The types and rates of second malignancies following cancers commonly seen in older adults are described and the literature on these malignancies is reviewed.
Keywords: Neoplasms, Second primary, Geriatrics, Antineoplastic agents, Neoplasms, Radiation induced, Lymphoma, Carcinoma
Abstract
The U.S. population is aging, life expectancy is increasing, and cancer is a disease associated with aging. Advances in screening and therapeutics have led to a growing number of cancer survivors who are at risk for the development of secondary malignancies. Although the risks for the development of second malignancies following a first diagnosis of cancer are well described for survivors of childhood malignancies, there are fewer data for malignancies common in older adults. With the aging of the U.S. population, and with improving survival statistics in many adult malignancies, there is an increasing need to identify those second malignancies that might develop in the older adult survivor of cancer. In this paper, we describe the types and rates of second malignancies following cancers commonly seen in older adults and review the literature on these malignancies. Comparisons are made between older and younger adults with regard to the risks for developing treatment-related cancers with different modalities. Recommendations for early detection of second malignancies are summarized, though there remains an unmet need for evidence-based guidelines for screening for second malignancies in the older adult in particular.
Introduction
The number of cases of cancer in the U.S. is increasing, with >1.5 million estimated new cases in 2010, compared with 1.3 million cases estimated in 2005 [1]. This is a result, for the most part, of the aging of the population. The absolute number of cases diagnosed in those individuals aged ≥65 years is expected to double by 2030 [2]. Approximately 21% of men and 15% of women will develop cancer at some time between their 50th and 70th birthdays [3]. Both the increase in number of cases and the increase in survival rates are expected to contribute to a growing number of cancer survivors in the coming decades.
It is well established that the risk for a new malignancy is higher in those who have already survived one or more cancers than in the general population [4–7]. Second cancers make up as many as 14% of all incident malignancies in the U.S. [5, 7, 8]. Adult survivors of childhood cancer have up to six times the lifetime risk of developing a new malignancy than the general population [9, 10]. However, the overall frequency of second cancers is highest among those diagnosed with their first cancer at the age of 50–69 years (16.4% by 25 years of follow-up) [10].
Although adults who develop a first cancer at age ≥70 years have not been shown to be at higher risk, on average, than the general population for the development of a new cancer, it is thought that the lack of a higher risk seen among adults with a first diagnosis at age ≥70 years may be a result of underreporting of second malignancies in older patients, who may be suboptimally followed after first diagnosis [10, 11]. Additional possible reasons for equivalent or lower rates of second cancers in older adults may include a lesser susceptibility to the mutagenic effect of radiation, a lower likelihood of association with a genetic cancer syndrome, more competing causes of mortality, generally fewer remaining years to develop a second cancer, and more indolent sporadic cancers than in the younger adult population requiring less aggressive treatment. Another possibility is that age is occasionally a surrogate for era of treatment. Older adults who are long-term survivors of cancer were generally treated in an earlier time period with different survival rates, which could affect perceived second malignancy rates. Parsing out to what extent these factors play a role has rarely been done and makes it difficult to examine the impact of second malignancies among older adults.
Genetic cancer syndromes, shared risk factors, and treatment of the first cancer may predispose to second malignancies [12]. Below we describe second malignancies that are common among older adults.
Overview of Treatment-Related Second Malignancies in Older Adults
Second malignancies may be related to prior chemotherapy, radiation therapy, hormonal therapy, or a combination of effects of more than one of these modalities. For a patient to be susceptible to a second malignancy, that patient needs to survive long enough following diagnosis and treatment of the first cancer for new carcinogenesis to take place. Hence, second malignancies are best reported for cancers and/or treatment modalities that have high long-term survivorship numbers and rates, such as breast and prostate cancers. Conversely, patients with aggressive cancers such as lung cancer have poor survival rates, leading to a small relative number of survivors of lung cancer among the population of cancer survivors, despite the high incidence of lung cancer in the population [13]. Furthermore, less common cancers, such as Hodgkin's lymphoma (HL) and testicular cancer, are associated with the development of second malignancies largely because of the high curability of these diseases and relatively young age of many patients at diagnosis, and hence the large number of person-years of follow-up [8].
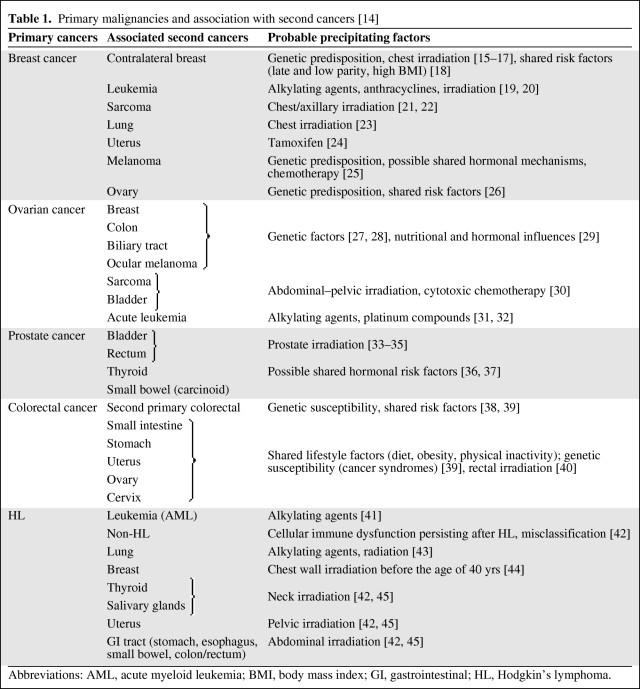
Solid tumors, as a whole, are more common sequelae of cancer treatment than hematologic malignancies. There are some cancers that are particularly prone to develop after certain types of primary cancer. Examples of these include breast cancer following HL, bladder cancer following prostate cancer, and sarcoma following breast cancer. An abridged list of new malignancies associated with specific primary cancers can be seen in Table 1 [14–45].
Table 1.
Primary malignancies and association with second cancers [14]
Abbreviations: AML, acute myeloid leukemia; BMI, body mass index; GI, gastrointestinal; HL, Hodgkin's lymphoma.
Importantly, treatment-related second malignancies are often more aggressive and less responsive to treatment than malignancies not related to treatment, even in older adults. For example, the median survival time of patients with therapy-related acute myeloid leukemia (AML) is only 8–10 months, with a 5-year survival rate <10% [46]. This is compared with a 5-year relative survival rate of 23% in all adult patients with AML [47].
Breast Cancer Survivors
In survivors of a first breast cancer, the risk for a subsequent contralateral breast cancer is approximately two- to fivefold greater [15, 16]. Some of the greater risk is a result of pre-existing risk factors, and radiation therapy may also contribute, though results of trials are conflicting. In a study by Boice et al. [15], the overall relative risk (RR) for contralateral breast cancer was not greater in women treated with radiation therapy than in women with breast cancer who did not receive radiation. However, in women aged <45 years at the time of radiation therapy, the RR was significantly higher (RR, 1.59; 95% confidence interval [CI], 1.07–2.36), consistent with the hypothesis that older women are not as likely to develop radiation-associated breast cancer [15]. In a large Danish case–control study, there was no significant difference in the risk for contralateral breast cancer in women who were irradiated versus those who were not, regardless of age at treatment, and the locations of second cancers were evenly distributed across the breast. Areas of the contralateral breast near the radiation field would be more likely to develop a tumor if there had been a true radiation effect [16]. In an Early Breast Cancer Trialists' Collaborative Group report, which combined 42,000 women from 78 clinical trials and reported on 15-year survival outcomes, there was a significantly higher risk for contralateral breast cancer (rate ratio, 1.18; standard error, 0.06; p = .002) in those who were irradiated, even among women aged ≥50 years at the time of randomization [17]. And in a study by Gao et al. [18], women aged >55 years at the time of initial diagnosis of breast cancer had a significantly higher risk for developing contralateral breast cancer than women aged 45–55 years (RR, 1.15; p = .04). Additionally, the risk associated with radiation therapy was apparent in both younger (age <45 years) and older (age ≥55 years) women, with women aged ≥55 years 15% more likely to develop contralateral breast cancer if they received radiation than if they did not [18].
Other solid malignancies are also more likely following radiation treatment for breast cancer. Women who received radiation treatment for breast cancer in one population-based study were found to have a significantly greater risk for developing lung cancer after 10 years (RR, 1.62; 95% CI, 1.05–2.54) and a higher risk for esophageal cancer after 15 years (RR, 2.19; 95% CI, 1.10–4.62) than those who did not receive radiation, although no breakdown of risk by age was performed [23]. The risk for second cancers appears higher with radiation given postmastectomy, wherein the irradiated field is more extensive, where radiation given postlumpectomy has less certain effects [48]. Sarcoma risk is higher in survivors of breast cancer, though the overall risk remains low, and these cancers appear to be related to prior radiation because the tumors occur most often in the irradiated field [21, 22].
In survivors of breast cancer, alkylating agents have been associated with a higher risk for subsequent leukemia. In one study, those who received alkylating agents had a 10-fold higher risk for subsequent AML than those who received neither chemotherapy nor radiation [19]. The dose intensity of alkylating agents, specifically cyclophosphamide, is an independent determinant of future hematologic malignancy risk. In a study of patients with breast cancer that combined results from multiple National Surgical Adjuvant Breast and Bowel Project (NSABP) studies, a dose-intense arm of cyclophosphamide (2,400 mg/m2 every 3 weeks for either two or four cycles) when combined with standard-dose doxorubicin (60 mg/m2) was associated with a 5-year risk for the development of AML of 1.01% (95% CI, 0.63%–1.62%), compared with a risk of 0.21% in those who received standard dosing of each agent (cyclophosphamide, 600 mg/m2; doxorubicin, 60 mg/m2; every 21 days for four cycles) [20].
Older age may predispose to a higher risk for hematologic malignancy following treatment for breast cancer. In a study by Patt et al. [49], women aged ≥66 years at the time of a breast cancer diagnosis showed a 10-year absolute risk for developing AML after adjuvant chemotherapy of 1.8%, versus a 1.2% 10-year risk if they did not receive chemotherapy (hazard ratio, 1.53; 95% CI, 1.14–2.06). Myeloid growth factors are increasingly being used as supportive medications in the treatment of many primary malignancies. Whether or not these factors have a contributory role to the development of secondary myeloid malignancies remains a topic of some debate. Hershman et al. [50] recently performed a population-based study of 5,510 women aged ≥65 years treated with chemotherapy for breast cancer. Those authors found that the use of G-CSF was associated with a risk for subsequent myelodysplastic syndrome (MDS) or AML that was double that of those who did not receive the growth factor [50]. However, Patt et al. [49] found that G-CSF use within the first year of diagnosis did not convey a greater risk for subsequent AML in older women. In addition, in a randomized trial of dose-dense chemotherapy (doxorubicin and cyclophosphamide followed by docetaxel [AC-T] or doxorubicin, docetaxel, and cyclophosphamide [ATC] given every 2 weeks with G-CSF support) in comparison with conventional chemotherapy (the same doses of AC-T or ATC given every 3 weeks without G-CSF support), there was no difference in the risk for AML or MDS after a median follow-up of 36 months [51].
Endocrine therapy with tamoxifen for breast cancer has a protective effect against contralateral breast cancer, with a 32% lower 5-year risk for patients with hormone-positive or unknown hormone status cancers, as calculated in one meta-analysis [52]. However, tamoxifen conveys a higher risk for subsequent endometrial cancer, as confirmed in the NSABP P-1 study (RR for tamoxifen versus placebo, 2.53; 95% CI, 1.35–4.97). Those authors noted that the greater risk occurred predominantly in women aged ≥50 years [24]. There has been debate over whether tamoxifen-related endometrial cancers are more or less aggressive than those unrelated to tamoxifen [8]. In a study comparing women with breast cancer who developed endometrial cancer with or without prior tamoxifen use, Saadat et al. [53] found that, although tamoxifen use was associated with more aggressive tissue subtypes, there was no difference in histologic grade or stage, and there was no difference in the endometrial cancer–related survival outcome because most cases were amenable to surgery. This finding mirrored the result in the NSABP P-1 study [24].
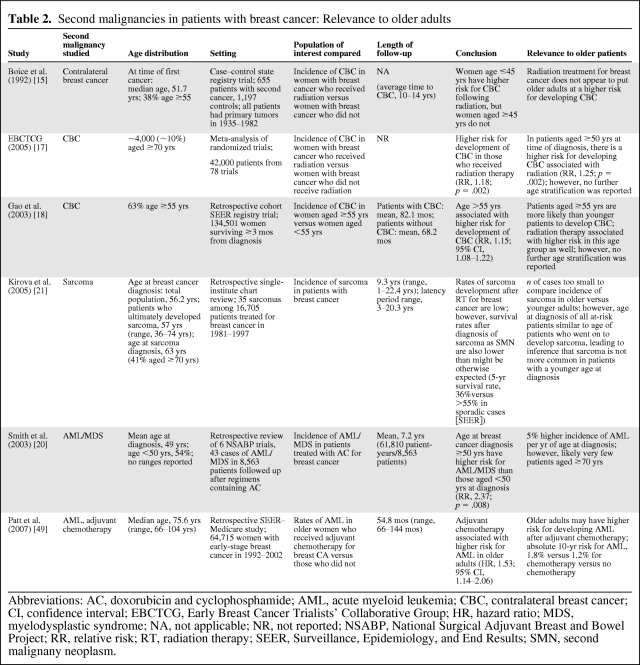
There are limitations in interpreting the relevance of the aforementioned studies to older adults because there is heterogeneity in how patient ages are reported, and multivariate analyses taking into account age and other potential risk factors are rarely possible with small sample sizes. Table 2 provides a summary of how studies evaluating second malignancies in breast cancer may be applied to older adult populations.
Table 2.
Second malignancies in patients with breast cancer: Relevance to older adults
Abbreviations: AC, doxorubicin and cyclophosphamide; AML, acute myeloid leukemia; CBC, contralateral breast cancer; CI, confidence interval; EBCTCG, Early Breast Cancer Trialists' Collaborative Group; HR, hazard ratio; MDS, myelodysplastic syndrome; NA, not applicable; NR, not reported; NSABP, National Surgical Adjuvant Breast and Bowel Project; RR, relative risk; RT, radiation therapy; SEER, Surveillance, Epidemiology, and End Results; SMN, second malignany neoplasm.
Ovarian Cancer Survivors
Most ovarian cancer patients present with relatively late stage disease, and thus the overall 5-year survival rate is only 46% [1]. However, in women who survive ≥5 years following ovarian cancer, the risk for developing a second malignancy is high, with a 31% higher risk than in the general population [54]. The cancers most often seen after ovarian cancer are breast, colorectal, and bladder cancer and leukemia [55]. Breast and colon cancer are most often seen after ovarian cancer in younger women, largely because of the prevalence of genetic syndromes, such as BRCA mutation and hereditary nonpolyposis colorectal cancer, that predispose to these tumors and ovarian cancer [27, 29, 55]. Some rare tumors have also been associated with ovarian cancer, including biliary tract cancer and both the ocular and skin variants of malignant melanoma, and are also thought to be a result of genetic syndromes such as BRCA-2 mutation [26, 54, 56].
However, an excess risk for second malignancies has been seen in women diagnosed with primary cancer of the ovary at age 50–69 years as well, and these second cancers appear more likely to be a result of treatment. In particular, the risks for bladder, soft tissue, and bone cancers are substantially higher in women who received radiation, and these risks become most apparent ≥10 years following the initial radiation therapy [54, 57].
The risk with chemotherapy in the development of second cancers is also apparent across all age groups. The risk for the subsequent development of both AML and acute lymphocytic leukemia have been shown to be significantly higher in women following a primary diagnosis of ovarian cancer, even in women aged ≥70 years at the time of their primary diagnosis and in women who did not receive radiation [54]. Historically, this greater risk was attributed to alkylating chemotherapeutic agents, such as melphalan and chlorambucil [32], but these agents have largely fallen out of use in the treatment of ovarian cancer. More recently, platinum-based chemotherapy, which is routinely used in ovarian cancer treatment, has also been associated with a higher late risk for leukemia. This was demonstrated in a large registry case–control study of women who developed leukemia after ovarian cancer. Of note, 71% of patients who developed leukemia were aged ≥60 years at the time of their ovarian cancer diagnosis, whereas 29% were aged ≥70 years [31].
Prostate Cancer Survivors
There is an elevated risk for a second cancer after pelvic irradiation for prostate cancer, but the attributable magnitude of the radiation-related risk remains a topic of debate. In a population-based study using Surveillance, Epidemiology, and End Results (SEER) data, Neugut et al. [33] found that there was a slightly, but statistically significant, higher risk for bladder cancer among prostate cancer survivors treated with radiotherapy (RR, 1.5; 95% CI, 1.1–2.0), but there was no difference in the risk for rectal cancer or hematologic malignancies. The median age at diagnosis of prostate cancer was 69.2 years in the radiation group and 73.2 years in the unradiated group [33]. Chrouser et al. [34], in a retrospective review of the Mayo Clinic Cancer Registry, did not find a higher risk for bladder cancer after radiation for prostate cancer (mean age at diagnosis of prostate cancer, 70.5 years). However, in men who received adjuvant radiation after radical prostatectomy, there was a statistically significant greater risk (RR, 5.01; 95% CI, 1.04–14.61), likely because of more of the bladder being exposed to radiation in the postoperative setting [34]. In a population-based study using SEER data, Brenner et al. [35] compared men with prostate cancer treated with radiation therapy alone with men treated using surgery alone. They found that those who received external-beam radiation had a slightly, but significantly, higher risk for solid tumors on the whole, with the excess risk largely a result of more cases of bladder, rectum, lung, and connective tissue cancers. The men studied had a mean age of 71 years at the time of diagnosis of prostate cancer, and a mean age of 75.3–77.0 years at the time of diagnosis of the second malignancy [35]. Moon et al. [58], using a more recent SEER database search, found that there was a significantly higher risk for developing second cancers at several sites following external-beam radiation for prostate cancer, including the bladder, rectum, colon, brain, and lung. The greater risk at sites distant from the radiation field was thought to be a result of radiation scatter. However, the risk difference was small, with odds ratios of 1.25 for lung to 1.85 for colon. Age at diagnosis was not associated with any difference in the odds ratio on multivariate analysis. Additionally, men who received radiation in the form of radioactive isotopes or implants did not have a higher risk for second cancers [58].
Colorectal Cancer Survivors
In a large SEER study, it was found that adults diagnosed with colon cancer at age ≥70 years did not have a higher risk for developing new malignancies as a whole when compared with an age-matched general population [38]. This is in contrast to younger adults, who had higher absolute risks for second malignancies that were higher the younger the age at diagnosis. On the other hand, adults aged >70 years did have a significantly higher risk than the age-matched general population of developing cancer of the small intestine (observed/expected ratio [O/E], 2.42) or rectum (O/E, 1.2) or a new colon primary (O/E, 1.33) [38]. Adults diagnosed with colon cancer at age 60–69 years additionally had elevated risks for developing new cancers of the esophagus, stomach, kidney, and oropharynx [38]. The higher risk for digestive system cancer following colon cancer is not likely a result of radiation treatment because colon cancer is rarely treated with radiation. The most likely cause for the higher risk for digestive cancer is shared risk factors, such as obesity, diet, and low activity levels [39].
Rectal cancer was also not associated with higher rates of most second malignancies in older adults in the largest SEER study done to date. Although adults diagnosed with rectal cancer at age 50–69 years had higher risks for lung, kidney, and digestive system cancers, adults aged >70 years only had a higher risk for colon cancer, and this excess risk was lower in magnitude than in younger adults (O/E, 1.58 versus 1.29 for age 50–69 years versus age >70 years) [38]. However, patients with rectal cancer who received radiation had higher risks for bladder, uterine, and lung cancers, particularly ≥10 years after the initial cancer diagnosis [38]. Studies using other cancer registries have shown greater risks for prostate cancer following a diagnosis of rectal cancer as well [59, 60]. Radiation is a less likely cause because the effect was seen prior to the routine adoption of radiation for rectal cancer. The higher risk is rather thought to be a result of greater cancer surveillance, causing discovery of cancers that might otherwise have remained occult [60].
Survivors of HL
In a retrospective analysis, second malignancies were the most common cause of death by 15 years after treatment in long-term survivors of HL [61]. At a median of 12 years, survivors of HL had a 4.6-fold higher risk for developing a new cancer than in the general population, and an excess risk of 1% per year [8]. Although HL is most common in younger adults, it is often overlooked that there is a bimodal age distribution, with a peak incidence again emerging at ages >55 years. A review of 1,319 consecutive patients with HL showed that patients aged <20 years at diagnosis had an RR of 10.7 for developing a second cancer (95% CI, 7.8–14.4), compared with those aged ≥50 years at diagnosis, who only had an RR of 2.4 (95% CI, 1.6–3.4) [62]. Adults diagnosed with HL at age >60 years have a higher risk for developing a second malignancy than the age-matched general population, particularly, lung cancer and leukemia [42].
In all age groups, solid tumors account for ∼75% of second malignancies following HL [62]. In one large registry case–control study of patients treated with alkylating agents for HL, there was a higher risk for lung cancer even from chemotherapy alone, with an RR of 4.2 (95% CI, 2.1–8.8). Of note, 51% of patients who went on to develop lung cancer were aged >50 years at the time of the HL diagnosis, with 20% aged >60 years. Patients aged ≥55 years at diagnosis, in particular, had a stronger association of lung cancer with alkylating chemotherapy as well as radiation (RR for alkylating agents, 6.5; 95% CI, 2.3–22; RR for radiation ≥5 Gy, 10.2; 95% CI, 2.9–43) [43].
Radiation-associated solid tumors after HL are well studied. These tend to develop 5–9 years after initial exposure, with a higher risk persisting for ≥30 years [45]. Tumors usually develop within or around the edges of prior radiation fields [8]. There is a significant radiation dose–response relationship in the development of lung and breast cancer after radiation for HL [43, 44].
Development of breast cancer is relatively common after chest or mediastinal radiation therapy. The risk in young female survivors of HL also persists into older age. In a study on subsequent malignancies after childhood HL (median age at diagnosis, 11 years), the 30-year cumulative incidence of female breast cancer was 16.9% (95% CI, 9.4%–24.5%), which represented a >50-fold higher risk over the age-matched general population [63]. Other studies have demonstrated an absolute 20- to 25-year risk of 4.2%–34% [64]. However, the risk for breast cancer decreases with increasing age at the time of radiation, because of age-related lesser susceptibility of tissues to the effects of radiation. Women diagnosed with HL at age >50 years have much lower rates of second breast or thyroid cancer than younger survivors of HL [7].
Alkylating agents tend to decrease the risk for subsequent breast neoplasms after HL. In a large, international, case–control study, patients who received both chemotherapy and radiation therapy had only about half the risk for later developing breast cancer of those who received radiation alone [44]. The lower risk is likely associated with the high frequency of premature menopause after receiving alkylating agents, implying that ovarian hormones play a role in breast tumorigenesis [44].
Hematologic malignancies such as MDS and AML occur in survivors of HL in particular. One study of 1,939 patients with a history of HL showed a 20-year risk for the development of MDS or AML of 6% in a predominantly younger cohort (11.6% of patients aged ≥60 years) [41]. This high risk is likely primarily a result of the high cumulative doses of alkylating agents used in most regimens until recently [41]. Although not demonstrated as such in HL patients, data from breast cancer suggest that the risk for AML or MDS increases with age at initial treatment with alkylating agents, which could make the risk seen in HL even higher in patients diagnosed at older ages. With the replacement of alkylating regimens by other regimens, however, the leukemogenic risk associated with HL treatment has been substantially reduced, though the effect on risk in older adults has not been well studied [65].
Survivors of non-HL
The effect of radiation on second malignancies in non-HL (NHL) is not as well defined as in HL. In one population-based cohort study using data from the SEER database, there was no difference in the overall risk for second malignancies between patients who received radiation and those who did not; however, there was a higher risk for sarcoma, breast cancer, and mesothelioma in patients who received radiation. The average age at NHL diagnosis was 61 years and the average follow-up was 60 months. Patients who developed second malignancies tended to be older, with a mean age at NHL diagnosis of 65 years and an average age at second tumor diagnosis of 70 years. The authors noted that the patients who did not receive radiation had a lower risk for developing breast cancer than the general population, possibly because of the protective effects of alkylating chemotherapy, whereas those who were irradiated approached the endemic rate [66].
Greater risks for specific solid tumors after chemotherapy alone for NHL have been reported. In one case–control study, patients with NHL treated with cyclophosphamide had a 4.5-fold higher risk for developing bladder and kidney cancer than those who did not receive the drug, and the risk was dependent on the cumulative dose [30]. In a retrospective cohort study conducted by the British Collaborative Group, 1,274 patients treated with chemotherapy alone (usually with cyclophosphamide, doxorubicin, vincristine, and prednisone, the CHOP regimen) had greater risks for lung cancer (RR, 1.9; 95% CI, 1.1–3.3) and colorectal cancer (RR, 2.1; 95% CI, 1.1–3.6) over the median follow-up of 5 years [67]. A large European prospective cohort trial followed patients who received doxorubicin, cyclophosphamide, vindesine, bleomycin, and prednisone (the ACVBP regimen) for NHL for a median of 72 months. The RR for lung cancer was significantly greater than the age-matched general population for men (RR, 2.45; 95% CI, 1.48–3.83) but not for women [68]. However, both the European and British trials did not show a higher risk for all solid tumors taken as a whole [67, 68].
The risk for hematologic malignancies following chemotherapy for NHL remains higher than that of the general population, especially when the regimen includes an alkylating agent. The European trial above showed an RR for MDS or AML after ACVBP over the 72 month follow-up of 5.65 (95% CI, 1.54–14.5) in men, and among women, it was even higher at 19.9 (95% CI, 7.98–41.0) [68]. In the British study, the RR for leukemia was 10.5 (95% CI, 5.0–19.3) after chemotherapy alone, compared with estimated rates in the general population [67].
Screening for Second Malignancies in Older Adults
A number of screening recommendations exist for the development of second malignancies after a wide variety of cancers commonly seen in older adults. The most comprehensive guidelines for screening exist among cancer survivors with a higher risk for developing breast cancer. Additional screening recommendations exist for survivors of cancers of the breast, prostate, colon, and lung and HL, but these guidelines remain incomplete, are generally not based on consistent evidence, and do not take age into account [13].
The National Comprehensive Cancer Network (NCCN) recommends that any woman who has had thoracic irradiation for any malignancy undergo annual mammography and biannual to annual clinical breast exams, and suggests annual breast magnetic resonance imaging as an adjunct to these tests [69]. Additionally, any woman with a history of breast cancer is recommended to undergo a history and physical exam every 3–6 months for 5 years, and then annually, accompanied by an annual mammogram. It is recommended that any woman on tamoxifen therapy undergo a yearly gynecologic exam [70]. The American Society of Clinical Oncology (ASCO) echoes the NCCN in the need for regular breast exams and mammography. ASCO guidelines recommend that all women with a history of breast cancer undergo annual gynecologic examinations and women taking tamoxifen are recommended to report any vaginal bleeding to their gynecologist. ASCO guidelines do not recommend routine blood tests or other forms of imaging as part of routine monitoring following breast cancer [71].
The NCCN additionally has specific guidelines for screening following HL. The NCCN recommends a yearly complete blood count, yearly thyroid function tests if patients received neck irradiation, yearly chest imaging (x-ray or computed tomography scan) for patients at risk for lung cancer, and yearly breast exams as above [72]. For survivors of colorectal cancer, the NCCN recommends colonoscopy at 1 year and then age-appropriate screening for breast, cervical, and prostate cancer, with no changes particular to colon cancer survivors [73]. While the NCCN does not give recommendations regarding surveillance for second cancers following prostate cancer, some authors recommend that all patients receiving prostate irradiation undergo endoscopic evaluation for rectal cancer starting 5 years later [74].
Notably, age and frailty are not taken into account in the above recommendations. Hence, we propose a rational approach to determine whether or not there exists a benefit to screening for a given patient following any particular malignancy. Walter and Covinsky propose a guide for primary cancer screening in older adults that takes into account life expectancy, the risk for a cancer death, and the benefits of early detection using screening. The authors suggest in-depth use of life-expectancy tables, including physician assessment of the relative health of the patient, in the determination of life expectancy. For example, 25% of 75-year-old women will live >17 additional years, 50% will live at least 11.9 years, and 25% will live <6.8 years. By assigning patients to quartiles using functional status, comorbidities, frailty, and other factors, a physician could better predict life expectancy and hence better predict whether or not a patient is likely to benefit from screening [75]. Although developed for primary screening in older adults, this guide may be applied to screening for second cancers as well, though the ability to quantify the benefits of screening may be less well established and thus more difficult for the clinician to estimate. We suggest that, in screening for second cancers in older adults, clinicians attempt to quantify each of the following for the second malignancy for which the patient is at risk: (a) the risk for developing the cancer, (b) the risk for death from the second cancer, (c) the likely benefit of screening to the risk for death from the cancer, (d) the life expectancy of the patient based on quartile assignment, and (e) the values and preferences of the individual patient.
Regardless, it remains critical that primary care physicians and geriatricians have as much specific cancer history and cancer treatment history as possible when caring for older adults with a history of cancer and determining screening and prevention strategies because they may be more involved in the patient's daily care than the treating oncologist.
Conclusions
The risks for both hematologic and solid malignancies are significantly greater following a wide variety of primary tumors even among older adults. The higher risk may be a result of a genetic predisposition to multiple cancers or shared risk factors such as tobacco, or be related to the treatment of the original cancer. Radiation therapy seems to confer higher risks for both solid and hematologic malignancies after many primary cancers, particularly in organs near and around the original radiation field, though the risks appear to be lower in patients radiated at older ages. Chemotherapy may confer a higher future risk for leukemia, particularly when alkylating agents are used, though platinum agents have also been implicated, and these risks may be higher in older adults. The combination of radiation and chemotherapy often increases the risk for solid tumors more than either modality alone, with the notable exception that alkylating agents actually decrease the risk for the future development of breast cancer likely because of their ovarian suppressive function. The association of myeloid growth factors with future hematologic malignancies remains a topic of dispute; however, there is no definitive evidence of an association from randomized trials. Tamoxifen, even while decreasing the risk for contralateral breast cancer, increases the risk for subsequent endometrial cancer. Screening recommendations for second malignancies exist following some cancers, but remain incomplete and are not specific for older adults. Further evidence-based guidelines are needed for early detection and treatment of second malignancies in older adults, and physicians caring for this population should integrate age, health status, projected life expectancy, and patient preferences when deciding upon screening and prevention measures.
Acknowledgments
The authors would like to thank Smita Bhatia, M.D., M.P.H., for her guidance and help in the review of earlier versions of the manuscript.
Footnotes
- (C/A)
- consulting/advisory relationship
- (RF)
- Research funding
- (E)
- Employment
- (H)
- Honoraria received
- (OI)
- Ownership interests
- (IP)
- Intellectual property rights/inventor/patent holder
Author Contributions
Conception/Design: Ari M. VanderWalde, Arti Hurria
Provision of study material or patients: Arti Hurria
Collection and/or assembly of data: Ari M. VanderWalde, Arti Hurria
Data analysis and interpretation: Ari M. VanderWalde, Arti Hurria
Manuscript writing: Ari M. VanderWalde, Arti Hurria
Final approval of manuscript: Ari M. VanderWalde, Arti Hurria
References
- 1.Jemal A, Siegel R, Xu J, et al. Cancer statistics, 2010. CA Cancer J Clin. 2010;60:277–300. doi: 10.3322/caac.20073. [DOI] [PubMed] [Google Scholar]
- 2.Edwards BK, Howe HL, Ries LA, et al. Annual report to the nation on the status of cancer, 1973–1999, featuring implications of age and aging on U.S. cancer burden. Cancer. 2002;94:2766–2792. doi: 10.1002/cncr.10593. [DOI] [PubMed] [Google Scholar]
- 3.Ries LAG, Melbert D, Krapcho M, et al., editors. SEER Cancer Statistics Review 1975–2005. Bethesda, MD: National Cancer Institute; 2008. [accessed September 9, 2008]. p. 31. Based on November 2007 SEER data submission, posted to the SEER Web site, 2008. Available at http://seer.cancer.gov/csr/1975_2005. [Google Scholar]
- 4.Dong C, Hemminki K. Second primary neoplasms in 633,964 cancer patients in Sweden, 1958–1996. Int J Cancer. 2001;93:155–161. doi: 10.1002/ijc.1317. [DOI] [PubMed] [Google Scholar]
- 5.Travis LB. The epidemiology of second primary cancers. Cancer Epidemiol Biomarkers Prev. 2006;15:2020–2026. doi: 10.1158/1055-9965.EPI-06-0414. [DOI] [PubMed] [Google Scholar]
- 6.Ghelani D, Saliba R, Lima M. Secondary malignancies after hematopoietic stem cell transplantation. Crit Rev Oncol Hematol. 2005;56:115–126. doi: 10.1016/j.critrevonc.2005.03.014. [DOI] [PubMed] [Google Scholar]
- 7.Allan JM, Travis LB. Mechanisms of therapy-related carcinogenesis. Nat Rev Cancer. 2005;5:943–955. doi: 10.1038/nrc1749. [DOI] [PubMed] [Google Scholar]
- 8.Ng AV, Travis LB. Second primary cancers: An overview. Hematol Oncol Clin North Am. 2008;22:271–289. doi: 10.1016/j.hoc.2008.01.007. [DOI] [PubMed] [Google Scholar]
- 9.Landier W, Bhatia S. Cancer survivorship: A pediatric perspective. The Oncologist. 2008;13:1181–1192. doi: 10.1634/theoncologist.2008-0104. [DOI] [PubMed] [Google Scholar]
- 10.Fraumeni JF, Jr, Curtis RE, Edwards BK, et al. Chapter 1: Introduction. In: Curtis R, Freedman D, Ron E, et al., editors. New Malignancies Among Cancer Survivors: SEER Cancer Registries, 1973–2000. Bethesda, MD: National Cancer Institute; 2006. pp. 1–9. NIH Publ. No. 05–5302. [Google Scholar]
- 11.Mauch PM, Kalish LA, Marcus KC, et al. Long-term survival in Hodgkin's disease relative impact of mortality, second tumors, infection, and cardiovascular disease. Cancer J Sci Am. 1995;1:33–42. [PubMed] [Google Scholar]
- 12.Travis LB, Rabkin CS, Brown LM, et al. Cancer survivorship—genetic susceptibility and second primary cancers: Research strategies and recommendations. J Natl Cancer Inst. 2006;98:15–25. doi: 10.1093/jnci/djj001. [DOI] [PubMed] [Google Scholar]
- 13.Hewitt M, Greenfield S, Stovall G, editors. Washington DC: The National Academies Press; 2006. From Cancer Patient to Cancer Survivor: Lost in Transition. National Cancer Policy Board Institute of Medicine and National Research Council of the National Archives; pp. 23–65. [Google Scholar]
- 14.Curtis R, Freedman D, Ron E, et al., editors. NIH Publ. No. 05–5302. Bethesda, MD: National Cancer Institute; 2006. New Malignancies Among Cancer Survivors: SEER Cancer Registries, 1973–2000; pp. 1–482. [Google Scholar]
- 15.Boice JD, Jr, Harvey EB, Blettner M, et al. Cancer in the contralateral breast after radiotherapy for breast cancer. N Engl J Med. 1992;326:781–785. doi: 10.1056/NEJM199203193261201. [DOI] [PubMed] [Google Scholar]
- 16.Storm HH, Andersson M, Boice JD, Jr, et al. Adjuvant radiotherapy and risk of contralateral breast cancer. J Natl Cancer Inst. 1992;84:1245–1250. doi: 10.1093/jnci/84.16.1245. [DOI] [PubMed] [Google Scholar]
- 17.Early Breast Cancer Trialists' Collaborative Group (EBCTCG) Effects of radiotherapy and of differences in the extent of surgery for early breast cancer on local recurrence and 15-year survival: An overview of the randomised trials. Lancet. 2005;366:2087–2106. doi: 10.1016/S0140-6736(05)67887-7. [DOI] [PubMed] [Google Scholar]
- 18.Gao X, Fisher SG, Emami B. Risk of second primary cancer in the contralateral breast in women treated for early-stage breast cancer: A population-based study. Int J Radiat Oncol Biol Phys. 2003;56:1038–1045. doi: 10.1016/s0360-3016(03)00203-7. [DOI] [PubMed] [Google Scholar]
- 19.Curtis RE, Boice JD, Jr, Stovall M, et al. Risk of leukemia after chemotherapy and radiation treatment for breast cancer. N Engl J Med. 1992;326:1745–1751. doi: 10.1056/NEJM199206253262605. [DOI] [PubMed] [Google Scholar]
- 20.Smith RE, Bryant J, DeCillis A, et al. Acute myeloid leukemia and myelodysplastic syndrome after doxorubicin-cyclophosphamide adjuvant therapy for operable breast cancer: The National Surgical Adjuvant Breast and Bowel Project Experience. J Clin Oncol. 2003;21:1195–1204. doi: 10.1200/JCO.2003.03.114. [DOI] [PubMed] [Google Scholar]
- 21.Kirova YM, Vilcoq JR, Asselain B, et al. Radiation-induced sarcomas after radiotherapy for breast carcinoma: A large-scale single-institution review. Cancer. 2005;104:856–863. doi: 10.1002/cncr.21223. [DOI] [PubMed] [Google Scholar]
- 22.Rubino C, de Vathaire F, Shamsaldin A, et al. Radiation dose, chemotherapy, hormonal treatment and risk of second cancer after breast cancer treatment. Br J Cancer. 2003;89:840–846. doi: 10.1038/sj.bjc.6601138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Roychoudhuri R, Evans H, Robinson D, et al. Radiation-induced malignancies following radiotherapy for breast cancer. Br J Cancer. 2004;91:868–872. doi: 10.1038/sj.bjc.6602084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Fisher B, Costantino JP, Wickerham DL, et al. Tamoxifen for prevention of breast cancer: Report of the National Surgical Adjuvant Breast and Bowel Project P-1 Study. J Natl Cancer Inst. 1998;90:1371–1388. doi: 10.1093/jnci/90.18.1371. [DOI] [PubMed] [Google Scholar]
- 25.Schaapveld M, Visser O, Louwman MJ, et al. Risk of new primary nonbreast cancers after breast cancer treatment: A Dutch population-based study. J Clin Oncol. 2008;26:1239–1246. doi: 10.1200/JCO.2007.11.9081. [DOI] [PubMed] [Google Scholar]
- 26.The Breast Cancer Linkage Consortium. Cancer risks in BRCA2 mutation carriers. J Natl Cancer Inst. 1999;91:1310–1316. doi: 10.1093/jnci/91.15.1310. [DOI] [PubMed] [Google Scholar]
- 27.Li AJ, Karlan BY. Genetic factors in ovarian carcinoma. Curr Oncol Rep. 2001;3:27–32. doi: 10.1007/s11912-001-0039-y. [DOI] [PubMed] [Google Scholar]
- 28.Lynch HT, Lanspa S, Smyrk T, et al. Hereditary nonpolyposis colorectal cancer (Lynch syndromes I & II). Genetics, pathology, natural history, and cancer control, Part I. Cancer Genet Cytogenet. 1991;53:143–160. doi: 10.1016/0165-4608(91)90093-a. [DOI] [PubMed] [Google Scholar]
- 29.Weinberg DS, Newschaffer CJ, Topham A. Risk for colorectal cancer after gynecologic cancer. Ann Intern Med. 1999;131:189–193. doi: 10.7326/0003-4819-131-3-199908030-00005. [DOI] [PubMed] [Google Scholar]
- 30.Travis LB, Curtis RE, Glimelius B, et al. Bladder and kidney cancer following cyclophosphamide therapy for non-Hodgkin's lymphoma. J Natl Cancer Inst. 1995;87:524–530. doi: 10.1093/jnci/87.7.524. [DOI] [PubMed] [Google Scholar]
- 31.Travis LB, Holowaty EJ, Bergfeldt K, et al. Risk of leukemia after platinum-based chemotherapy for ovarian cancer. N Engl J Med. 1999;340:351–357. doi: 10.1056/NEJM199902043400504. [DOI] [PubMed] [Google Scholar]
- 32.Kaldor JM, Day NE, Pettersson F, et al. Leukemia following chemotherapy for ovarian cancer. N Engl J Med. 1990;322:1–6. doi: 10.1056/NEJM199001043220101. [DOI] [PubMed] [Google Scholar]
- 33.Neugut AI, Ahsan H, Robinson E, et al. Bladder carcinoma and other second malignancies after radiotherapy for prostate carcinoma. Cancer. 1997;79:1600–1604. doi: 10.1002/(sici)1097-0142(19970415)79:8<1600::aid-cncr24>3.0.co;2-0. [DOI] [PubMed] [Google Scholar]
- 34.Chrouser K, Leibovich B, Bergstralh E, et al. Bladder cancer risk following primary and adjuvant external beam radiation for prostate cancer. J Urol. 2005;174:107–110. doi: 10.1097/01.ju.0000163459.57305.a1. discussion 110–111. [DOI] [PubMed] [Google Scholar]
- 35.Brenner DJ, Curtis RE, Hall EJ, et al. Second malignancies in prostate carcinoma patients after radiotherapy compared with surgery. Cancer. 2000;88:398–406. doi: 10.1002/(sici)1097-0142(20000115)88:2<398::aid-cncr22>3.0.co;2-v. [DOI] [PubMed] [Google Scholar]
- 36.Scélo G, Boffetta P, Hemminki K, et al. Associations between small intestine cancer and other primary cancers: An international population-based study. Int J Cancer. 2006;118:189–196. doi: 10.1002/ijc.21284. [DOI] [PubMed] [Google Scholar]
- 37.Ronckers CM, McCarron P, Ron E. Thyroid cancer and multiple primary tumors in the SEER cancer registries. Int J Cancer. 2005;117:281–288. doi: 10.1002/ijc.21064. [DOI] [PubMed] [Google Scholar]
- 38.Mysliwiec PA, Cronin KA, Schatzkin A. Chapter 5: New malignancies following cancer of the colon, rectum, and anus. In: Curtis R, Freedman D, Ron E, et al., editors. New Malignancies Among Cancer Survivors: SEER Cancer Registries, 1973–2000. NIH Publ No 05–5302. Bethesda, MD: National Cancer Institute; 2006. pp. 111–144. [Google Scholar]
- 39.Giovannucci E. Modifiable risk factors for colon cancer. Gastroenterol Clin North Am. 2002;31:925–943. doi: 10.1016/s0889-8553(02)00057-2. [DOI] [PubMed] [Google Scholar]
- 40.Kendal WS, Nicholas G. A population-based analysis of second primary cancers after irradiation for rectal cancer. Am J Clin Oncol. 2007;30:333–339. doi: 10.1097/01.coc.0000258084.55036.9e. [DOI] [PubMed] [Google Scholar]
- 41.van Leeuwen FE, Klokman WJ, Hagenbeek A, et al. Second cancer risk following Hodgkin's disease: A 20-year follow-up study. J Clin Oncol. 1994;12:312–325. doi: 10.1200/JCO.1994.12.2.312. [DOI] [PubMed] [Google Scholar]
- 42.Dores GM, Coté TR, Travis LB. Chapter 16: New malignancies following Hodgkin lymphoma, non-Hodgkin lymphoma, and myeloma. In: Curtis R, Freedman D, Ron E, et al., editors. New Malignancies Among Cancer Survivors: SEER Cancer Registries, 1973–2000. NIH Publ No 05–5302. Bethesda, MD: National Cancer Institute; 2006. pp. 397–436. [Google Scholar]
- 43.Travis LB, Gospodarowicz M, Curtis RE, et al. Lung cancer following chemotherapy and radiotherapy for Hodgkin's disease. J Natl Cancer Inst. 2002;94:182–192. doi: 10.1093/jnci/94.3.182. [DOI] [PubMed] [Google Scholar]
- 44.Travis LB, Hill DA, Dores GM, et al. Breast cancer following radiotherapy and chemotherapy among young women with Hodgkin disease. JAMA. 2003;290:465–475. doi: 10.1001/jama.290.4.465. [DOI] [PubMed] [Google Scholar]
- 45.Hodgson DC, Gilbert ES, Dores GM, et al. Long-term solid cancer risk among 5-year survivors of Hodgkin's lymphoma. J Clin Oncol. 2007;25:1489–1497. doi: 10.1200/JCO.2006.09.0936. [DOI] [PubMed] [Google Scholar]
- 46.Rund D, Krichevsky S, Bar-Cohen S, et al. Therapy-related leukemia: Clinical characteristics and analysis of new molecular risk factors in 96 adult patients. Leukemia. 2005;19:1919–1928. doi: 10.1038/sj.leu.2403947. [DOI] [PubMed] [Google Scholar]
- 47.Howlader N, Noone A, Krapcho M, et al., editors. SEER Cancer Statistics Review. Bethesda, MD: National Cancer Institute; 1975–2008. [accessed August 23, 2011]. Based on November 2010 SEER data submission, posted to the SEER Web site, 2011. Available at http://seer.cancer.gov/csr/1975_2008. [Google Scholar]
- 48.Deutsch M, Land SR, Begovic M, et al. The incidence of lung carcinoma after surgery for breast carcinoma with and without postoperative radiotherapy. Results of National Surgical Adjuvant Breast and Bowel Project (NSABP) clinical trials B-04 and B-06. Cancer. 2003;98:1362–1368. doi: 10.1002/cncr.11655. [DOI] [PubMed] [Google Scholar]
- 49.Patt DA, Duan Z, Fang S, et al. Acute myeloid leukemia after adjuvant breast cancer therapy in older women: Understanding risk. J Clin Oncol. 2007;25:3871–3876. doi: 10.1200/JCO.2007.12.0832. [DOI] [PubMed] [Google Scholar]
- 50.Hershman D, Neugut AI, Jacobson JS, et al. Acute myeloid leukemia or myelodysplastic syndrome following use of granulocyte colony-stimulating factors during breast cancer adjuvant chemotherapy. J Natl Cancer Inst. 2007;99:196–205. doi: 10.1093/jnci/djk028. [DOI] [PubMed] [Google Scholar]
- 51.Citron ML, Berry DA, Cirrincione C, et al. Randomized trial of dose-dense versus conventionally scheduled and sequential versus concurrent combination chemotherapy as postoperative adjuvant treatment of node-positive primary breast cancer: First report of Intergroup Trial C9741/Cancer and Leukemia Group B Trial 9741. J Clin Oncol. 2003;21:1431–1439. doi: 10.1200/JCO.2003.09.081. [DOI] [PubMed] [Google Scholar]
- 52.Early Breast Cancer Trialists' Collaborative Group (EBCTCG) Effects of chemotherapy and hormonal therapy for early breast cancer on recurrence and 15-year survival: An overview of the randomised trials. Lancet. 2005;365:1687–1717. doi: 10.1016/S0140-6736(05)66544-0. [DOI] [PubMed] [Google Scholar]
- 53.Saadat M, Truong PT, Kader HA, et al. Outcomes in patients with primary breast cancer and a subsequent diagnosis of endometrial cancer: Comparison of cohorts treated with and without tamoxifen. Cancer. 2008;110:31–37. doi: 10.1002/cncr.22734. [DOI] [PubMed] [Google Scholar]
- 54.Freedman DM, Curtis RE, Travis LB, et al. Chapter 9: New malignancies following cancer of the uterine corpus or ovary. In: Curtis R, Freedman D, Ron E, et al., editors. New Malignancies Among Cancer Survivors: SEER Cancer Registries, 1973–2000. NIH Publ No 05–5302. Bethesda, MD: National Cancer Institute; 2006. pp. 231–256. [Google Scholar]
- 55.Travis LB, Curtis RE, Boice JD, Jr, et al. Second malignant neoplasms among long-term survivors of ovarian cancer. Cancer Res. 1996;56:1564–1570. [PubMed] [Google Scholar]
- 56.Houlston RS, Damato BE. Genetic predisposition to ocular melanoma. Eye (Lond) 1999;13:43–46. doi: 10.1038/eye.1999.9. [DOI] [PubMed] [Google Scholar]
- 57.Kaldor JM, Day NE, Kittelmann B, et al. Bladder tumours following chemotherapy and radiotherapy for ovarian cancer: A case-control study. Int J Cancer. 1995;63:1–6. doi: 10.1002/ijc.2910630102. [DOI] [PubMed] [Google Scholar]
- 58.Moon K, Stukenborg GJ, Keim J, et al. Cancer incidence after localized therapy for prostate cancer. Cancer. 2006;107:991–998. doi: 10.1002/cncr.22083. [DOI] [PubMed] [Google Scholar]
- 59.McCredie M, Macfarlane GJ, Bell J, et al. Second primary cancers after cancers of the colon and rectum in New South Wales, Australia, 1972–1991. Cancer Epidemiol Biomarkers Prev. 1997;6:155–160. [PubMed] [Google Scholar]
- 60.Hoar SK, Wilson J, Blot WJ, et al. Second cancer following cancer of the digestive system in Connecticut, 1935–82. Natl Cancer Inst Monogr. 1985;68:49–82. [PubMed] [Google Scholar]
- 61.Hoppe RT. Hodgkin's disease: Complications of therapy and excess mortality. Ann Oncol. 1997;8(suppl 1):115–118. [PubMed] [Google Scholar]
- 62.Ng AK, Bernardo MV, Weller E, et al. Second malignancy after Hodgkin disease treated with radiation therapy with or without chemotherapy: Long-term risks and risk factors. Blood. 2002;100:1989–1996. doi: 10.1182/blood-2002-02-0634. [DOI] [PubMed] [Google Scholar]
- 63.Bhatia S, Yasui Y, Robison LL, et al. High risk of subsequent neoplasms continues with extended follow-up of childhood Hodgkin's disease: Report from the Late Effects Study Group. J Clin Oncol. 2003;21:4386–4394. doi: 10.1200/JCO.2003.11.059. [DOI] [PubMed] [Google Scholar]
- 64.Travis LB, Fossa SD, Schonfeld SJ, et al. Second cancers among 40,576 testicular cancer patients: Focus on long-term survivors. J Natl Cancer Inst. 2005;97:1354–1365. doi: 10.1093/jnci/dji278. [DOI] [PubMed] [Google Scholar]
- 65.Brusamolino E, Baio A, Orlandi E, et al. Long-term events in adult patients with clinical stage IA-IIA nonbulky Hodgkin's lymphoma treated with four cycles of doxorubicin, bleomycin, vinblastine, and dacarbazine and adjuvant radiotherapy: A single-institution 15-year follow-up. Clin Cancer Res. 2006;12:6487–6493. doi: 10.1158/1078-0432.CCR-06-1420. [DOI] [PubMed] [Google Scholar]
- 66.Tward JD, Wendland MM, Shrieve DC, et al. The risk of secondary malignancies over 30 years after the treatment of non-Hodgkin lymphoma. Cancer. 2006;107:108–115. doi: 10.1002/cncr.21971. [DOI] [PubMed] [Google Scholar]
- 67.Mudie NY, Swerdlow AJ, Higgins CD, et al. Risk of second malignancy after non-Hodgkin's lymphoma: A British Cohort Study. J Clin Oncol. 2006;24:1568–1574. doi: 10.1200/JCO.2005.04.2200. [DOI] [PubMed] [Google Scholar]
- 68.André M, Mounier N, Leleu X, et al. Second cancers and late toxicities after treatment of aggressive non-Hodgkin lymphoma with the ACVBP regimen: A GELA cohort study on 2837 patients. Blood. 2004;103:1222–1228. doi: 10.1182/blood-2003-04-1124. [DOI] [PubMed] [Google Scholar]
- 69.National Comprehensive Cancer Network (NCCN) Clinical Practice Guidelines in Oncology. Breast Cancer Screening and Diagnosis, Version. [accessed April 12, 2011]. Available at http://www.nccn.org/professionals/physician_gls/pdf/breast-screening.pdf.
- 70.National Comprehensive Cancer Network (NCCN) Clinical Practice Guidelines in Oncology. Breast Cancer, Version 2.2011. [accessed April 12, 2011]. Available at http://www.nccn.org/professionals/physician_gls/pdf/breast.pdf.
- 71.Khatcheressian JL, Wolff AC, Smith TJ, et al. American Society of Clinical Oncology 2006 update of the breast cancer follow-up and management guidelines in the adjuvant setting. J Clin Oncol. 2006;24:5091–5097. doi: 10.1200/JCO.2006.08.8575. [DOI] [PubMed] [Google Scholar]
- 72.National Comprehensive Cancer Network (NCCN) Clinical Practice Guidelines in Oncology. Hodgkin Lymphoma, Version 2.2011. [accessed May 6, 2011]. Available at http://www.nccn.org/professionals/physician_gls/pdf/hodgkins.pdf. [DOI] [PubMed]
- 73.National Comprehensive Cancer Network (NCCN) Clinical Practice Guidelines in Oncology. Colon Cancer, Version 3.2011. [accessed May 6, 2011.)]. Available at http://www.nccn.org/professionals/physician_gls/pdf/colon.pdf.
- 74.Grady WM, Russell K. Ionizing radiation and rectal cancer: Victims of our own success. Gastroenterology. 2005;128:1114–1117. doi: 10.1053/j.gastro.2005.02.036. [DOI] [PubMed] [Google Scholar]
- 75.Walter LC, Covinsky KE. Cancer screening in elderly patients: A framework for individualized decision making. JAMA. 2001:2750–2756. doi: 10.1001/jama.285.21.2750. [DOI] [PubMed] [Google Scholar]