is comparable with that reported using first-line single-agent trastuzumab, as illustrated earlier. A phase III randomized study compared first-line paclitaxel plus lapatinib with placebo in MBC [46]. Fifteen percent of patients had HER-2+ disease. In this HER-2+ subgroup, the ORR was superior—63.3% in the paclitaxel plus lapatinib group versus 37.8% in the paclitaxel alone group (p = .023). The TTP was longer in the combination group (36.4 weeks versus 25.1 weeks; p = .005). In HER-2− patients, no significant differences were found. Subsequent analysis found no correlation between EGFR overexpression and responsiveness to lapatinib [47], concordant with other studies [43].
Antibody–Drug Conjugate
Trastuzumab-DM1 (T-DM1) is an antibody–drug conjugate in which trastuzumab is linked to DM1, an antimicrotubule agent, enabling additional delivery of the cytotoxic to targeted cells. T-DM1 showed activity against trastuzumab-resistant tumor cells [48].
A phase II study of T-DM1 in MBC patients pretreated with chemotherapy and trastuzumab and/or lapatinib [49] showed an ORR of 25%. T-DM1 was generally well tolerated, with side effects of fatigue, thrombocytopenia, epistaxis, and conjunctivitis.
HER Dimerization Inhibitor
Pertuzumab is a recombinant humanized monoclonal antibody that binds to the dimerization epitope of HER-2, blocking its heterodimerization with EGFR and HER-3. Based on preclinical evidence that the combination of trastuzumab and pertuzumab, targeting distinct extracellular HER-2 domains, has synergistic activity in breast cancer cell lines [50], phase II studies were conducted. Patients progressing on trastuzumab-containing regimens received the combination of trastuzumab and pertuzumab. Results demonstrated an ORR of 18%–24% [51, 52]. Pertuzumab monotherapy was less active than the two antibodies combined [53]. Toxicities were mild, with the most common being diarrhea, fatigue, and nausea, but asymptomatic reduction in the ejection fraction was reported.
Pertuzumab and T-DM1 bind different subdomains of HER-2, thus their logical combination was investigated in a phase Ib/II trial [54]. The patients had all been exposed to trastuzumab. Nine partial responses were noted.
Multi-HER TKIs
Neratinib is an orally administered irreversible pan-HER TKI. A phase I study established its activity in patients with advanced solid tumors, including some trastuzumab-pretreated HER-2–overexpressing breast cancer patients [55]. Neratinib demonstrated activity in both trastuzumab-naïve and trastuzumab-resistant HER-2+ breast cancer patients, with ORRs of 56% and 24%, respectively, in a phase II trial [56]. Diarrhea was the most common adverse effect but was manageable.
In a phase I/II study, neratinib plus trastuzumab yielded an ORR of 27% and median PFS interval of 19 weeks in patients with advanced breast cancer progressing on trastuzumab [57]. The combination of neratinib with vinorelbine resulted in ORRs of 25% and 43% in trastuzumab-resistant, lapatinib-pretreated and lapatinib-naïve patients, respectively [58]. In another phase I/II study, the combination of neratinib and weekly paclitaxel resulted in an ORR of 69% in the first- to fourth-line setting [59].
Afatinib is another orally available irreversible EGFR and HER-2 TKI. In a phase II study, four of 21 evaluable trastuzumab-resistant or trastuzumab-intolerant patients derived a partial response [60].
Inhibitors of mTOR
Single-agent temsirolimus resulted in a clinical benefit rate of 37% in advanced breast cancer patients pretreated with an anthracycline and/or taxane in a phase II study [61], although these patients were unstratified by HER-2 status and only 17% had prior trastuzumab exposure. In patients evaluable for HER-2 status in the same study, no response was observed in HER-2− patients whereas the ORR was 15% in HER-2+ patients.
An important phase I study first provided preliminary clinical data that trastuzumab resistance could possibly be reversed by everolimus, an orally administered mTOR inhibitor [62]. In that trial, heavily pretreated patients with progression on prior trastuzumab and taxane therapy were administered everolimus in combination with trastuzumab and paclitaxel. Five of seven evaluable patients had a partial response. The most common toxicities were neutropenia and stomatitis. The investigators then studied the combination in a multicenter phase II setting; interim results showed an ORR of 20% [63]. These results were consistent with those of two other studies in trastuzumab-refractory patients, one investigating everolimus plus trastuzumab and vinorelbine [64] and the other investigating everolimus plus trastuzumab [65].
Notably, two molecular complexes of mTOR, mTOR complex 1 (mTORC1) and mTORC2, exist, depending on the associated protein—raptor or rictor, respectively. The rapamycin analogs temsirolimus and everolimus inhibit only mTORC1, and treatment may lead to paradoxical feedback activation of AKT and eukaryotic translation factor 4E, a downstream effector of mTOR [66]. Dual mTORC1–mTORC2 inhibitors and dual PI3K–mTOR inhibitors may negate this feedback mechanism and are currently in early clinical development (Table 2).
EGFR Inhibitors
Gefitinib and erlotinib are EGFR TKIs still under investigation in the treatment of MBC, but the overall results have been disappointing. Combinations of gefitinib or erlotinib with taxanes resulted in an ORR on the order of 50%–60% [67, 68], but these results are not interpretable without single-agent arms. Cetuximab and panitumumab are monoclonal antibodies that bind to EGFR. Both have not demonstrated reproducible and clinically relevant activity in MBC patients. These results are consistent with preclinical data suggesting preferential phosphorylation of HER-3 over EGFR in HER-2–amplified breast cancer tissues [29].
HSP90 Inhibitors
HSP90 is a molecular chaperone that protects and stabilizes client proteins, such as EGFR, HER-2 (including p95HER-2), and their downstream proteins PI3K and AKT. HSP90 inhibitors, namely, tanespimycin, alvespimycin, and BIIB021, are currently in phase I/II development. In a phase II study of HER-2–overexpressing MBC patients progressing on adjuvant or one line of trastuzumab-based treatment for MBC, the combination of tanespimycin and trastuzumab achieved an RR of 24% [69].
IGF-1R Inhibitors
Cixutumumab (IMC-A12) is a monoclonal antibody that blocks IGF-1R signaling, implicated in trastuzumab resistance. The N0733 trial is accruing to investigate the efficacy and safety of the addition of cixutumumab to lapatinib and capecitabine in HER-2+ patients progressing on prior trastuzumab therapy [70]. BMS-754807, a small molecule inhibitor of IGF-1R, is in phase I of clinical development.
PI3K and AKT Inhibitors
Some PI3K inhibitors are also concomitant inhibitors of mTOR. They not only counteract constitutive activation of the PI3K–AKT pathway, but also prevent reactivation of the same pathway mediated by feedback mechanisms. A multitude of PI3K inhibitors exists. Wortmannin and LY294002 are the first in the class, but neither progressed to clinical evaluation because of poor stability and selectivity. Newer generation compounds are in phase I clinical trials. For example, XL147, GDC0941, GSK1059615, and PX866 inhibit PI3K, whereas XL765, NVP-BEZ235, and SF1126 inhibit both PI3K and mTOR [71]. MK-2206 is a selective AKT inhibitor currently in phase I development.
Continuation of Trastuzumab Beyond Progression
Modest clinical benefit is observed when trastuzumab is continued beyond progression. This observation suggests that such tumors remain dependent on HER-2 signaling.
In one study, HER-2+ breast cancer patients progressing on adjuvant or first-line trastuzumab-based treatment for MBC were randomized to capecitabine with trastuzumab or capecitabine alone at the same dose [72]. The TTP were 8.2 months with the combination and 5.6 months in the monotherapy group (p = .034). The OS times were 25.5 months and 20.4 months, respectively, although the difference was not statistically significant. That study suggested that trastuzumab might still be continued upon disease progression with added clinical benefit.
In the EGF104900 study, HER-2+ MBC patients progressing on prior trastuzumab-containing regimens were assigned to lapatinib alone or in combination with trastuzumab [73]. Patients in the combination arm had a significantly longer PFS interval (p = .008) and higher clinical benefit rate (24.7% versus 12.4%; p = .01). A trend for a longer OS time was also observed. The phase II TBCRC003 study is evaluating lapatinib plus trastuzumab in two cohorts of patients—treatment naïve and pretreated with trastuzumab [74]. Its clinical and biomarker results are awaited, but preliminary reports suggested a trend toward a better outcome with trastuzumab plus lapatinib in trastuzumab-resistant EGFR-amplified patients [37]. These findings also lend support to the role of the continuation of trastuzumab beyond progression.
Future Research
Correlation Between Laboratory and Clinical Trastuzumab Resistance Mechanisms
Research is needed to clarify the correlation between the molecular mechanisms that define the distinction between trastuzumab-resistant and trastuzumab-refractory disease. Many mechanisms are derived from laboratory experiments and lack substantive in vivo confirmation. It is also not clear whether intrinsic and acquired mechanisms are distinct or otherwise overlapping. Moreover, the intertumor prevalence and intratumor clonal frequency of many of the reported mechanisms of trastuzumab resistance have not been conclusively defined in HER-2–overexpressing MBC patients. Assessment of intratumor heterogeneity and the frequency of subdominant clones harboring drug-resistant mechanisms is becoming increasingly important, as emphasized from work demonstrating that c-MET amplification occurs in subpopulations of non-small cell lung cancer cells prior to drug exposure and the acquisition of resistance initiated by MET-driven activation of the PI3K pathway [75]. Prospective biomarker-driven clinical trials are required to formally define and accurately quantify the frequency of these events, and new molecular techniques using functional genomics RNA interference screening approaches and massively parallel tumor sequencing analyses [76, 77] might help reveal unexplained resistance mechanisms in the remaining patients.
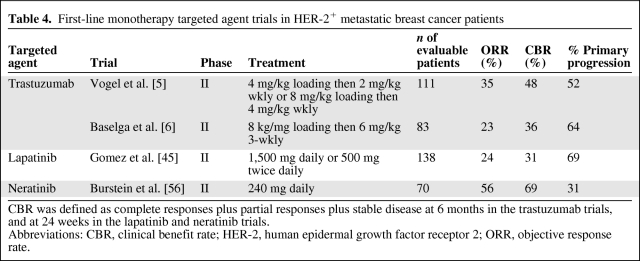
Optimal First-Line Targeted Treatment for HER-2+ MBC Patients
Primary resistance to HER-2–directed therapy occurs less frequently with neratinib than with trastuzumab and lapatinib (Table 4). This confirms that the majority of HER-2+ breast cancers are actually HER-2 dependent and sensitive to HER-2–directed manipulation, although concomitant blockade of multiple HER family members is likely necessary.
Table 4.
First-line monotherapy targeted agent trials in HER-2+ metastatic breast cancer patients
CBR was defined as complete responses plus partial responses plus stable disease at 6 months in the trastuzumab trials, and at 24 weeks in the lapatinib and neratinib trials.
Abbreviations: CBR, clinical benefit rate; HER-2, human epidermal growth factor receptor 2; ORR, objective response rate.
Molecular Mechanisms of Lapatinib Resistance
Resistance to lapatinib is another emerging problem yet to be unraveled. The ORR to lapatinib of >20% in treatment-naïve HER-2+ breast cancer patients [45], compared with <5% in trastuzumab-pretreated patients [43], suggests significant overlapping crossresistance mechanisms between trastuzumab and lapatinib. For example, HER-3 signaling is important in resistance to both drugs. In vitro and in xenografts treated with HER TKIs, inhibition of negative feedback mediated by AKT increases membrane HER-3 expression and phosphorylation [78]. On the other hand, other established trastuzumab resistance mechanisms, such as activated PI3K–AKT signaling through PTEN loss or PIK3CA mutation [27] and IGF-1R signaling [34], have been reported not to confer resistance to lapatinib in cell lines. Similarly, p95HER-2 expression did not affect the response to lapatinib [18]. Lapatinib resistance mechanisms distinct from trastuzumab resistance also include HER-2 kinase domain mutations such as T789I [79], enhanced estrogen signaling [80], and P-glycoprotein expression [81].
HER-2 Testing and Biomarkers
In this context, especially with the financial implications associated with targeted agents, the accurate testing of HER-2 overexpression and the development of novel methods to predict HER-2–PI3K–AKT pathway activation are of paramount importance, in order to facilitate patient selection and to improve the clinical benefit derived from HER-targeting agents. HER-2 testing should preferably be performed in central or quality-assured laboratories. Furthermore, HER-2 is necessary but not sufficient to predict response to HER-2–targeted agents. p95HER-2 expression, PTEN status, and PIK3CA mutation should be prospectively evaluated as predictive biomarkers of resistance to trastuzumab therapy. Retrospective identification of a gene-expression signature specific for trastuzumab resistance was recently reported [82], but large prospective studies are necessary to validate these findings.
Conclusions
Trastuzumab-resistant and trastuzumab-refractory HER-2–overexpressing MBC remain dependent on HER-2 signaling in many cases, displaying the hallmarks of “oncogene addiction.” Monotherapy activity of alternative HER-2–directed therapies and the clinical benefit observed following continued trastuzumab beyond progression support the HER-2–driven nature of these cancers. It is now clear that the oncogenic potential of HER-2 is largely dependent on the activated PI3K pathway [29], which can be exploited rationally as a target to overcome trastuzumab resistance and improve clinical efficacy.
Molecular and clinical research is essential to improve the outcome of HER-2–overexpressing MBC patients that might ultimately impact adjuvant HER-2 treatment strategies. Prospective analysis of HER-2 pathway resistance mechanisms through clinical trials with robust tissue collection procedures, before and after acquisition of resistance, integrated with next-generation sequencing technologies and consistent definitions is identified as a priority area for development. Development of biomarkers predictive of treatment response or resistance is of utmost importance in order to optimize the health economic costs of HER-2–targeted strategies, to develop new therapeutic approaches for patients with intrinsic resistance to HER-2 pathway manipulation, and to enhance treatment stratification and patient outcome.
Acknowledgment
Dr. Ava Kwong is now Visiting Associate Professor, Department of Oncology, Stanford University School of Medicine.
Footnotes
- (C/A)
- consulting/advisory relationship
- (RF)
- Research funding
- (E)
- Employment
- (H)
- Honoraria received
- (OI)
- Ownership interests
- (IP)
- Intellectual property rights/inventor/patent holder
Author Contributions
Conception/Design: Charles Swanton, Thomas Yau, Hilda Wong
Financial support: Raymond Liang
Provision of study material or patients: Charles Swanton, Thomas Yau, Hilda Wong
Collection and/or assembly of data: Charles Swanton, Thomas Yau, Hilda Wong, Ava Kwong, Raymond Liang
Data analysis and interpretation: Charles Swanton, Thomas Yau, Hilda Wong, Roland Leung, Joanne Chiu, Raymond Liang
Manuscript writing: Charles Swanton, Thomas Yau, Hilda Wong, Roland Leung, Ava Kwong, Joanne Chiu, Raymond Liang
Final approval of manuscript: Charles Swanton, Thomas Yau, Hilda Wong, Roland Leung, Ava Kwong, Joanne Chiu, Raymond Liang
References
- 1.Ross JS, Fletcher JA. The HER-2/neu oncogene in breast cancer: Prognostic factor, predictive factor, and target for therapy. Stem Cells. 1998;16:413–428. doi: 10.1002/stem.160413. [DOI] [PubMed] [Google Scholar]
- 2.Hynes NE, Lane HA. ERBB receptors and cancer: The complexity of targeted inhibitors. Nat Rev Cancer. 2005;5:341–354. doi: 10.1038/nrc1609. [DOI] [PubMed] [Google Scholar]
- 3.Valabrega G, Montemurro F, Aglietta M. Trastuzumab: Mechanism of action, resistance and future perspectives in HER2-overexpressing breast cancer. Ann Oncol. 2007;18:977–984. doi: 10.1093/annonc/mdl475. [DOI] [PubMed] [Google Scholar]
- 4.Junttila TT, Akita RW, Parsons K, et al. Ligand-independent HER2/HER3/PI3K complex is disrupted by trastuzumab and is effectively inhibited by the PI3K inhibitor GDC-0941. Cancer Cell. 2009;15:429–440. doi: 10.1016/j.ccr.2009.03.020. [DOI] [PubMed] [Google Scholar]
- 5.Vogel CL, Cobleigh MA, Tripathy D, et al. Efficacy and safety of trastuzumab as a single agent in first-line treatment of HER2-overexpressing metastatic breast cancer. J Clin Oncol. 2002;20:719–726. doi: 10.1200/JCO.2002.20.3.719. [DOI] [PubMed] [Google Scholar]
- 6.Baselga J, Carbonell X, Castañeda-Soto NJ, et al. Phase II study of efficacy, safety, and pharmacokinetics of trastuzumab monotherapy administered on a 3-weekly schedule. J Clin Oncol. 2005;23:2162–2171. doi: 10.1200/JCO.2005.01.014. [DOI] [PubMed] [Google Scholar]
- 7.Slamon DJ, Leyland-Jones B, Shak S, et al. Use of chemotherapy plus a monoclonal antibody against HER2 for metastatic breast cancer that overexpresses HER2. N Engl J Med. 2001;344:783–792. doi: 10.1056/NEJM200103153441101. [DOI] [PubMed] [Google Scholar]
- 8.Bria E, Cuppone F, Fornier M, et al. Cardiotoxicity and incidence of brain metastases after adjuvant trastuzumab for early breast cancer: The dark side of the moon? A meta-analysis of the randomized trials. Breast Cancer Res Treat. 2008;109:231–239. doi: 10.1007/s10549-007-9663-z. [DOI] [PubMed] [Google Scholar]
- 9.Dahabreh IJ, Linardou H, Siannis F, et al. Trastuzumab in the adjuvant treatment of early-stage breast cancer: A systematic review and meta-analysis of randomized controlled trials. The Oncologist. 2008;13:620–630. doi: 10.1634/theoncologist.2008-0001. [DOI] [PubMed] [Google Scholar]
- 10.Yau T, Swanton C, Chua S, et al. Incidence, pattern and timing of brain metastases among patients with advanced breast cancer treated with trastuzumab. Acta Oncol. 2006;45:196–201. doi: 10.1080/02841860500486630. [DOI] [PubMed] [Google Scholar]
- 11.Burrell RA, Juul N, Johnston SR, et al. Targeting chromosomal instability and tumour heterogeneity in HER2-positive breast cancer. J Cell Biochem. 2010;111:782–790. doi: 10.1002/jcb.22781. [DOI] [PubMed] [Google Scholar]
- 12.Scaltriti M, Rojo F, Ocaña A, et al. Expression of p95HER2, a truncated form of the HER2 receptor, and response to anti-HER2 therapies in breast cancer. J Natl Cancer Inst. 2007;99:628–638. doi: 10.1093/jnci/djk134. [DOI] [PubMed] [Google Scholar]
- 13.Molina MA, Codony-Servat J, Albanell J, et al. Trastuzumab (Herceptin), a humanized anti-Her2 receptor monoclonal antibody, inhibits basal and activated Her2 ectodomain cleavage in breast cancer cells. Cancer Res. 2001;61:4744–4749. [PubMed] [Google Scholar]
- 14.Colomer R, Montero S, Lluch A, et al. Circulating HER2 extracellular domain and resistance to chemotherapy in advanced breast cancer. Clin Cancer Res. 2000;6:2356–2362. [PubMed] [Google Scholar]
- 15.Scott GK, Robles R, Park JW, et al. A truncated intracellular HER2/neu receptor produced by alternative RNA processing affects growth of human carcinoma cells. Mol Cell Biol. 1993;13:2247–2257. doi: 10.1128/mcb.13.4.2247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sàez R, Molina MA, Ramsey EE, et al. p95HER-2 predicts worse outcome in patients with HER-2-positive breast cancer. Clin Cancer Res. 2006;12:424–431. doi: 10.1158/1078-0432.CCR-05-1807. [DOI] [PubMed] [Google Scholar]
- 17.Sperinde J, Jin X, Banerjee J, et al. Quantitation of p95HER2 in paraffin sections by using a p95-specific antibody and correlation with outcome in a cohort of trastuzumab-treated breast cancer patients. Clin Cancer Res. 2010;16:4226–4235. doi: 10.1158/1078-0432.CCR-10-0410. [DOI] [PubMed] [Google Scholar]
- 18.Scaltriti M, Chandarlapaty S, Prudkin L, et al. Clinical benefit of lapatinib-based therapy in patients with human epidermal growth factor receptor 2-positive breast tumors coexpressing the truncated p95HER2 receptor. Clin Cancer Res. 2010;16:2688–2695. doi: 10.1158/1078-0432.CCR-09-3407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Chandarlapaty S, Scaltriti M, Angelini P, et al. Inhibitors of HSP90 block p95-HER2 signaling in trastuzumab-resistant tumors and suppress their growth. Oncogene. 2010;29:325–334. doi: 10.1038/onc.2009.337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Nagata Y, Lan KH, Zhou X, et al. PTEN activation contributes to tumor inhibition by trastuzumab, and loss of PTEN predicts trastuzumab resistance in patients. Cancer Cell. 2004;6:117–127. doi: 10.1016/j.ccr.2004.06.022. [DOI] [PubMed] [Google Scholar]
- 21.Berns K, Horlings HM, Hennessy BT, et al. A functional genetic approach identifies the PI3K pathway as a major determinant of trastuzumab resistance in breast cancer. Cancer Cell. 2007;12:395–402. doi: 10.1016/j.ccr.2007.08.030. [DOI] [PubMed] [Google Scholar]
- 22.Fujita T, Doihara H, Kawasaki K, et al. PTEN activity could be a predictive marker of trastuzumab efficacy in the treatment of ErbB2-overexpressing breast cancer. Br J Cancer. 2006;94:247–252. doi: 10.1038/sj.bjc.6602926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Saal LH, Holm K, Maurer M, et al. PIK3CA mutations correlate with hormone receptors, node metastasis, and ERBB2, and are mutually exclusive with PTEN loss in human breast carcinoma. Cancer Res. 2005;65:2554–2559. doi: 10.1158/0008-5472-CAN-04-3913. [DOI] [PubMed] [Google Scholar]
- 24.Bachman KE, Argani P, Samuels Y, et al. The PIK3CA gene is mutated with high frequency in human breast cancers. Cancer Biol Ther. 2004;3:772–775. doi: 10.4161/cbt.3.8.994. [DOI] [PubMed] [Google Scholar]
- 25.Chakrabarty A, Rexer BN, Wang SE, et al. H1047R phosphatidylinositol 3-kinase mutant enhances HER2-mediated transformation by heregulin production and activation of HER3. Oncogene. 2010;29:5193–5203. doi: 10.1038/onc.2010.257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Dupont J, Laenkholm A, Knoop A, et al. PIK3CA Mutations can be acquired during tumor progression in breast cancer [abstract 6023]. Cancer Res; Presented at the Thirty-Second Annual CTRC-AACR San Antonio Breast Cancer Symposium; San Antonio, Texas; December 10–13, 2009. 2009. p. 69. http://dx.doi.org/10.1158/0008-5472.SABCS-09-6023. [Google Scholar]
- 27.O'Brien NA, Browne BC, Chow L, et al. Activated phosphoinositide 3-kinase/AKT signaling confers resistance to trastuzumab but not lapatinib. Mol Cancer Ther. 2010;9:1489–1502. doi: 10.1158/1535-7163.MCT-09-1171. [DOI] [PubMed] [Google Scholar]
- 28.Lu CH, Wyszomierski SL, Tseng LM, et al. Preclinical testing of clinically applicable strategies for overcoming trastuzumab resistance caused by PTEN deficiency. Clin Cancer Res. 2007;13:5883–5888. doi: 10.1158/1078-0432.CCR-06-2837. [DOI] [PubMed] [Google Scholar]
- 29.Lee-Hoeflich ST, Crocker L, Yao E, et al. A central role for HER3 in HER2-amplified breast cancer: Implications for targeted therapy. Cancer Res. 2008;68:5878–5887. doi: 10.1158/0008-5472.CAN-08-0380. [DOI] [PubMed] [Google Scholar]
- 30.Lu Y, Zi X, Pollak M. Molecular mechanisms underlying IGF-I-induced attenuation of the growth-inhibitory activity of trastuzumab (Herceptin) on SKBR3 breast cancer cells. Int J Cancer. 2004;108:334–341. doi: 10.1002/ijc.11445. [DOI] [PubMed] [Google Scholar]
- 31.Browne BC, Crown J, Venkatesan N, et al. Inhibition of IGF1R activity enhances response to trastuzumab in HER-2-positive breast cancer cells. Ann Oncol. 2011;22:68–73. doi: 10.1093/annonc/mdq349. [DOI] [PubMed] [Google Scholar]
- 32.Nahta R, Yuan LX, Zhang B, et al. Insulin-like growth factor-I receptor/human epidermal growth factor receptor 2 heterodimerization contributes to trastuzumab resistance of breast cancer cells. Cancer Res. 2005;65:11118–11128. doi: 10.1158/0008-5472.CAN-04-3841. [DOI] [PubMed] [Google Scholar]
- 33.Huang X, Gao L, Wang S, et al. Heterotrimerization of the growth factor receptors erbB2, erbB3, and insulin-like growth factor-I receptor in breast cancer cells resistant to Herceptin. Cancer Res. 2010;70:1204–1214. doi: 10.1158/0008-5472.CAN-09-3321. [DOI] [PubMed] [Google Scholar]
- 34.Nahta R, Yuan LX, Du Y, et al. Lapatinib induces apoptosis in trastuzumab-resistant breast cancer cells: Effects on insulin-like growth factor I signaling. Mol Cancer Ther. 2007;6:667–674. doi: 10.1158/1535-7163.MCT-06-0423. [DOI] [PubMed] [Google Scholar]
- 35.Moasser MM, Basso A, Averbuch SD, et al. The tyrosine kinase inhibitor ZD1839 (“Iressa”) inhibits HER2-driven signaling and suppresses the growth of HER2-overexpressing tumor cells. Cancer Res. 2001;61:7184–7188. [PubMed] [Google Scholar]
- 36.Ritter CA, Perez-Torres M, Rinehart C, et al. Human breast cancer cells selected for resistance to trastuzumab in vivo overexpress epidermal growth factor receptor and ErbB ligands and remain dependent on the ErbB receptor network. Clin Cancer Res. 2007;13:4909–4919. doi: 10.1158/1078-0432.CCR-07-0701. [DOI] [PubMed] [Google Scholar]
- 37.Krop IE, Flores L, Najita JS, et al. The role of EGFR amplification in trastuzumab resistance: A correlative analysis of TBCRC003 [abstract 528]. J Clin Oncol; Presented at the 47th ASCO Annual Meeting; June 3–7, 2011; Chicago, Illinois. 2011. [Google Scholar]
- 38.Nagy P, Friedländer E, Tanner M, et al. Decreased accessibility and lack of activation of ErbB2 in JIMT-1, a Herceptin-resistant, MUC4-expressing breast cancer cell line. Cancer Res. 2005;65:473–482. [PubMed] [Google Scholar]
- 39.Wang SE, Xiang B, Guix M, et al. Transforming growth factor beta engages TACE and ErbB3 to activate phosphatidylinositol-3 kinase/Akt in ErbB2-overexpressing breast cancer and desensitizes cells to trastuzumab. Mol Cell Biol. 2008;28:5605–5620. doi: 10.1128/MCB.00787-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Valabrega G, Montemurro F, Sarotto I, et al. TGFα expression impairs Trastuzumab-induced HER2 downregulation. Oncogene. 2005;24:3002–3010. doi: 10.1038/sj.onc.1208478. [DOI] [PubMed] [Google Scholar]
- 41.Gijsen M, King P, Perera T, et al. Upregulation of ADAM proteases and HER ligands through a feedback loop mediates acquired resistance to trastuzumab in HER2-amplified breast cancer. Breast Cancer Res. 2010;12(suppl 1):O2. [Google Scholar]
- 42.Blackwell KL, Pegram MD, Tan-Chiu E, et al. Single-agent lapatinib for HER2-overexpressing advanced or metastatic breast cancer that progressed on first- or second-line trastuzumab-containing regimens. Ann Oncol. 2009;20:1026–1031. doi: 10.1093/annonc/mdn759. [DOI] [PubMed] [Google Scholar]
- 43.Burstein HJ, Storniolo AM, Franco S, et al. A phase II study of lapatinib monotherapy in chemotherapy-refractory HER2-positive and HER2-negative advanced or metastatic breast cancer. Ann Oncol. 2008;19:1068–1074. doi: 10.1093/annonc/mdm601. [DOI] [PubMed] [Google Scholar]
- 44.Cameron D, Casey M, Press M, et al. A phase III randomized comparison of lapatinib plus capecitabine versus capecitabine alone in women with advanced breast cancer that has progressed on trastuzumab: Updated efficacy and biomarker analyses. Breast Cancer Res Treat. 2008;112:533–543. doi: 10.1007/s10549-007-9885-0. [DOI] [PubMed] [Google Scholar]
- 45.Gomez HL, Doval DC, Chavez MA, et al. Efficacy and safety of lapatinib as first-line therapy for ErbB2-amplified locally advanced or metastatic breast cancer. J Clin Oncol. 2008;26:2999–3005. doi: 10.1200/JCO.2007.14.0590. [DOI] [PubMed] [Google Scholar]
- 46.Di Leo A, Gomez HL, Aziz Z, et al. Phase III, double-blind, randomized study comparing lapatinib plus paclitaxel with placebo plus paclitaxel as first-line treatment for metastatic breast cancer. J Clin Oncol. 2008;26:5544–5552. doi: 10.1200/JCO.2008.16.2578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Press MF, Finn RS, Cameron D, et al. HER-2 gene amplification, HER-2 and epidermal growth factor receptor mRNA and protein expression, and lapatinib efficacy in women with metastatic breast cancer. Clin Cancer Res. 2008;14:7861–7870. doi: 10.1158/1078-0432.CCR-08-1056. [DOI] [PubMed] [Google Scholar]
- 48.Lewis Phillips GD, Li G, Dugger DL, et al. Targeting HER2-positive breast cancer with trastuzumab-DM1, an antibody-cytotoxic drug conjugate. Cancer Res. 2008;68:9280–9290. doi: 10.1158/0008-5472.CAN-08-1776. [DOI] [PubMed] [Google Scholar]
- 49.Vogel C, Burris HA, Limentani S, et al. A phase II study of trastuzumab-DM1 (T-DM1), a HER2 antibody-drug conjugate (ADC), in patients (pts) with HER2+ metastatic breast cancer (MBC): Final results [abstract 1017]. J Clin Oncol; Presented at the 45th ASCO Annual Meeting; May 29–June 2, 2009; Orlando, Florida. 2009. [Google Scholar]
- 50.Nahta R, Hung MC, Esteva FJ. The HER-2-targeting antibodies trastuzumab and pertuzumab synergistically inhibit the survival of breast cancer cells. Cancer Res. 2004;64:2343–2346. doi: 10.1158/0008-5472.can-03-3856. [DOI] [PubMed] [Google Scholar]
- 51.Portera CC, Walshe JM, Rosing DR, et al. Cardiac toxicity and efficacy of trastuzumab combined with pertuzumab in patients with trastuzumab-insensitive human epidermal growth factor receptor 2-positive metastatic breast cancer. Clin Cancer Res. 2008;14:2710–2716. doi: 10.1158/1078-0432.CCR-07-4636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Baselga J, Gelmon KA, Verma S, et al. Phase II trial of pertuzumab and trastuzumab in patients with human epidermal growth factor receptor 2-positive metastatic breast cancer that progressed during prior trastuzumab therapy. J Clin Oncol. 2010;28:1138–1144. doi: 10.1200/JCO.2009.24.2024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Cortés J, Baselga J, Petrella T, et al. Pertuzumab monotherapy following trastuzumab-based treatment: Activity and tolerability in patients with advanced HER2- positive breast cancer [abstract 1022]. J Clin Oncol; Presented at the 45th ASCO Annual Meeting; May 29–June 2, 2009; Orlando, Florida. 2009. [Google Scholar]
- 54.Miller K, Gianni L, Andre F, et al. A phase Ib/II trial of trastuzumab-DM1 (T-DM1) with pertuzumab (P) for women with HER2-positive, locally advanced or metastatic breast cancer (BC) who were previously treated with trastuzumab (T) [abstract 1012]. J Clin Oncol; Presented at the 46th ASCO Annual Meeting; June 4–8, 2010; Chicago, Illinois. 2010. [Google Scholar]
- 55.Wong K.-K, Fracasso PM, Bukowski RM, et al. A phase I study with neratinib (HKI-272), an irreversible pan ErbB receptor tyrosine kinase inhibitor, in patients with solid tumors. Clin Cancer Res. 2009;15:2552–2558. doi: 10.1158/1078-0432.CCR-08-1978. [DOI] [PubMed] [Google Scholar]
- 56.Burstein HJ, Sun Y, Dirix LY, et al. Neratinib, an irreversible ErbB receptor tyrosine kinase inhibitor, in patients with advanced ErbB2-positive breast cancer. J Clin Oncol. 2010;28:1301–1307. doi: 10.1200/JCO.2009.25.8707. [DOI] [PubMed] [Google Scholar]
- 57.Swaby R, Blackwell K, Jiang Z, et al. Neratinib in combination with trastuzumab for the treatment of advanced breast cancer: A phase I/II study [abstract 1004]. J Clin Oncol; Presented at the 45th ASCO Annual Meeting; May 29–June 2, 2009; Orlando, Florida. 2009. [Google Scholar]
- 58.Awada A, Dirix L, Beck J, et al. Safety and efficacy of neratinib (HKI-272) in combination with vinorelbine in ERBB2+ metastatic breast cancer (MBC) [abstract 145P]. Ann Oncol; Presented at the IMPAKT Breast Cancer Conference; May 5–8, 2010; Brussels, Belgium. 2010. p. iv62. [Google Scholar]
- 59.Chow L, Gupta S, Hershman DL, et al. Safety and efficacy of neratinib (HKI-272) in combination with paclitaxel in ERBB2+ metastatic breast cancer (MBC) [abstract 144P]. Ann Oncol; Presented at the IMPAKT Breast Cancer Conference; May 5–8, 2010; Brussels, Belgium. 2010. p. iv62. [Google Scholar]
- 60.Hickish T, Wheatley D, Lin N, et al. Use of BIBW 2992, a novel irreversible EGFR/HER2 tyrosine kinase inhibitor (TKI), to treat patients with HER2-positive metastatic breast cancer after failure of treatment with trastuzumab [abstract 1023]. J Clin Oncol; Presented at the 45th ASCO Annual Meeting; May 29–June 2, 2009; Orlando, Florida. 2009. [Google Scholar]
- 61.Chan S, Scheulen ME, Johnston S, et al. Final results of a phase 2 study of single agent CCI-779 in locally advanced or metastatic breast cancer failing prior anthracycline and/or taxane regimens [abstract 346] Breast Cancer Res Treat. 2003;82:S82. [Google Scholar]
- 62.Andre F, Campone M., Hurvitz SA, et al. Multicenter phase I clinical trial of daily and weekly RAD001 in combination with weekly paclitaxel and trastuzumab in patients with HER2-overexpressing metastatic breast cancer with prior resistance to trastuzumab [abstract 1003]. Proc Am Soc Clin Oncol; Presented at the 44th ASCO Annual Meeting; May 30–June 3, 2008; Chicago, Illinois. 2008. [Google Scholar]
- 63.Dalenc F, Campone M, Hupperets P, et al. Everolimus in combination with weekly paclitaxel and trastuzumab in patients with HER2-overexpressing metastatic breast cancer with prior resistance to trastuzumab and taxanes: A multicentre phase II clinical trial [abstract 1013]. J Clin Oncol; Presented at the 46th ASCO Annual Meeting; June 4–8, 2010; Chicago, Illinois. 2010. [Google Scholar]
- 64.Dieras V, Gianni L, Jerusalem GH, et al. Multicenter phase I clinical trial of daily and weekly RAD001 (everolimus) in combination with vinorelbine and trastuzumab in patients with HER2-overexpressing metastatic breast cancer with prior resistance to trastuzumab [abstract 253]. Presented at the 2009 American Society of Clinical Oncology Breast Cancer Symposium; September 5–7, 2009; Washington, D.C.. [Google Scholar]
- 65.Morrow PH, Wulf GM, Booser DJ, et al. Phase I/II trial of everolimus (RAD001) and trastuzumab in patients with trastuzumab-resistant, HER2-overexpressing breast cancer [abstract 1014]. J Clin Oncol; Presented at the 46th ASCO Annual Meeting; June 4–8, 2010; Chicago, Illinois. 2010. [Google Scholar]
- 66.Wang X, Yue P, Kim YA, et al. Enhancing mammalian target of rapamycin (mTOR)-targeted cancer therapy by preventing mTOR/raptor inhibition-initiated, mTOR/rictor-independent Akt activation. Cancer Res. 2008;68:7409–7418. doi: 10.1158/0008-5472.CAN-08-1522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Cresta S, Perotti A, Merlini L, et al. Combination of paclitaxel and two schedules of gefitinib in patients with metastatic breast cancer. J Clin Oncol. 2006;24:10599. [Google Scholar]
- 68.Kaur H, Silverman P, Singh D, et al. Toxicity and outcome data in a phase II study of weekly docetaxel in combination with erlotinib in recurrent and/or metastatic breast cancer (MBC) J Clin Oncol. 2006;24:10623. [Google Scholar]
- 69.Modi S, Sugarman S, Stopeck A, et al. Phase II trial of the Hsp90 inhibitor tanespimycin (Tan) + trastuzumab (T) in patients (pts) with HER2-positive metastatic breast cancer (MBC) [abstract 1027]. J Clin Oncol; Presented at the 44th ASCO Annual Meeting; May 30–June 3, 2008; Chicago, Illinois. 2008. [Google Scholar]
- 70.Haluska P, Reinholz MM, Dueck AC, et al. N0733: Phase II trial of capecitabine and lapatinib plus or minus cixutumumab in HER2-positive breast cancer [abstract TPS 129]. J Clin Oncol; Presented at the 46th ASCO Annual Meeting; June 4–8, 2010; Chicago, Illinois. 2010. [Google Scholar]
- 71.Markman B, Atzori F, Pérez-García J, et al. Status of PI3K inhibition and biomarker development in cancer therapeutics. Ann Oncol. 2010;21:683–691. doi: 10.1093/annonc/mdp347. [DOI] [PubMed] [Google Scholar]
- 72.von Minckwitz G, du Bois A, Schmidt M, et al. Trastuzumab beyond progression in human epidermal growth factor receptor 2-positive advanced breast cancer: A German Breast Group 26/Breast International Group 03–05 study. J Clin Oncol. 2009;27:1999–2006. doi: 10.1200/JCO.2008.19.6618. [DOI] [PubMed] [Google Scholar]
- 73.Blackwell KL, Burstein HJ, Storniolo AM, et al. Randomized study of lapatinib alone or in combination with trastuzumab in women with ErbB2-positive, trastuzumab-refractory metastatic breast cancer. J Clin Oncol. 2010;28:1124–1130. doi: 10.1200/JCO.2008.21.4437. [DOI] [PubMed] [Google Scholar]
- 74.Lin NU, Mayer A, Hobday TJ, et al. TBCRC 003: Phase II trial of trastuzumab (T) and lapatinib (L) in patients with HER2+ metastatic breast cancer (MBC) [abstract TPS 132]. J Clin Oncol; Presented at the 46th ASCO Annual Meeting; June 4–8, 2010; Chicago, Illinois. 2010. [Google Scholar]
- 75.Turke AB, Zejnullahu K, Wu YL, et al. Preexistence and clonal selection of MET amplification in EGFR mutant NSCLC. Cancer Cell. 2010;17:77–88. doi: 10.1016/j.ccr.2009.11.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Juul N, Szallasi Z, Eklund AC, et al. Assessment of an RNA interference screen-derived mitotic and ceramide pathway metagene as a predictor of response to neoadjuvant paclitaxel for primary triple-negative breast cancer: A retrospective analysis of five clinical trials. Lancet Oncol. 2010;11:358–365. doi: 10.1016/S1470-2045(10)70018-8. [DOI] [PubMed] [Google Scholar]
- 77.Swanton C, Larkin JM, Gerlinger M, et al. Predictive biomarker discovery through the parallel integration of clinical trial and functional genomics datasets. Genome Med. 2010;2:53. doi: 10.1186/gm174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Sergina NV, Rausch M, Wang D, et al. Escape from HER-family tyrosine kinase inhibitor therapy by the kinase-inactive HER3. Nature. 2007;445:437–441. doi: 10.1038/nature05474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Trowe T, Boukouvala S, Calkins K, et al. EXEL-7647 inhibits mutant forms of ErbB2 associated with lapatinib resistance and neoplastic transformation. Clin Cancer Res. 2008;14:2465–2475. doi: 10.1158/1078-0432.CCR-07-4367. [DOI] [PubMed] [Google Scholar]
- 80.Xia W, Bacus S, Hegde P, et al. A model of acquired autoresistance to a potent ErbB2 tyrosine kinase inhibitor and a therapeutic strategy to prevent its onset in breast cancer. Proc Natl Acad Sci U S A. 2006;103:7795–7800. doi: 10.1073/pnas.0602468103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Polli JW, Humphreys JE, Harmon KA, et al. The role of efflux and uptake transporters in [N-{3-chloro-4-[(3-fluorobenzyl)oxy]phenyl}-6-[5-({[2-(methylsulfonyl)ethyl]amino}methyl)-2-furyl]-4-quinazolinamine (GW572016, lapatinib) disposition and drug interactions. Drug Metab Dispos. 2008;36:695–701. doi: 10.1124/dmd.107.018374. [DOI] [PubMed] [Google Scholar]
- 82.Khoury T, Kanehira K, Wang D, et al. Breast carcinoma with amplified HER2: A gene expression signature specific for trastuzumab resistance and poor prognosis. Mod Pathol. 2010;23:1364–1378. doi: 10.1038/modpathol.2010.125. [DOI] [PubMed] [Google Scholar]
- 83.Perez-Tenorio G, Alkhori L, Olsson B, et al. PIK3CA mutations and PTEN loss correlate with similar prognostic factors and are not mutually exclusive in breast cancer. Clin Cancer Res. 2007;13:3577–3584. doi: 10.1158/1078-0432.CCR-06-1609. [DOI] [PubMed] [Google Scholar]